Progression-Free Survival and Treatment-Free Interval in Head and Neck Cancer with Long-Term Response to Nivolumab: Timing of Active Discontinuation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definitions
2.3. Statistical Analysis
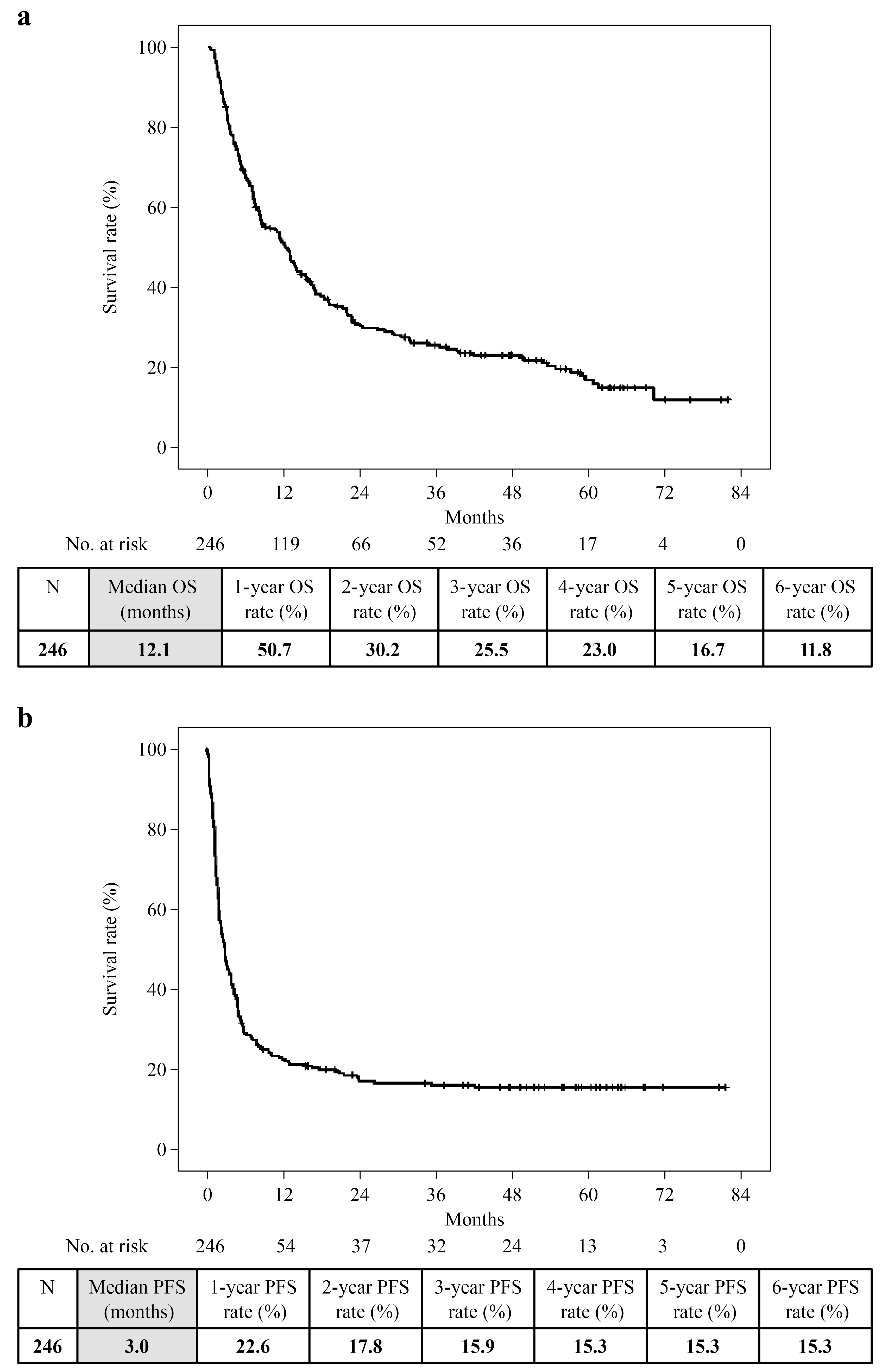
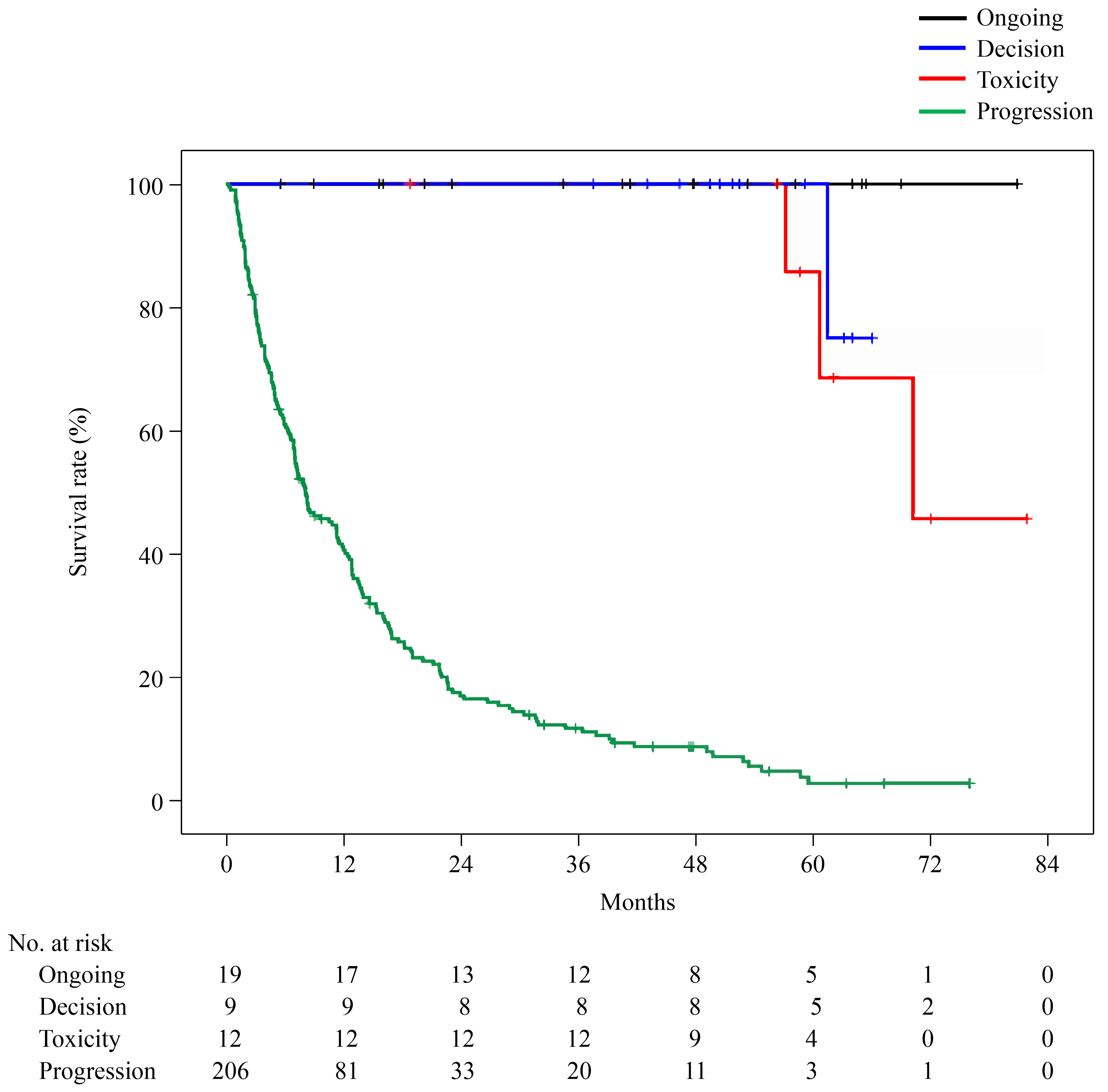
3. Results
3.1. Baseline Patient Characteristics
3.2. Clinical Outcomes Analysis
3.3. Frequency of irAEs
3.4. Duration of Nivolumab Treatment and TFI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, D.; Hoffman, G.R. Outcome of head and neck cancer patients who did not receive curative-intent treatment. J. Oral Maxillofac. Surg. 2017, 75, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, L.P.; Carvalho, A.L. Natural history of untreated head and neck cancer. Eur. J. Cancer 2000, 36, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomized, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Head and Neck Cancers Version 1.2024. Available online: www.nccn.org/Home (accessed on 30 March 2024).
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs. investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Yasumatsu, R.; Masuda, M.; Yamauchi, M.; Wakasaki, T.; Hashimoto, K.; Jiromaru, R.; Manako, T.; Nakagawa, T. Five-year follow-up of patients with head and neck cancer treated with nivolumab and long-term responders for over two years. In Vivo 2022, 36, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Stöth, M.; Meyer, T.; Gehrke, T.; Hagen, R.; Scheich, M.; Hackenberg, S.; Scherzad, A. Discontinuation of anti-programmed cell death protein 1 immune checkpoint inhibition after complete remission in head and neck squamous cell carcinoma: A case report and literature review. Oncol. Lett. 2023, 26, 489. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lee, J.-S.; Ciuleanu, T.-E.; Caro, R.B.; Nishio, M.; Urban, L.; Audigier-Valette, C.; Lupinacci, L.; Sangha, R.; Pluzanski, A.; et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non–small-cell lung cancer in checkmate 227. J. Clin. Oncol. 2022, 41, 1200–1212. [Google Scholar] [CrossRef]
- De Risi, I.; Sciacovelli, A.M.; Guida, M. Checkpoint inhibitors immunotherapy in metastatic melanoma: When to stop treatment? Biomedicines 2022, 10, 2424. [Google Scholar] [CrossRef]
- Tahara, M.; Kiyota, N.; Nibu, K.I.; Akamatsu, A.; Hoshino, T.; Hayashi, R. Real-world safety and effectiveness of nivolumab for recurrent or metastatic head and neck cancer in Japan: A post-marketing surveillance. Int. J. Clin. Oncol. 2021, 26, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Shi, Y.; Lv, S.; Dai, T.; Zhang, F.; Liu, S.; Wu, B. Nivolumab vs. Pembrolizumab for Treatment of US Patients with Platinum-Refractory Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma A Network Meta-Analysis and Cost-effectiveness Analysis. JAMA Netw. Open 2021, 4, e218065. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Saito, Y.; Kogure, Y.; Mizuno, K.; Ito, Y.; Tabata, M.; Kanai, T.; Murakami, K.; Koya, J.; Kataoka, K. Pan-Cancer Comparative and Integrative Analyses of Driver Alterations Using Japanese and International Genomic Databases. Cancer Discov. 2024, 14, 786–803. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Kish, J.K.; Korytowsky, B.; Radtchenko, J.; Singh, P.; Shaw, J.; Feinberg, B. Real-world treatment patterns, cost of care and effectiveness of therapies for patients with squamous cell carcinoma of head and neck pre and post approval of immuno-oncology agents. J. Med. Econ. 2020, 23, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Yasumatsu, R.; Masuda, M.; Toh, S.; Wakasaki, T.; Hashimoto, K.; Jiromaru, R.; Manako, T.; Nakagawa, T. Inflammation-based Prognostic Score as a Prognostic Biomarker in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma Treated with Nivolumab Therapy. In Vivo 2022, 36, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Yasumatsu, R.; Wakasaki, T.; Hashimoto, K.; Nakashima, K.; Manako, T.; Taura, M.; Matsuo, M.; Nakagawa, T. Monitoring the neutrophil-to-lymphocyte ratio may be useful for predicting the anticancer effect of nivolumab in recurrent or metastatic head and neck cancer. Head Neck 2019, 41, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Taguchi, Y.; Kitabayashi, T.; Sato, N.; Kaya, H.; Abe, T.; Endo, T.; Suzuki, H.; Kawasaki, Y.; Yamada, T. Serum albumin as an independent predictor of long-term survival in patients with recurrent and metastatic head and neck squamous cell carcinoma treated with nivolumab. J. Clin. Med. 2024, 13, 2456. [Google Scholar] [CrossRef] [PubMed]
- Vacher, L.; Bernadach, M.; Molnar, I.; Passildas-Jahanmohan, J.; Dubray-Longeras, P. The efficacy of immune checkpoint inhibitors following discontinuation for long-term response or toxicity in advanced or metastatic non-small-cell lung cancers: A retrospective study. Health Sci. Rep. 2024, 7, e1825. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Zambrana, F.; Carril-Ajuria, L.; Gómez de Liaño, A.; Martinez Chanza, N.; Manneh, R.; Castellano, D.; de Velasco, G. Complete response and renal cell carcinoma in the immunotherapy era: The paradox of good news. Cancer Treat. Rev. 2021, 99, 102239. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Flores, R.; Samlowski, W.; Perez, L. Elective Checkpoint Inhibitor Discontinuation in Metastatic Solid Tumor Patients: A Case Series. Ann. Case Rep. 2022, 7, 894. [Google Scholar] [CrossRef]
- Davies, M.A. Is it safe to stop anti-PD-1 immunotherapy in patients with metastatic melanoma who achieve a complete response? J. Clin. Oncol. 2020, 38, 1645–1647. [Google Scholar] [CrossRef]
- Amoroso, V.; Gallo, F.; Alberti, A.; Paloschi, D.; Ferrari Bravo, W.; Esposito, A.; Cosentini, D.; Grisanti, S.; Pedersini, R.; Petrelli, F.; et al. Immune-related adverse events as potential surrogates of immune checkpoint inhibitors’ efficacy: A systematic review and meta-analysis of randomized studies. ESMO Open 2023, 8, 100787. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Yasumatsu, R.; Masuda, M.; Toh, S.; Wakasaki, T.; Hashimoto, K.; Taura, M.; Uchi, R.; Nakagawa, T. Relationship between immune-related adverse events and the long-term outcomes in recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab. Oral Oncol. 2020, 101, 104525. [Google Scholar] [CrossRef] [PubMed]
- Aiken, A.H.; Farley, A.; Baugnon, K.L.; Corey, A.; El-Deiry, M.; Duszak, R.; Beitler, J.; Hudgins, P.A. Implementation of a Novel Surveillance Template for Head and Neck Cancer: Neck Imaging Reporting and Data System (NI-RADS). J. Am. Coll. Radiol. 2016, 13, 743–746.e1. [Google Scholar] [CrossRef] [PubMed]
- Gougis, P.; Jochum, F.; Abbar, B.; Dumas, E.; Bihan, K.; Lebrun-Vignes, B.; Moslehi, J.; Spano, J.-P.; Laas, E.; Hotton, J.; et al. Clinical spectrum and evolution of immune-checkpoint inhibitors toxicities over a decade-a worldwide perspective. EClinicalMedicine 2024, 70, 102536. [Google Scholar] [CrossRef] [PubMed]
- Bilger, G.; Girard, N.; Doubre, H.; Levra, M.G.; Giroux-Leprieur, E.; Giraud, F.; Decroisette, C.; Carton, M.; Massiani, M.A. Discontinuation of immune checkpoint inhibitor (ICI) above 18 months of treatment in real-life patients with advanced non-small cell lung cancer (NSCLC): INTEPI, a multicentric retrospective study. Cancer Immunol. Immunother. 2022, 71, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, D.M.; Garon, E.B.; Chandler, J.; McCleod, M.; Hussein, M.; Jotte, R.; Horn, L.; Daniel, D.B.; Keogh, G.; Creelan, B.; et al. Continuous versus 1-year fixed-duration nivolumab in previously treated advanced non–small-cell lung cancer: CheckMate 153. J. Clin. Oncol. 2020, 38, 3863–3873. [Google Scholar] [CrossRef]
- Gauci, M.-L.; Lanoy, E.; Champiat, S.; Caramella, C.; Ammari, S.; Aspeslagh, S.; Varga, A.; Baldini, C.; Bahleda, R.; Gazzah, A.; et al. Long-term survival in patients responding to anti-PD-1/PD-L1 therapy and disease outcome upon treatment discontinuation. Clin. Cancer Res. 2019, 25, 946–956. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Metzenmacher, M.; Kessler, L.; Umutlu, L.; Aigner, C.; Karl, K.O.; Grünwald, V.; Eberhardt, W.E.E.; Fendler, W.P.; Herrmann, K.; et al. Complete metabolic response in patients with advanced non-small cell lung cancer with prolonged response to immune checkpoint inhibitor therapy. J. Immunother. Cancer 2021, 9, e002262. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.-W.; Kim, M.; Lee, Y.; Ahn, H.K.; Cho, J.H.; Kim, I.H.; Lee, Y.-G.; Shin, S.-H.; Park, S.E.; et al. Long-term outcomes in patients with advanced and/or metastatic non–small cell lung cancer who completed 2 years of immune checkpoint inhibitors or achieved a durable response after discontinuation without disease progression: Multicenter, real-world data (KCSG LU20-11). Cancer 2022, 128, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Asahina, H.; Honjo, O.; Tanaka, H.; Honda, R.; Oizumi, S.; Nakamura, K.; Takamura, K.; Hommura, F.; Kawai, Y.; et al. Prognostic factors in patients with advanced non-small cell lung cancer after long-term anti-PD-1 therapy (HOT1902). Lung Cancer 2021, 156, 12–19. [Google Scholar] [CrossRef] [PubMed]
| Total (N = 246) | Ongoing (N = 19) | Discontinuation | p Value | |||
|---|---|---|---|---|---|---|
| Decision (N = 9) | Toxicity (N = 12) | Progression (N = 206) | ||||
| Sex | ||||||
| Male Female | 195 51 | 14 5 | 5 4 | 11 1 | 165 41 | 0.193 1 |
| Age | ||||||
| <75 years ≥75 years | 206 40 | 14 5 | 8 1 | 9 3 | 175 31 | 0.393 1 |
| Median (range) | 66 (23–87) | 61 (23–78) | 65 (50–80) | 63 (54–82) | 66 (24–87) | 0.601 2 |
| PS | ||||||
| 0–1 2–4 | 209 37 | 18 1 | 9 0 | 12 0 | 170 36 | 0.161 1 |
| Primary site | ||||||
| Nasopharynx Oropharynx Hypopharynx Larynx Oral cavity Sinonasal tract External auditory canal Salivary gland Primary unknown | 9 40 65 17 70 30 10 2 3 | 0 3 5 1 3 4 1 0 2 | 1 1 2 0 2 3 0 0 0 | 0 2 5 2 3 0 0 0 0 | 8 34 53 14 62 23 9 2 1 | 0.191 1 |
| Platinum | ||||||
| Sensitive Resistant | 57 189 | 4 15 | 4 5 | 2 10 | 47 159 | 0.479 1 |
| PD-L1 | ||||||
| Positive 1–20 ≥21 Negative Not measured | 29 42 10 165 | 3 6 0 10 | 0 2 1 6 | 0 0 0 12 | 26 34 9 137 | 0.316 1 |
| Total (N = 246) | Ongoing (N = 19) | Discontinuation | |||
|---|---|---|---|---|---|
| Decision (N = 9) | Toxicity (N = 12) | Progression (N = 206) | |||
| Duration of nivolumab treatment (median), months | 0.03–81.9 (2.9) | 5.6–81.9 (41.8) | 4.0–70.1 (36.8) | 0.03–50.3 (18.9) | 0.03–42.9 (2.0) |
| Treatment-free interval (median), months | — | — | 0.6–61.6 (15.1) | 2.8–64.8 (30.6) | Second-line treatment or best supportive care |
| irAE incidence irAE incidence over 2 years | 29.7% (73/246) 1.2% (3/246) | 42.1% (8/19) 0.0% (0/19) | 44.4% (4/9) 11.1% (1/9) | 100% (12/12) 8.3% (1/12) | 23.8% (49/206) 0.5% (1/206) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, M.; Masuda, M.; Yamauchi, M.; Hashimoto, K.; Kogo, R.; Sato, M.; Masuda, S.; Nakagawa, T. Progression-Free Survival and Treatment-Free Interval in Head and Neck Cancer with Long-Term Response to Nivolumab: Timing of Active Discontinuation. Cancers 2024, 16, 2527. https://doi.org/10.3390/cancers16142527
Matsuo M, Masuda M, Yamauchi M, Hashimoto K, Kogo R, Sato M, Masuda S, Nakagawa T. Progression-Free Survival and Treatment-Free Interval in Head and Neck Cancer with Long-Term Response to Nivolumab: Timing of Active Discontinuation. Cancers. 2024; 16(14):2527. https://doi.org/10.3390/cancers16142527
Chicago/Turabian StyleMatsuo, Mioko, Muneyuki Masuda, Moriyasu Yamauchi, Kazuki Hashimoto, Ryunosuke Kogo, Masanobu Sato, Shogo Masuda, and Takashi Nakagawa. 2024. "Progression-Free Survival and Treatment-Free Interval in Head and Neck Cancer with Long-Term Response to Nivolumab: Timing of Active Discontinuation" Cancers 16, no. 14: 2527. https://doi.org/10.3390/cancers16142527
APA StyleMatsuo, M., Masuda, M., Yamauchi, M., Hashimoto, K., Kogo, R., Sato, M., Masuda, S., & Nakagawa, T. (2024). Progression-Free Survival and Treatment-Free Interval in Head and Neck Cancer with Long-Term Response to Nivolumab: Timing of Active Discontinuation. Cancers, 16(14), 2527. https://doi.org/10.3390/cancers16142527





