Development of a Bladder Cancer-on-a-Chip Model to Assess Bladder Cancer Cell Invasiveness
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture
2.2. Design and Fabrication of the Chips
2.3. Introduction of the Cells and the Chip Setup
2.4. Assessment of Bladder Cancer Cell Invasion
2.5. Use of Adenovirus-GFP and a Quantum Dot-Based Tracker to Support the Cell Visualization
2.6. Immunofluorescence Analyses, Antibodies
2.7. ATN-161 Treatments
2.8. Statistical Analysis
3. Results
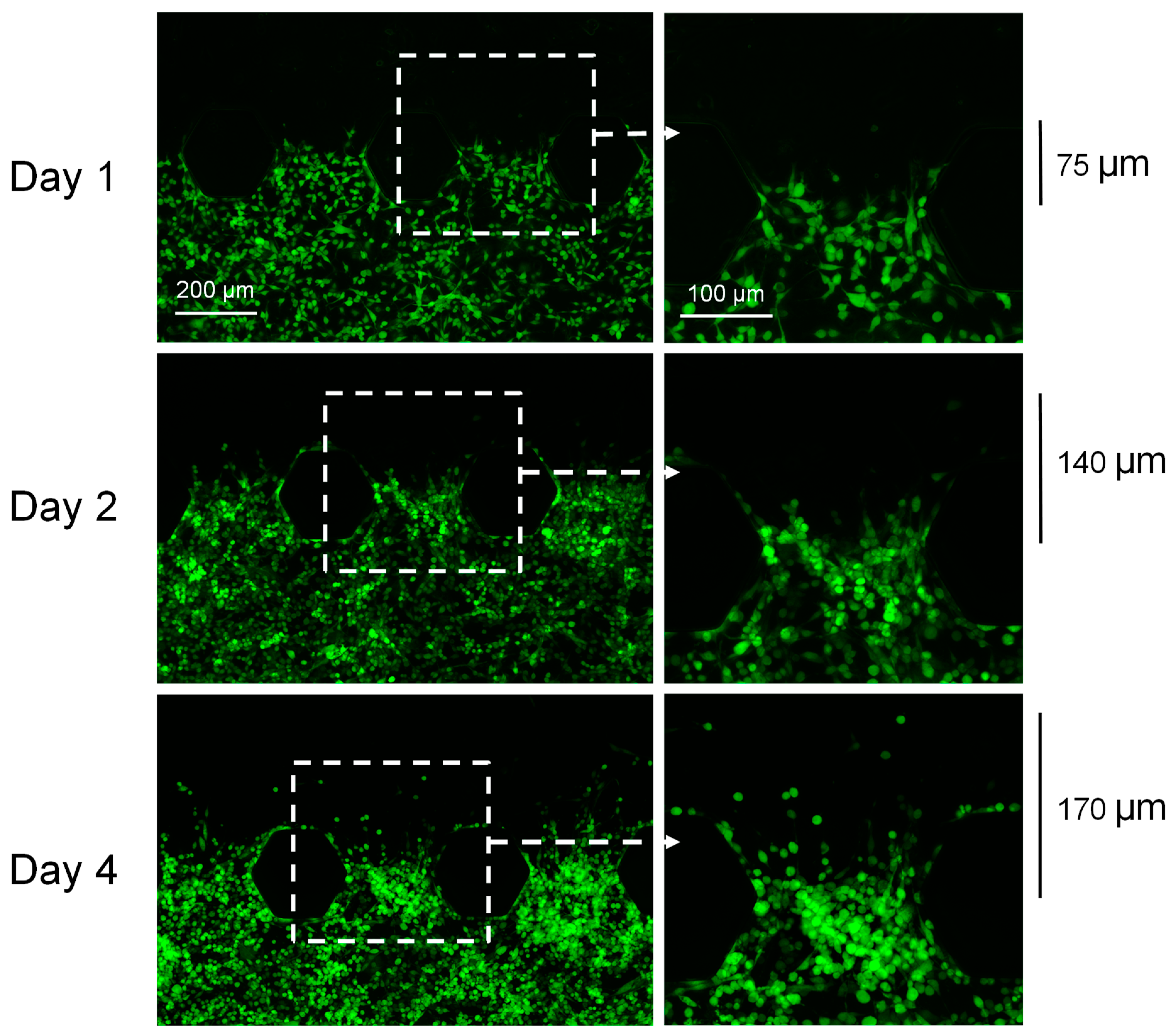
3.1. Visualization of the Bladder Cancer Cells within the Chips: Cells Can Remain Viable within the Chips for at Least 10 Days
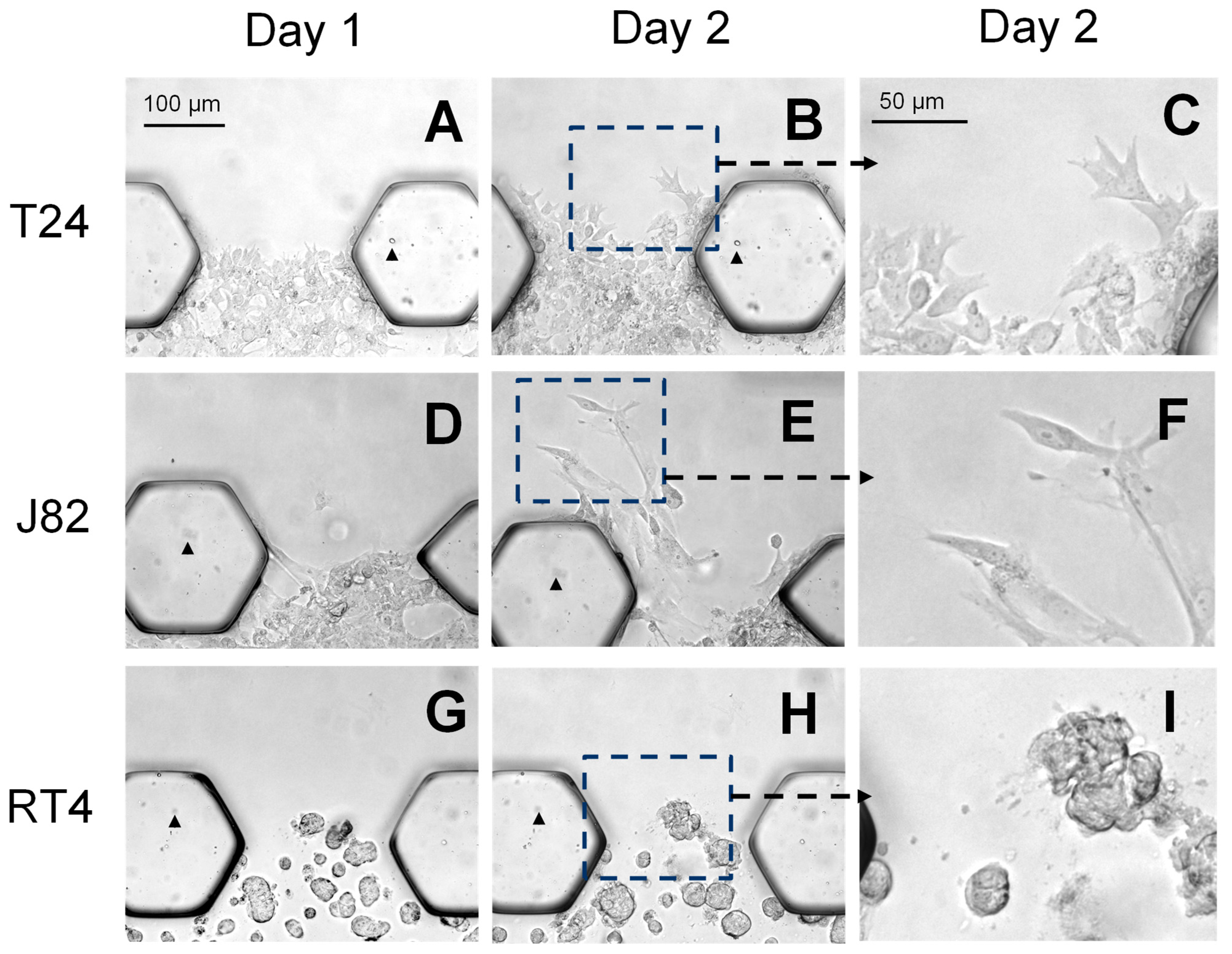
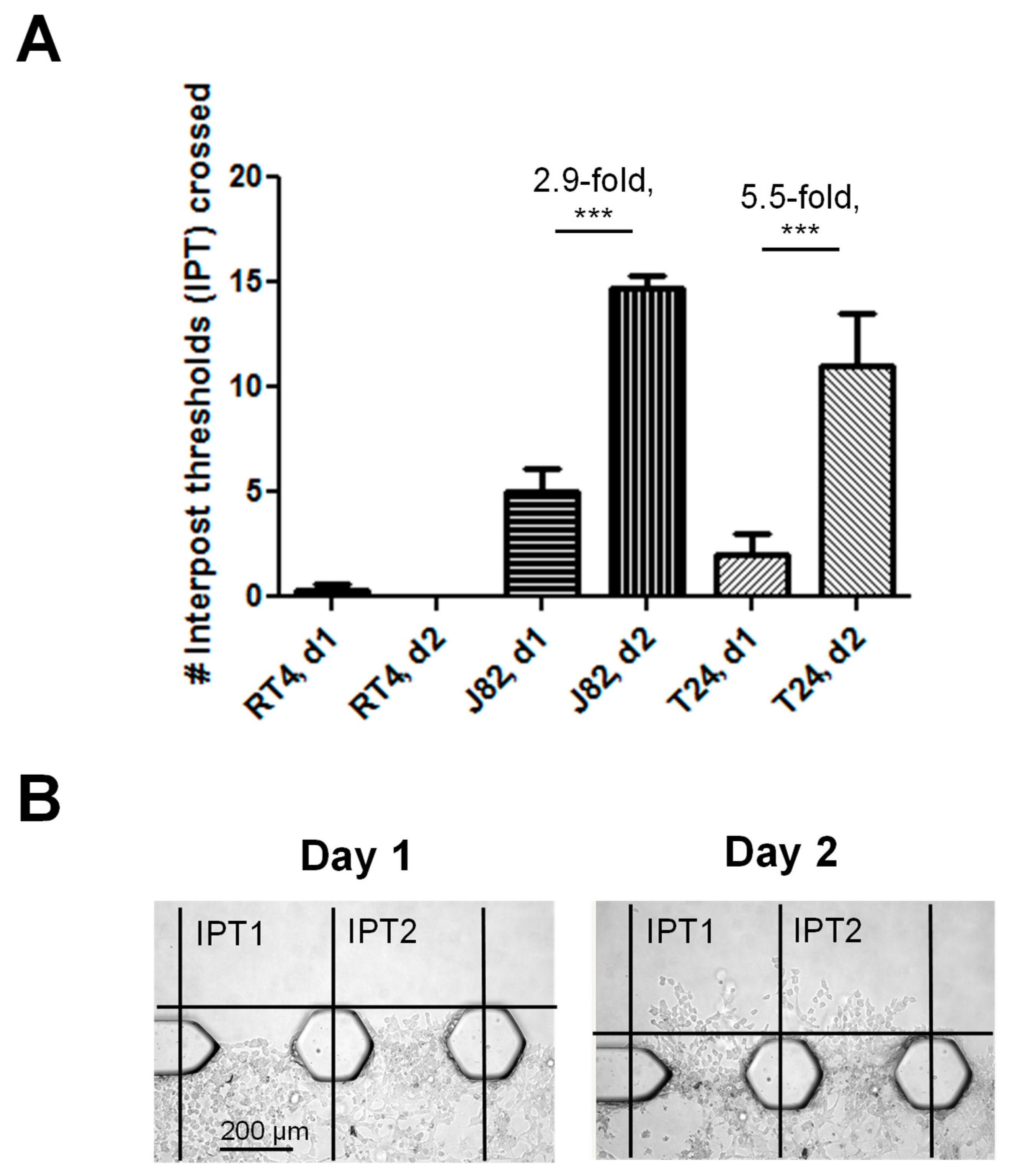
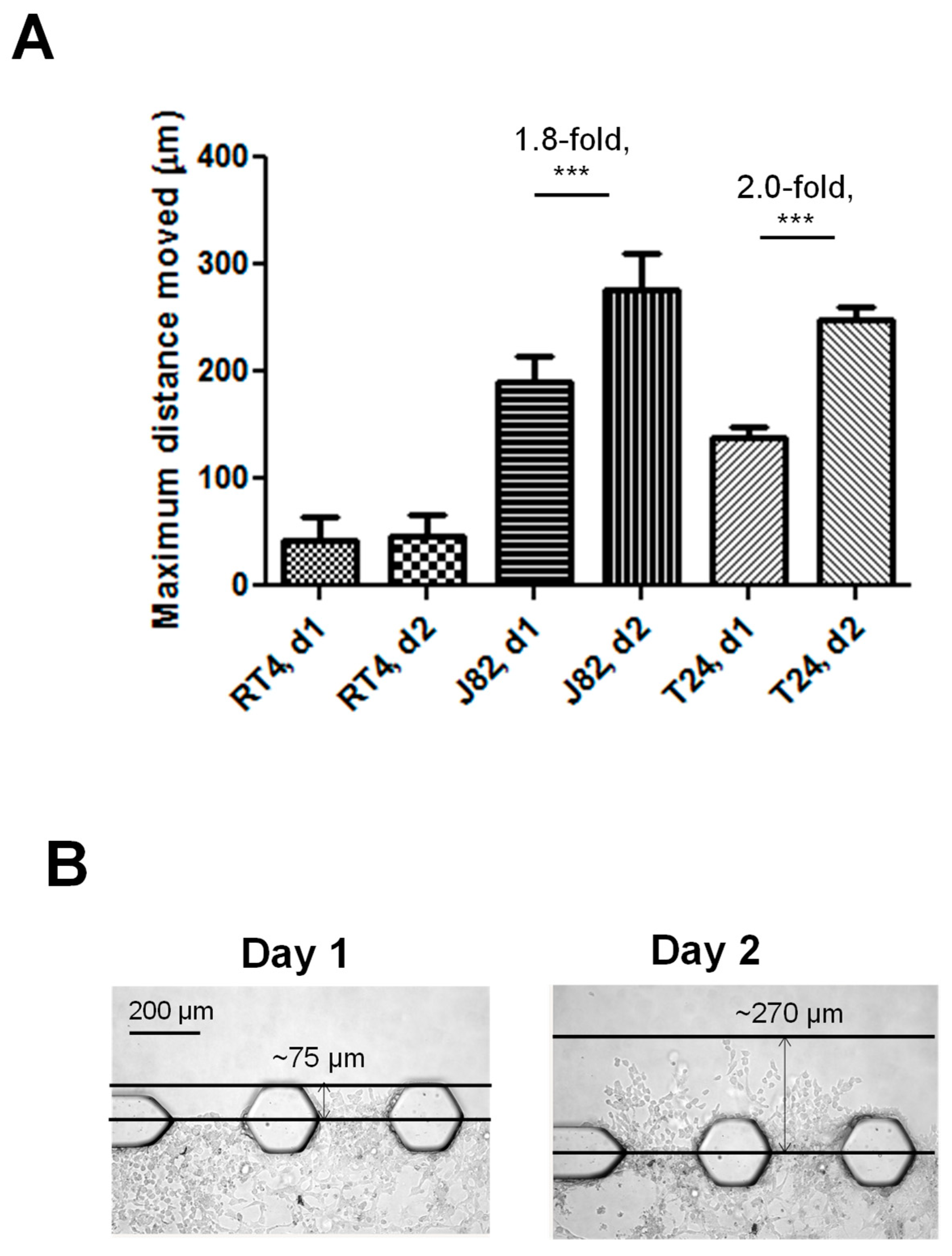
3.2. The Bladder Cancer-on-a-Chip Model Can Be Used to Assess and Quantify the Bladder Cancer Cell Invasiveness
3.3. GFP-Adenovirus and Qdots Can Be Used to Further Support the Visualization of the Bladder Cancer Cells within the Chips
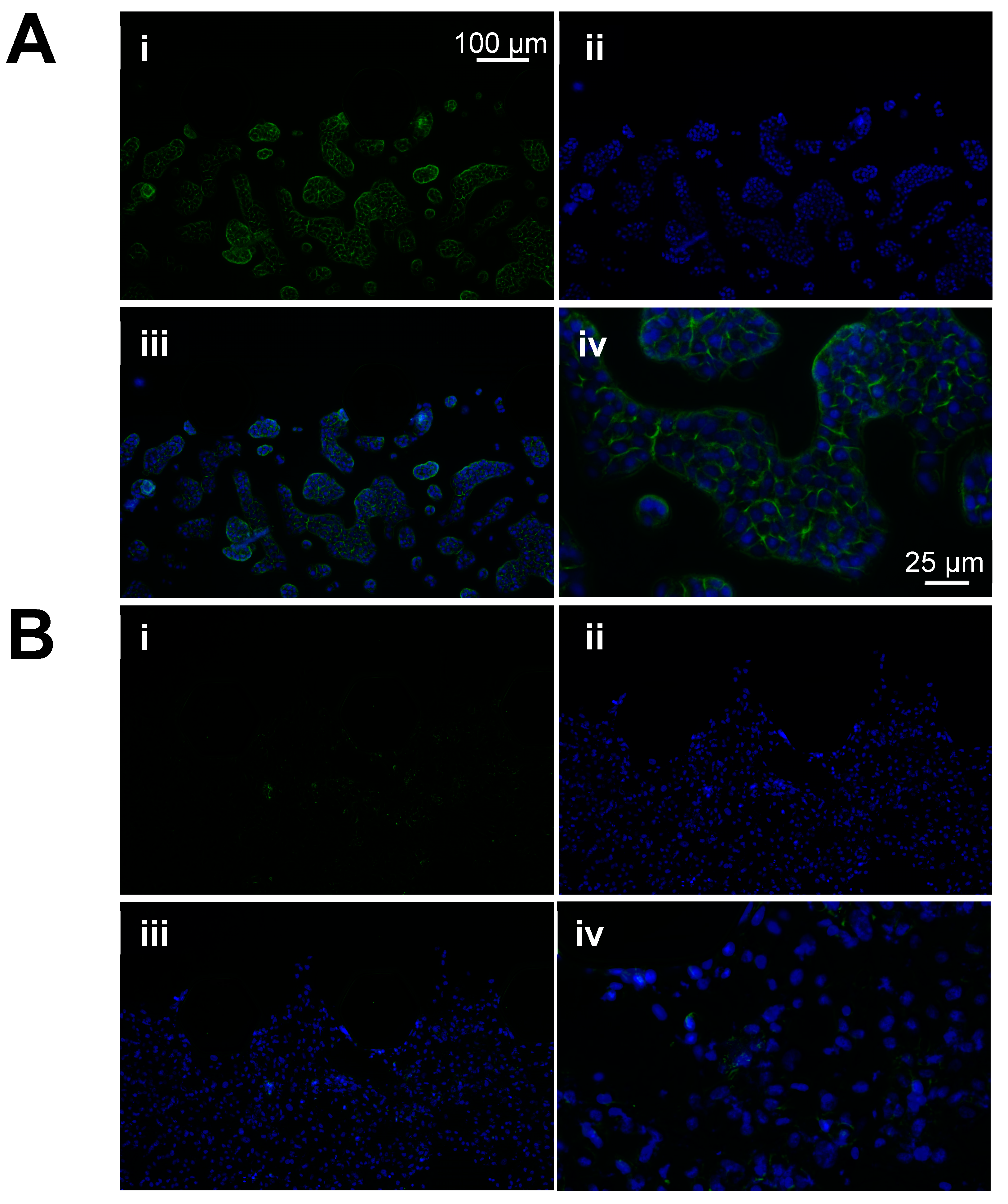
3.4. Immunofluorescence Analyses Confirmed That E-Cadherin Expression Is Lower in Muscle-Invasive Bladder Cancer Cells Compared to Non-Muscle-Invasive Bladder Cancer Cells
3.5. Treatment of the J82 Cells with ATN-161, an α5β1 Integrin Inhibitor, Causes a Dose-Dependent Decrease in the Cell Invasiveness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Bladder Cancer Prognosis and Survival Rates 2023. Available online: https://www.cancer.gov/types/bladder/survival (accessed on 15 March 2024).
- Petrelli, F.; Coinu, A.; Cabiddu, M.; Ghilardi, M.; Vavassori, I.; Barni, S. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: A meta-analysis. Eur. Urol. 2014, 65, 350–357. [Google Scholar] [CrossRef]
- von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Burke, K.P.; Van Allen, E.M. Genomic Approaches to Understanding Response and Resistance to Immunotherapy. Clin. Cancer Res. 2016, 22, 5642–5650. [Google Scholar] [CrossRef]
- Li, P.; Hao, S.; Ye, Y.; Wei, J.; Tang, Y.; Tan, L.; Liao, Z.; Zhang, M.; Li, J.; Gui, C.; et al. Identification of an Immune-Related Risk Signature Correlates With Immunophenotype and Predicts Anti-PD-L1 Efficacy of Urothelial Cancer. Front. Cell Dev. Biol. 2021, 9, 646982. [Google Scholar] [CrossRef] [PubMed]
- Gandalovičová, A.; Rosel, D.; Fernandes, M.; Veselý, P.; Heneberg, P.; Čermák, V.; Petruželka, L.; Kumar, S.; Sanz-Moreno, V.; Brábek, J. Migrastatics-Anti-metastatic and Anti-invasion Drugs: Promises and Challenges. Trends Cancer 2017, 3, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Raudenska, M.; Petrlakova, K.; Jurinakova, T.; Leischner Fialova, J.; Fojtu, M.; Jakubek, M.; Rosel, D.; Brabek, J.; Masarik, M. Engine shutdown: Migrastatic strategies and prevention of metastases. Trends Cancer 2023, 9, 293–308. [Google Scholar] [CrossRef]
- Tosca, E.M.; Ronchi, D.; Facciolo, D.; Magni, P. Replacement, Reduction, and Refinement of Animal Experiments in Anticancer Drug Development: The Contribution of 3D In Vitro Cancer Models in the Drug Efficacy Assessment. Biomedicines 2023, 11, 1058. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.; Cai, C.; Zhang, H.; Shen, H.; Han, Y. Patient-derived xenograft models in cancer therapy: Technologies and applications. Signal Transduct. Target. Ther. 2023, 8, 160. [Google Scholar] [CrossRef]
- Jin, J.; Yoshimura, K.; Sewastjanow-Silva, M.; Song, S.; Ajani, J.A. Challenges and Prospects of Patient-Derived Xenografts for Cancer Research. Cancers 2023, 15, 4352. [Google Scholar] [CrossRef]
- Cao, U.M.N.; Zhang, Y.; Chen, J.; Sayson, D.; Pillai, S.; Tran, S.D. Microfluidic Organ-on-A-chip: A Guide to Biomaterial Choice and Fabrication. Int. J. Mol. Sci. 2023, 24, 3232. [Google Scholar] [CrossRef]
- Zhang, X.; Karim, M.; Hasan, M.M.; Hooper, J.; Wahab, R.; Roy, S.; Al-Hilal, T.A. Cancer-on-a-Chip: Models for Studying Metastasis. Cancers 2022, 14, 648. [Google Scholar] [CrossRef] [PubMed]
- Ozer, L.Y.; Fayed, H.S.; Ericsson, J.; Al Haj Zen, A. Development of a cancer metastasis-on-chip assay for high throughput drug screening. Front. Oncol. 2023, 13, 1269376. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Jouybar, M.; de Winde, C.M.; Wolf, K.; Friedl, P.; Mebius, R.E.; den Toonder, J.M.J. Cancer-on-chip models for metastasis: Importance of the tumor microenvironment. Trends Biotechnol. 2024, 42, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Imparato, G.; Urciuolo, F.; Netti, P.A. Organ on Chip Technology to Model Cancer Growth and Metastasis. Bioengineering 2022, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T.; Frey, B.; Fellner, M.; Herrmann, M.; Fabry, B. Integrin α5β1 facilitates cancer cell invasion through enhanced contractile forces. J. Cell Sci. 2011, 124, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, H.; Luo, C.; Xiao, H.; Zou, X.; Zou, J.; Zhang, G. Targeting integrin α5β1 in urological tumors: Opportunities and challenges. Front. Oncol. 2023, 13, 1165073. [Google Scholar] [CrossRef]
- Knowles, L.M.; Zewe, J.; Malik, G.; Parwani, A.V.; Gingrich, J.R.; Pilch, J. CLT1 targets bladder cancer through integrin α5β1 and CLIC3. Mol. Cancer Res. 2013, 11, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Balci, M.G.; Tayfur, M. Loss of E-cadherin expression in recurrent non-invasive urothelial carcinoma of the bladder. Int. J. Clin. Exp. Pathol. 2018, 11, 4163–4168. [Google Scholar]
- Yu, Q.; Zhang, K.; Wang, X.; Liu, X.; Zhang, Z. Expression of transcription factors snail, slug, and twist in human bladder carcinoma. J. Exp. Clin. Cancer Res. 2010, 29, 119. [Google Scholar] [CrossRef]
- Yun, S.J.; Kim, W.-J. Role of the Epithelial-Mesenchymal Transition in Bladder Cancer: From Prognosis to Therapeutic Target. Korean J. Urol. 2013, 54, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ansari, J.A.; Maurya, N.; Mandhani, A.; Agrawal, V.; Garg, M. Epithelial-To-Mesenchymal Transition and Its Correlation With Clinicopathologic Features in Patients With Urothelial Carcinoma of the Bladder. Clin. Genitourin. Cancer 2017, 15, e187–e197. [Google Scholar] [CrossRef] [PubMed]
- McConkey, D.J.; Choi, W.; Marquis, L.; Martin, F.; Williams, M.B.; Shah, J.; Svatek, R.; Das, A.; Adam, L.; Kamat, A.; et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009, 28, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Liang, C.; Zhu, J.; Xu, A.; Zhao, K.; Hua, Y.; Zhang, J.; Chen, W.; Suo, C.; Zhang, C.; et al. Prognostic role of matrix metalloproteinases in bladder carcinoma: A systematic review and meta-analysis. Oncotarget 2017, 8, 32309–32321. [Google Scholar] [CrossRef] [PubMed]
- Kudelski, J.; Tokarzewicz, A.; Gudowska-Sawczuk, M.; Mroczko, B.; Chłosta, P.; Bruczko-Goralewska, M.; Mitura, P.; Młynarczyk, G. The Significance of Matrix Metalloproteinase 9 (MMP-9) and Metalloproteinase 2 (MMP-2) in Urinary Bladder Cancer. Biomedicines 2023, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Steele, T.M.; Talbott, G.C.; Sam, A.; Tepper, C.G.; Ghosh, P.M.; Vinall, R.L. Obatoclax, a BH3 Mimetic, Enhances Cisplatin-Induced Apoptosis and Decreases the Clonogenicity of Muscle Invasive Bladder Cancer Cells via Mechanisms That Involve the Inhibition of Pro-Survival Molecules as Well as Cell Cycle Regulators. Int. J. Mol. Sci. 2019, 20, 1285. [Google Scholar] [CrossRef] [PubMed]
- Kassem, T.; Sarkar, T.; Nguyen, T.; Saha, D.; Ahsan, F. 3D Printing in Solid Dosage Forms and Organ-on-Chip Applications. Biosensors 2022, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Al-Hilal, T.A.; Keshavarz, A.; Kadry, H.; Lahooti, B.; Al-Obaida, A.; Ding, Z.; Li, W.; Kamm, R.; McMurtry, I.F.; Lahm, T.; et al. Pulmonary-arterial-hypertension (PAH)-on-a-chip: Fabrication, validation and application. Lab. Chip 2020, 20, 3334–3345. [Google Scholar] [CrossRef]
- Nguyen, T.; Ho, L.; Moinuddin, S.M.; Sarkar, T.; Saha, D.; Ahsan, F. Multicellular Cell Seeding on a Chip: New Design and Optimization towards Commercialization. Biosensors 2022, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Sarkar, T.; Tran, T.; Moinuddin, S.M.; Saha, D.; Ahsan, F. Multilayer Soft Photolithography Fabrication of Microfluidic Devices Using a Custom-Built Wafer-Scale PDMS Slab Aligner and Cost-Efficient Equipment. Micromachines 2022, 13, 1357. [Google Scholar] [CrossRef]
- Patel, B.; Gauvin, R.; Absar, S.; Gupta, V.; Gupta, N.; Nahar, K.; Khademhosseini, A.; Ahsan, F. Computational and bioengineered lungs as alternatives to whole animal, isolated organ, and cell-based lung models. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L733–L747. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tu, C.; Fiandalo, M.V.; Pop, E.; Stocking, J.J.; Azabdaftari, G.; Li, J.; Wei, H.; Ma, D.; Qu, J.; Mohler, J.L.; et al. Proteomic Analysis of Charcoal-Stripped Fetal Bovine Serum Reveals Changes in the Insulin-like Growth Factor Signaling Pathway. J. Proteome Res. 2018, 17, 2963–2977. [Google Scholar] [CrossRef] [PubMed]
- Nwabo Kamdje, A.H.; Seke Etet, P.F.; Kipanyula, M.J.; Vecchio, L.; Tagne Simo, R.; Njamnshi, A.K.; Lukong, K.E.; Mimche, P.N. Insulin-like growth factor-1 signaling in the tumor microenvironment: Carcinogenesis, cancer drug resistance, and therapeutic potential. Front. Endocrinol. 2022, 13, 927390. [Google Scholar] [CrossRef] [PubMed]
- Stivarou, T.; Patsavoudi, E. Extracellular Molecules Involved in Cancer Cell Invasion. Cancers 2015, 7, 238–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, W.; Bai, X.; Wang, H.; Wang, X.; Xiao, H.; Li, Y. Androgen dihydrotestosterone promotes bladder cancer cell proliferation and invasion via EPPK1-mediated MAPK/JUP signalling. Cell Death Dis. 2023, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Metalli, D.; Lovat, F.; Tripodi, F.; Genua, M.; Xu, S.Q.; Spinelli, M.; Alberghina, L.; Vanoni, M.; Baffa, R.; Gomella, L.G.; et al. The insulin-like growth factor receptor I promotes motility and invasion of bladder cancer cells through Akt- and mitogen-activated protein kinase-dependent activation of paxillin. Am. J. Pathol. 2010, 176, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Summerhayes, I.C.; Franks, L.M. Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro. J. Natl. Cancer Inst. 1979, 62, 1017–1023. [Google Scholar] [PubMed]
- Loskog, A.; Tötterman, T.H.; Böhle, A.; Brandau, S. In vitro activation of cancer patient-derived dendritic cells by tumor cells genetically modified to express CD154. Cancer Gene Ther. 2002, 9, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.S.; Lattime, E.C. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 2003, 63, 2150–2157. [Google Scholar]
- O’Donnell, M.A.; Luo, Y.; Hunter, S.E.; Chen, X.; Hayes, L.L.; Clinton, S.K. The essential role of interferon-gamma during interleukin-12 therapy for murine transitional cell carcinoma of the bladder. J. Urol. 2004, 171, 1336–1342. [Google Scholar] [CrossRef]
- Buchanan, B.C.; Yoon, J.Y. Microscopic Imaging Methods for Organ-on-a-Chip Platforms. Micromachines 2022, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Bracke, M.E.; Van Roy, F.M.; Mareel, M.M. The E-cadherin/catenin complex in invasion and metastasis. Curr. Top. Microbiol. Immunol. 1996, 213 Pt 1, 123–161. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, P.; Gao, Y.; Gu, L.; Chen, L.; Fan, Y.; Zhang, F.; Zhang, X. Reduced E-cadherin expression is correlated with poor prognosis in patients with bladder cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 62489–62499. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.M.; Carvalho, V.; Rodrigues, R.O.; Pinho, D.; Teixeira, S.; Moita, A.; Hori, T.; Kaji, H.; Lima, R.; Minas, G. Organ-on-a-Chip Platforms for Drug Screening and Delivery in Tumor Cells: A Systematic Review. Cancers 2022, 14, 935. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.; Kang, S.J.; Choi, Y.W.; Choi, S.Y.; Park, J.Y.; Chang, I.H. Establishment of Three-Dimensional Bioprinted Bladder Cancer-on-a-Chip with a Microfluidic System Using Bacillus Calmette-Guérin. Int. J. Mol. Sci. 2021, 22, 8887. [Google Scholar] [CrossRef] [PubMed]
- Tak, S.; Han, G.; Leem, S.H.; Lee, S.Y.; Paek, K.; Kim, J.A. Prediction of anticancer drug resistance using a 3D microfluidic bladder cancer model combined with convolutional neural network-based image analysis. Front. Bioeng. Biotechnol. 2023, 11, 1302983. [Google Scholar] [CrossRef] [PubMed]
- Agüero, E.I.; Belgorosky, D.; García-Silva, J.I.; Booth, R.; Lerner, B.; Pérez, M.S.; Eiján, A.M. Microdevices for cancer stem cell culture as a predictive chemotherapeutic response platform. J. Mol. Med. 2023, 101, 1465–1475. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Ho, J.N.; Kim, D.; Lee, S.; Jeon, J.S. 3D vascularized microphysiological system for investigation of tumor-endothelial crosstalk in anti-cancer drug resistance. Biofabrication 2023, 15, 045016. [Google Scholar] [CrossRef]
- Liu, P.F.; Cao, Y.W.; Zhang, S.D.; Zhao, Y.; Liu, X.G.; Shi, H.Q.; Hu, K.Y.; Zhu, G.Q.; Ma, B.; Niu, H.T. A bladder cancer microenvironment simulation system based on a microfluidic co-culture model. Oncotarget 2015, 6, 37695–37705. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Chi, B.H.; Yoo, J.J.; Ju, Y.M.; Whang, Y.M.; Chang, I.H. Structure establishment of three-dimensional (3D) cell culture printing model for bladder cancer. PLoS ONE 2019, 14, e0223689. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Lu, Y.; Xiong, Y.; Zhan, X.; Liu, T.; Tang, M.; Xie, A.; Liu, X.; Fu, B. Advances in the bladder cancer research using 3D culture models. Bladder 2023, 10, e21200005. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.X.; Bos, P.D.; Massague, J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer 2009, 9, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.D.; Ashby, W.J.; Lewis, J.D.; Zijlstra, A. Targeting tumor cell motility to prevent metastasis. Adv. Drug Deliv. Rev. 2011, 63, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Moshksayan, K.; Kashaninejad, N.; Warkiani, M.E.; Lock, J.G.; Moghadas, H.; Firoozabadi, B.; Saidi, M.S.; Nguyen, N.-T. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens. Actuators B Chem. 2018, 263, 151–176. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The use of 3-D cultures for high-throughput screening: The multicellular spheroid model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef]
- Ho, W.J.; Pham, E.A.; Kim, J.W.; Ng, C.W.; Kim, J.H.; Kamei, D.T.; Wu, B.M. Incorporation of multicellular spheroids into 3-D polymeric scaffolds provides an improved tumor model for screening anticancer drugs. Cancer Sci. 2010, 101, 2637–2643. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Pan, E.; Bogumil, D.; Cortessis, V.; Yu, S.; Nieva, J. A Systematic Review of the Efficacy of Preclinical Models of Lung Cancer Drugs. Front. Oncol. 2020, 10, 591. [Google Scholar] [CrossRef]
- Popova, N.V.; Jücker, M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Passaniti, A.; Kleinman, H.K.; Martin, G.R. Matrigel: History/background, uses, and future applications. J. Cell Commun. Signal 2022, 16, 621–626. [Google Scholar] [CrossRef]
- Grabe-Heyne, K.; Henne, C.; Mariappan, P.; Geiges, G.; Pöhlmann, J.; Pollock, R.F. Intermediate and high-risk non-muscle-invasive bladder cancer: An overview of epidemiology, burden, and unmet needs. Front. Oncol. 2023, 13, 1170124. [Google Scholar] [CrossRef]
- Zhu, J.; Ji, L.; Chen, Y.; Li, H.; Huang, M.; Dai, Z.; Wang, J.; Xiang, D.; Fu, G.; Lei, Z.; et al. Organoids and organs-on-chips: Insights into predicting the efficacy of systemic treatment in colorectal cancer. Cell Death Discov. 2023, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Bouquerel, C.; Dubrova, A.; Hofer, I.; Phan, D.T.T.; Bernheim, M.; Ladaigue, S.; Cavaniol, C.; Maddalo, D.; Cabel, L.; Mechta-Grigoriou, F.; et al. Bridging the gap between tumor-on-chip and clinics: A systematic review of 15 years of studies. Lab. Chip 2023, 23, 3906–3935. [Google Scholar] [CrossRef]
- Omorphos, N.P.; Piedad, J.C.P.; Vasdev, N. Guideline of guidelines: Muscle-invasive bladder cancer. Turk. J. Urol. 2021, 47, S71–S78. [Google Scholar] [CrossRef]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 244–258. [Google Scholar] [CrossRef]
- Wong, V.K.; Ganeshan, D.; Jensen, C.T.; Devine, C.E. Imaging and Management of Bladder Cancer. Cancers 2021, 13, 1396. [Google Scholar] [CrossRef]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Diagnostic performance of MRI for prediction of muscle-invasiveness of bladder cancer: A systematic review and meta-analysis. Eur. J. Radiol. 2017, 95, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Shao, S.X.; Cao, Y.W.; Yang, X.C.; Shi, H.Q.; Wang, Y.L.; Xue, S.Y.; Wang, X.S.; Niu, H.T. The study of energy metabolism in bladder cancer cells in co-culture conditions using a microfluidic chip. Int. J. Clin. Exp. Med. 2015, 8, 12327–12336. [Google Scholar] [PubMed]
- Truong, D.D.; Kratz, A.; Park, J.G.; Barrientos, E.S.; Saini, H.; Nguyen, T.; Pockaj, B.; Mouneimne, G.; LaBaer, J.; Nikkhah, M. A Human Organotypic Microfluidic Tumor Model Permits Investigation of the Interplay between Patient-Derived Fibroblasts and Breast Cancer Cells. Cancer Res. 2019, 79, 3139–3151. [Google Scholar] [CrossRef] [PubMed]



| Cell Line | Stage | Grade | Notes |
|---|---|---|---|
| T24, NMIBC | pTa | G3 | Female |
| J82, MIBC | pT3 | G3 | Male |
| RT4, NMIBC | pT1 | G1 | Male |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ewell, D.J.; Vue, N.; Moinuddin, S.M.; Sarkar, T.; Ahsan, F.; Vinall, R.L. Development of a Bladder Cancer-on-a-Chip Model to Assess Bladder Cancer Cell Invasiveness. Cancers 2024, 16, 2657. https://doi.org/10.3390/cancers16152657
Ewell DJ, Vue N, Moinuddin SM, Sarkar T, Ahsan F, Vinall RL. Development of a Bladder Cancer-on-a-Chip Model to Assess Bladder Cancer Cell Invasiveness. Cancers. 2024; 16(15):2657. https://doi.org/10.3390/cancers16152657
Chicago/Turabian StyleEwell, Desiree J., Nita Vue, Sakib M. Moinuddin, Tanoy Sarkar, Fakhrul Ahsan, and Ruth L. Vinall. 2024. "Development of a Bladder Cancer-on-a-Chip Model to Assess Bladder Cancer Cell Invasiveness" Cancers 16, no. 15: 2657. https://doi.org/10.3390/cancers16152657
APA StyleEwell, D. J., Vue, N., Moinuddin, S. M., Sarkar, T., Ahsan, F., & Vinall, R. L. (2024). Development of a Bladder Cancer-on-a-Chip Model to Assess Bladder Cancer Cell Invasiveness. Cancers, 16(15), 2657. https://doi.org/10.3390/cancers16152657








