Simple Summary
Lung cancer is the leading cause of cancer deaths worldwide. Surgery, with or without neoadjuvant or adjuvant chemotherapy has historically been the mainstay of treatment for resectable non-small-cell lung cancer (rNSCLC). However, as many as half of those treated still die of the disease. Immune checkpoint inhibitors are changing the landscape of treatment both in the neoadjuvant and adjuvant rNSCLC settings in many countries. In this review and analysis, outcomes among patients treated in the neoadjuvant setting (i.e., prior to surgery) with the immune checkpoint inhibitor nivolumab combined with chemotherapy were compared to those of patients treated with the historical standard of care treatments, using data from published studies. The results of this evidence synthesis show that neoadjuvant nivolumab when combined with chemotherapy improves the likelihood of event-free survival and a pathological complete response for patients relative to traditional treatments, specifically, surgery, alone or in combination with neoadjuvant or adjuvant (i.e., after surgery) chemotherapy, and neoadjuvant chemoradiotherapy.
Abstract
Background: This study aimed to estimate the relative efficacy of neoadjuvant nivolumab in combination with chemotherapy (neoNIVO + CT) compared to relevant treatments amongst resectable non-metastatic non-small-cell lung cancer (rNSCLC) patients. Methods: Treatment comparisons were based on a network meta-analysis (NMA) using randomized clinical trial data identified via systematic literature review (SLR). The outcomes of interest were event-free survival (EFS) and pathological complete response (pCR). NeoNIVO + CT was compared to neoadjuvant chemotherapy (neoCT), neoadjuvant chemoradiotherapy (neoCRT), adjuvant chemotherapy (adjCT), and surgery alone (S). Due to the potential for effect modification by stage, all-stage and stage-specific networks were considered. Fixed-effect (FE) and random-effects Bayesian NMA models were run (EFS = hazard ratios [HR]; pCR = odds ratios [OR]; 95% credible intervals [CrI]). Results: Sixty-one RCTs were identified (base case = 9 RCTs [n = 1978 patients]). In the all-stages FE model, neoNIVO + CT had statistically significant EFS improvements relative to neoCT (HR = 0.68 [95% CrI: 0.49, 0.94]), S (0.59 [0.42, 0.82]), adjCT (0.66 [0.45, 0.96]), but not relative to neoCRT (HR = 0.77 [0.52, 1.16]). NeoNIVO + CT (5 RCTs) had statistically significant higher odds of pCR relative to neoCT (OR = 12.53 [5.60, 33.82]) and neoCRT (7.15 [2.31, 24.34]). Stage-specific model findings were consistent. CONCLUSIONS: This NMA signals improved EFS and/or pCR of neoNIVO + CT relative to comparators among patients with rNSCLC.
1. Introduction
Lung cancer is the leading cause of cancer deaths worldwide [1]. Surgery with curative intent is the cornerstone of treatment for resectable (stage I, II and select III) non-small-cell lung cancer (rNSCLC) [2,3,4]. However, 30 to 55% of patients who undergo curative surgery have recurrence and ultimately die of their disease [5,6]. Historically, surgery can be followed by adjuvant therapy with platinum-based chemotherapy, which has been shown to improve overall survival (OS); however, the absolute 5-year survival benefit of adjuvant therapy is moderate compared with observation alone [7,8,9]. Neoadjuvant chemotherapy has also been associated with a moderate survival benefit; a 2014 meta-analysis found that rates of 5-year recurrence-free survival (RFS) with neoadjuvant chemotherapy followed by surgery were slightly higher than for surgery alone (36% vs. 30%) [10]. Radiation as an add-on to neoadjuvant chemotherapy is a standard approach in many institutions when a lobectomy is feasible; however, its clinical benefit has not been established [11,12].
The emergence of immune checkpoint inhibitors has revolutionized the treatment of various cancers, including rNSCLC [13,14,15,16,17,18,19,20,21]. Neoadjuvant treatment with nivolumab in combination with chemotherapy (neoNIVO + CT) has been approved in many countries based on findings from the randomized, open-label, phase 3 CheckMate 816 (CM816) clinical trial which compared neoNIVO + CT to neoadjuvant chemotherapy (neoCT). NeoNIVO + CT has now become the standard of care for patients with rNSCLC in many countries. Other immunotherapies (IOs) have been explored in the perioperative and adjuvant settings, including but not limited to pembrolizumab (PEMBRO), durvalumab (DURVA), and atezolizumab (ATEZO).
Given the rapidly changing treatment landscape in the rNSCLC space, which is shifting towards IO-based treatment strategies for non-oncogene addicted rNSCLC, it is important to understand the place of neoadjuvant IO relative to conventional treatments such as surgery alone (S), neoCT, adjuvant chemotherapy (adjCT), and neoadjuvant chemoradiotherapy (neoCRT). The objective of this study was to compare event-free survival (EFS), and pathological complete response (pCR) associated with neoNIVO + CT against conventional comparators amongst patients with stage IB-IIIA rNSCLC by means of a systematic literature review (SLR) and network meta-analysis (NMA).
2. Materials and Methods
The study was conducted according to a pre-specified research protocol developed in alignment with good practice guidelines for SLR and NMA [22,23].
2.1. SLR and NMA Eligibility
A systematic search of the literature was conducted using Embase, MEDLINE, and the Cochrane Central Register of Controlled trials through Ovid, complemented by a search of major conference proceedings in the last two years (Tables S1–S4). The SLR was designed to capture randomized controlled trials (RCTs) based on pre-defined broad inclusion criteria (Table S5). For the base case NMA, eligibility was limited to studies that evaluated the therapies of interest (neoNIVO + CT, neoCT, neoCRT, adjCT, S) among patient populations that were deemed resectable at study randomization (which was required to occur prior to surgery or neoadjuvant treatment); chemotherapy-based regimens were restricted to those involving 3rd generation platinum-based doublets. While perioperative IO-based regimens and adjuvant IO-based regimens were of interest, they were either not available at the time of the SLR conduct (e.g., perioperative IO), or were not relevant for the NMA due to known differences in study design (e.g., ATEZO administered after surgery and a course of adjCT; explored in a separate analysis) [24]. A detailed description of the NMA selection criteria is defined in Table S6.
Abstract and full text screening were conducted by two experienced reviewers. Arbitration was provided by a third reviewer to resolve any disagreements. Reasons for exclusion were recorded at abstract and full text screening. The study selection process was documented following the Preferred Reporting of Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram [22]. Data extraction was conducted by a single reviewer using a customized Microsoft Excel® form, and all the extracted data were independently verified and validated by a second reviewer. Kaplan–Meier (KM) curves were digitized using digitizing software (DigitizeIt v2.5.3) [25]. Included studies were critically appraised by a single reviewer using the Cochrane risk-of-bias tool for randomized trials (RoB 2) [26]. The systematic review did not register in a public registry.
2.2. Target Populations
Several target populations were defined for the NMA. First, a broadly defined target population was considered: patients with stage IB (tumour size ≥ 4 cm) to IIIA (AJCC TNM 7th edition) rNSCLC (hereafter referenced as IB-IIIA), which aligns with the inclusion criteria in CM816. All NMA-eligible RCTs were included in this network, under the core assumption that, within stages IB to IIIA, disease stage does not modify relative treatment effect between the regimens of interest (additional details in Table S7).
Second, more narrowly defined stage-specific target populations were considered, including: (1) an early stage (IB-II) population; (2) a stage IIIA population (spanning all relevant nodal status within stage IIIA); and (3) a stage IIIA population with mediastinal lymph node metastasis (IIIA N2 disease). NMA-eligible RCTs were included in these stage-specific networks if the eligibility criteria of the RCT—or available subgroup data—aligned with those target populations. For the stage IIIA N2 network, an exception was made for CM816, as nodal status was not recorded in this RCT; as a result, stage IIIA data (not restricted to N2) was used. The stages IB–IIIA and stage-specific models were considered base case models onto which the sensitivity analyses were applied.
The European Medicines Agency (EMA) approved neoNIVO + CT for its use among rNSCLC patients with high risk of recurrence (i.e., stage II to IIIA) and PD-L1 expression ≥1% [27]. As such, two additional PD-L1 expression level ≥1% specific populations were defined: (1) PD-L1 ≥ 1% in a stage IB-IIIA population, so as to not break randomization in CM816 (hereinafter described as PD-L1 specific), and (2) PD-L1 ≥ 1% in a stage II-IIIA population to align with the EMA indication (hereinafter described as EMA-target). Both target populations also addressed the potential for effect modification by levels of PD-L1 expression for comparisons involving IOs, as PD-L1-specific inputs were used for studies evaluating an IO-based regimen [28]. In general, inputs were aligned with the target population (i.e., subgroup-specific data were used where available).
2.3. Data Preparation
HRs were used to inform the EFS NMA; whenever HRs were not reported but KMs were available, HRs were estimated with Cox proportional hazard models applied to individual patient-level data (IPD) reconstructed using the methods described by Rogula et al. and Guyot et al. [29,30]. For the binary endpoint pCR, the counts of patients who achieved pCR and the total sample size in each arm were used as model inputs. For multi-arm trials involving two similar regimens (e.g., two neoCRT arms and one neoCT arm), the two arms were pooled where possible. Reconstructed IPD were used to pool EFS data, and counts were summed for pCR.
2.4. Quantitative Evidence Synthesis
For EFS, the assumption of proportional hazards was tested using the reconstructed IPD (Table S8). Fixed-effect (FE) and random-effects (RE) Bayesian NMA models were run (Table S8). HRs were reported for EFS, and odds ratios (OR) were reported for pCR; Bayesian 95% credible intervals (CrI) were computed. NeoCT was used as the reference treatment in the analyses. Absolute estimates of EFS at 2, 3, and 5 years were generated by applying the computed HRs to assumed hazards for the reference treatment (modeled as a lognormal parametric curve fitted to the neoCT arm from CM816). Similarly, absolute percentages of patients achieving pCR were estimated for each treatment by applying the computed ORs to the observed odds of pCR in the neoCT arm from CM816, converted from odds to percentages using standard functions.
All analyses were performed using R version 4.3.0 and WinBUGS version 1.4.3.
2.5. Sensitivity Analysis
Two sensitivity analyses were conducted. First, eligibility was expanded to include RCTs that randomized patients after surgical resection; this enabled the inclusion of additional evidence (e.g., both potentially resectable and completely resected patients). Second, eligible comparators were expanded to include RCTs involving 2nd generation platinum-based chemotherapies. Many of these treatments are no longer included in the National Comprehensive Cancer Network (NCCN) or European Society for Medical Oncology (ESMO) guidelines but may be used in clinical practice albeit to a lesser degree [2,3,31]. Sensitivity analyses were applied to the base case EFS models.
The full list of evaluated models is presented in Table S9.
3. Results
3.1. Evidence Base
The SLR was executed on 15 November 2022, and yielded 12,863 abstracts, of which 61 RCTs met the broader SLR eligibility criteria. From these, a total of 18 RCTs met the NMA PICOS criteria. The PRISMA diagram is presented in Figure S1. Among these, nine RCTs were included across the various target-population-specific and endpoint-specific base case models. Of note, although perioperative IO regimens were not included in the NMA PICOS for this study, the NADIM II RCT was included, as the pCR data generated following receipt of neoNIVO + CT in the study was considered relevant to the pCR models in this NMA, as the estimates are not affected by the receipt of adjuvant nivolumab. Five RCTs were added to the EFS base case set for the sensitivity analyses expanding to second generation chemotherapies (total = 13 RCTs); and four were added to the EFS base case set in the sensitivity analysis expanding to RCTs involving randomization after surgery (total = 12 RCTs). The stage-specific target populations for the base case, across various endpoints, were informed by four RCTs (stage IB-II); three RCTs (stage IIIA); and five RCTs (stage IIIA N2).
Characteristics of the 18 studies included in the NMA are presented in Table 1 and the baseline characteristics of the base case studies are presented in Figure 1. Median age ranged from 56 to 65 years. There was some heterogeneity in terms of the proportion of males (range: 61% to 89%), regimen characteristics (cisplatin-based or carboplatin-based regimens), number of treatment cycles (two vs. three), concurrent vs. sequential radiotherapy, squamous histology (range: 16% to 60%), stage (including staging criteria used) and post-surgical therapy amongst studies with a surgery-alone arm.

Table 1.
Characteristics of included studies.
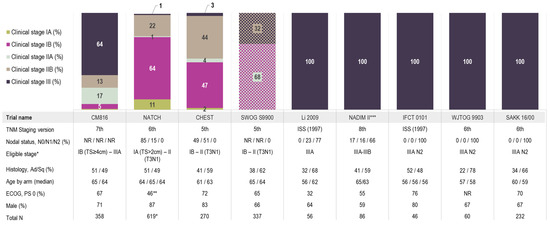
Figure 1.
Stage distribution and key patient characteristics across the base case studies. * One patient was included in NATCH despite having stage IV disease (T4N0); ** 1% in NATCH had ECOG Performance status of 2; *** NADIM II included stage IIIA to IIIB patients based on the 8th edition for TNM staging which aligns with stage IIIA of the 7th edition as they did not include T4N2 or anyone with N3 disease. Abbreviations: PS, performance status; TS, tumour size. Note: SWOG S9900 was the only study that reported staging in two distinct groups (IB/IIA and IIB/IIIA) and as such, is visualized distinctly from the rest of the studies; 68% of patients had stage IB/IIA disease (presented in a purple and light sage cross-hatch pattern) and 32% of patients had stage IIB/III disease (presented in a light brown and dark navy cross-hatch pattern).
The RCTs were considered to have some concerns of bias. Seven base case RCTs were open-label, while the remaining two studies did not report their blinding status. The methods of randomization were poorly reported in some of the studies and pre-specified analysis plans were often not available, especially amongst older trials (Figure S2).
3.2. Proportional Hazard Assessment and Model Selection
There was no strong evidence of violation of the proportional hazard assumption of the evidence base for EFS (Figure S3). As such, an analysis relying on the assumption of a constant hazard ratio was deemed appropriate.
Due to the sparse evidence base, the between-study standard deviation could not be estimated with precision in the RE model. As such, the fixed-effect model results are presented for all endpoints and networks. The width of the 95% CrIs of the outputs from the RE model were highly influenced by the choice of prior on the between-study standard deviation; models using informed prior distributions are presented in the Tables S10–S15; Figures S4–S7.
3.3. Relative Efficacy for Event-Free Survival
The eight RCTs included in the analysis of EFS formed a connected network of evidence in the stage IB-IIIA target population (Figure 2A), including data from 1978 patients and 18 trial arms (17 effective arms due to the pooling of two neoCRT arms in IFCT 0101). All networks of evidence were primarily star-shaped, with one closed loop formed by one three-arm trial (NATCH) in the stage IB-IIIA and stage IB-II networks (Figure 2A,B). This network geometry precluded inconsistency assessment. Data inputs and endpoint definitions informing the EFS NMA are provided in Table S10.
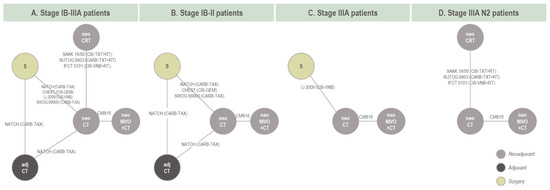
Figure 2.
Network of evidence for base case EFS models. Note: For studies involving neoCT, neoCRT, or S, see baseline characteristics regarding protocol-defined use of post-surgical therapy. Amongst the four base case studies which included a surgery alone arm, surgery was not followed by discretionary adjCT apart from Li 2009. Both Li 2009 and NATCH were also the only two studies that allowed for adjuvant radiotherapy following surgery in some cases. EMA-target network is not reflected in this diagram; exclusions relating to the EMA-target network are available in Supplementary Material. Networks of evidence used in the sensitivity analyses are available in the Supplementary Material. Abbreviations: adj, adjuvant; CARB, carboplatin; CIS, cisplatin; CT, chemotherapy; CRT, chemoradiotherapy; GEM, gemcitabine; neo, neoadjuvant; NIVO, nivolumab; RT, radiotherapy; S, surgery; VNB, vinorelbine; TAX, paclitaxel; TXT, docetaxel.
For the target population of patients with stage IB-IIIA, neoNIVO + CT was associated with statistically significant improvements in EFS relative to neoCT (HR = 0.68 [95% CrI: 0.49, 0.94]), S (0.59 [0.42, 0.82]), and adjCT (0.66 [0.45, 0.96]) (Figure 3). For the comparison to neoCRT, neoNIVO + CT trended towards being more efficacious but did not reach statistical significance (HR = 0.77 [0.52, 1.16]). For target populations having a more advanced stage (stage IIIA, and stage IIIA N2), the conclusions were similar for comparisons against neoCT, S and neoCRT, with stronger magnitudes of effect favouring neoNIVO + CT. The adjCT comparator was not included in these networks due to lack of available data. In the early-stage target population (IB-II), neoNIVO + CT still trended towards being more efficacious consistent with the all-stages (IB-IIIA) network (for common comparators), yet HRs were closer to the null value of 1 and did not reach statistical significance. The findings from the stage IB-IIIA PD-L1 ≥ 1% target population were relatively more favourable for neoNIVO + CT compared to the stage IB-IIIA base case model. Tabular summaries of HRs between all treatments in the stage IB-IIIA base case network are available in Table S11.
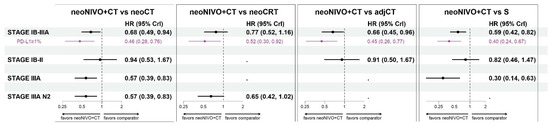
Figure 3.
Event-free survival hazard ratio estimates for neoNIVO + CT vs. all relevant comparators across base case and the PD-L1 specific models (fixed-effect model estimates). Note: the results associated with PD-L1 ≥ 1% specific target population conducted within the stage IB-IIIA network are illustrated in purple. Sensitivity results are available in Figure S4; the EMA-target results are available in Tables S16–S18; Figures S8–S10. Abbreviations: adj, adjuvant; CrI, credible interval, CT, chemotherapy; CRT, chemoradiotherapy; HR, hazard ratio; neo, neoadjuvant; NIVO, nivolumab; S, surgery.
Anchored on an assumed EFS curve for the neoCT arm, absolute EFS estimates at 2-year, 3-year, and 5-year timepoints are available for each comparator in Table S12. For neoNIVO + CT, 5-year EFS ranged from 43% (stage IIIA and stage IIIA N2 target population) to 46% (stage IB-IIIA target population), whereas for S alone, 5-year EFS ranged from 6% (stage IIIA) to 38% (stage IB-II).
The findings from the sensitivity analyses were consistent with the base case models (Figure S4). The inputs and results for the EMA-target model are presented in Tables S16–S18; Figures S8–S10, and the model outputs were closely aligned with those from the PD-L1 ≥ 1% stage IB-IIIA model.
3.4. Relative Efficacy for Pathological Complete Response
The networks of evidence for pCR in the stage IB-IIIA and stage IIIA N2 target population are presented in Figure 4A. As all studies comparing neoCRT vs. neoCT were conducted among patients with stage IIIA N2, the main difference between these two models was the input from CM816 (intent-to-treat vs. stage IIIA OR, respectively) and NADIM II (intent-to-treat vs. stage III N2 OR). Five RCTs informed the stage IB-IIIA/IIIA N2 network, including data from 780 patients and 11 trial arms, resulting in ten effective arms as two neoCRT arms were pooled into a single arm (IFCT 0101). For the stage IIIA target population, only two studies informed a connection between neoNIVO + CT and neoCT (Figure 4B), and therefore a pairwise meta-analysis of direct evidence was conducted. For the stage IB-II target population, no quantitative evidence synthesis was conduced as the evidence base was only informed by CM816. Data informing the pCR NMA, along with pCR definitions are available in Table S13.
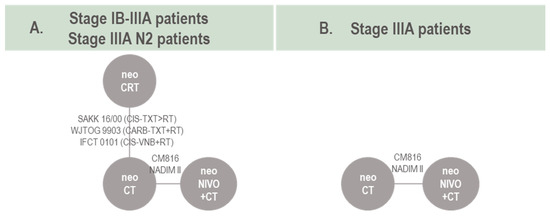
Figure 4.
Network of evidence for pCR models. Note: for studies involving neoCT or neoCRT (e.g., neoCT vs. S) see baseline characteristics summary. NADIM II was included in the pCR analysis as the intervention arm (perioperative nivolumab combined with neoadjuvant chemotherapy) can effectively be considered comparable to neoadjuvant nivolumab combined with neoadjuvant chemotherapy as pCR outcomes are collected prior to receipt of any adjuvant therapy. Abbreviations: CARB, carboplatin; CIS, cisplatin; CT, chemotherapy; CRT, chemoradiotherapy; neo, neoadjuvant; NIVO, nivolumab; TXT, docetaxel; VNB, vinorelbine.
For the target population of patients with stage IB-IIIA, neoNIVO + CT was associated with improvements in pCR relative to neoCT (OR = 12.53 [5.60, 33.82]) and neoCRT (7.15 [2.31, 24.34]) (Figure 5). Estimates of relative improvement were greater in the stage IIIA and IIIA N2 target populations. Anchored on the neoCT arm of CM816, the proportions achieving pCR for neoNIVO + CT were 20%, 14% and 19% in the stage IB-IIIA, stage IIIA and stage IIIA N2 target populations, respectively (Table S14). Tabular summaries of ORs between all treatments in the stage IB-IIIA base case network are available in Table S15.
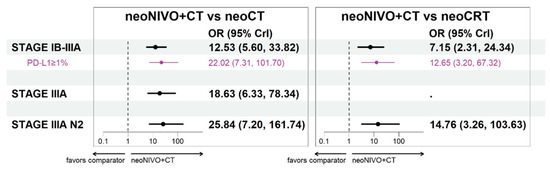
Figure 5.
Pathological complete response odds ratio estimates for neoNIVO + CT vs. all relevant comparators across base case and the PD-L1 specific models (fixed-effect model estimates). Note: The stage IB-II cases are not represented in the figure as they were only informed by CheckMate 816 (odds ratio: 6.85; 95% confidence interval: 1.89, 24.76) and as such, no NMA was conducted for this stage. Abbreviations: CrI, credible interval; CT, chemotherapy; CRT, chemoradiotherapy; neo, neoadjuvant; NIVO, nivolumab; OR, odds ratio.
Findings from the target populations that used inputs from the PD-L1 ≥ 1% CM816 population had a larger magnitude of effect for neoNIVO + CT compared to the base case model. As shown in Tables S16–S18; Figures S8–S10, the EMA-target model closely aligned with the results of the stage IB-IIIA PD-L1 ≥ 1% model.
3.5. Safety
Safety outcomes were not included in the NMA due to limited data and large numbers of zero cells; for completeness, however, a tabular summary of the overall occurrence of adverse events and adverse events leading to discontinuation across the base case studies in the NMA are included in Tables S19 and S20.
4. Discussion
The positive findings from CM816 have established neoNIVO + CT as a new standard of care for patients with rNSCLC. Our study provides evidence of improved EFS and pCR with neoNIVO + CT relative to conventional therapies, namely neoCT, adjCT, and S alone. These results help to establish the value of a neoadjuvant IO treatment strategy and provide a benchmark against which to contrast emerging evidence from other IO-based regimens.
Results from the more narrowly defined populations suggest that the greatest clinical benefit associated with neoNIVO + CT relative to conventional therapies may be among patients with stage IIIA and patients whose tumours express PD-L1 (even though numerical benefits were also observed among patients with stage IB-II and PD-L1 < 1%) [32]. However, limitations in subgroup sizes and statistical power in CM816 warrant caution in interpreting these findings. Additional evidence from observational studies as well as a longer follow up for CM816, particularly relevant for earlier stage patients, could help elucidate which subgroups are likely to have a meaningful clinical benefit from neoNIVO + CT.
This NMA also provided additional evidence with respect to the superiority of neoadjuvant therapy relative to S alone or adjCT. It was already well understood that, while only moderately more efficacious [34], neoadjuvant chemotherapy helped overcome the limitations of adjCT, namely, higher completion rates of planned chemotherapy cycles [34,51], and avoidance of systemic treatment delays experienced by patients receiving adjCT [52,53,54]. However, in clinical practice, some physicians or patients may historically have considered bypassing neoadjuvant therapy to avoid the risk of surgery being delayed or cancelled, opting for a surgery-first strategy. The emergence of early IO therapy in the rNSCLC space has provided an opportunity to treat micrometastatic disease and enhance the immune response when bulk tumour and tumour antigens are present, and has resulted in a boost in the efficacy of the neoadjuvant treatment strategy, relative to neoCT, as demonstrated by CM816 [9,32,55]. The compelling results of this NMA have further helped establish neoNIVO + CT as a superior strategy to S alone and adjCT, in alignment with NCCN recommendations that all patients who are IO-eligible should be considered for neoadjuvant IO-based treatments.
This NMA has provided inconclusive evidence on the relative efficacy of neoNIVO + CT relative to neoCRT among patients with stage IIIA, for which neoCRT is recommended [2]. The findings within the stage-specific IIIA N2 population from the current NMA indicate that the odds of pCR are 14 times higher, and that the EFS risk is 35% lower with neoNIVO + CT relative to neoCRT; however, the corresponding estimates of EFS benefit were not statistically significant. In other indications, evidence suggests that adding radiotherapy to neoCT may reduce the risk of locoregional recurrence relative to neoCT alone, via its localized delivery, but does not reduce the risk of distant metastases [56]. In addition, no studies to date have demonstrated an OS benefit of neoCRT relative to neoCT. In terms of tolerability, the addition of radiotherapy to neoCT could potentially impact the fitness of patients to proceed to surgery after neoadjuvant treatment. For these reasons, the role of neoCRT as a treatment option for rNSCLC patients is uncertain (except among patients for which IO-based therapies are contraindicated) especially as the treatment landscape changes with the introduction of IO-based therapies.
While the relative safety of neoNIVO + CT could not be investigated via the NMA due to several challenges (AEs being reported for some agents and not others due to differences in the mechanism of action, sparse data with large numbers of zero cells, and non-reporting), evidence from CM816 indicates that the addition of nivolumab to neoCT is not associated with a higher incidence or greater severity of AEs [20]. Two of the studies in the NMA evidence base reported the proportion of patients experiencing any AEs of grades 3 to 4; SAK 16/00 reported fewer grade 3–4 CT-related AEs in the neoCRT arm (45%) than in the neoCT arm (60%) and CHEST reported fewer grade 3–4 AEs in the surgery-alone arm (11%) compared to the neoCT arm (41%) [35,40]. In addition, surgery-related AEs such as surgical 90-day morbidity/mortality and delay or cancellation of surgery due to toxicity were not captured in the current review as they were not pre-defined fields but are important to consider when interpreting the efficacy-focused findings of our analysis. In CM816, while the percentage of patients with delayed surgery was similar in the two treatment groups (21% and 18% in the neoNIVO + CT and neoCT arms, respectively), patients treated with neoNIVO + CT had shorter operations, had higher rates of minimally invasive surgery (30% vs. 22%), lower rates of pneumonectomies (17% vs. 25%) and experienced fewer conversions to open surgery (11% vs. 16%) compared to patients treated with neoCT; these differences were most notable in the stage IIIA cohort [20].
The findings presented in this analysis are consistent with other meta-analyses. For the comparison of adjCT to S, several meta-analyses have been conducted [7,51,57,58]; however, these were primarily based on evidence from RCTs that only enrolled completely resected patients, based on post-surgical eligibility assessments. Our sensitivity analysis also captured such studies, although these do not necessarily reflect the relative effect in a potentially resectable population. Disease-free or recurrence-free survival effect estimates for adjCT vs. S in those previous meta-analyses ranged from 0.67 (0.56, 0.81) to 0.84 (0.78, 0.91) depending on study eligibility, which aligns with our EFS HR sensitivity analysis estimate of 0.76 for adjCT vs. S and was stronger than the HR in our base case that excluded RCTs restricted to completely resected patients (HR of 0.89). The 2014 meta-analysis reported by the NSCLC Meta-analysis Collaborative Group estimated an RFS HR of 0.85 (95% CI: 0.76, 0.94) for the comparison of neoCT vs. S; highly consistent with the ones produced by our NMAs (0.86, 95% CrI: 0.78, 0.96) [10].
This study was not without limitations. First, there was some heterogeneity in the evidence base in terms of stage, regimen characteristics, gender distribution, and histology. With respect to stage, no conclusive evidence has been identified as to whether it conveys treatment effect modification in the rNSCLC space. However, an attempt was made to address heterogeneity through consideration of stage-specific models, but the significant evolution in clinical staging and progress in surgical techniques during the study timeline could have led to discrepancies in the baseline stage inclusions that do not completely match current understanding of rNSCLC. It was not possible to control for these important variables. With respect to regimen characteristics, gender distribution and histology, while these characteristics may be prognostic among patients with rNSCLC, they are not known treatment-effect modifiers, so are unlikely to have introduced bias to the NMA. Future work can be conducted using a multi-level network meta-regression, as described by Phillippo et al. 2020, to adjust for all heterogeneous prognostic and effect-modifying variables in the evidence base [59]. Second, the base case NMA was informed by a small number of studies, with the largest network of evidence being informed by at most three studies per available connection. Some of the analyses presented relied on subgroup data (e.g., stage-specific data from all-stage trials). It is important to note that these studies may not be powered to detect treatment effects amongst these subgroups. In addition, if trials were not stratified by these subgroups upon randomization, patient characteristics may not necessarily be balanced between study arms within these subgroups, which may introduce bias. For both reasons, analyses relating to the specific target populations should be interpreted with caution. Third, due to the paucity of data amongst the base case trials, relevant outcomes such as major pathologic response (MPR), could not be included in this evidence synthesis. In the case of pCR, where there were limited data, an indirect comparison versus neoCRT could not be drawn for the stage IB-II, or stage IIIA target populations; however, neoCRT is most relevant to the target population of stage IIIA N2 for which an indirect comparison was possible. Similarly, and perhaps most notably, OS was not evaluated as it is an ongoing endpoint in CM816 and will be evaluated at a later time; however, there is evidence to suggest a positive association between pCR or EFS with OS [60,61,62,63,64,65]. Fourth, within the stage IIIA N2 specific analysis, nodal status was not captured in one study (CM816), as such, stage IIIA data were used as inputs in this model. Additionally, N2 patients are considered to be a heterogeneous population [66], and the studies informing the N2-specific models seldomly reported details related to type of N2 disease (i.e., single station vs. multi-station). Similarly, heterogeneity could have been introduced due to the complexity of defining the criteria for ‘potentially resectable’ particularly among IIIA N2 patients. In a study conducted by Mainguene et al. 2022, concordance of defining resectability was 70% between two different multidisciplinary tumour board sessions, further highlighting the need for more specific assessment criteria [66]. This known lack of concordance could lead to differences in the proportion of patients across trials that are less likely to respond to treatment (i.e., those who could have met non-resectability criteria); these differences can result in biased relative effect estimates that could not be measured or adjusted for. Finally, while other immunotherapy regimens such as ATEZO, DURVA, and PEMBRO are rapidly changing the treatment landscape, they were not included in this analysis because they were not approved and/or were not relevant due to known differences in study conduct [67,68,69,70]. Future analyses to include comparisons with adjuvant IO-therapies will need to adjust for differences in their study designs and differences in enrolled patient populations. Methodology has been proposed to reduce biases associated with differences in trial design with respect to the time of randomization and patient eligibility, such as those between CM816 and IMpower010/Keynote-091 [24]. Similarly, perioperative immunotherapy, including perioperative NIVO was not included as a comparator of interest also due to lack of regulatory approval at the time of analysis. The findings from CM816 and our NMA provide supporting evidence that early introduction of IO can mount an immune system response while the tumour is still present. Findings from CM816 have motivated the exploration of perioperative IO strategies, as evaluated in NADIM II (which was included in our base case pCR analysis) and CheckMate 77T (an ongoing clinical trial of perioperative NIVO plus neoCT published after the SLR cut-off date). NADIM II found that perioperative NIVO combined with neoCT reduced the risk of PFS by 53% compared to neoCT alone [71]. CM77T demonstrated statistically significant EFS improvement with perioperative NIVO combined with neoCT versus neoCT alone (HR: 0.58; 95% CI: 0.42–0.81) [72]. It will be important for future analyses to compare perioperative IO regimens with established treatments in rNSCLC, including neoNIVO + CT to help elucidate the clinical relevance of the additional adjuvant immunotherapy component.
5. Conclusions
This NMA provides evidence that neoNIVO + CT confers added clinical benefit in terms of EFS and pCR relative to neoCT, S, and adjCT amongst patients with rNSCLC. These findings are restricted by limited data, including the shorter follow-up from CM816. Updates to this analysis will be conducted with more mature data from CM816, which may mitigate some of the uncertainty associated with the estimates. Similarly, the impact of neoNIVO + CT on OS in relation to existing treatment options will need to be assessed once mature data are available.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16132492/s1, Table S1: Search strategy (MEDLINE)—Database(s): Ovid MEDLINE(R) and Epub Ahead of Print, In-Process and Other Non-Indexed Citations and Daily. Search was last run on 15 November 2022; Table S2: Search strategy (EMBASE)—Database(s): Embase. Search was last run on 15 November 2022; Table S3: Search strategy (Cochrane)—Database(s): EBM Reviews—Cochrane Central Register of Controlled Trials. Search was last run on 15 November 2022; Table S4: Conferences searched; Table S5: SLR PICOS; Table S6: NMA PICOS; Table S7: Evidence for and against effect modification of stage on relative effect size; Table S8: Methods for evidence synthesis; Table S9: Full list of models; Figure S1: PRISMA; Figure S2: Risk of bias assessment; Figure S3: Kaplan–Meier curves of EFS for base case: All stages, potentially resectable, third generation chemotherapies; Table S10: Data inputs and definitions for event-free survival in the base case; Table S11: Pairwise event-free survival hazard ratio estimates in the base case stage IB-IIIA model (fixed-effect model estimates); Table S12: Event-free survival percentages at 2, 3, and 5 years anchored on neoCT in the base case models (fixed-effect model estimates); Figure S4: Event-free survival hazard ratio estimates for neoNIVO + CT vs. all relevant comparators across all models (base case and sensitivity models); Figure S5: Network of evidence for EFS models with studies included in the sensitivity analyses; Figure S6: Event-free survival hazard ratio estimates for neoNIVO + CT vs. all relevant comparators across the base case and the PD-L1 specific models (random-effects model estimates); Table S13: Data inputs and definitions for pathological complete response in the base case; Table S14: Pathological complete response percentages anchored on neoCT in the base case models (fixed effect model estimates); Table S15: Pairwise pathological complete response odds ratio estimates in the base case stage IB-III model (fixed-effect model estimates); Figure S7: Pathological complete response odd ratio estimates for neoNIVO + CT vs. all relevant comparators across the base case and the PD-L1 specific models (random-effects model estimates); Table S16: Baseline patient characteristics across EFS evidence base for trials included in the stage II-IIIA PD ≥ 1% model; Table S17—EFS NMA inputs evidence base for stage II-IIIA PD ≥ 1% model; Figure S8: Event-free survival hazard ratio estimates for neoNIVO + CT vs. all relevant comparators for stage II-IIIA PD ≥ 1% model (fixed-effect model estimates); Figure S9: Event-free survival hazard ratio estimates for neoNIVO + CT vs. all relevant comparators for stage II-IIIA PD ≥ 1% model (random-effects model estimates); Table S18: Pathological complete response NMA inputs evidence base for stage II-IIIA PD ≥ 1% model; Figure S10: Pathological complete response odds ratio estimates for neoNIVO + CT vs. all relevant comparators for stage II-IIIA PD ≥ 1% model (fixed-effect model estimates); Table S19: Proportion of patients with grade 3 to 4 adverse events; Table S20: Proportion of patients with AEs leading to treatment discontinuation. References [7,8,10,20,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,52,58,73] are also cited in the supplementary materials.
Author Contributions
N.G. provided clinical expertise and oversight for the study. M.B., S.G. and G.L.-O. contributed to the writing and to the design and interpretation of the analysis. B.R. performed the data analysis and contributed to the writing, design, and interpretation of the analysis. S.L., L.V. and M.A.C. contextualized the findings and contributed to the design and interpretation of the analysis. M.T., N.V., J.M.L. and J.S. provided clinical insights and contextualized the findings. N.W. and W.W.Y. conducted the systematic literature review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Bristol Myers Squibb. The APC was funded by Bristol Myers Squibb.
Institutional Review Board Statement
Not applicable (only previously published data were used in this study).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets produced during this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Stefano Lucherini, Lien Vo, Mohammad A Chaudhary, Mia Tran, and Nebibe Varol are employees of Bristol Myers Squibb. Mariam Besada, Basia Rogula, and Greta Lozano-Ortega are employees of Broadstreet HEOR which was contracted by Bristol Myers Squibb for the conduct of this study. Sarah Goring was contracted to support Broadstreet HEOR with the conduct of this study. Nathalie Waser is an employee of ICON which were also contracted by Bristol Myers Squibb for the conduct of this study. Nicolas Girard declares research grants/support from Abbvie, Amgen, AstraZeneca, Beigene, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Gilead, Hoffmann-La Roche, Janssen, LeoPharma, Lilly, Merck Sharp & Dohme, Novartis, Sivan; Consultative services for Abbvie, Amgen, AstraZeneca, Beigene, Bristol Myers Squibb, Daiichi-Sankyo, Gilead, Ipsen, Hoffmann-La Roche, Janssen, LeoPharma, Merck Sharp & Dohme, Mirati, Novartis, Pfizer, Sanofi, Takeda; Participation on a data safety monitoring board for Hoffmann-La Roche; employment of a family member with AstraZeneca. Jay M Lee declares research grants/support from Bristol Myers Squibb, Genentech, Novartis, Roche; advisory board/consultant with AstraZeneca, Bristol Myers Squibb, Foundation Medicine Institute, Genentech, IDEOlogy Health, Merck, Novartis, Regeneron Pharmaceuticals, Roche; steering committee with Genentech, Merck, Novartis, Roche; speakers bureau with AstraZeneca, Bristol Myers Squibb, DAVA Oncology, ecancer, Genentech, Medscape, Roche, Targeted Oncology; and patents with University of California Los Angeles. Jonathon Spicer declares research grants from AstraZeneca, BMS, Merck, Roche, CLS Therapeutics, Protalix Biotherapeutics, Pfizer, Regeneron; consulting, advisory role or honoraria with AstraZeneca, Merck, Roche, BMS, Novartis, Chemocentryx, Amgen, Protalix Biotherapeutics, Xenetic Biosciences, Regeneron, Eisai, Peerview, OncLive, Medscape, Pfizer; and clinical trial leadership role with BMS, Novartis, Roche, Merck, AstraZeneca.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- NCC Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer; Version 3; NCC: Atlanta, GA, USA, 2023. [Google Scholar]
- Postmus, P.; Kerr, K.; Oudkerk, M.; Senan, S.; Waller, D.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- PDQ Adult Treatment Editorial Board. PDQ Non-Small Cell Lung Cancer Treatment. 2021. Available online: https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq (accessed on 2 December 2021).
- Taylor, M.D.; Nagji, A.S.; Bhamidipati, C.M.; Theodosakis, N.; Kozower, B.D.; Lau, C.L.; Jones, D.R. Tumor recurrence after complete resection for non-small cell lung cancer. Ann. Thorac. Surg. 2012, 93, 1813–1820; discussion 1820–1821. [Google Scholar] [CrossRef] [PubMed]
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, R.; Auperin, A.; Burdett, S.; Higgins, J.P.; Johnson, D.H.; Le Chevalier, T.; Le Pechoux, C.; Parmar, M.K.; Pignon, J.P.; Souhami, R.L.; et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet 2010, 375, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.A.; Ding, K.; Seymour, L.; Twumasi-Ankrah, P.; Graham, B.; Gandara, D.; Johnson, D.H.; Kesler, K.A.; Green, M.; Vincent, M.; et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: Updated survival analysis of JBR-10. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Uprety, D.; Mandrekar, S.J.; Wigle, D.; Roden, A.C.; Adjei, A.A. Neoadjuvant immunotherapy for NSCLC: Current concepts and future approaches. J. Thorac. Oncol. 2020, 15, 1281–1297. [Google Scholar] [CrossRef] [PubMed]
- NSCLC Meta-Analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: A systematic review and meta-analysis of individual participant data. Lancet 2014, 383, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Albain, K.S.; Swann, R.S.; Rusch, V.W.; Turrisi, A.T., 3rd; Shepherd, F.A.; Smith, C.; Chen, Y.; Livingston, R.B.; Feins, R.H.; Gandara, D.R.; et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet 2009, 374, 379–386. [Google Scholar] [CrossRef]
- Spicer, J.D.; Shewale, J.B.; Nelson, D.B.; Mitchell, K.G.; Bott, M.J.; Vallières, E.; Wilshire, C.L.; Vaporciyan, A.A.; Swisher, S.G.; Jones, D.R.; et al. Multimodality Therapy for N2 Non-Small Cell Lung Cancer: An Evolving Paradigm. Ann. Thorac. Surg. 2019, 107, 277–284. [Google Scholar] [CrossRef]
- Zinner, R.; Axelrod, R.; Solomides, C.C.; Cowan, S.; Leiby, B.; Bhatia, A.K.; Sundermeyer, M.L.; Hooper, D.C.; Harshyne, L.; Lu-Yao, G.L. Neoadjuvant nivolumab (N) plus cisplatin (C)/pemetrexed (P) or cisplatin/gemcitabine (G) in resectable NSCLC. J. Clin. Oncol. 2020, 38, 9051. [Google Scholar] [CrossRef]
- Yang, C.-F.J.; McSherry, F.; Mayne, N.R.; Wang, X.; Berry, M.F.; Tong, B.; Harpole, D.H., Jr.; D’Amico, T.A.; Christensen, J.D.; Ready, N.E. Surgical outcomes after neoadjuvant chemotherapy and ipilimumab for non-small cell lung cancer. Ann. Thorac. Surg. 2018, 105, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Cai, L.; Chen, S.; Jiang, Y. The safety and efficacy of neoadjuvant programmed death 1 inhibitor therapy with surgical resection in stage IIIA non-small cell lung cancer. Ann. Transl. Med. 2021, 9, 486. [Google Scholar] [CrossRef] [PubMed]
- Tfayli, A.; Al Assaad, M.; Fakhri, G.; Akel, R.; Atwi, H.; Ghanem, H.; El Karak, F.; Farhat, F.; Al Rabi, K.; Sfeir, P. Neoadjuvant chemotherapy and Avelumab in early stage resectable nonsmall cell lung cancer. Cancer Med. 2020, 9, 8406–8411. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.A.; Gainor, J.F.; Awad, M.M.; Chiuzan, C.; Grigg, C.M.; Pabani, A.; Garofano, R.F.; Stoopler, M.B.; Cheng, S.K.; White, A. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Wang, J.; Wu, J.; Chen, S.; Li, J.; Liu, J.; Chen, Q.; Jiang, Y. Neoadjuvant pembrolizumab with chemotherapy for the treatment of stage IIB–IIIB resectable lung squamous cell carcinoma. J. Thorac. Dis. 2021, 13, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Domine, M.; Majem, M.; Rodriguez-Abreu, D.; Martinez-Marti, A.; Carpeño, J.D.C. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, T.; Luo, Z.; Tong, L.; Dong, X.; Zhang, Y.; Afzal, M.Z.; Correale, P.; Liu, H.; Jiang, T. Neoadjuvant programmed cell death protein 1 inhibitors combined with chemotherapy in resectable non-small cell lung cancer: An open-label, multicenter, single-arm study. Transl. Lung Cancer Res. 2021, 10, 1020–1028. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Ades, A.; Caldwell, D.M.; Reken, S.; Welton, N.J.; Sutton, A.J.; Dias, S. Evidence synthesis for decision making 7: A reviewer’s checklist. Med. Decis. Mak. 2013, 33, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Goring, S.; Rogula, B.; Lucherini, S.; Vo, L.; Lozano-Ortega, G.; Besada, M.; Chaudhary, M.; Varol, N.; Lam, P.; Girard, N. P15 Indirect Treatment Comparisons of Time-to-Event Outcomes with Mis-Matched “Time Zero”: Methodology and Application in Resectable Non-Small Cell Lung Cancer. Value Health 2023, 26, S4. [Google Scholar] [CrossRef]
- Bormann, I. DigitizeIt. 2020 [2.5.3]. Available online: https://www.digitizeit.de (accessed on 5 March 2022).
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventionsb; Wiley: Hoboken, NJ, USA, 2019; pp. 205–228. [Google Scholar]
- European Medicines Agency. OPVIDO® (Nivolumab). 2023. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/opdivo (accessed on 1 December 2023).
- Xu, Y.; Wan, B.; Chen, X.; Zhan, P.; Zhao, Y.; Zhang, T.; Liu, H.; Afzal, M.Z.; Dermime, S.; Hochwald, S.N.; et al. The association of PD-L1 expression with the efficacy of anti- PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: A meta-analysis of randomized controlled trials. Transl. Lung Cancer Res. 2019, 8, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Rogula, B.; Lozano-Ortega, G.; Johnston, K.M. A method for reconstructing individual patient data from kaplan-meier survival curves that incorporate marked censoring times. MDM Policy Pract. 2022, 7, 23814683221077643. [Google Scholar] [CrossRef]
- Guyot, P.; Ades, A.; Ouwens, M.J.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef]
- Waser, N.; Vo, L.; McKenna, M.; Penrod, J.; Goring, S. Real-world treatment patterns in resectable (stages I–III) non-small-cell lung cancer: A systematic literature review. Future Oncol. 2022, 18, 1519–1530. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Girard, N.; Provencio, M.; Lu, S.; Wang, C.; Awad, M.M.; Mitsudomi, T.; Felip, E.; Swanson, S.J.; et al. Neoadjuvant nivolumab plus platinum-doublet chemotherapy for resectable NSCLC: 3-year update from CheckMate 816. In Proceedings of the European Lung Cancer Congress 2023, Copenhagen, Denmark, 29 March–1 April 2023. [Google Scholar]
- Provencio, M.; Nadal, E.; Gonzáles-Larriba, J.L.; Martinez-Marti, A.; Bernabé, R.; Bosch-Barrera, J.; Casal-Rubio, J.; Calvo, V.; Insa, A.; Ponce, S.; et al. Nivolumab + chemotherapy (CT) vs. CT as neoadjuvant treatment for resectable stage IIIA-B non-small cell lung cancer (NSCLC): NADIM II trial. In Proceedings of the ASCO, Chicago, IL, USA, 3–7 June 2022. [Google Scholar]
- Felip, E.; Rosell, R.; Maestre, J.A.; Rodríguez-Paniagua, J.M.; Morán, T.; Astudillo, J.; Alonso, G.; Borro, J.M.; González-Larriba, J.L.; Torres, A.; et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3138–3145. [Google Scholar] [CrossRef]
- Scagliotti, G.V.; Pastorino, U.; Vansteenkiste, J.F.; Spaggiari, L.; Facciolo, F.; Orlowski, T.M.; Maiorino, L.; Hetzel, M.; Leschinger, M.; Visseren-Grul, C. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Pisters, K.M.; Vallieres, E.; Crowley, J.J.; Franklin, W.A.; Bunn, P.A., Jr.; Ginsberg, R.J.; Putnam, J.B., Jr.; Chansky, K.; Gandara, D. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non–small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J. Clin. Oncol. 2010, 28, 1843–1849. [Google Scholar] [CrossRef]
- Li, J.; YU, L.C.; Chen, P.; SHI, S.B.; DAI, C.H.; WU, J.R. Randomized controlled trial of neoadjuvant chemotherapy with cisplatin and vinorelbine in patients with stage IIIA non-small cell lung cancer in China. Asia-Pac. J. Clin. Oncol. 2009, 5, 87–94. [Google Scholar] [CrossRef]
- Girard, N.; Mornex, F.; Douillard, J.Y.; Bossard, N.; Quoix, E.; Beckendorf, V.; Grunenwald, D.; Amour, E.; Milleron, B. Is neoadjuvant chemoradiotherapy a feasible strategy for stage IIIA-N2 non-small cell lung cancer? Mature results of the randomized IFCT-0101 phase II trial. Lung Cancer 2010, 69, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Tada, H.; Mitsudomi, T.; Kudoh, S.; Senba, H.; Matsui, K.; Saka, H.; Kurata, T.; Nishimura, Y.; Fukuoka, M. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer 2012, 118, 6126–6135. [Google Scholar] [CrossRef] [PubMed]
- Pless, M.; Stupp, R.; Ris, H.B.; Stahel, R.A.; Weder, W.; Thierstein, S.; Gerard, M.A.; Xyrafas, A.; Früh, M.; Cathomas, R.; et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: A phase 3 randomised trial. Lancet 2015, 386, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, Q.; Jian, H.; Zhou, Z.; Cheng, B.; Lu, S.; Liao, M. Long-term results of a randomized controlled trial evaluating preoperative chemotherapy in resectable non-small cell lung cancer. OncoTargets Ther. 2013, 6, 645–650. [Google Scholar]
- Depierre, A.; Milleron, B.; Moro-Sibilot, D.; Chevret, S.; Quoix, E.; Lebeau, B.; Braun, D.; Breton, J.-L.; Lemarié, E.; Gouva, S. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J. Clin. Oncol. 2002, 20, 247–253. [Google Scholar] [PubMed]
- Gilligan, D.; Nicolson, M.; Smith, I.; Groen, H.; Dalesio, O.; Goldstraw, P.; Hatton, M.; Hopwood, P.; Manegold, C.; Schramel, F. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: Results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007, 369, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Tsuchiya, R.; Mori, T.; Tada, H.; Ichinose, Y.; Koike, T.; Kato, H.; Lung Cancer Surgical Study Group of the Japan Clinical Oncology Group. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J. Thorac. Cardiovasc. Surg. 2003, 125, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Gómez-Codina, J.; Camps, C.; Javier Sánchez, J.; Maestre, J.; Padilla, J.; Cantó, A.; Abad, A.; Roig, J. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: A 7-year assessment of a randomized controlled trial. Lung Cancer 1999, 26, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Gómez-Codina, J.; Camps, C.; Maestre, J.; Padille, J.; Cantó, A.; Mate, J.L.; Li, S.; Roig, J.; Olazábal, A.; et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N. Engl. J. Med. 1994, 330, 153–158. [Google Scholar] [CrossRef]
- Winton, T.; Livingston, R.; Johnson, D.; Rigas, J.; Johnston, M.; Butts, C.; Cormier, Y.; Goss, G.; Inculet, R.; Vallieres, E.; et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N. Engl. J. Med. 2005, 352, 2589–2597. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Rosell, R.; De Lena, M.; Carpagnano, F.; Ramlau, R.; Gonzáles-Larriba, J.L.; Grodzki, T.; Pereira, J.R.; Le Groumellec, A.; Lorusso, V. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB–IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006, 7, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.; Sun, H.-b.; Ye, X.; Zhang, B.-b.; Yang, H.; Fang, Q.; Li, P.; Wang, S.-y. Adjuvant carboplatin-based chemotherapy in resected stage IIIA-N2 non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.M.; Herndon, J.E.; II, M.A.M.; Johnstone, D.W.; Johnson, E.A.; Harpole, D.H.; Gillenwater, H.H.; Watson, D.M.; Sugarbaker, D.J.; Schilsky, R.L. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J. Clin. Oncol. 2008, 26, 5043–5051. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Harris, G.; Patel, A.; Adachi, I.; Edmonds, L.; Song, F. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: Systematic review and indirect comparison meta-analysis of randomized trials. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2009, 4, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.M.; Shepherd, F.A.; Peng, Y.; Darling, G.; Li, G.; Kong, W.; Biagi, J.J.; Mackillop, W.J. Time to adjuvant chemotherapy and survival in non-small cell lung cancer: A population-based study. Cancer 2013, 119, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Kehl, K.L.; Zahrieh, D.; Yang, P.; Hillman, S.L.; Tan, A.D.; Sands, J.M.; Oxnard, G.R.; Gillaspie, E.A.; Wigle, D.; Malik, S.; et al. Rates of Guideline-Concordant Surgery and Adjuvant Chemotherapy Among Patients with Early-Stage Lung Cancer in the US ALCHEMIST Study (Alliance A151216). JAMA Oncol. 2022, 8, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Tsuboi, M.; Brunelli, A. Surgical perspective on neoadjuvant immunotherapy in non-small cell lung cancer. Ann. Thorac. Surg. 2022, 114, 1505–1515. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Pardoll, D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020, 367, eaax0182. [Google Scholar] [CrossRef] [PubMed]
- Sittenfeld, S.; Pham, Y.; Reddy, C.; Obi, E.; Kruse, M.; Al-Hilli, Z.; Cherian, S.; Shah, C.; Tendulkar, R. Ten Year Outcomes of Locoregional and Distant Recurrence for T1-2N1 Breast Cancer with or without Post-Mastectomy Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, E7. [Google Scholar] [CrossRef]
- Pang, L.-L.; Gan, J.-D.; Huang, Y.-H.; Liao, J.; Lv, Y.; Ali, W.A.-S.; Zhang, L.; Fang, W.-F. Investigation of the optimal platinum-based regimen in the postoperative adjuvant chemotherapy setting for early-stage resected non-small lung cancer: A Bayesian network meta-analysis. BMJ Open 2022, 12, e057098. [Google Scholar] [CrossRef]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Phillippo, D.M.; Dias, S.; Ades, A.E.; Belger, M.; Brnabic, A.; Schacht, A.; Saure, D.; Kadziola, Z.; Welton, N.J. Multilevel network meta-regression for population-adjusted treatment comparisons. J. R. Stat. Soc. Ser. A 2020, 183, 1189–1210. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Chaft, J.E.; William, W.N., Jr.; Rusch, V.; Pisters, K.M.; Kalhor, N.; Pataer, A.; Travis, W.D.; Swisher, S.G.; Kris, M.G. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: Proposal for the use of major pathological response as a surrogate endpoint. Lancet. Oncol. 2014, 15, e42–e50. [Google Scholar] [CrossRef] [PubMed]
- Nadler, E.; Vasudevan, A.; Wentworth, C.; Robert, N.; Penrod, J.R.; Fiore, J.; Vo, L. Real-world relationship of early end points to survival end points in patients with resectable non-small-cell lung cancer. Future Oncol. 2023, 19, 1785–1800. [Google Scholar] [CrossRef] [PubMed]
- Pataer, A.; Kalhor, N.; Correa, A.M.; Raso, M.G.; Erasmus, J.J.; Kim, E.S.; Behrens, C.; Lee, J.J.; Roth, J.A.; Stewart, D.J.; et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2012, 7, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Rosner, S.; Liu, C.; Forde, P.M.; Hu, C. Association of Pathologic Complete Response and Long-Term Survival Outcomes Among Patients Treated with Neoadjuvant Chemotherapy or Chemoradiotherapy for NSCLC: A Meta-Analysis. JTO Clin. Res. Rep. 2022, 3, 100384. [Google Scholar] [CrossRef] [PubMed]
- Waser, N.; Quintana, M.; Schweikert, B.; Chaft, J.; Berry, L.; Adam, A.; Vo, L.; Penrod, J.; Fiore, J.; Berry, D.; et al. Pathologic response in resectable non-small cell lung cancer: A systematic literature review and meta-analysis. J. Natl. Cancer Inst. Cancer Spectr. 2024, 8, pkae021. [Google Scholar]
- William, W.N., Jr.; Pataer, A.; Kalhor, N.; Correa, A.M.; Rice, D.C.; Wistuba, I.I.; Heymach, J.; Lee, J.J.; Kim, E.S.; Munden, R.; et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2013, 8, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Mainguene, J.; Basse, C.; Girard, P.; Beaucaire-Danel, S.; Cao, K.; Brian, E.; Grigoroiu, M.; Gossot, D.; Luporsi, M.; Perrot, L.; et al. Surgical or medical strategy for locally-advanced, stage IIIA/B-N2 non-small cell lung cancer: Reproducibility of decision-making at a multidisciplinary tumor board. Lung Cancer 2022, 163, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Heymach, J.; Harpole, D.; Mitsudomi, T. AEGEAN: A phase 3 trial of neoadjuvant durvalumab+ chemotherapy followed by adjuvant durvalumab in patients with resectable NSCLC. Zentralbl. Chir. 2023, 148, S87. [Google Scholar] [CrossRef]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Wakelee, H.A.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; He, J.; Gao, S.; Chen, K.-N.; Dooms, C.A.; Majem, M. KEYNOTE-671: Randomized, double-blind, phase 3 study of pembrolizumab or placebo plus platinum-based chemotherapy followed by resection and pembrolizumab or placebo for early stage NSCLC. J. Clin. Oncol. 2023, 41, LBA100. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; González-Larriba, J.L.; Martínez-Martí, A.; Bernabé, R.; Bosch-Barrera, J.; Casal-Rubio, J.; Calvo, V.; Insa, A.; Ponce, S.; et al. Perioperative Nivolumab and Chemotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 504–513. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. A Study of Neoadjuvant Chemotherapy Plus Nivolumab Versus Neoadjuvant Chemotherapy Plus Placebo, Followed by Surgical Removal and Adjuvant Treatment with Nivolumab or Placebo for Participants with Surgically Removable Early Stage Non-small Cell Lung Cancer. 2023. Available online: https://www.clinicaltrials.gov/study/NCT04025879 (accessed on 1 February 2024).
- Woods, B.S.; Hawkins, N.; Scott, D.A. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: A tutorial. BMC Med. Res. Methodol. 2010, 10, 54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).