Patterns and Frequency of Pathogenic Germline Mutations among Patients with Newly-Diagnosed Endometrial Cancer: The Jordanian Exploratory Cancer Genetics (Jo-ECAG) Endometrial Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
Statistical Analysis
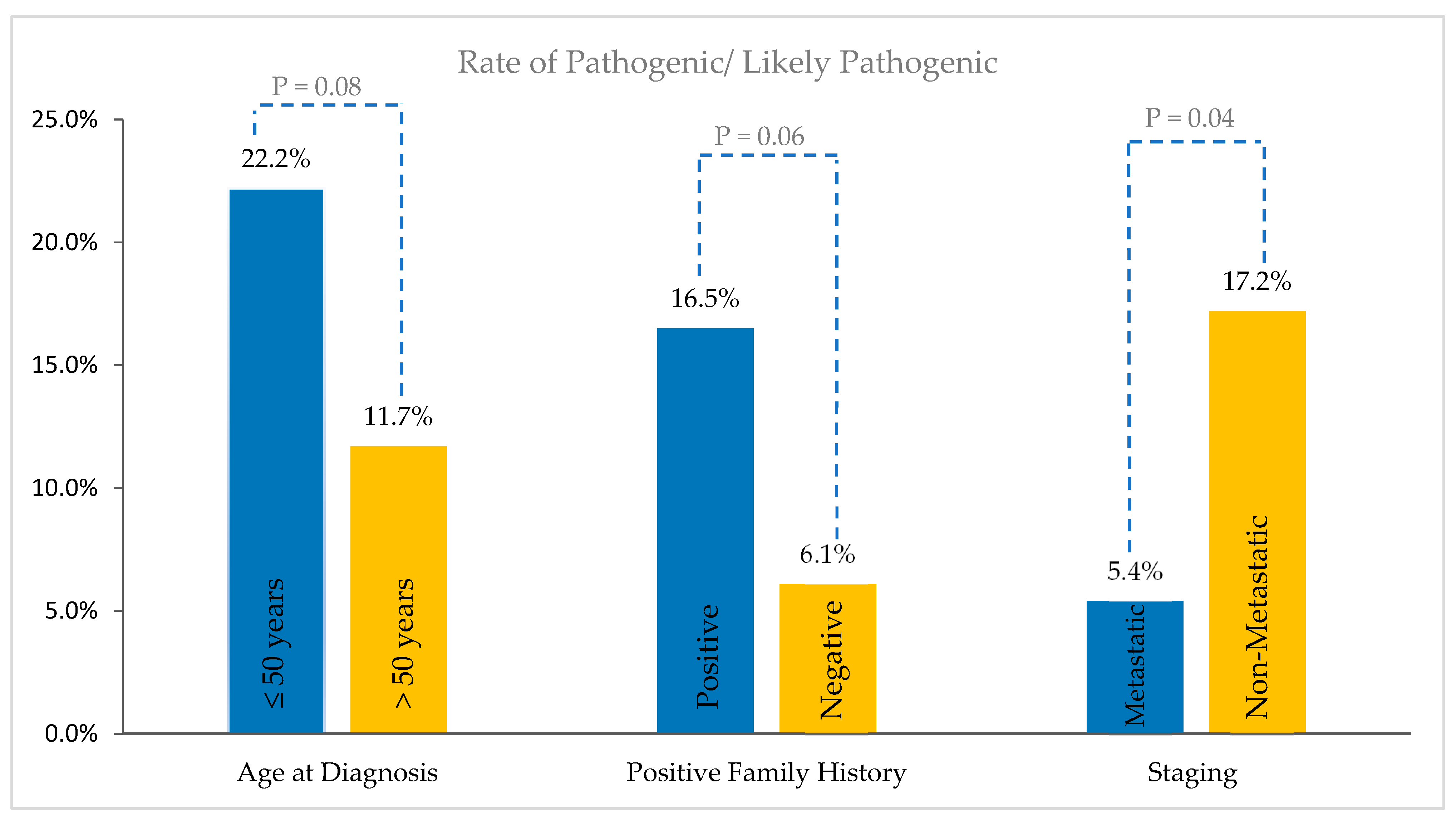
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.who.int/today (accessed on 23 May 2024).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Resnick, K.E.; Hampel, H.; Fishel, R.; Cohn, D.E. Current and Emerging Trends in Lynch Syndrome Identification in Women with Endometrial Cancer. Gynecol. Oncol. 2009, 114, 128–134. [Google Scholar] [CrossRef]
- Matoba, Y.; Devins, K.M.; Milane, L.; Manning, W.B.; Mazina, V.; Yeku, O.O.; Rueda, B.R. High-Grade Endometrial Cancer: Molecular Subtypes, Current Challenges, and Treatment Options. Reprod. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ballová, Z.; Gašparová, P.; Sitáš, M.; Dosedla, E. A new perspective on Endometrial Carcinoma classification and management strategies in context of molecular subtypes. Ceska Gynekol. 2024, 89, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.; Blom, R.; Högberg, T.; Simonsen, E. Death Rate and Recurrence Pattern among 841 Clinical Stage I Endometrial Cancer Patients with Special Reference to Uterine Papillary Serous Carcinoma. Gynecol. Oncol. 1993, 51, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.N.; Boruta, D.; Olawaiye, A.B.; Gehrig, P.A. Uterine Papillary Serous Carcinoma: Epidemiology, Pathogenesis and Management. Curr. Opin. Obstet. Gynecol. 2010, 22, 21–29. [Google Scholar] [CrossRef]
- Liontos, M.; Svarna, A.; Theofanakis, C.; Fiste, O.; Andrikopoulou, A.; Kaparelou, M.; Koutsoukos, K.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A.; et al. What Has Changed in the Management of Uterine Serous Carcinomas? Two Decades of Experience. Curr. Oncol. 2021, 28, 4862–4873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, T.J.; Desai, N.B.; DeLair, D.; Kollmeier, M.A.; Makker, V.; Leitao, M.M.; Abu-Rustum, N.R.; Alektiar, K.M. Comparison of Outcomes in Early-Stage Uterine Clear Cell Carcinoma and Serous Carcinoma. Brachytherapy 2019, 18, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Devlin, L.A.; Morrison, P.J. Inherited Gynaecological Cancer Syndromes. Obstetr. Gynaecol. 2008, 10, 9–15. [Google Scholar] [CrossRef][Green Version]
- Daniels, M.S.; Lu, K.H. Genetic Predisposition in Gynecologic Cancers. Semin. Oncol. 2016, 43, 543–547. [Google Scholar] [CrossRef]
- Kehoe, S.M.; Kauff, N.D. Screening and Prevention of Hereditary Gynecologic Cancers. Semin. Oncol. 2007, 34, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.; Bethanpowell, C.; Kauff, N.; Cass, I.; Chen, L.; Lu, K.; Mutch, D.; Berchuck, A.; Karlan, B.; Herzog, T. Society of Gynecologic Oncologists Education Committee Statement on Risk Assessment for Inherited Gynecologic Cancer Predispositions. Gynecol. Oncol. 2007, 107, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Scott, J.L.; Gilks, C.B.; Daniels, M.S.; Sun, C.C.; Lu, K.H. Testing Women With Endometrial Cancer to Detect Lynch Syndrome. J. Clin. Oncol. 2011, 29, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.C.; Yang, E.J.; Muto, M.G.; Feltmate, C.M.; Berkowitz, R.S.; Horowitz, N.S.; Syngal, S.; Yurgelun, M.B.; Chittenden, A.; Hornick, J.L.; et al. Universal Screening for Mismatch-Repair Deficiency in Endometrial Cancers to Identify Patients with Lynch Syndrome and Lynch-like Syndrome. Int. J. Gynecol. Pathol. 2017, 36, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Jumaah, A.S.; Salim, M.M.; Al-Haddad, H.S.; McAllister, K.A.; Yasseen, A.A. The Frequency of POLE-Mutation in Endometrial Carcinoma and Prognostic Implications: A Systemic Review and Meta-Analysis. J. Pathol. Transl. Med. 2020, 54, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Travaglino, A.; Raffone, A.; Stradella, C.; Esposito, R.; Moretta, P.; Gallo, C.; Orlandi, G.; Insabato, L.; Zullo, F. Impact of Endometrial Carcinoma Histotype on the Prognostic Value of the TCGA Molecular Subgroups. Arch. Gynecol. Obstet. 2020, 301, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Uterine Neoplasm. Version 2.2024. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1473 (accessed on 20 May 2024).
- McAlpine, J.N.; Chiu, D.S.; Nout, R.A.; Church, D.N.; Schmidt, P.; Lam, S.; Leung, S.; Bellone, S.; Wong, A.; Brucker, S.Y.; et al. Evaluation of Treatment Effects in Patients with Endometrial Cancer and POLE Mutations: An Individual Patient Data Meta-analysis. Cancer 2021, 127, 2409–2422. [Google Scholar] [CrossRef]
- Mutch, D.; Denny, L.; Quinn, M. Hereditary Gynecologic Cancers. Int. J. Gynecol. Obste 2014, 124, 189–192. [Google Scholar] [CrossRef]
- Brown, G.J.E.; St. John, D.J.B.; Macrae, F.A.; Aittomäki, K. Cancer Risk in Young Women at Risk of Hereditary Nonpolyposis Colorectal Cancer: Implications for Gynecologic Surveillance. Gynecol. Oncol. 2001, 80, 346–349. [Google Scholar] [CrossRef]
- Davidson, S.A. Hereditary Gynecologic Cancer Syndromes. Postgrad. Obstetr. Gynecol. 2010, 30, 1–7. [Google Scholar] [CrossRef]
- Randall, L.M.; Pothuri, B. The Genetic Prediction of Risk for Gynecologic Cancers. Gynecol. Oncol. 2016, 141, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, C.; Clarke, B.; Bosse, T. Gynaecological Neoplasms in Common Familial Syndromes (Lynch and HBOC). Pathology 2018, 50, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, S.E.; Kobayashi, Y.; Anderson, M.J.; Yang, S.; Desmond, A.J.; Mills, M.A.; Nilsen, G.B.; Jacobs, K.B.; Monzon, F.A.; Kurian, A.W.; et al. A Systematic Comparison of Traditional and Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Genes in More Than 1000 Patients. J. Mol. Diagn. 2015, 17, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Nykamp, K.; Anderson, M.; Powers, M.; Garcia, J.; Herrera, B.; Ho, Y.-Y.; Kobayashi, Y.; Patil, N.; Thusberg, J.; Westbrook, M.; et al. Sherloc: A Comprehensive Refinement of the ACMG–AMP Variant Classification Criteria. Genet. Med. 2017, 19, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1503 (accessed on 15 May 2024).
- Couch, F.J.; Nathanson, K.L.; Offit, K. Two Decades after BRCA: Setting Paradigms in Personalized Cancer Care and Prevention. Science 2014, 343, 1466–1470. [Google Scholar] [CrossRef] [PubMed]
- Theobald, K.A.; Susswein, L.R.; Marshall, M.L.; Roberts, M.E.; Mester, J.L.; Speyer, D.; Williams, R.N.W.; Knapke, S.C.; Solomon, S.R.; Murphy, P.D.; et al. Utility of Expedited Hereditary Cancer Testing in the Surgical Management of Patients with a New Breast Cancer Diagnosis. Ann. Surg. Oncol. 2018, 25, 3556–3562. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Bang, Y.-J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S.; Puzanov, I.; Mehnert, J.M.; Aung, K.L.; Lopez, J.; et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1–Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Barak, F.; Milgrom, R.; Laitman, Y.; Gemer, O.; Rabinovich, A.; Piura, B.; Anteby, E.; Baruch, G.B.; Korach, J.; Friedman, E. The Rate of the Predominant Jewish Mutations in the BRCA1, BRCA2, MSH2 and MSH6 Genes in Unselected Jewish Endometrial Cancer Patients. Gynecol. Oncol. 2010, 119, 511–515. [Google Scholar] [CrossRef]
- Gasparri, M.; Bellaminutti, S.; Farooqi, A.; Cuccu, I.; Di Donato, V.; Papadia, A. Endometrial Cancer and BRCA Mutations: A Systematic Review. J. Clin. Med. 2022, 11, 3114. [Google Scholar] [CrossRef]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Redmond, C.K.; Kavanah, M.; Cronin, W.M.; Vogel, V.; Robidoux, A.; Dimitrov, N.; Atkins, J.; et al. Tamoxifen for Prevention of Breast Cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 1998, 90, 1371–1388. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, M.L.; Taghavi, K.; Fiacco, E.; Zuber, V.; Di Micco, R.; Gazzetta, G.; Valentini, A.; Mueller, M.D.; Papadia, A.; Gentilini, O.D. Risk-Reducing Bilateral Salpingo-Oophorectomy for BRCA Mutation Carriers and Hormonal Replacement Therapy: If It Should Rain, Better a Drizzle than a Storm. Medicina 2019, 55, 415. [Google Scholar] [CrossRef]
- Machado, F.; Rodríguez, J.R.; León, J.P.H.; Rodríguez, J.R.; Parrilla, J.J.; Abad, L. Tamoxifen and Endometrial Cancer. Is Screening Necessary? A Review of the Literature. Eur. J. Gynaecol. Oncol. 2005, 26, 257–265. [Google Scholar] [PubMed]
- Kotsopoulos, J.; Gronwald, J.; Karlan, B.Y.; Huzarski, T.; Tung, N.; Moller, P.; Armel, S.; Lynch, H.T.; Senter, L.; Eisen, A.; et al. Hormone Replacement Therapy After Oophorectomy and Breast Cancer Risk Among BRCA1 Mutation Carriers. JAMA Oncol. 2018, 4, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- King, M.C.; Wieand, S.; Hale, K.; Lee, M.; Walsh, T.; Owens, K.; Tait, J.; Ford, L.; Dunn, B.K.; Costantino, J.; et al. Tamoxifen and Breast Cancer Incidence among Women with Inherited Mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA 2001, 286, 2251–2256. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, T.; Coosemans, A.; Morina, M.; Timmerman, D.; Amant, F. Screening for Uterine Tumours. Best. Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.M.; Liou, S.; Ford, J.M.; Berek, J.S.; Pai, R.K.; Longacre, T.A. Lynch Syndrome Screening Should Be Considered for All Patients with Newly Diagnosed Endometrial Cancer. Am. J. Surg. Pathol. 2014, 38, 1501–1509. [Google Scholar] [CrossRef]
- Walsh, C.S.; Blum, A.; Walts, A.; Alsabeh, R.; Tran, H.; Koeffler, H.P.; Karlan, B.Y. Lynch Syndrome among Gynecologic Oncology Patients Meeting Bethesda Guidelines for Screening. Gynecol. Oncol. 2010, 116, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.E.; Aronson, M.; Pollett, A.; Eiriksson, L.R.; Oza, A.M.; Gallinger, S.; Lerner-Ellis, J.; Alvandi, Z.; Bernardini, M.Q.; MacKay, H.J.; et al. Performance Characteristics of Screening Strategies for Lynch Syndrome in Unselected Women with Newly Diagnosed Endometrial Cancer Who Have Undergone Universal Germline Mutation Testing. Cancer 2014, 120, 3932–3939. [Google Scholar] [CrossRef]
- Manchanda, R.; Saridogan, E.; Abdelraheim, A.; Johnson, M.; Rosenthal, A.N.; Benjamin, E.; Brunell, C.; Side, L.; Gessler, S.; Jacobs, I.; et al. Annual Outpatient Hysteroscopy and Endometrial Sampling (OHES) in HNPCC/Lynch Syndrome (LS). Arch. Gynecol. Obstet. 2012, 286, 1555–1562. [Google Scholar] [CrossRef]
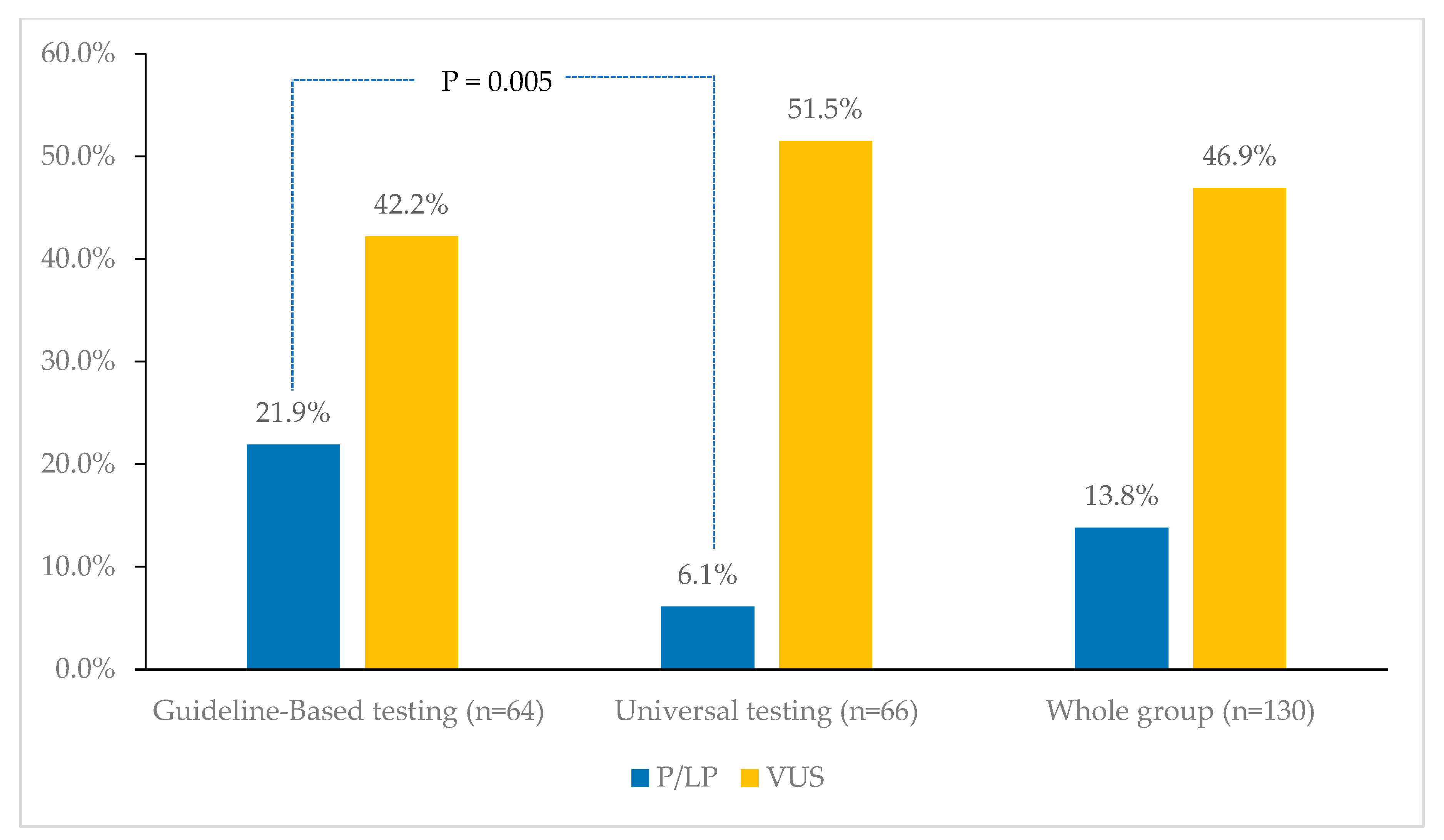
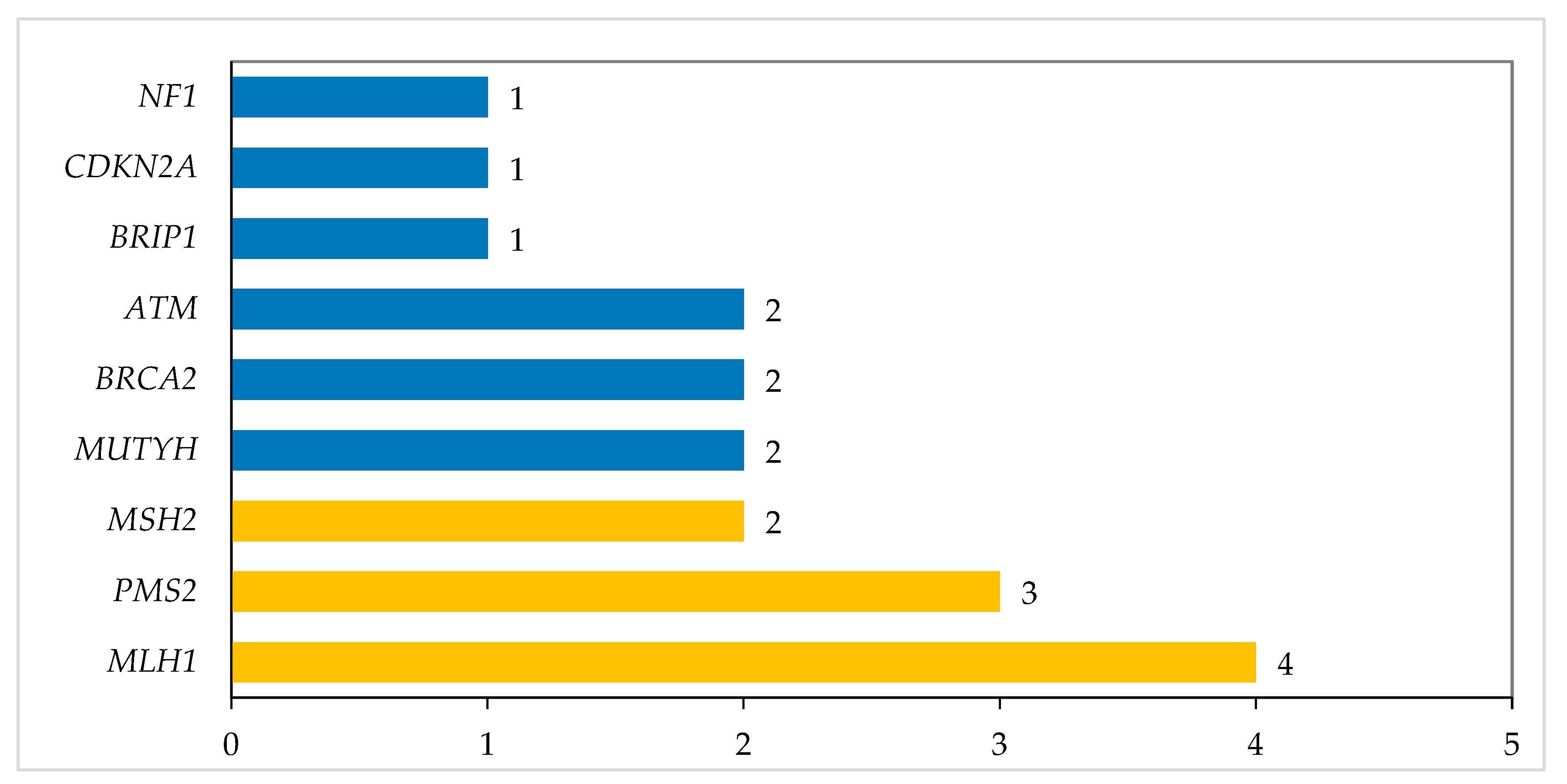

| Characteristics | Number | Percentage | |
|---|---|---|---|
| Age at diagnosis (years) | Median (range) | 60 | |
| ≤ 50 years | 27 | 20.8 | |
| > 50 years | 103 | 79.2 | |
| Positive family history | 97 | 74.6 | |
| Nationality | Jordanians | 128 | 98.5 |
| Non-Jordanians | 2 | 1.5 | |
| Pathology | Endometrioid adenocarcinoma | 74 | 56.9 |
| Serous endometrial carcinoma | 20 | 15.4 | |
| Carcinosarcoma | 10 | 7.7 | |
| Clear cell carcinoma | 9 | 6.9 | |
| Others | 17 | 13.1 | |
| Metastases | Present | 37 | 28.5 |
| Absent | 93 | 71.5 | |
| MMR Status | PMS2 | 30 | 23.1 |
| MLH1 | 22 | 16.9 | |
| MSH2 | 4 | 3.1 | |
| MSH6 | 5 | 3.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Razeq, H.; Bani Hani, H.; Sharaf, B.; Tamimi, F.; Khalil, H.; Abu Sheikha, A.; Alkyam, M.; Abdel-Razeq, S.; Ghatasheh, T.; Radaideh, T.; et al. Patterns and Frequency of Pathogenic Germline Mutations among Patients with Newly-Diagnosed Endometrial Cancer: The Jordanian Exploratory Cancer Genetics (Jo-ECAG) Endometrial Study. Cancers 2024, 16, 2543. https://doi.org/10.3390/cancers16142543
Abdel-Razeq H, Bani Hani H, Sharaf B, Tamimi F, Khalil H, Abu Sheikha A, Alkyam M, Abdel-Razeq S, Ghatasheh T, Radaideh T, et al. Patterns and Frequency of Pathogenic Germline Mutations among Patients with Newly-Diagnosed Endometrial Cancer: The Jordanian Exploratory Cancer Genetics (Jo-ECAG) Endometrial Study. Cancers. 2024; 16(14):2543. https://doi.org/10.3390/cancers16142543
Chicago/Turabian StyleAbdel-Razeq, Hikmat, Hira Bani Hani, Baha Sharaf, Faris Tamimi, Hanan Khalil, Areej Abu Sheikha, Mais Alkyam, Sarah Abdel-Razeq, Tala Ghatasheh, Tala Radaideh, and et al. 2024. "Patterns and Frequency of Pathogenic Germline Mutations among Patients with Newly-Diagnosed Endometrial Cancer: The Jordanian Exploratory Cancer Genetics (Jo-ECAG) Endometrial Study" Cancers 16, no. 14: 2543. https://doi.org/10.3390/cancers16142543
APA StyleAbdel-Razeq, H., Bani Hani, H., Sharaf, B., Tamimi, F., Khalil, H., Abu Sheikha, A., Alkyam, M., Abdel-Razeq, S., Ghatasheh, T., Radaideh, T., & Khater, S. (2024). Patterns and Frequency of Pathogenic Germline Mutations among Patients with Newly-Diagnosed Endometrial Cancer: The Jordanian Exploratory Cancer Genetics (Jo-ECAG) Endometrial Study. Cancers, 16(14), 2543. https://doi.org/10.3390/cancers16142543






