Locally Advanced Cervical Cancer: Neoadjuvant Treatment versus Standard Radio-Chemotherapy—An Updated Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Search Method
2.2. Study Selection
2.3. Statistical Analysis
2.4. Quality Assessment
3. Results
3.1. Studies’ Characteristics
3.2. Treatment Modalities
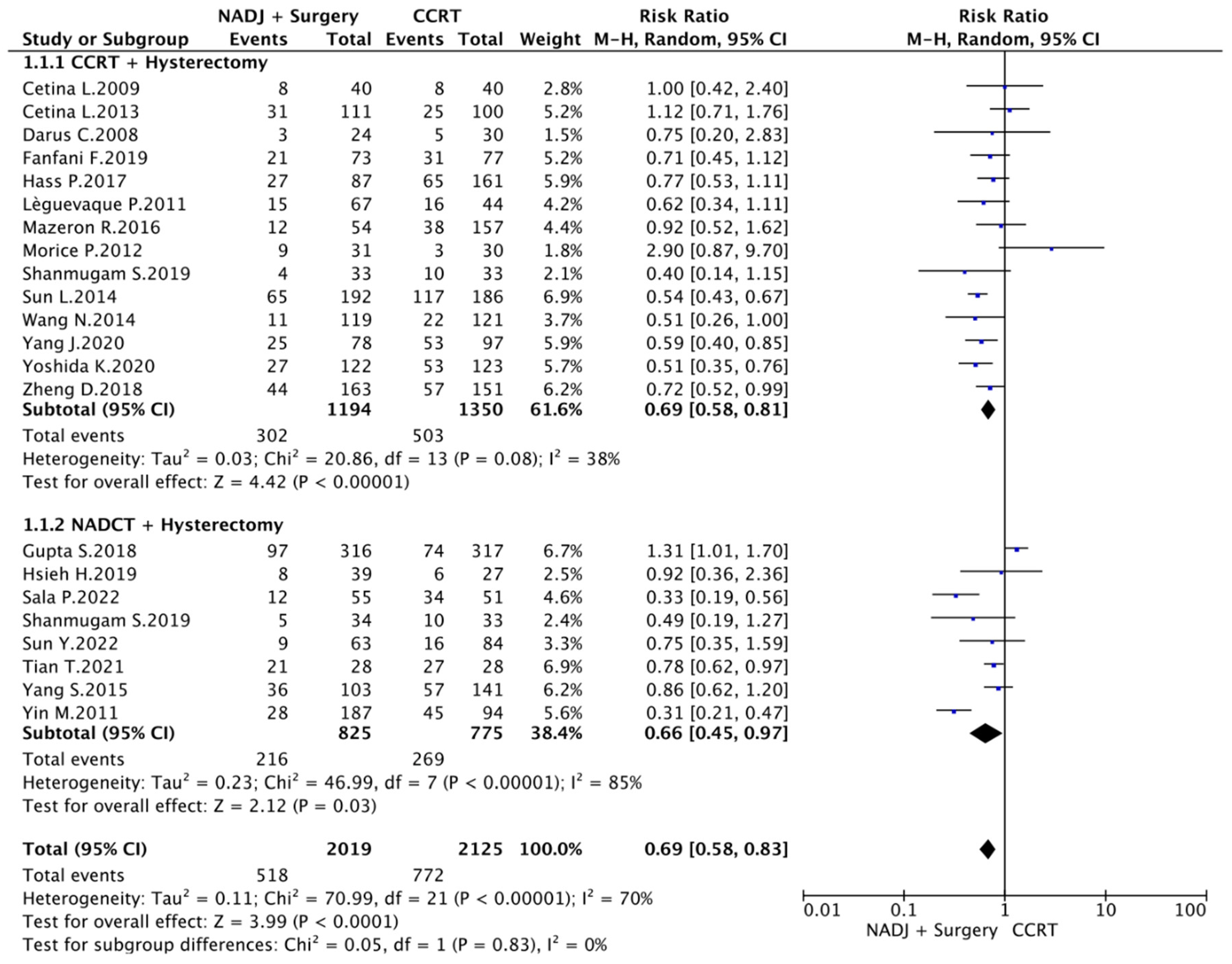
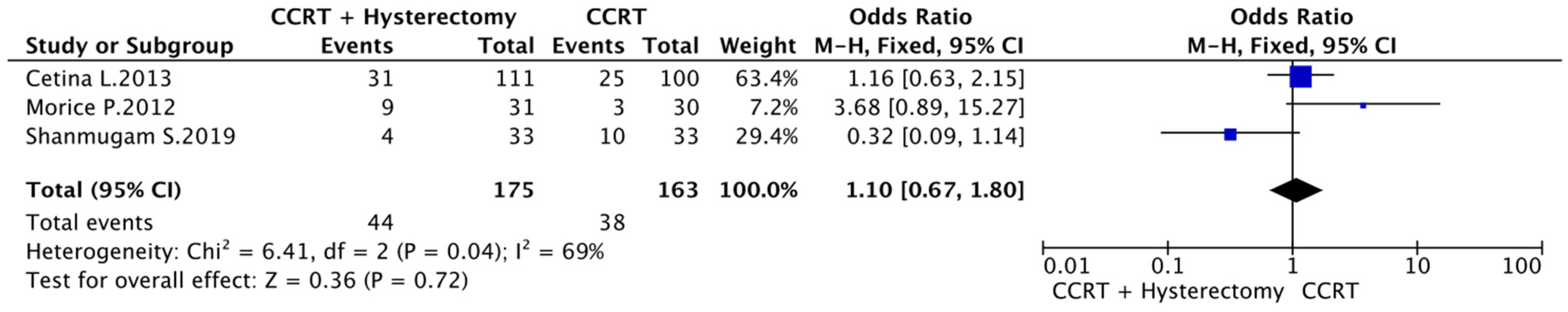
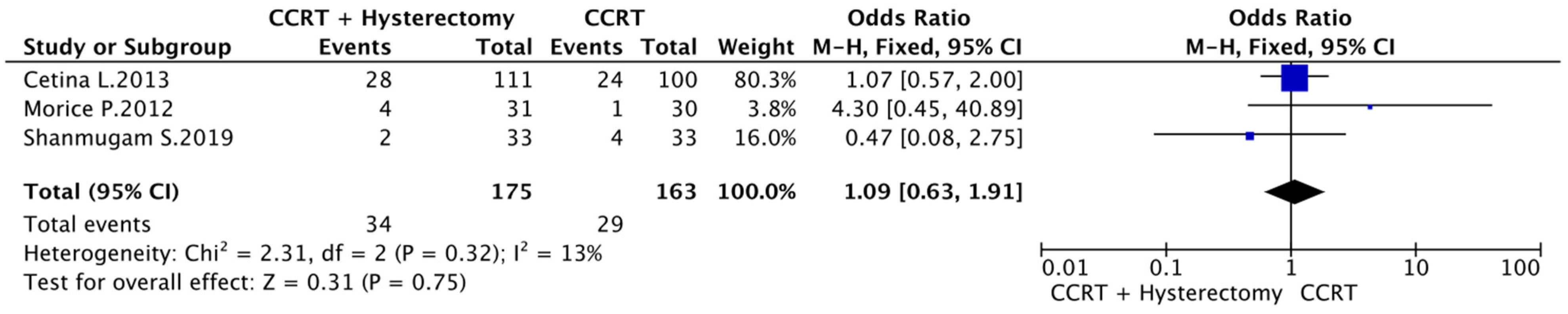
3.3. CCRT vs. CCRT plus Hysterectomy
3.4. CCRT vs. NACT plus Surgery
3.5. Complete Response Rate, Toxicity and Type of Recurrence
3.6. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S.; Zigliani, L.; Odicino, F. Revised FIGO staging for carcinoma of the cervix. Int. J. Gynecol. Obstet. 2009, 105, 107–108. [Google Scholar] [CrossRef]
- Quinn, M.A.; Benedet, J.L.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.T.; Heintz, A.P.M.; Ngan, H.Y.S.; Pecorelli, S. Carcinoma of the Cervix Uteri. Int. J. Gynaecol. Obstet. 2006, 95, S43–S103. [Google Scholar] [CrossRef] [PubMed]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B.; Muderspach, L.I.; Chafe, W.E.; Suggs, C.L.; Walker, J.L.; Gersell, D. Cisplatin, Radiation, and Adjuvant Hysterectomy Compared with Radiation and Adjuvant Hysterectomy for Bulky Stage IB Cervical Carcinoma. N. Engl. J. Med. 1999, 340, 1154–1161. [Google Scholar] [CrossRef]
- Peters, W.A.; Liu, P.Y.; Barrett, R.J.; Stock, R.J.; Monk, B.J.; Berek, J.S.; Alberts, D.S. Concurrent Chemotherapy and Pelvic Radiation Therapy Compared with Pelvic Radiation Therapy Alone as Adjuvant Therapy After Radical Surgery in High-Risk Early-Stage Cancer of the Cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Nunes de Arruda, F.; da Costa, S.; Bonadio, R.; Dornellas, A.; Pereira, D.; de Bock, G.; Del Pilar Estevez Diz, M. Quality of life of locally advanced cervical cancer patients after neoadjuvant chemotherapy followed by chemoradiation versus chemoradiation alone (CIRCE trial): A randomized phase II trial. Int. J. Gynecol. Cancer 2020, 30, 749–756. [Google Scholar] [CrossRef]
- Gupta, S.; Maheshwari, A.; Parab, P.; Mahantshetty, U.; Hawaldar, R.; Sastri, S.; Kerkar, R.; Engineer, R.; Tongaonkar, H.; Ghosh, J.; et al. Neoadjuvant Chemotherapy Followed by Radical Surgery Versus Concomitant Chemotherapy and Radiotherapy in Patients with Stage IB2, IIA, or IIB Squamous Cervical Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Ferrandina, G.; Legge, F.; Fagotti, A.; Fanfani, F.; Distefano, M.; Morganti, A.; Cellini, N.; Scambia, G. Preoperative concomitant chemoradiotherapy in locally advanced cervical cancer: Safety, outcome, and prognostic measures. Gynecol. Oncol. 2007, 107 (Suppl. S1), S127–S132. [Google Scholar] [CrossRef]
- Ferrandina, G.; Lucidi, A.; Paglia, A.; Corrado, G.; Macchia, G.; Tagliaferri, L.; Fanfani, F.; Morganti, A.G.; Valentini, V.; Scambia, G. Role of comorbidities in locally advanced cervical cancer patients administered preoperative chemoradiation: Impact on outcome and treatment-related complications. Eur. J. Surg. Oncol. EJSO 2012, 38, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Meder, C.H.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients with Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Li, H.; Peng, B. Neoadjuvant and Adjuvant Treatments Compared to Concurrent Chemoradiotherapy for Patients with Locally Advanced Cervical Cancer: A Bayesian Network Meta-Analysis. Front. Oncol. 2022, 12, 745522. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Berek, J.S.; Fredes, M.C.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The clavien-dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Chaimani, A.; Higgins, J.P.T.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical Tools for Network Meta-Analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef]
- Kansagara, D.; O’Neil, M.N.; Zakher, B.; Motu’apuaka, M.; Paynter, R. Quality Assessment Criteria for Observational Studies, Based on the Newcastle-Ottawa Scale. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK476448/table/appc.t4 (accessed on 27 March 2022).
- Shanmugam, S.; Govindasamy, G.; Hussain, S.A.; Mani, J.G. Comparison of Neoadjuvant Chemotherapy Followed by Radical Hysterectomy and Neoadjuvant Chemoradiation Followed by Radical Hysterectomy with Concurrent Chemoradiation in Locally Advanced Carcinoma Cervix (FIGO Stages IB2, IIA2, IIB): Interim Results of a Randomized Control Study. J. S. Asian Fed. Obstet. Gynaecol. 2019, 11, 35–43. [Google Scholar] [CrossRef]
- Albert, A.; Allbright, R.; Lee, A.; Vijayakumar, S. Preoperative chemoradiation followed by hysterectomy for cervical cancer: Patterns of care and survival in a large, hospital database. J. Gynecol. Oncol. 2019, 30, e41. [Google Scholar] [CrossRef] [PubMed]
- Cetina, L.; González-Enciso, A.; Cantú, D.; Coronel, J.; Pérez-Montiel, D.; Hinojosa, J.; Serrano, A.; Rivera, L.; Poitevin, A.; Mota, A.; et al. Brachytherapy versus radical hysterectomy after external beam chemoradiation with gemcitabine plus cisplatin: A randomized, phase III study in IB2-IIB cervical cancer patients. Ann. Oncol. 2013, 24, 2043–2047. [Google Scholar] [CrossRef] [PubMed]
- Cetina, L.; Garcia-Arias, A.; Candelaria, M.; Cantú, D.; Rivera, L.; Coronel, J.; Bazan-Perkins, B.; Flores, V.; Gonzalez, A.; Dueñas-González, A. Brachytherapy versus radical hysterectomy after external beam chemoradiation: A non-randomized matched comparison in IB2-IIB cervical cancer patients. World J. Surg. Oncol. 2009, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Chereau, E.; De la Hosseraye, C.; Ballester, M.; Monnier, L.; Rouzier, R.; Touboul, E.; Darai, E. The role of completion surgery after concurrent radiochemotherapy in locally advanced stages IB2-IIB cervical cancer. Anticancer Res. 2013, 33, 1661–1666. [Google Scholar]
- Darus, C.J.; Callahan, M.B.; Nguyen, Q.-N.; Pastore, L.M.; Schneider, B.F.; Rice, L.W.; Jazaeri, A.A. Chemoradiation with and without adjuvant extrafascial hysterectomy for IB2 cervical carcinoma. Int. J. Gynecol. Cancer 2008, 18, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Fanfani, F.; Monterossi, G.; Vizza, E.; Corrado, G. Chemoradiation versus neoadjuvant chemoradiation followed by radical surgery for FIGO stage III cervical cancer: Analysis of complications and 3-year survival. Gynecol. Oncol. 2015, 137, 30. [Google Scholar] [CrossRef]
- Hass, P.; Eggemann, H.; Costa, S.D.; Ignatov, A. Adjuvante Hysterektomie nach Radiochemotherapie bei lokal fortgeschrittenem Zervixkarzinom. Strahlenther. Onkol. 2017, 193, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.Y.; Huang, J.W.; Lu, C.H.; Lin, J.C.; Wang, L. Definite chemoradiotherapy is a competent treatment option in FIGO stage IB2 cervical cancer compared with radical surgery +/− neoadjuvant chemotherapy. J. Formos. Med. Assoc. 2019, 118, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Lèguevaque, P.; Motton, S.; Delannes, M.; Querleu, D.; Soulé-Tholy, M.; Tap, G.; Houvenaeghel, G. Completion surgery or not after concurrent chemoradiotherapy for locally advanced cervical cancer? Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Mazeron, R.; Gouy, S.; Chargari, C.; Del Campo, E.R.; Dumas, I.; Mervoyer, A.; Genestie, C.; Bentivegna, E.; Balleyguier, C.; Pautier, P.; et al. Post radiation hysterectomy in locally advanced cervical cancer: Outcomes and dosimetric impact. Radiother. Oncol. 2016, 120, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Rouanet, P.; Rey, A.; Romestaing, P.; Houvenaeghel, G.; Boulanger, J.C.; Leveque, J.; Cowen, D.; Mathevet, P.; Malhaire, J.P.; et al. Results of the GYNECO 02 Study, an FNCLCC Phase III Trial Comparing Hysterectomy with No Hysterectomy in Patients with a (Clinical and Radiological) Complete Response After Chemoradiation Therapy for Stage IB2 or II Cervical Cancer. Oncologist 2012, 17, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.S.; Kang, S.B.; Kim, K.T.; Chang, K.H.; Kim, J.W.; Kim, J.H. Efficacy of different types of treatment in FIGO stage IB2 cervical cancer in Korea: Results of a multicenter retrospective Korean study (KGOG-1005). Int. J. Gynecol. Cancer 2007, 17, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Sala, P.; Bogliolo, S.; Barra, F.; Fazio, A.; Maramai, M.; Cassani, C.; Gardella, B.; Babilonti, L.; Giannelli, F.; Mammoliti, S.; et al. Neoadjuvant Chemotherapy Followed by Radical Surgery versus Concurrent Chemo-Radiotherapy in the Treatment of Locally Advanced Cervical Cancer: A Multicenter Retrospective Analysis. J. Investig. Surg. 2022, 35, 308–314. [Google Scholar] [CrossRef]
- Li, S.; Sheng, X.; Jiang, J.; Li, X.; Liu, N.; Liu, Y.; Zhang, T.; Li, D.; Zhang, X.; Wei, P. Surgical morbidity and oncologic results after concurrent chemoradiation therapy for advanced cervical cancer. Int. J. Gynecol. Obstet. 2014, 125, 111–115. [Google Scholar] [CrossRef]
- Sun, Y.; Li, G.; Hai, P.; Cao, Y.; Han, P.; Liu, Y.; Wen, J.; Wang, Y.; Cheng, X.; Ren, F. The comparative study for survival outcome of locally advanced cervical cancer treated by neoadjuvant arterial interventional chemotherapy or intravenous chemotherapy followed by surgery or concurrent chemoradiation. World J. Surg. Oncol. 2022, 20, 389. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Gao, X.; Ju, Y.; Ding, X.; Ai, Y. Comparison of the survival outcome of neoadjuvant therapy followed by radical surgery with that of concomitant chemoradiotherapy in patients with stage IB2–IIIB cervical adenocarcinoma. Arch. Gynecol. Obstet. 2021, 303, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, W.-W.; Li, J.-P.; Liu, J.-Y.; Zhou, Y.-C.; Zhang, Y.; Hu, J.; Huang, Y.-H.; Chen, Y.; Wei, L.-C.; et al. Comparison of concurrent chemoradiotherapy followed by radical surgery and high-dose-rate intracavitary brachytherapy: A retrospective study of 240 patients with FIGO stage IIB cervical carcinoma. OncoTargets Ther. 2014, 7, 91–100. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Cao, D.; Shen, K.; Ma, J.; Zhang, F. Completion hysterectomy after chemoradiotherapy for locally advanced adeno-type cervical carcinoma: Updated survival outcomes and experience in post radiation surgery. J. Gynecol. Oncol. 2020, 31, e16. [Google Scholar] [CrossRef] [PubMed]
- SYang, S.; Gao, Y.; Sun, J.; Xia, B.; Liu, T.; Zhang, H.; Li, Q.; Xiao, M.; Zhang, Y. Neoadjuvant chemotherapy followed by radical surgery as an alternative treatment to concurrent chemoradiotherapy for young premenopausal patients with FIGO stage IIB squamous cervical carcinoma. Tumor Biol. 2015, 36, 4349–4356. [Google Scholar] [CrossRef]
- Yin, M.; Zhao, F.; Lou, G.; Zhang, H.; Sun, M.; Li, C.; Hou, Y.; Li, X.; Meng, F.; Chen, X. The long-term efficacy of neoadjuvant chemotherapy followed by radical hysterectomy compared with radical surgery alone or concurrent chemoradiotherapy on locally advanced-stage cervical cancer. Int. J. Gynecol. Cancer 2011, 21, 92–99. [Google Scholar] [CrossRef]
- Yoshida, K.; Kajiyama, H.; Yoshihara, M.; Tamauchi, S.; Ikeda, Y.; Yoshikawa, N.; Nishino, K.; Niimi, K.; Suzuki, S.; Kikkawa, F. The role of additional hysterectomy after concurrent chemoradiation for patients with locally advanced cervical cancer. Int. J. Clin. Oncol. 2020, 25, 384–390. [Google Scholar] [CrossRef]
- Zheng, D.; Mou, H.-P.; Diao, P.; Li, X.-M.; Zhang, C.-L.; Jiang, J.; Chen, J.-L.; Wang, L.-S.; Wang, Q.; Zhou, G.-Y.; et al. Chemoradiotherapy in Combination with Radical Surgery Is Associated with Better Outcome in Cervical Cancer Patients. Oncotarget 2017, 9, 2866–2875. [Google Scholar] [CrossRef]
- Piver, M.S.; Rutledge, F.; Smith, J.P. Five classes of extended hysterectomy for women with cervical cancer. Obstet. Gynecol. 1974, 44, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Querleu, D.; Cibula, D.; Abu-Rustum, N.R. 2017 Update on the Querleu–Morrow Classification of Radical Hysterectomy. Ann Surg Oncol. 2017, 24, 3406–3412. [Google Scholar] [CrossRef]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent Cisplatin-Based Radiotherapy and Chemotherapy for Locally Advanced Cervical Cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic Radiation with Concurrent Chemotherapy Compared with Pelvic and Para-Aortic Radiation for High-Risk Cervical Cancer. N. Engl. J. Med. 1999, 340, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Green, J.A.; Kirwan, J.M.; Tierney, J.F.; Symonds, P.; Fresco, L.; Collingwood, M.; Williams, C.J. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet 2001, 358, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Stutz, E.; Liu, M.; Rogers, S.; Klingbiel, D.; Siebenhüner, A.; Singh, S.; Bodis, S. Concurrent chemoradiotherapy vs. radiotherapy alone in locally advanced cervix cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2017, 145, 374–385. [Google Scholar] [CrossRef]
- Ronsini, C.; Anchora, L.P.; Restaino, S.; Fedele, C.; Arciuolo, D.; Teodorico, E.; Bizzarri, N.; Zannoni, G.F.; Ferrandina, G.; Scambia, G.; et al. The role of semiquantitative evaluation of lympho-vascular space invasion in early stage cervical cancer patients. Gynecol. Oncol. 2021, 162, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W.T.; Kohler, M.F. Is lymph vascular space involvement an independent prognostic factor in early cervical cancer? Gynecol. Oncol. 2004, 92, 525–529. [Google Scholar] [CrossRef]
- Ronsini, C.; De Franciscis, P.; Carotenuto, R.M.; Pasanisi, F.; Cobellis, L.; Colacurci, N. The Oncological Implication of Sentinel Lymph Node in Early Cervical Cancer: A Meta-Analysis of Oncological Outcomes and Type of Recurrences. Medicina 2022, 58, 1539. [Google Scholar] [CrossRef]
- Carter, J.; Sonoda, Y.; Aburustum, N. Reproductive concerns of women treated with radical trachelectomy for cervical cancer. Gynecol. Oncol. 2007, 105, 13–16. [Google Scholar] [CrossRef]
- Ronsini, C.; Solazzo, M.C.; Molitierno, R.; De Franciscis, P.; Pasanisi, F.; Cobellis, L.; Colacurci, N. Fertility-Sparing Treatment for Early-Stage Cervical Cancer ≥2 cm: Can One Still Effectively Become a Mother? A Systematic Review of Fertility Outcomes. Ann. Surg. Oncol. 2023, 30, 5587–5596. [Google Scholar] [CrossRef] [PubMed]
- Ronsini, C.; Solazzo, M.C.; Bizzarri, N.; Ambrosio, D.; La Verde, M.; Torella, M.; Carotenuto, R.M.; Cobellis, L.; Colacurci, N.; De Franciscis, P. Fertility-Sparing Treatment for Early-Stage Cervical Cancer ≥2 cm: A Problem with a Thousand Nuances—A Systematic Review of Oncological Outcomes. Ann. Surg. Oncol. 2022, 29, 8346–8358. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Kim, S.N.; Chae, S.H.; Kim, J.E.; Lee, S.J. Impact of adjuvant hysterectomy on prognosis in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy: A meta-analysis. J. Gynecol. Oncol. 2018, 29, e25. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Liang, Z.; Zhang, C.; Zhang, H.; Liu, X. The effect of surgery on the survival status of patients with locally advanced cervical cancer after radiotherapy/chemoradiotherapy: A meta-analysis. BMC Cancer 2018, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Lu, C.; Yu, Z.; Gao, L. Chemoradiotherapy alone vs. chemoradiotherapy and hysterectomy for locally advanced cervical cancer: A systematic review and updated meta-analysis. Oncol. Lett. 2020, 21, 160. [Google Scholar] [CrossRef] [PubMed]
- Fanfani, F.; Fagotti, A.; Ferrandina, G.; Raspagliesi, F.; Ditto, A.; Cerrotta, A.M.; Morganti, A.; Smaniotto, D.; Scambia, G. Neoadjuvant Chemoradiation Followed by Radical Hysterectomy in FIGO Stage IIIB Cervical Cancer: Feasibility, Complications, and Clinical Outcome. Int. J. Gynecol. Cancer 2009, 19, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Ferrandina, G.; Chiantera, V.; Fagotti, A.; Fanfani, F.; Ercoli, A.; Legge, F.; Costantini, B.; Alletti, S.G.; Bottoni, C.; et al. Laparoscopic Radical Hysterectomy After Concomitant Chemoradiation in Locally Advanced Cervical Cancer: A Prospective Phase II Study. J. Minim. Invasive Gynecol. 2015, 22, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.E.; Bertrand, M.M.; Gutowski, M.; Mourregot, A.; Fabbro, M.; Saint-Aubert, B.; Quenet, F.; Gourgou, S.; Kerr, C.; Rouanet, P. Total laparoscopic radical hysterectomy for locally advanced cervical carcinoma (stages IIB, IIA and bulky stages IB) after concurrent chemoradiation therapy: Surgical morbidity and oncological results. Gynecol. Oncol. 2009, 114, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ronsini, C.; Köhler, C.; De Franciscis, P.; La Verde, M.; Mosca, L.; Solazzo, M.C.; Colacurci, N. Laparo-assisted vaginal radical hysterectomy as a safe option for Minimal Invasive Surgery in early stage cervical cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2022, 166, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Vizza, E.; Corrado, G.; Mancini, E.; Vici, P.; Sergi, D.; Baiocco, E.; Patrizi, L.; Saltari, M.; Pomati, G.; Cutillo, G. Laparoscopic versus robotic radical hysterectomy after neoadjuvant chemotherapy in locally advanced cervical cancer: A case control study. Eur. J. Surg. Oncol. EJSO 2015, 41, 142–147. [Google Scholar] [CrossRef]
- Benedetti-Panici, P.; Greggi, S.; Colombo, A.; Amoroso, M.; Smaniotto, D.; Giannarelli, D.; Amunni, G.; Raspagliesi, F.; Zola, P.; Mangioni, C.; et al. Neoadjuvant Chemotherapy and Radical Surgery Versus Exclusive Radiotherapy in Locally Advanced Squamous Cell Cervical Cancer: Results from the Italian Multicenter Randomized Study. J. Clin. Oncol. 2002, 20, 179–188. [Google Scholar] [CrossRef]
- Zou, W.; Han, Y.; Zhang, Y.; Hu, C.; Feng, Y.; Zhang, H.; Wang, J. Neoadjuvant chemotherapy plus surgery versus concurrent chemoradiotherapy in stage IB2-IIB cervical cancer: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0225264. [Google Scholar] [CrossRef]
| Comparative Studies | ||||||
|---|---|---|---|---|---|---|
| Name | Country | Study Design | Study Year | FIGO Stage | N of Partecipant (NADJ/CCRT) | Mean FUP * Months |
| Albert A., 2019 [18] | USA | Retrospective Case-Control Monocentric Study | 2010–2014 | IB2 IIA | 1546 (139/1407) | 33.3 |
| Cetina L., 2013 [19] | Mexico | Prospective Case-Control Monocentric Study | 2004–2009 | IB2 IIA2 IIB | 211 (111/100) | 36 |
| Cetina L., 2009 [20] | Mexico | Retrospective matched Control Monocentric Study | 1999–2003 | IB2 IIA IIB | 140 (40/100) | 29 |
| Chereau E., 2013 [21] | France | Retrospective Case-Control Monocentric Study | 2002–2012 | IB2 II | 80 (46/34) | 30.7 |
| Darus C., 2008 [22] | USA | Retrospective Case-Control Monocentric Study | 1994–2004 | IB2 | 54 (24/30) | 46.8 |
| Fanfani F., 2019 [23] | Italy | Retrospective Case-Control Multicentric Study | 1999–2013 | IIIA IIIB | 150 (73/77) | 40 |
| Gupta S., 2018 [7] | India | Randomized Control Monocentric Trial | 2003–2015 | IB2 IIA IIB | 633 (316/317) | 58.5 |
| Hass P., 2017 [24] | Germany | Retrospective Case-Control Multicentric Study | 2003–2011 | IB2-IVA | 248 (87/161) | 57 |
| Hsieh H., 2019 [25] | Republic of China | Retrospective Case-Control Monocentric Study | 2002–2016 | IB2 | 66 ^ (39/27) | 66.2 |
| Lèguevaque P., 2011 [26] | France | Retrospective Case Control Multicentric Study | 1989–2006 | IB1-IVA | 111 (67/44) | - |
| Mazeron R., 2016 [27] | Russia | Retrospective Case-Control Monocentric Study | 2004–2008 | IB1 IB2 IIA IIB | 211 (54/157) | 57,4 |
| Morice P., 2012 [28] | France | Randomized Control Multicentric Trial | 2003–2006 | IB2 II | 61 (31/30) | 44 |
| Ryu H., 2007 [29] | Korea | Retrospective Observational Multicentric Study | 1995–2005 | IB2 | 132 ^ (81/51) | 120 |
| Sala P., 2022 [30] | Italy | Retrospective Case-Control Multicentric Study | 2006–2018 | IB2-IVA | 106 (55/51) | 33 |
| Shanmugam S., 2019 [17] | India | Randomized Control Monocentric Trial | 2014–2018 | IB2 IIA2 IIB | 100 (34/33/33) | 28 |
| Sun L., 2014 [31] | China | Retrospective Case-Control Monocentric Study | 1992–2012 | IIB III IVA | 378 (192/186) | 190 |
| Sun Y., 2022 [32] | China | Retrospective Case-Control Multicentric Study | 2013–2019 | IB2 IIA2 IIB | 147 (63/84) | 60 |
| Tian T., 2021 [33] | China | Retrospective Matched Control Monocentric Study | 2013–2017 | IB2 IIA2 IIB IIIB | 56 a (28/28) | - |
| Wang N., 2014 [34] | China | Prospective Case-Control Monocentric Study | 2004–2011 | IIB | 240 (119/121) | 33 |
| Yang J., 2020 [35] | China | Retrospective Case-Control Monocentric Study | 2004–2018 | IB IIA IIB III | 175 (78/97) | 20.5 |
| Yang S., 2015 [36] | China | Retrospective Case-Control Monocentric Study | 2007–2009 | IIB | 244 (103/141) | 67 |
| Yin M., 2011 [37] | China | Retrospective Case-Control Monocentric Study | 2000–2005 | IB2 IIA IIB | 281 (187/94) | 82.8 |
| Yoshida K., 2020 [38] | Japan | Retrospective Matched Case-Control Monocentric Study | 2005–2015 | IB2 IIA IIB | 245 (122/123) | 64.8 |
| Zheng D., 2018 [39] | China | Retrospective Case-Control Monocentric Study | 2008–2013 | IB2 IIB | 314 (163/151) | 60 |
| NADJ | CCRT | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | NADJ Treatment | 3Y DFS (%) | 3Y OS (%) | 4.5Y DFS (%) | 4.5Y OS (%) | FU | 3Y DFS (%) | 3Y OS (%) | 4.5Y DFS (%) | 4.5Y OS (%) | FU | P |
| Albert A., 2019 [18] | Radiation | - | - | - | 82.2% | - | - | - | - | 74.9% | 33 | 0.036 |
| Cetina L., 2013 [19] | 71.7 | 74.5 | - | - | 36 | 74.8 | 76.3 | - | - | 36 | 0.186 (3YDFS) 0.236 | |
| Cetina L., 2009 [20] | - | - | 78% | 78% | 26 | - | - | 78% | 78% | 22 | NS | |
| Chereau E., 2013 [21] | - | - | 86.9% | - | - | 85.3% | 30.7 | NS | ||||
| Darus C., 2008 [22] | - | - | 83,3 | 87.5 | 46.8 | - | - | 86.6 | 83.3 | 46.8 | 0.7 (DFS) 0.8 (OS) | |
| Fanfani F., 2019 [23] | 71.23 | 63.6 | - | - | 39 | 59.7 | 63.6 | - | - | 41 | 0.686 (3YDFS) 0.675 | |
| Gupta S., 2018 [7] | Chemotherapy | - | - | 69.3 | 75.4 | 58.5 | - | - | 76.7 | 74.7 | 58.5 | 0.038 (3YDFS) 0.870 |
| Hass P., 2017 [24] | - | - | 68.9 | - | 53 | - | - | 59.6 | - | 53 | - | |
| Hsieh H., 2019 [25] | - | - | 79.3 | 94.1 | 83.5 | - | - | 79.5 | 80.1 | 63.3 | 0.401 (4.5YDFS) 0.197 | |
| Lèguevaque P., 2011 [26] | - | - | 77.6 | 83.5 | - | - | - | 65.9 | 79.5 | - | 0.558 (4.5DFS) 0.296 | |
| Mazeron R., 2016 [27] | - | - | 77.7 | 83.3 | 67.9 | - | - | 75.8 | 78.9 | 42.1 | 0.926 (4.5DFS) 0.630 | |
| Morice P., 2012 [28] | 72 | 86 | - | - | 44 | 89 | 97 | - | - | 44 | NS (3YDFS) NS | |
| Ryu H., 2007 [29] | - | - | - | 90 | 120 | - | - | - | 83 | 120 | NS | |
| Sala P., 2022 [30] | - | - | 77.4 | 93.8 | 33 | - | - | 33.4 | 56.5 | 33 | <0.001 (4.5DFS) 0.003 | |
| Shanmugam S., 2019 [17] | 85 88 | 100 94 | - | - | 28 | 70 | 88 | - | - | 28 | 0.571 | |
| Sun L., 2014 [31] | 83.2 | 72.2 | 66.1 | 47.9 | 190 | 54.1 | 45.9 | 37.1 | 32.2 | 190 | <0.005 | |
| Sun Y., 2022 [32] | 90.5 | 95.2 | 86.1 | 89.9 | 60 | 89.3 | 95.2 | 80.6 | 89.9 | 60 | 0.849 (3YDFS) 0.816 (3YOS) >0.05 (4.5YDFS7OS) | |
| Tian T., 2021 [33] | - | - | 25 a | 25 b | - | - | - | 4 a | 4 b | - | 0.00015 (4.5YDFS) 0.00014 | |
| Wang N., 2014 [34] | 91 | 94.9 | - | - | 36 | 81.8 | 84.6 | - | - | 30 | 0.049 (3YDFS) 0.011 | |
| Yang J., 2020 [35] | 67.9 | 92.3 | - | - | 28 | 45.4 | 67 | - | - | 16 | 0.002 (3YDFS) 0.002 | |
| Yin M., 2011 [37] | - | - | 65 | 78.6 | 67 | - | - | 59.4 | 74.5 | 67 | 0.456 (4.5YDFS) 0.637 | |
| Yoshida K., 2020 [38] | - | - | 85 | 88.67 | 82.8 | - | - | 52 | 64.37 | 82.8 | <0.0001 (4.5DFS) <0.0001 | |
| Zheng D., 2018 [39] | - | - | 78.3 a | 87.7 b | 64.8 | - | 56.9 a | 66.2 b | 64.8 | 0.027 (4.5DFS) 0.017 | ||
| Yin M., 2011 [37] | 77.3 | 87.1 | 73.3 | 81.7 | 60 | 67.2 | 72.8 | 62.4 | 67.3 | 60 | 0.01 (3–4.5YDFS) 0.001 (3–4.5OS) | |
| Name | EBRT (Gy) | BRT Yes/No (Gy) | ADJ Surgery | Pelvic Lymphadenectomy Yes/No |
|---|---|---|---|---|
| Albert A., 2019 [18] | 60 | NA | SH | NA |
| Cetina L., 2013 [19] | 50.4 | No | RH | Yes |
| Cetina L., 2009 [20] | 50 | No a | RH | Yes |
| Chereau E., 2013 [21] | 40 | Yes (20) | SH/RH | Yes |
| Darus C., 2008 [22] | 45 | Yes (30) | SH | No |
| Fanfani F., 2019 [23] | 50 | Yes (30) | RS | Yes |
| Hass P., 2017 [24] | 50.4 | No | RS | No b |
| Lèguevaque P., 2011 [26] | 45 | Yes (15) | SH/RH | Yes |
| Mazeron R., 2016 [27] | 40–50.4 | Yes (20) | SH/RH | No |
| Morice P., 2012 [28] | 45 | Yes (15) | SH/RH | Yes c |
| Shanmugam S., 2019 [17] | 50 | No | RH | NA |
| Sun L., 2014 [31] | 45–50 | Yes (45–55) | SH/RH | No |
| Wang N., 2014 [34] | 40–50 | No | RH | Yes |
| Yang J., 2020 [35] | NA | Yes | SH | No |
| Yoshida K., 2020 [38] | 39.6 | No | RH | Yes |
| Zheng D., 2018 [39] | 46–50 | Yes (25–30) | RH | Yes |
| Name | Drugs | N of Cycle | ADJ Surgery | Pelvic Lymphadenectomy Yes/No |
|---|---|---|---|---|
| Gupta S., 2018 [7] | Paclitaxel + Carboplatin | 3 | RH | Yes |
| Hsieh H., 2019 [25] | Cisplatin | 6 | RH | Yes |
| Ryu H., 2007 [29] | NA | NA | RH | NA |
| Sala P., 2022 [30] | Platinum based combination | 3 | RH | Yes |
| Shanmugam S., 2019 [17] | Paclitaxel + Cisplatin | 3 | RH | Yes |
| Sun Y., 2022 [32] | Paclitaxel + Platinum | 2 | RH | Yes |
| Tian T., 2021 [33] | Paclitaxel + Cisplatin | 2–3 | RH | Yes |
| Yang S., 2015 [36] | cisplatin/nedaplatin/carboplatin + pac-litaxel | 1–3 | RH | Yes |
| Yin M., 2011 [37] | Platinum-based com-bination | 2–3 | RH | Yes |
| Name | NADJ CR (%) | CCRT CR (%) | p |
|---|---|---|---|
| Cetina L., 2009 [20] | 22 (55%) | 34(85%) | 0.2 |
| Cetina L., 2013 [19] | 62 (72%) | - | - |
| Darus C., 2008 [22] | 13 (67%) | - | |
| Chereau E., 2013 [21] | 27 (12.4%) | - | - |
| Fanfani F., 2019 [23] | 41.5% | 38.9% | 0.6 |
| Hass P., 2017 [24] | 40 (40.6%) | 81 (50.3%) | 0.5 |
| Hsieh H., 2019 [25] | 5 (12.8%) | - | |
| Shanmugam S., 2019 [17] | 24 (35.8%) | 24 (72%) | 0.001 |
| Sun L., 2014 [31] | 165 (85.9%) | - | - |
| Wang N., 2014 [34] | 33 (27.73%) | - | - |
| Yang J., 2020 [35] | - | 28 (35.9%) | |
| Yoshida K., 2020 [38] | 12 (24%) | 26(34.2%) | 0.2 |
| Name | NADJ Early Complication Rate (%) | NADJ Late Complication Rate (%) | Tot. % |
|---|---|---|---|
| Cetina L., 2013 [19] | 0 | 5 (5.8%) | 5.8 |
| Cetina L., 2009 [20] | - | 9 (22.5%) | 22.5 |
| Chereau E., 2013 [21] | - | - | 6.4 |
| Fanfani F., 2019 [23] | 5 (6.8%) | 3 (4.1%) | 10.9 |
| Hass P., 2017 [24] | 2 (2.2%) | 2.2 | |
| Mazeron R., 2016 [27] | 11 (20.3%) | 20.3 | |
| Yang J., 2020 [35] | 5 (6.4%) | - | 6.4 |
| Yoshida K., 2020 [38] | 14 (28%) | 28 |
| Name | NADJ Recurrence Rate (%), n | Tot. | CCRT Recurrence Rate (%), n | Tot. | p |
|---|---|---|---|---|---|
| Cetina L., 2009 [20] | 8 (20%) | 40 | 8 (20%) | 40 | 1 |
| Cetina L., 2013 [19] | 13 (11.7%) | 86 | 15 (15%) | 86 | 0.9 |
| Darus C., 2008 [22] | 3 (12.5%) | 24 | 5 (16.6%) | 30 | ns |
| Fanfani F., 2019 [23] | 21 (28.7) | 73 | 31 (40.2) | 77 | <0.001 |
| Gupta S., 2018 [7] | 59 (18.6%) | 316 | 43 (13.56%) | 317 | 0.08 |
| Hass P., 2017 [24] | 27 (31%) | 87 | 65 (40.3%) | 161 | 0.4 |
| Hsieh H., 2019 [25] | 7 (17.9%) | 39 | 11 (40.7%) | 27 | ns |
| Lèguevaque P., 2011 [26] | 15 (2.4%) | 67 | 16 (36.4%) | 44 | 0.01 |
| Mazeron R., 2016 [27] | 14 (29.5%) | 54 | 36 (22.9%) | 157 | 0.6 |
| Morice P., 2012 [28] | 8 (25.8%) | 31 | 4 (13.3%) | 30 | 0.2 |
| Sun L., 2014 [31] | 32 (16.7%) | 192 | 59 (31.7%) | 186 | 0.0006 |
| Sun Y., 2022 [32] | 6 (9.5%) | 63 | 12 (14.2%) | 84 | 0.3 |
| Tian T., 2021 [33] | 21 | 28 | 27 | 28 | 0.02 |
| Wang N., 2014 [34] | 11 (9.24) | 119 | 22 (18.18) | 121 | 0.06 |
| Yang J., 2020 [35] | 16 (33.33%) | 78 | 45 (50.56%) | 97 | 0.025 |
| Yang S., 2015 [36] | 59 (57.28%) | 103 | 37 (26.24%) | 141 | 0.3 |
| Yin M., 2011 [37] | 28 | 187 | 45 | 94 | >0.05 |
| Yoshida K., 2020 [38] | 15 (30%) | 50 | 28 (36.8%) | 76 | 0.4 |
| Zheng D., 2018 [39] | 48 (29.4%) | 163 | 58 (38.4%) | 151 | 0.009 |
| Name | NADJ Recurrence Site (n) | CCRT Recurrence Site (n) | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| l | s | ls | Tot | FUP * | l | s | ls | Tot | FUP * | ||
| Cetina L., 2009 [20] | 6 | 0 | 2 | 8 | 26 | 6 | 1 | 1 | 8 | 22 | ns |
| Cetina L., 2013 [19] | 7 | 6 | 0 | 13 | 36 | 10 | 5 | 0 | 15 | 36 | 0.4 (l) 0.4 (s) |
| Darus C., 2008 [22] | 1 | - | - | 3 | 24 | 1 | 3 | 1 | 5 | 30 | ns |
| Fanfani F., 2019 [23] | 2 | 3 | - | 21 | 39 | 7 | 6 | - | 31 | 41 | <0.002 (l) 0.9 (s) |
| Gupta S., 2018 [7] | 39 | 11 | 20 | 89 | 58.5 | 19 | 22 | 24 | 73 | 58.5 | 0.01 (l) |
| Hass P., 2017 [24] | 20 | 7 | 0 | 27 | 58.5 | 44 | 21 | 0 | 65 | 58.5 | 0.5 (l) 0.2 (s) |
| Hsieh H., 2019 [25] | 7 | 4 | 0 | 11 | 83.5 | 3 | 4 | 0 | 7 | 63.3 | ns ns |
| Lèguevaque P., 2011 [26] | 6 | 9 | 0 | 15 | - | 9 | 7 | 0 | 16 | - | 0.3 (l) 0.3 (s) |
| Mazeron R., 2016 [27] | 1 | 15 | 0 | 16 | 67.9 | 11 | 41 | 0 | 52 | 42.1 | 0.1 (l) 0.1 (s) |
| Morice P., 2012 [28] | 1 | 2 | 5 | 8 | 44 | 2 | 1 | 0 | 3 | 44 | ns |
| Wang N., 2014 [34] | 5 | 7 | 1 | 13 | 36 | 8 | 13 | 0 | 22 | 30 | 0.5 (l) 0.2 (s) |
| Yang J., 2020 [35] | 4 | 16 | 6 | 26 | 28 | 12 | 19 | 14 | 45 | 16 | 0.2 (l) 0.2 (s) |
| Yang S., 2015 [36] | 17 | 13 | 7 | 37 | 67 | 33 | 18 | 8 | 59 | 67 | 0.6 (l) 0.6 (s) |
| Zheng D., 2018 [39] | 12 | 29 | 7 | 48 | 60 | 18 | 32 | 8 | 58 | 60 | 0.4 (l) 0.5 (s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronsini, C.; Solazzo, M.C.; Braca, E.; Andreoli, G.; Vastarella, M.G.; Cianci, S.; Capozzi, V.A.; Torella, M.; Cobellis, L.; De Franciscis, P. Locally Advanced Cervical Cancer: Neoadjuvant Treatment versus Standard Radio-Chemotherapy—An Updated Meta-Analysis. Cancers 2024, 16, 2542. https://doi.org/10.3390/cancers16142542
Ronsini C, Solazzo MC, Braca E, Andreoli G, Vastarella MG, Cianci S, Capozzi VA, Torella M, Cobellis L, De Franciscis P. Locally Advanced Cervical Cancer: Neoadjuvant Treatment versus Standard Radio-Chemotherapy—An Updated Meta-Analysis. Cancers. 2024; 16(14):2542. https://doi.org/10.3390/cancers16142542
Chicago/Turabian StyleRonsini, Carlo, Maria Cristina Solazzo, Eleonora Braca, Giada Andreoli, Maria Giovanna Vastarella, Stefano Cianci, Vito Andrea Capozzi, Marco Torella, Luigi Cobellis, and Pasquale De Franciscis. 2024. "Locally Advanced Cervical Cancer: Neoadjuvant Treatment versus Standard Radio-Chemotherapy—An Updated Meta-Analysis" Cancers 16, no. 14: 2542. https://doi.org/10.3390/cancers16142542
APA StyleRonsini, C., Solazzo, M. C., Braca, E., Andreoli, G., Vastarella, M. G., Cianci, S., Capozzi, V. A., Torella, M., Cobellis, L., & De Franciscis, P. (2024). Locally Advanced Cervical Cancer: Neoadjuvant Treatment versus Standard Radio-Chemotherapy—An Updated Meta-Analysis. Cancers, 16(14), 2542. https://doi.org/10.3390/cancers16142542