ENO2, a Glycolytic Enzyme, Contributes to Prostate Cancer Metastasis: A Systematic Review of Literature
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Data Source and Searches
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection
2.5. Qualitiy Assessment
2.6. Certainty of Evidence
3. Results
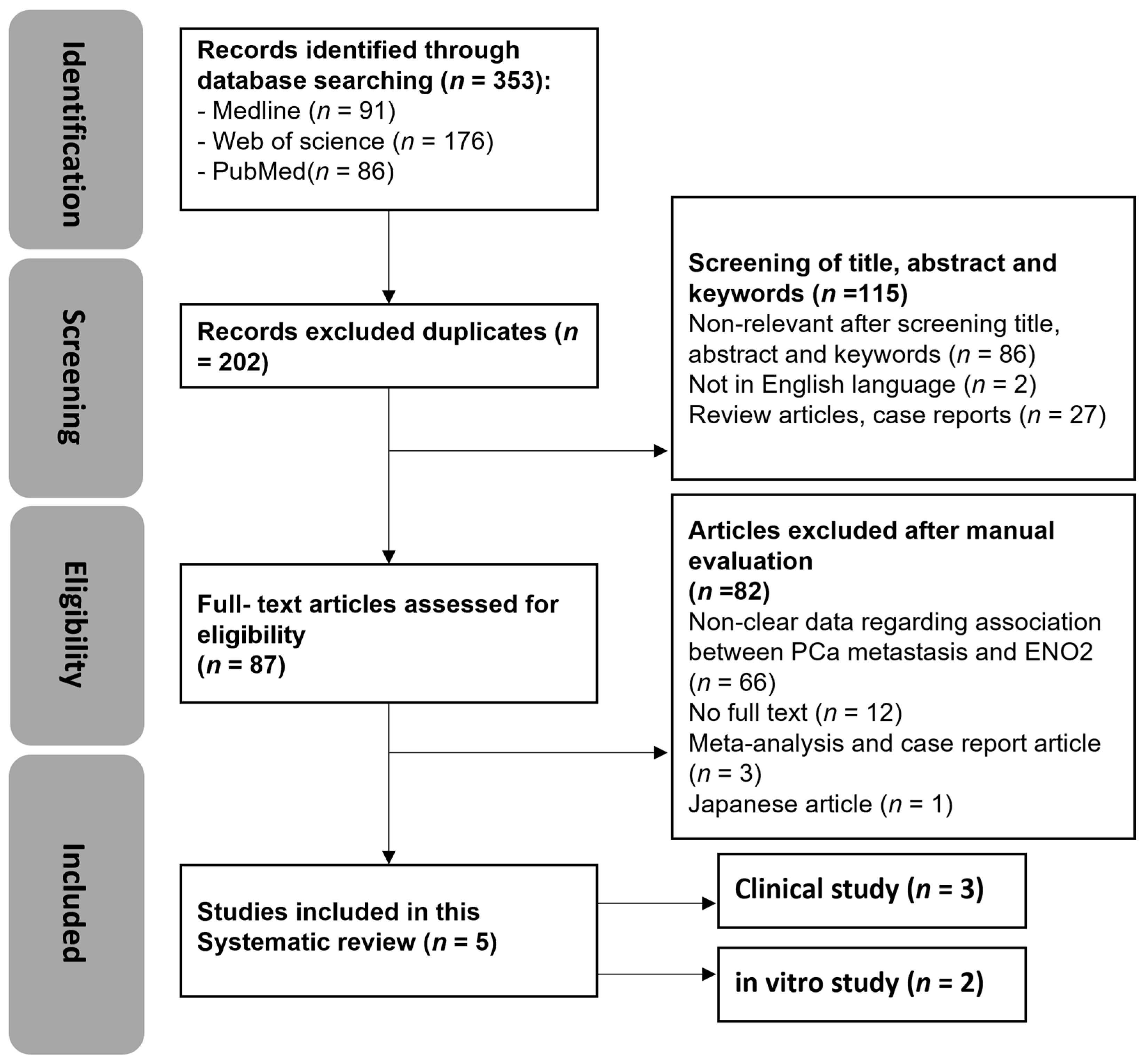
3.1. Search Results
3.2. Quality Assessment
3.3. Certainty of Evidence
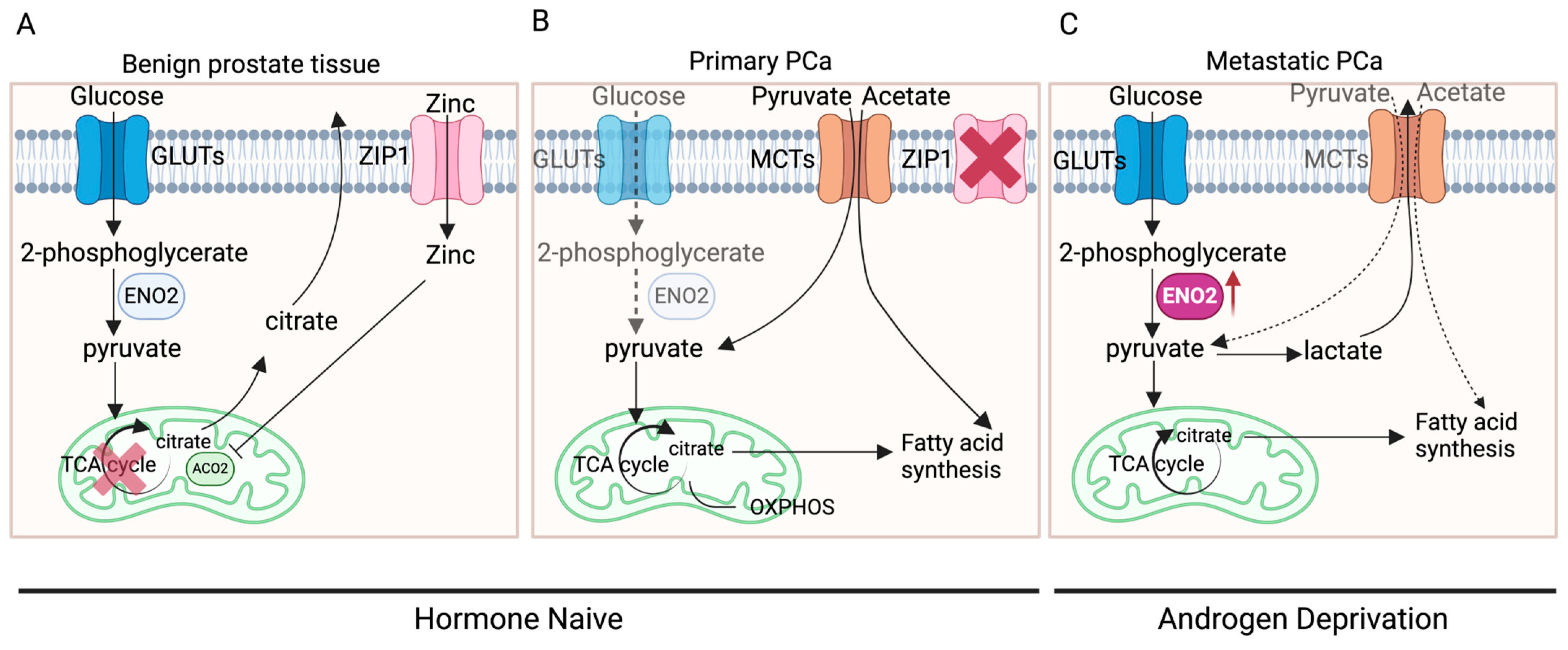
3.4. Narrative Synthesis
3.4.1. Clinical Studies
| Author | Year | Region | Study Type | No. of Patients | Mean Age (Years) | The Comparison Details | Comparison Outcome (n Number, p Value) |
|---|---|---|---|---|---|---|---|
| Kim et al. [18] | 2017 | USA | Bioinformatics (RNA-seq) | n = 86 | / | The influence of ENO2 expression in patients with primary, CRPC, or NEPC in a clinical setting |
|
| Kessel et al. [21] | 2020 | Germany | Cohort study (CTC enrichment and RT qPCR) | n = 19 | 68.8 years | The impact of ENO2 expression in blood samples obtained from either healthy individuals or mCRPC patients |
|
| Szarvas et al. [22] | 2021 | Germany | Cohort study (serum biomarker analysis) | n = 395 | 66 years (RP group) 71 years (DOC group) 73 years (ABI/ENZA group) | The effects of serum ENO2 protein level in patients who received radical prostatectomy or who received DOC or ABI/ENZA treatment in a clinical setting |
|
3.4.2. In Vitro Studies
| Author | Year | Region | Assay Type | Cell Line | Culture Conditions | The Comparison Details | Comparison Outcome |
|---|---|---|---|---|---|---|---|
| Bock et al. [23] | 2019 | Australia | RT-qPCR | PC3 | Monoculture medium: RPMI1640 + L-glutamine, 5%FBS + 1% P/S | The impact of ENO2 expression in vitro in PCa cell lines, which were either mono- or co-cultured with hOBMT in a medium with or without DHT |
|
| LNCaP | Co-culture medium: RPMI1640, 10%FBS (containing 0.6 nmol/L DHT) + 1%P/S (PCa-Norm) RPMI1640, 10%CSS + 1%P/S (PCa-AD) | ||||||
| C4-2B | RPMI1640, 10%FBS (containing 10 nmol/L DHT) + 1%P/S (PCa-DHT) | ||||||
| Bery et al. [17] | 2020 | Germany | RT-qPCR | PC3 | RPMI 1640, 5% FBS + 1% P/S | Expression levels of ENO2 in PCa cell lines of different types and origins |
|
| 22Rv1 | RPMI 1640, 10% FBS + 1% P/S | ||||||
| NCI-H660 | RPMI1640, 0.005 mg/mL insulin + 0.01 mg/mL transferrin + 30 nM sodium selenite + 10 nM β-estradiol + 2 mM 5% FBS + 1% P/S |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2PG | 2-Phosphoglycerate |
| ABI/ENZA | Abiraterone/enzalutamide |
| ACO2 | Aconitase 2 |
| ADT | Androgen deprivation therapy |
| AR | Androgen receptor |
| BC | Bladder cancer |
| BMP2 | Bone morphogenetic protein 2 |
| BRAF | B-Raf proto-oncogene, serine/threonine kinase |
| CCND1 | Cyclin D1 |
| CRC | Colorectal cancer |
| CRPC | Castrate-resistant PCa |
| CSS | Charcoal-stripped serum |
| CTCs | Circulating tumour cells |
| DHT | Dihydrotestosterone |
| DOC | Docetaxel |
| EMT | Epithelial mesenchymal transition |
| ENO2 | Enolase 2, Γ-enolase |
| GLAST | Glutamate–aspartate transporter |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| HNSCC | Head and neck squamous cell carcinoma |
| hOBMT | Human osteoblast-derived microtissues |
| ID1 | Inhibitor of DNA binding 1 |
| KLF12 | Kruppel-like factor 12 |
| KLK2 | Kallikrein-related peptidase 2 |
| KLK3 | Kallikrein-related peptidase 3 |
| LDH | Lactate dehydrogenase |
| ncRNAs | Non-coding RNAs |
| MAPK | Mitogen-activated protein kinase |
| mCRPC | Metastatic CRPC |
| MCT | Monocarboxylate transporters |
| NBL1 | DAN family BMP antagonist |
| NEPC | Neuroendocrine prostate cancer |
| NEtD | Transdifferentiation |
| OXPHOS | Oxidative phosphorylation |
| PCa | Prostate cancer |
| PDH | Pyruvate dehydrogenase |
| PDK | Pyruvate dehydrogenase kinase |
| PEP | Phosphoenolpyruvate |
| PI3K | Phosphatidylinositol 3-kinase |
| PK | Pyruvate kinase |
| PRISMA | Reporting Items for Systematic Reviews and Meta-Analyses |
| RP | Radical prostatectomy |
| SCLC | Small cell lung cancer cells |
| SLC39A1 | Solute carrier family 39 member 1 |
| TCA cycle | Tricarboxylic acid cycle, citric acid cycle |
| YAP1 | Yes1-associated transcriptional regulator |
| ZIP1 | Zinc transporter |
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.; McConnell, H.; Alonzi, R.; Maher, J. Using routinely collected data to stratify prostate cancer patients into phases of care in the United Kingdom: Implications for resource allocation and the cancer survivorship programme. Br. J. Cancer 2015, 112, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef]
- Cutruzzola, F.; Giardina, G.; Marani, M.; Macone, A.; Paiardini, A.; Rinaldo, S.; Paone, A. Glucose Metabolism in the Progression of Prostate Cancer. Front. Physiol. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Huang, Y.; Li, C.; Yin, Q.; Ying, J. Beyond ENO1, emerging roles and targeting strategies of other enolases in cancers. Mol. Ther. Oncolyt. 2023, 31, 100750. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Xiao, L.; Bode, A.M.; Dong, Z.; Cao, Y. Glycolytic genes in cancer cells are more than glucose metabolic regulators. J. Mol. Med. 2014, 92, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Yu, H.; Wang, K.; Chen, C.; Tang, J.; Han, F.; Mai, M.; Ye, K.; Lai, M.; Zhang, H. ENO2 Promotes Colorectal Cancer Metastasis by Interacting with the LncRNA CYTOR and Activating YAP1-Induced EMT. Cells 2022, 11, 2363. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, C.; Yang, J.; Zhao, Y.; Jia, H.; Xue, M.; Xu, D.; Yang, F.; Fu, D.; Wang, C.; et al. Insulin-like growth factor 1-induced enolase 2 deacetylation by HDAC3 promotes metastasis of pancreatic cancer. Signal Transduct. Target. Ther. 2020, 5, 53. [Google Scholar] [CrossRef]
- Gao, L.; Yang, F.; Tang, D.; Xu, Z.; Tang, Y.; Yang, D.; Sun, D.; Chen, Z.; Teng, Y. Mediation of PKM2-dependent glycolytic and non-glycolytic pathways by ENO2 in head and neck cancer development. J. Exp. Clin. Cancer Res. 2023, 42, 1. [Google Scholar] [CrossRef] [PubMed]
- Isgrò, M.A.; Bottoni, P.; Scatena, R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. Adv Exp. Med. Biol. 2015, 867, 125–143. [Google Scholar] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. OHAT Risk of Bias Rating Tool for Human and Animal Studies; National Institutes of Health: Bethesda, MD, USA, 2015.
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Carrasco, G.; Li, B.; Sophocleous, A.; Idris, A.I. TRAF6 as a potential target in advanced breast cancer: A systematic review, meta-analysis, and bioinformatics validation. Sci. Rep. 2023, 13, 4646. [Google Scholar] [CrossRef] [PubMed]
- Bery, F.; Cancel, M.; Chantome, A.; Guibon, R.; Bruyere, F.; Rozet, F.; Maheo, K.; Fromont, G. The Calcium-Sensing Receptor is A Marker and Potential Driver of Neuroendocrine Differentiation in Prostate Cancer. Cancers 2020, 12, 860. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jin, H.; Zhao, J.C.; Yang, Y.A.; Li, Y.; Yang, X.; Dong, X.; Yu, J. FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene 2017, 36, 4072–4080. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H.; Rickman, D.S.; Park, K.; Chae, S.S.; Sboner, A.; MacDonald, T.Y.; Wang, Y.; Sheikh, K.L.; Terry, S.; Tagawa, S.T.; et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011, 1, 487–495. [Google Scholar] [CrossRef]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.; Varambally, S.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef]
- Kessel, K.; Seifert, R.; Weckesser, M.; Roll, W.; Humberg, V.; Schlack, K.; Boegemann, M.; Bernemann, C.; Rahbar, K. Molecular analysis of circulating tumor cells of metastatic castration-resistant Prostate Cancer Patients receiving 177Lu-PSMA-617 Radioligand Therapy. Theranostics 2020, 10, 7645–7655. [Google Scholar] [CrossRef]
- Szarvas, T.; Csizmarik, A.; Fazekas, T.; Huttl, A.; Nyirady, P.; Hadaschik, B.; Grunwald, V.; Pullen, L.; Juranyi, Z.; Kocsis, Z.; et al. Comprehensive analysis of serum chromogranin A and neuron-specific enolase levels in localized and castration-resistant prostate cancer. Bju Int. 2021, 127, 44–55. [Google Scholar] [CrossRef]
- Bock, N.; Shokoohmand, A.; Kryza, T.; Rohl, J.; Meijer, J.; Tran, P.A.; Nelson, C.C.; Clements, J.A.; Hutmacher, D.W. Engineering osteoblastic metastases to delineate the adaptive response of androgen-deprived prostate cancer in the bone metastatic microenvironment. Bone Res. 2019, 7, 13. [Google Scholar] [CrossRef]
- Sramkoski, R.M.; Pretlow, T.G., 2nd; Giaconia, J.M.; Pretlow, T.P.; Schwartz, S.; Sy, M.S.; Marengo, S.R.; Rhim, J.S.; Zhang, D.; Jacobberger, J.W. A new human prostate carcinoma cell line, 22Rv1. Vitr. Cell. Dev. Biol. Anim. 1999, 35, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; You, Z. In vitro and in vivo model systems used in prostate cancer research. J. Biol. Methods 2015, 2, e17. [Google Scholar] [CrossRef] [PubMed]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Johnson, B.E.; Whang-Peng, J.; Naylor, S.L.; Zbar, B.; Brauch, H.; Lee, E.; Simmons, A.; Russell, E.; Nam, M.H.; Gazdar, A.F. Retention of Chromosome 3 in Extrapulmonary Small Cell Cancer Shown by Molecular and Cytogenetic Studies. JNCI J. Natl. Cancer Inst. 1989, 81, 1223–1228. [Google Scholar] [CrossRef]
- Mertz, K.D.; Setlur, S.R.; Dhanasekaran, S.M.; Demichelis, F.; Perner, S.; Tomlins, S.; Tchinda, J.; Laxman, B.; Vessella, R.L.; Beroukhim, R.; et al. Molecular characterization of TMPRSS2-ERG gene fusion in the NCI-H660 prostate cancer cell line: A new perspective for an old model. Neoplasia 2007, 9, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Yukimoto, R.; Nishida, N.; Hata, T.; Fujino, S.; Ogino, T.; Miyoshi, N.; Takahashi, H.; Uemura, M.; Satoh, T.; Hirofumi, Y.; et al. Specific activation of glycolytic enzyme enolase 2 in BRAF V600E-mutated colorectal cancer. Cancer Sci. 2021, 112, 2884–2894. [Google Scholar] [CrossRef]
- Lu, L.; Zha, Z.; Zhang, P.; Wang, P.; Liu, X.; Fang, X.; Weng, C.; Li, B.; Mao, H.; Wang, L.; et al. Neuron-specific enolase promotes stem cell-like characteristics of small-cell lung cancer by downregulating NBL1 and activating the BMP2/Smad/ID1 pathway. Oncogenesis 2022, 11, 21. [Google Scholar] [CrossRef]
- Chen, W.J.; Yang, W.; Gong, M.; He, Y.; Xu, D.; Chen, J.X.; Chen, W.J.; Li, W.Y.; Wang, Y.Q.; Dong, K.Q.; et al. ENO2 affects the EMT process of renal cell carcinoma and participates in the regulation of the immune microenvironment. Oncol. Rep. 2023, 49, 33. [Google Scholar] [CrossRef]
- Tang, C.; Wang, M.; Dai, Y.; Wei, X. Kruppel-like factor 12 suppresses bladder cancer growth through transcriptionally inhibition of enolase 2. Gene 2021, 769, 145338. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H. MiR-7-5p suppresses tumor metastasis of non-small cell lung cancer by targeting NOVA2. Cell. Mol. Biol. Lett. 2019, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zha, Z.; Zhang, P.; Li, D.; Liu, G. NSE, positively regulated by LINC00657-miR-93-5p axis, promotes small cell lung cancer (SCLC) invasion and epithelial-mesenchymal transition (EMT) process. Int. J. Med. Sci. 2021, 18, 3768–3779. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Cherukuri, M.K.; Choyke, P.L. Metabolic reprogramming in prostate cancer. Br. J. Cancer 2021, 125, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Plymate, S.R.; Sprenger, C.; Haffner, M.C. Starving lethal prostate cancer by targeting heat shock proteins and glycolytic enzymes. Cell Rep. Med. 2022, 3, 100493. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.-C.; Anderle, P.; Bürzle, M.; Suzuki, Y.; Freeman, M.; Hediger, M.; Kovacs, G. Zinc transporters in prostate cancer. Mol. Asp. Med. 2013, 34, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Bader, D.A.; McGuire, S.E. Tumour metabolism and its unique properties in prostate adenocarcinoma. Nat. Rev. Urol. 2020, 17, 214–231. [Google Scholar] [CrossRef]
- Prescott, J.L.; Blok, L.; Tindall, D.J. Isolation and androgen regulation of the human homeobox cDNA, NKX3.1. Prostate 1998, 35, 71–80. [Google Scholar] [CrossRef]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef]
- Vavere, A.L.; Kridel, S.J.; Wheeler, F.B.; Lewis, J.S. 1-11C-acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. J. Nucl. Med. 2008, 49, 327–334. [Google Scholar] [CrossRef]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(1)(3)C]pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.J.; Zafar, M.B.; Lai, Y.H.; Segall, G.M.; Terris, M.K. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology 2001, 57, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Payen, V.L.; Mina, E.; Van Hee, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Takenaga, K.; Koshikawa, N.; Akimoto, M.; Tatsumi, Y.; Lin, J.; Itami, M.; Nagase, H. MCT4 is induced by metastasis-enhancing pathogenic mitochondrial NADH dehydrogenase gene mutations and can be a therapeutic target. Sci. Rep. 2021, 11, 13302. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Schuster, S.; Bonhoeffer, S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001, 292, 504–507. [Google Scholar] [CrossRef]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zeng, F.; Richards, G.O.; Wang, N. ENO2, a Glycolytic Enzyme, Contributes to Prostate Cancer Metastasis: A Systematic Review of Literature. Cancers 2024, 16, 2503. https://doi.org/10.3390/cancers16142503
Zhou Y, Zeng F, Richards GO, Wang N. ENO2, a Glycolytic Enzyme, Contributes to Prostate Cancer Metastasis: A Systematic Review of Literature. Cancers. 2024; 16(14):2503. https://doi.org/10.3390/cancers16142503
Chicago/Turabian StyleZhou, Yuhan, Feier Zeng, Gareth Owain Richards, and Ning Wang. 2024. "ENO2, a Glycolytic Enzyme, Contributes to Prostate Cancer Metastasis: A Systematic Review of Literature" Cancers 16, no. 14: 2503. https://doi.org/10.3390/cancers16142503
APA StyleZhou, Y., Zeng, F., Richards, G. O., & Wang, N. (2024). ENO2, a Glycolytic Enzyme, Contributes to Prostate Cancer Metastasis: A Systematic Review of Literature. Cancers, 16(14), 2503. https://doi.org/10.3390/cancers16142503







