Breast Cancer Patient’s Outcomes after Neoadjuvant Chemotherapy and Surgery at 5 and 10 Years for Stage II–III Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient and Tumor Characteristics
3.2. Neoadjuvant Chemotherapy Outcomes
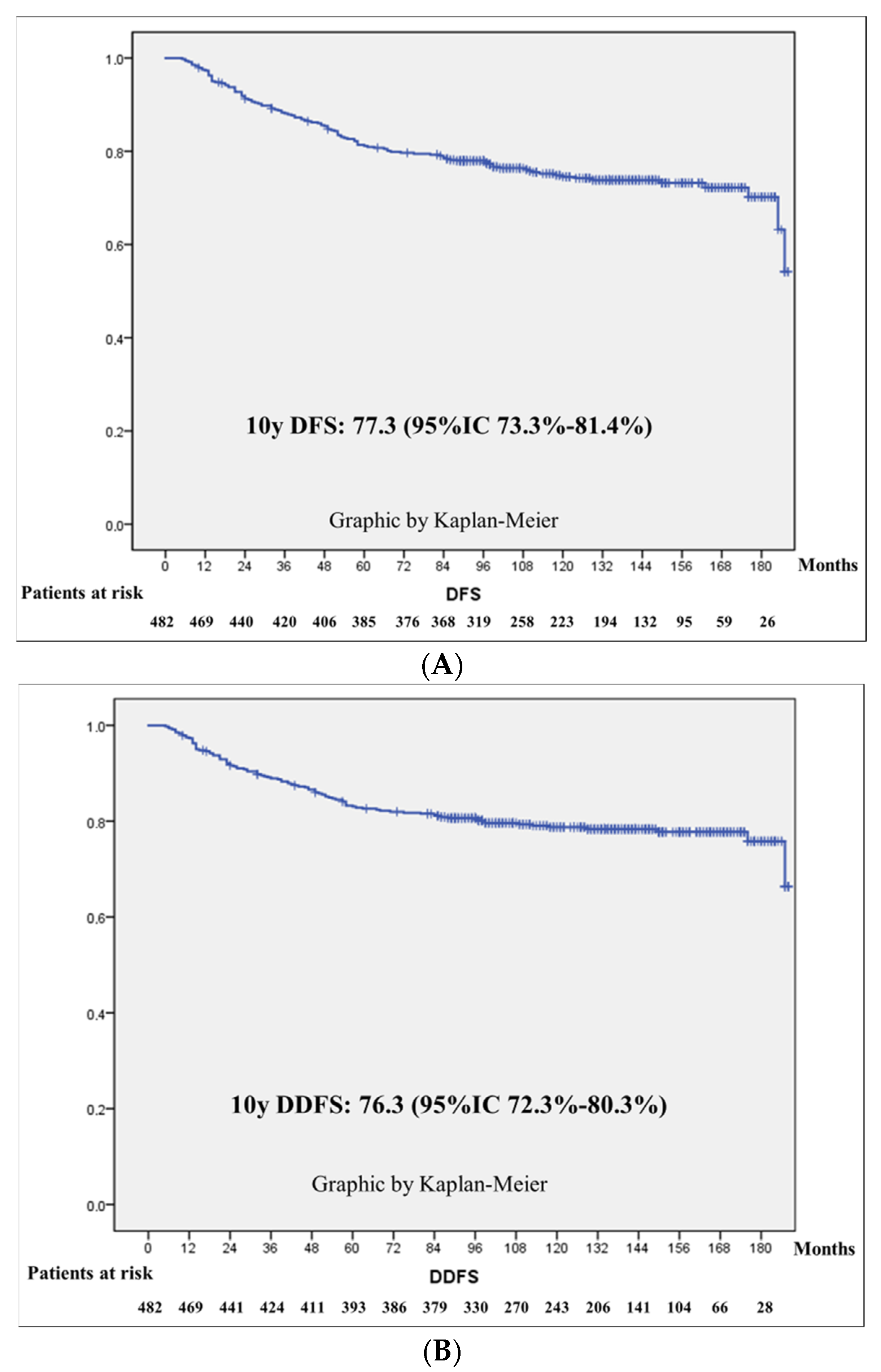
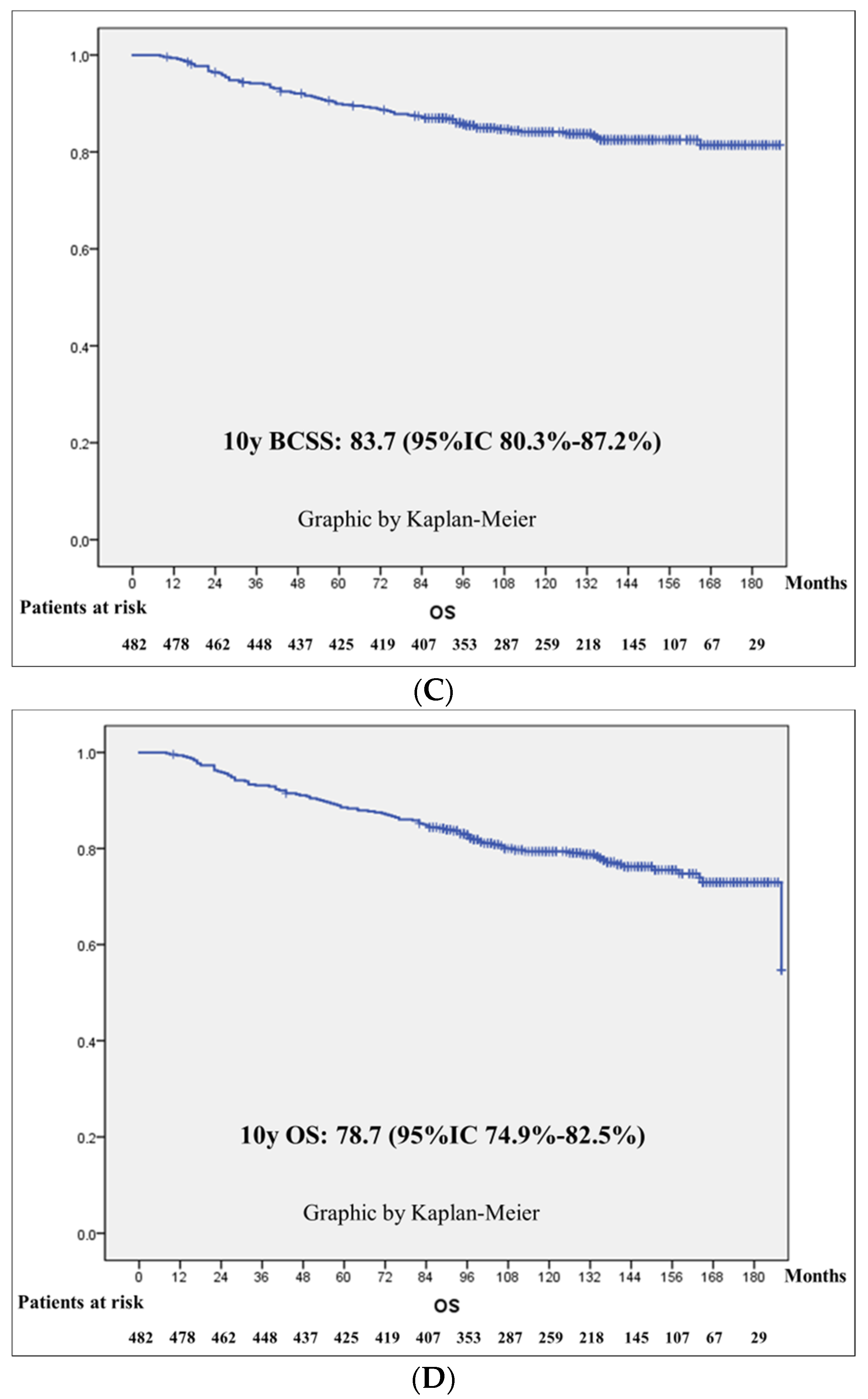
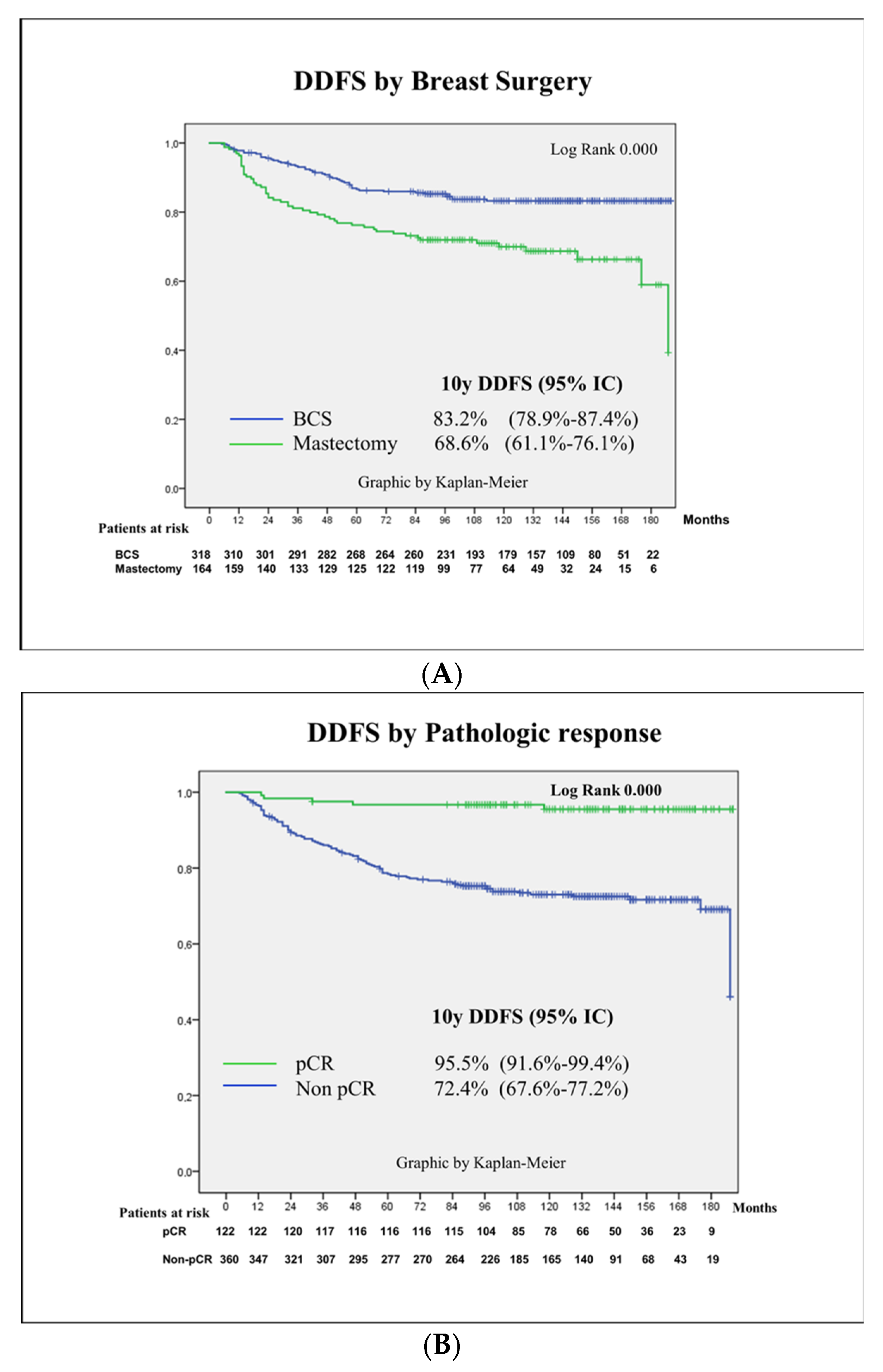
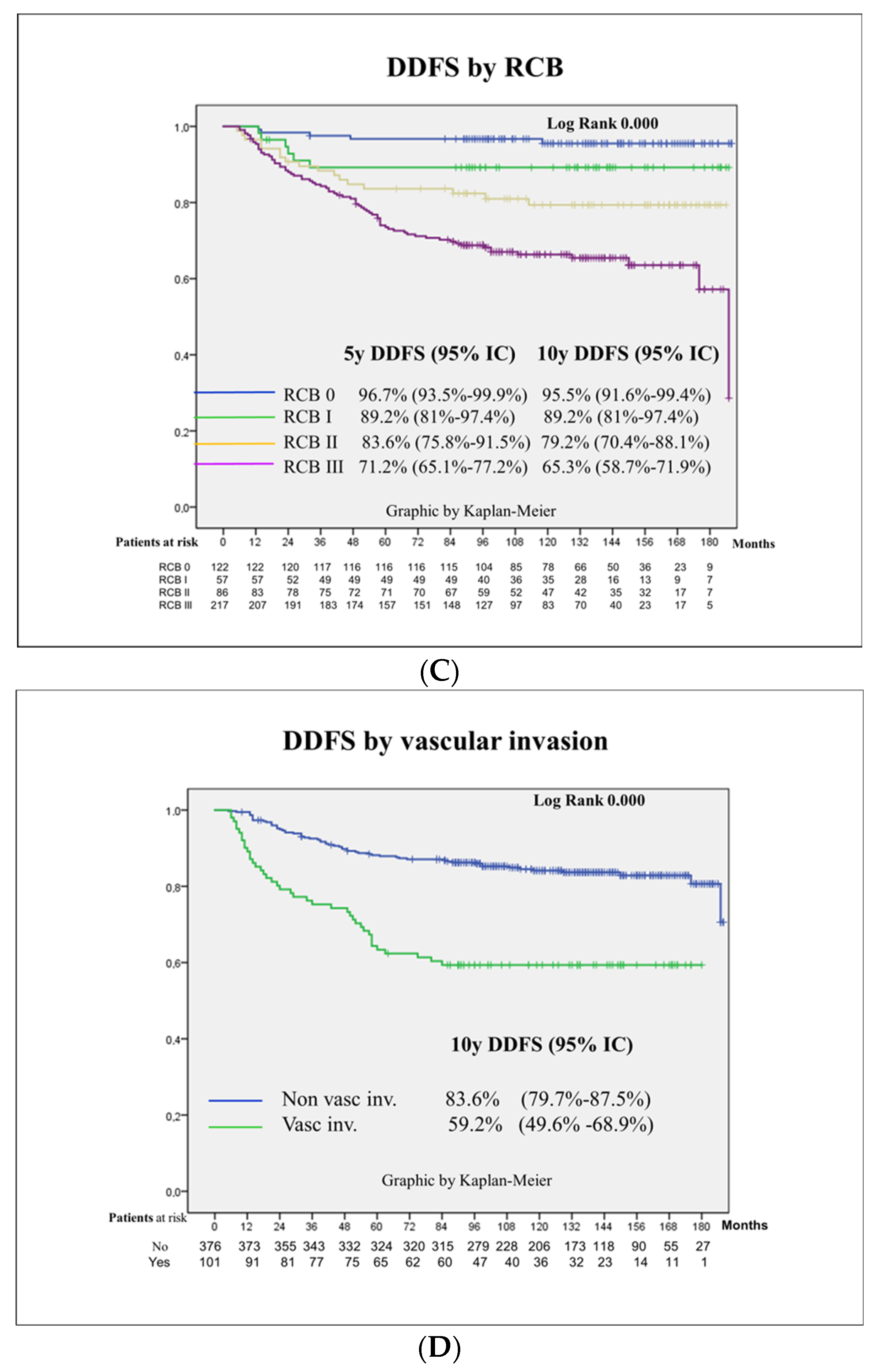
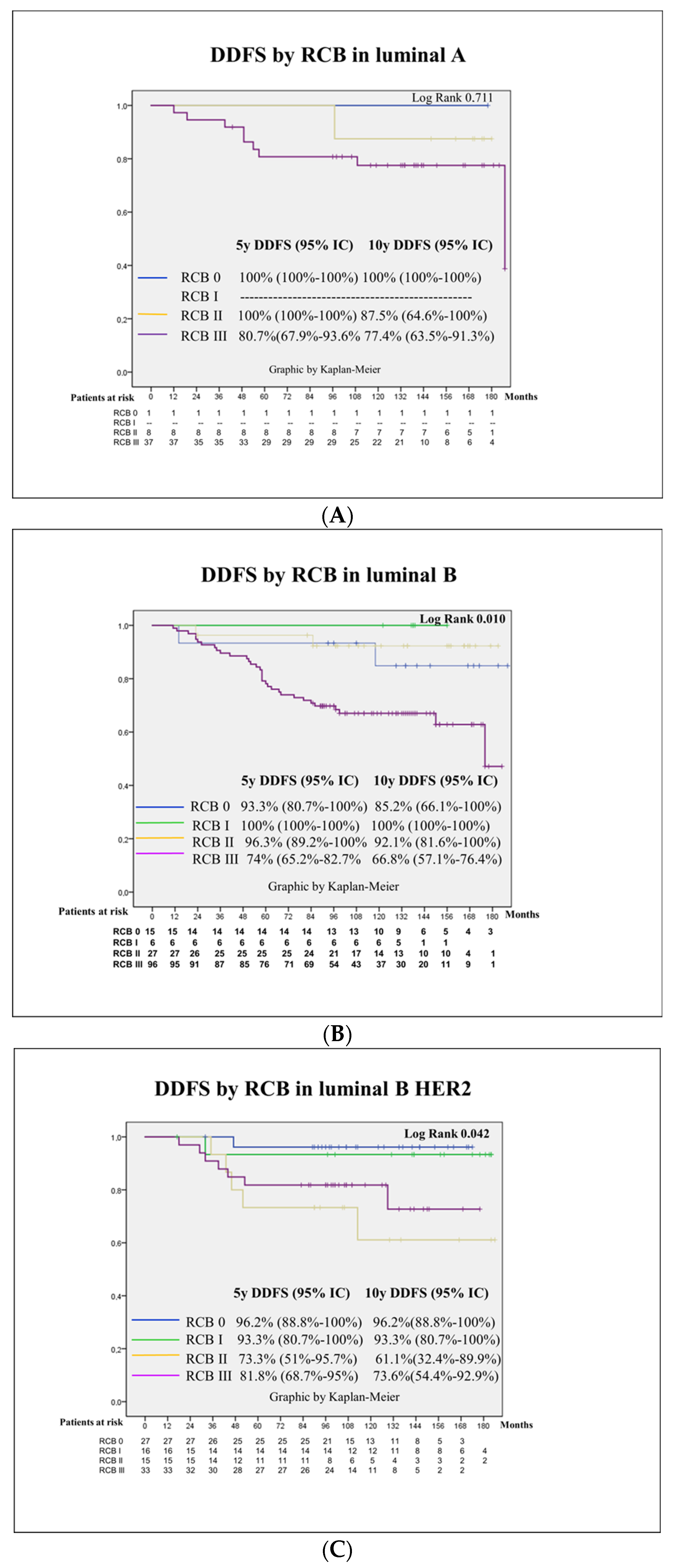
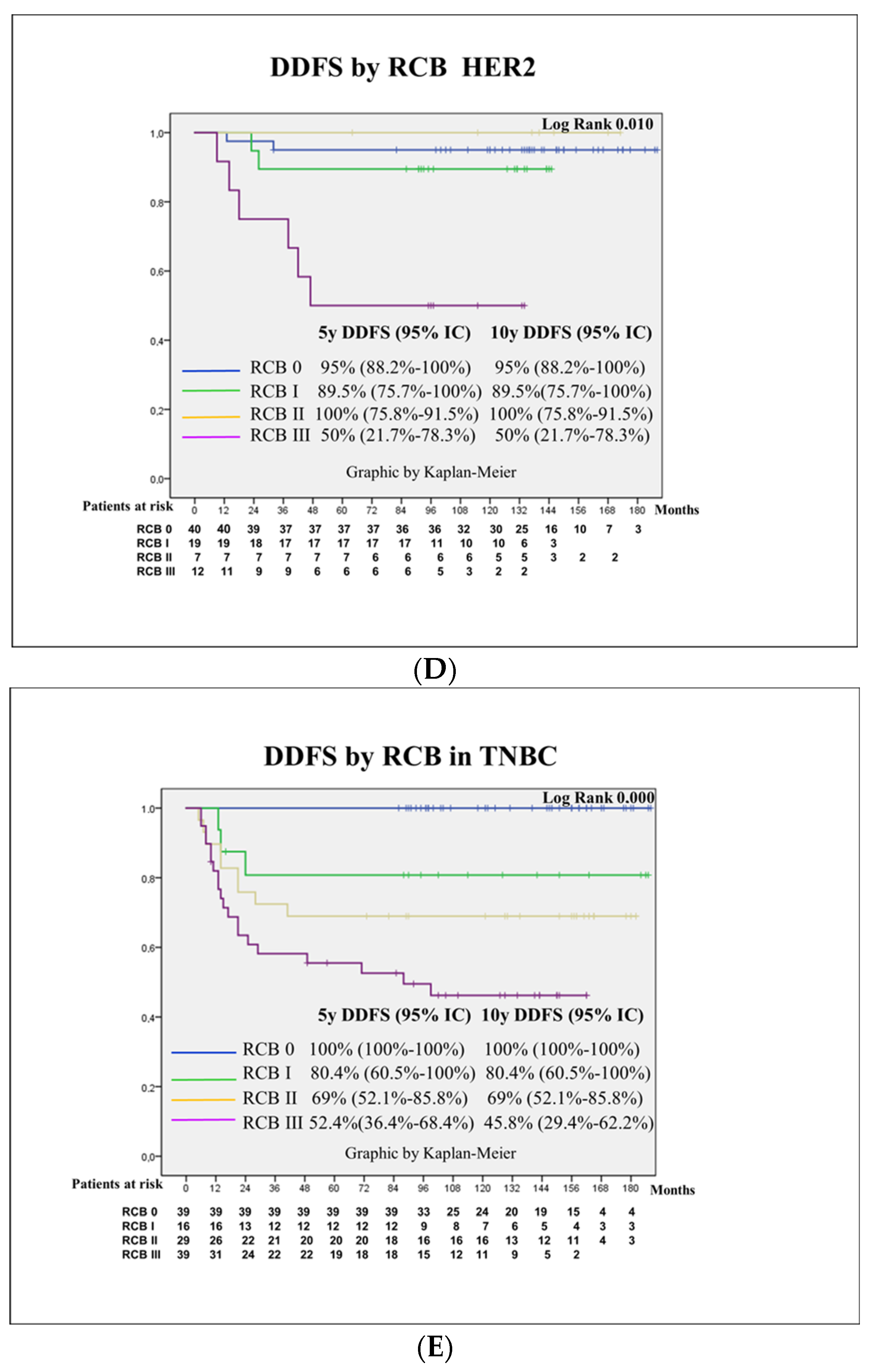
3.3. Survival Outcomes
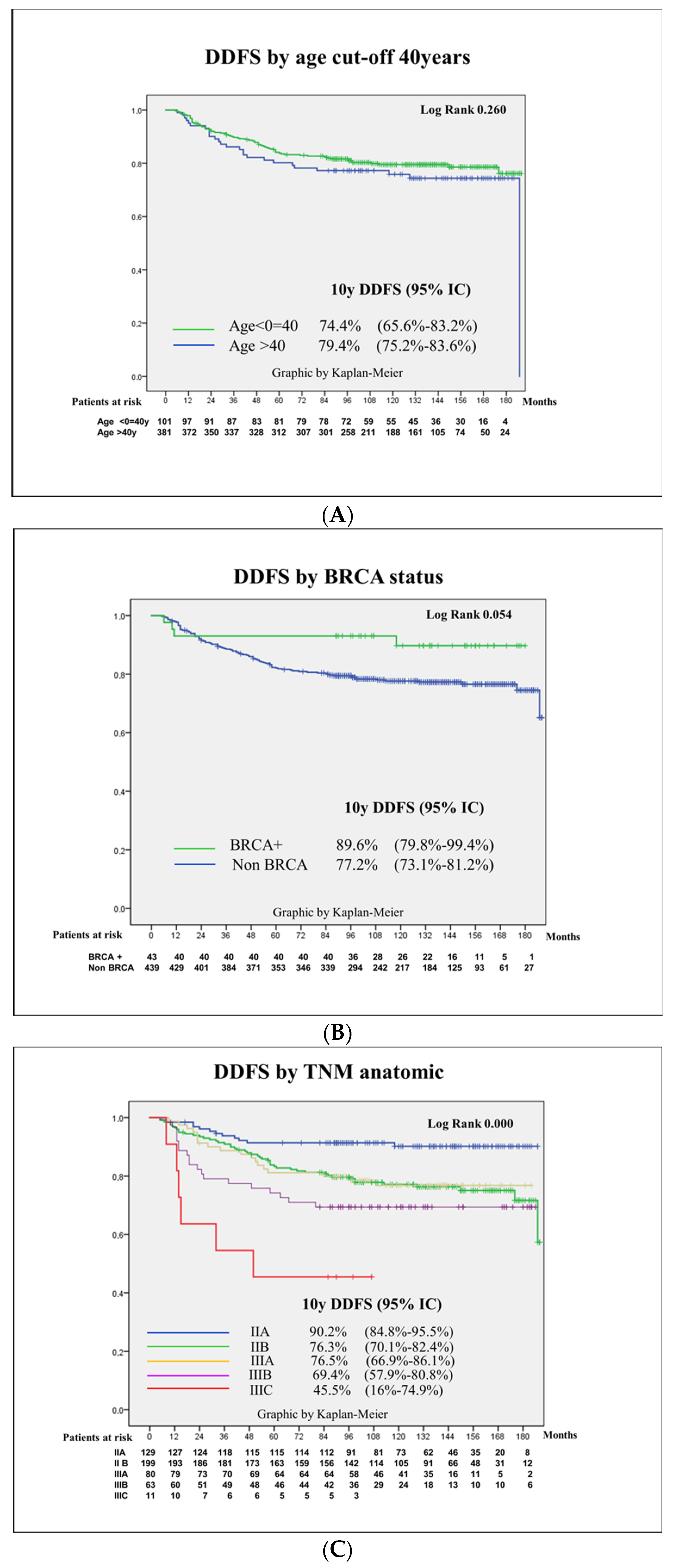
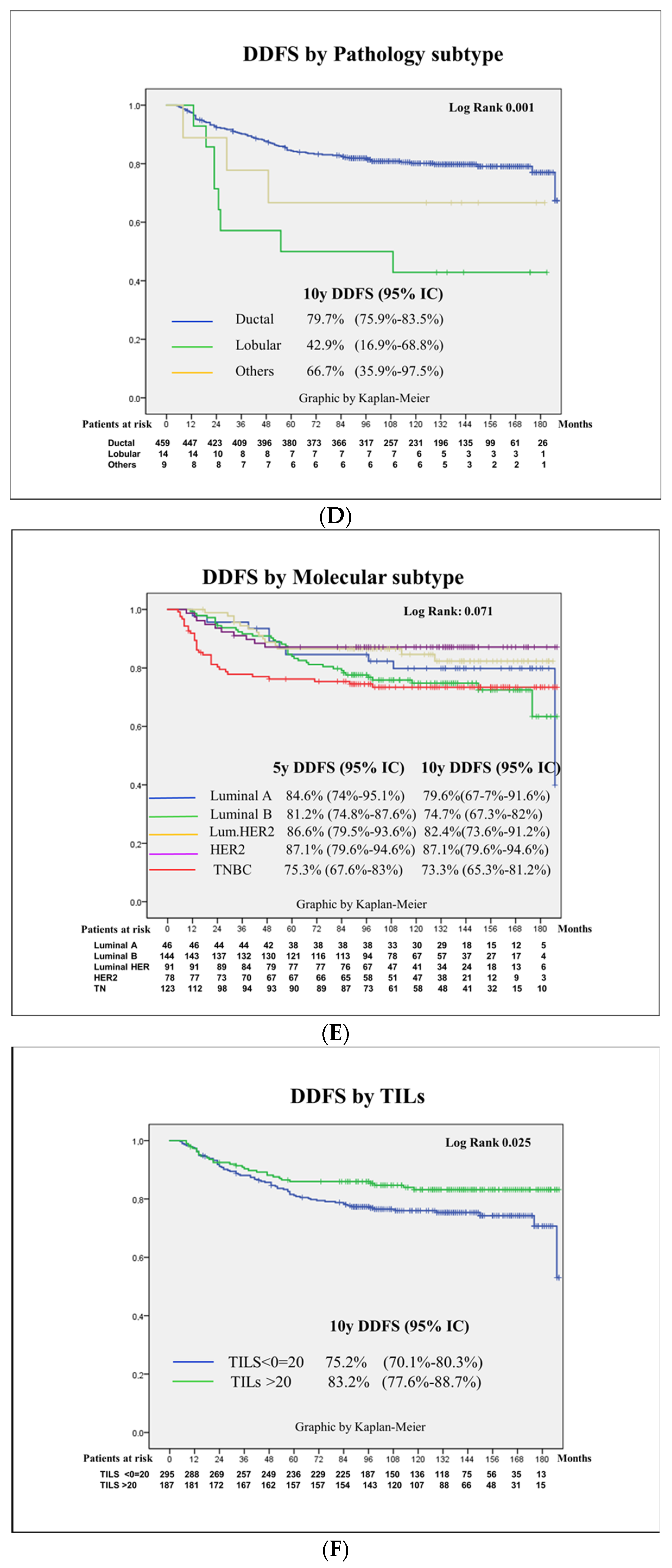
3.4. Prognostic Factors for Patient Survival
3.5. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NATC | Neoadjuvant chemotherapy |
| pCR | Pathological complete response |
| NRI | Neoadjuvant response index |
| RCB | Residual cancer burden |
| TNBC | Triple-negative breast cancer |
| AJCC | American Joint Committee on Cancer |
| ASCO | American Society of Clinical Oncology |
| cN0 | Clinical N0 |
| N+ | Node positive |
| RT | Radiotherapy |
| BMI | Body mass index |
| TILs | Tumor-infiltrating lymphocytes |
| DFS | Disease-free survival |
| DDFS | Distant disease-free survival |
| OS | Overall survival |
| BCSS | Breast-cancer-specific survival |
| SD | Standard deviation |
| CI | Confidence intervals |
| HR | Hazard ratio |
References
- Bonadonna, G.; Veronesi, U.; Brambilla, C.; Ferrari, L.; Luini, A.; Greco, M.; Bartoli, C.; de Yoldi, G.C.; Zucali, R.; Rilke, F.; et al. Primary chemotherapy to avoid Mastectomy in tumors with diameters of three centimeters or more. J. Natl. Cancer Inst. 1990, 82, 1539–1545. [Google Scholar] [CrossRef]
- Wolmark, N.; Wang, J.; Mamounas, E.; Bryant, J.; Fisher, B. Preoperative Chemotherapy in patients with operable Breast Cancer: Nine-years results from National Surgical Adjuvant Breast and Bowel Project B-18. J. Natl. Cancer Inst. Monogr. 2001, 30, 96–102. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the treatment of women with early breast cancer: Highlights of the st gallen international expert consensus on the primary therapy of early breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Tinterri, C.; Barbieri, E.; Sagona, A.; Bottini, A.; Canavese, G.; Gentile, D. De-Escalation Surgery in cT3-4 Breast Cancer Patients after Neoadjuvant Therapy: Predictors of Breast Conservation and Comparison of Long-Term Oncological Outcomes with Mastectomy. Cancers 2024, 16, 1169. [Google Scholar] [CrossRef] [PubMed]
- Spronk, P.E.R.; Volders, J.H.; van den Tol, P.; Smorenburg, C.H.; Vrancken Peeters, M.J.T.F.D. Breast conserving therapy after neoadjuvant chemotherapy; data from the Dutch Breast Cancer Audit. Eur. J. Surg. Oncol. 2019, 45, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Fancellu, A.; Houssami, N.; Sanna, V.; Porcu, A.; Ninniri, C.; Marinovich, M.L. Outcomes after breast-conserving surgery or mastectomy in patients with triple-negative breast cancer: Meta-analysis. Br. J. Surg. 2021, 108, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Werutsky, G.; Untch, M.; Hanusch, C.; Fasching, P.A.; Blohmer, J.U.; Seiler, S.; Denkert, C.; Tesch, H.; Jackisch, C.; Gerber, B.; et al. Locoregional recurrence risk after neoadjuvant chemotherapy: A pooled analysis of nine prospective neoadjuvant breast cancer trials. Eur. J. Cancer 2020, 130, 92–101. [Google Scholar] [CrossRef]
- Mukhtar, R.A.; Chau, H.; Woriax, H.; Piltin, M.; Ahrendt, G.; Tchou, J.; Yu, H.; Ding, Q.; Dugan, C.L.; Sheade, J.; et al. Breast Conservation Surgery and Mastectomy Have Similar Locoregional Recurrence after Neoadjuvant Chemotherapy: Results from 1462 Patients on the Prospective, Randomized I-SPY2 Trial. Ann. Surg. 2023, 278, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Arlow, R.L.; Paddock, L.E.; Niu, X.; Kirstein, L.; Haffty, B.G.; Goyal, S.; Kearney, T.; Toppmeyer, D.; Stroup, A.M.; Khan, A.J. Breast-conservation Therapy after Neoadjuvant Chemotherapy Does Not Compromise 10-Year Breast Cancer specific Mortality. Am. J. Clin. Oncol. Cancer Clin. Trials 2018, 41, 1246–1251. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Shelley Hwang, E.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Bear, H.D.; Anderson, S.; Smith, R.E.; Geyer, C.E.; Mamounas, E.P.; Fisher, B.; Brown, A.M.; Robidoux, A.; Margolese, R.; Kahlenberg, M.S.; et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National surgical adjuvant breast and bowel project protocol B-27. J. Clin. Oncol. 2006, 24, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Amoroso, V.; Gallo, F.; Bertaglia, V.; Simoncini, E.; Pedersini, R.; Ferrari, L.; Bottini, A.; Bruzzi, P.; Sormani, M.P. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: A meta-regression of 29 randomized prospective studies. J. Clin. Oncol. 2014, 32, 3883–3891. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Hosoda, M.; Yamamoto, M.; Taguchi, K.; Hatanaka, K.C.; Takakuwa, E.; Hatanaka, Y.; Matsuno, Y.; Yamashita, H. Prognostic significance of pathologic complete response and Ki67 expression after neoadjuvant chemotherapy in breast cancer. Breast Cancer 2015, 22, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Broglio, K.R.; Quintana, M.; Foster, M.; Olinger, M.; McGlothlin, A.; Berry, S.M.; Boileau, J.F.; Brezden-Masley, C.; Chia, S.; Dent, S.; et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes ameta-analysis. JAMA Oncol. 2016, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Sala, I.; Oriecuia, C.; De Pas, T.; Specchia, C.; Graffeo, R.; Pagan, E.; Queirolo, P.; Pennacchioli, E.; et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: Systematic review and meta-analysis. BMJ 2021, 375, e066381. [Google Scholar] [CrossRef] [PubMed]
- Ogston, K.N.; Miller, I.D.; Payne, S.; Hutcheon, A.W.; Sarkar, T.K.; Smith, I.; Schofield, A.; Heys, S.D. A new histological grading system to assess response of breast cancers to primary chemotherapy: Prognostic significance and survival. Breast 2003, 12, 320–327. [Google Scholar] [CrossRef]
- Rodenhuis, S.; Mandjes, I.A.M.; Wesseling, J.; van de Vijver, M.J.; Peeters, M.J.T.D.F.V.; Sonke, G.S.; Linn, S.C. A simple system for grading the response of breast cancer to neoadjuvant chemotherapy. Ann. Oncol. 2009, 21, 481–487. [Google Scholar] [CrossRef]
- Gentile, D.; Sagona, A.; De Carlo, C.; Fernandes, B.; Barbieri, E.; Di Maria Grimaldi, S.; Jacobs, F.; Vatteroni, G.; Scardina, L.; Biondi, E.; et al. Pathologic response and residual tumor cellularity after neo-adjuvant chemotherapy predict prognosis in breast cancer patients. Breast 2023, 69, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, V.; Provenzano, E.; Symmans, W.F.; Boughey, J.C.; Coles, C.; Curigliano, G.; Dixon, J.M.; Esserman, L.J.; Fastner, G.; Kuehn, T.; et al. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann. Oncol. 2015, 26, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Burstein, H.J.; Gnant, M.; Loibl, S.; Cameron, D.; Regan, M.M.; Denkert, C.; Poortmans, P.; Weber, W.P.; Thürlimann, B.; et al. Understanding breast cancer complexity to improve patient outcomes: The St Gallen International Consensus Conference for the Primary Therapy of Individuals with Early Breast Cancer 2023. Ann. Oncol. 2023, 34, 970–986. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalez, S.; Falo, C.; Pla, M.J.; Pernas, S.; Bajen, M.; Soler, T.; Ortega, R.; Quetglas, C.; Perez-Martin, X.; Fernandez Montoli, M.E.; et al. The Shift From Sentinel Lymph Node Biopsy Performed Either Before or After Neoadjuvant Systemic Therapy in the Clinical Negative Nodes of Breast Cancer Patients. Results, and the Advantages and Disadvantages of Both Procedures. Clin. Breast Cancer 2018, 18, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sheri, A.; Smith, I.E.; Johnston, S.R.; A’hern, R.; Nerurkar, A.; Jones, R.L.; Hills, M.; Detre, S.; Pinder, S.E.; Symmans, W.F.; et al. Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann. Oncol. 2015, 26, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.J.; Olivotto, I.A.; Parulekar, W.R.; Ackerman, I.; Chua, B.H.; Nabid, A.; Vallis, K.A.; White, J.R.; Rousseau, P.; Fortin, A.; et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N. Engl. J. Med. 2015, 373, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Poortmans, P.M.; Collette, S.; Kirkove, C.; Van Limbergen, E.; Budach, V.; Struikmans, H.; Collette, L.; Fourquet, A.; Maingon, P.; Valli, M.; et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N. Engl. J. Med. 2015, 373, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILS) in breast cancer: Recommendations by an International TILS Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Gourgou-Bourgade, S.; Cameron, D.; Poortmans, P.; Asselain, B.; Azria, D.; Cardoso, F.; A’Hern, R.; Bliss, J.; Bogaerts, J.; Bonnefoi, H.; et al. Guidelines for time-to-event end point definitions in breast cancer trials: Results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials). Ann. Oncol. 2015, 26, 873–879. [Google Scholar] [CrossRef]
- Asselain, B.; Barlow, W.; Bartlett, J.; Bergh, J.; Bergsten-Nordström, E.; Bliss, J.; Boccardo, F.; Boddington, C.; Bogaerts, J.; Bonadonna, G.; et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Falo, C.; Moreno, A.; Benito, E.; Lloveras, B.; Varela, M.; Serra, J.M.; Prieto, L.; Azpeitia, D.; Escobedo, A. Primary chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil in operable breast carcinoma. Cancer 2005, 103, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Hartkopf, A.D.; Gass, P.; Häberle, L.; Akpolat-Basci, L.; Hein, A.; Volz, B.; Taran, F.A.; Nabieva, N.; Pott, B.; et al. Efficacy of neoadjuvant pertuzumab in addition to chemotherapy and trastuzumab in routine clinical treatment of patients with primary breast cancer: A multicentric analysis. Breast Cancer Res. Treat. 2019, 173, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Pondé, N.F.; La Valle, G.; Del Mastro, L.; De Azambuja, E.; Lambertini, M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.D.; Toi, M.; O’Shaughnessy, J.; Rastogi, P.; Campone, M.; Neven, P.; Huang, C.-S.; Huober, J.; Jaliffe, G.G.; Cicin, I.; et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023, 24, 77–90. [Google Scholar] [CrossRef]
- Slamon, D.; Lipatov, O.; Nowecki, Z.; McAndrew, N.; Kukielka-Budny, B.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.-S.; Fasching, P.A.; Crown, J.; et al. Ribociclib plus Endocrine Therapy in Early Breast Cancer. N. Engl. J. Med. 2024, 390, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Buzdar, A.U.; Ibrahim, N.K.; Francis, D.; Booser, D.J.; Thomas, E.S.; Theriault, R.L.; Pusztai, L.; Green, M.C.; Arun, B.K.; Giordano, S.H.; et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J. Clin. Oncol. 2005, 23, 3676–3685. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Roman, L.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.A.; et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- Sikov, W.M.; Berry, D.A.; Perou, C.M.; Singh, B.; Cirrincione, C.T.; Tolaney, S.M.; Kuzma, C.S.; Pluard, T.J.; Somlo, G.; Port, E.R.; et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 2015, 33, 13–21. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B.; et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014, 15, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Sikov, W.M.; Huober, J.; Rugo, H.S.; Wolmark, N.; O’Shaughnessy, J.; Maag, D.; Untch, M.; Golshan, M.; Lorenzo, J.P.; et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann. Oncol. 2022, 33, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Prat, A.; Saura, C.; Pascual, T.; Hernando, C.; Muñoz, M.; Paré, L.; Farré, B.G.; Fernández, P.L.; Galván, P.; Chic, N.; et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 33–43. [Google Scholar] [CrossRef]
- Johnston, S.; Puhalla, S.; Wheatley, D.; Ring, A.; Barry, P.; Holcombe, C.; Boileau, J.F.; Provencher, L.; Robidoux, A.; Rimawi, M.; et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor–positive early breast cancer: Pallet trial. J. Clin. Oncol. 2019, 37, 178–189. [Google Scholar] [CrossRef]
- Gil-Gil, M.; Alba, E.; Gavilá, J.; de la Haba-Rodríguez, J.; Ciruelos, E.; Tolosa, P.; Candini, D.; Llombart-Cussac, A. The role of CDK4/6 inhibitors in early breast cancer. Breast 2021, 58, 160–169. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Barni, S. Response to neoadjuvant chemotherapy in ductal compared to lobular carcinoma of the breast: A meta-analysis of published trials including 1,764 lobular breast cancer. Breast Cancer Res. Treat. 2013, 142, 227–235. [Google Scholar] [CrossRef]
- Rajan, K.K.; Fairhurst, K.; Birkbeck, B.; Novintan, S.; Wilson, R.; Savović, J.; Holcombe, C.; Potter, S. Overall survival after mastectomy versus breast-conserving surgery with adjuvant radiotherapy for early-stage breast cancer: Meta-analysis. BJS Open 2024, 8, zrae040. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Li, D.; Zhao, Q.; Zhu, L.; Wu, T. A Meta-analysis of Randomized Controlled Trials Comparing Breast-Conserving Surgery and Mastectomy in Terms of Patient Survival Rate and Quality of Life in Breast Cancer. Int. J. Qual. Health Care 2024, 36, mzae043. [Google Scholar] [CrossRef]
| N: 482 | N (%) |
|---|---|
| Age 50 Years (SD 12.6) | |
| Age (years) | |
| ≤40 | 101 (21%) |
| >40 | 381 (79%) |
| Menopausal status | |
| Pre | 247 (51.2%) |
| Post | 235 (48.8%) |
| BRCA carriers | |
| Yes | 43 (8.9%) |
| No | 439 (91.1%) |
| TNM anatomic | |
| IIA | 129 (26.8) |
| IIB | 199 (41.3) |
| IIIA | 80 (16.6) |
| IIIB | 63 (13.1) |
| IIIC | 11 (2.3) |
| TNM prognostic | |
| IB | 50 (10.4) |
| IIA | 140 (29) |
| IIB | 111 (23) |
| IIIA | 48 (10) |
| IIIB | 102 (21.2) |
| IIIC | 31 (6.4) |
| Pathology subtype | |
| Ductal | 459 (95.2) |
| Lobular | 14 (2.9) |
| Others | 9 (1.9) |
| Grade | |
| I | 20 (4.1) |
| II | 194 (40.2) |
| III | 254 (52.7) |
| Ki 67 | |
| ≤30 | 203 (42.1) |
| >30 | 279 (57.9) |
| Molecular surrogate subtype | |
| Luminal A-like | 46 (9.5) |
| Luminal B-like | 144 (29.9) |
| LuminalBHER2 | 91 (18.9) |
| HER2 | 78 (16.2) |
| TNBC | 123 (25.5) |
| TILs | |
| ≤20 | 295 (61.2) |
| >20 | 187 (38.8) |
| N (%) | |
|---|---|
| pCR | |
| Yes | 122 (25.3) |
| No | 360 (74.7) |
| RCB | |
| 0 | 122 (25.3) |
| I | 57 (11.8) |
| II | 86 (17.8) |
| III | 217 (45) |
| Vascular invasion | |
| No | 376 (78) |
| Yes | 101 (21) |
| Missing | 5 (1) |
| Breast surgery | |
| Conservative | 318 (66) |
| Mastectomy | 164 (34) |
| Recurrences | |
| No | 356 (73.9) |
| Contralateral | 6 (1.2) |
| Local | 54 (11.2) |
| Systemic | 103 (21.4) |
| Deaths | |
| Breast cancer | 78 (16.2) |
| Other cancers | 9 (1.9) |
| Other causes | 21 (4.3) |
| Total | 108 (22.4) |
| Events | HR Univariate | HR Multivariate | p | ||
|---|---|---|---|---|---|
| Age (years) | 0.223 | p | |||
| ≤40 | 26 (25.7) | ||||
| >40 | 77 (20.2) | ||||
| Menopausal status | 1 | ||||
| Pre | 53 (21.5) | 0.9 (0.6–1.4) | |||
| Post | 50 (21.3) | Ref | |||
| BRCA carriers | 0.050 | ||||
| Yes | 4 (9.3) | 0.3 (0.1–1.05) | |||
| No | 99 (22.6) | Ref | |||
| TNM anatomic | 0.000 | ||||
| IIA | 12 (9.3) | Ref | |||
| IIB | 48 (24.1) | 2.6 (1.4–5.0) | |||
| IIIA | 18 (22.5) | 2.5 (1.2–5.3) | |||
| IIIB | 19 (30.2) | 3.8 (1.8–7.9) | |||
| IIIC | 6 (54.5) | 9.6 (3.6–25.9) | |||
| TNM prognostic | 0.000 | ||||
| IB | 7 (14) | Ref | |||
| IIA | 25 (17.9) | 1.2 (0.5–2.9) | |||
| IIB | 11 (9.9) | 0.6 (0.2–1.7) | |||
| IIIA | 17 (35.4) | 2.8 (1.1–6.8) | |||
| IIIB | 31 (30.4) | 2.4 (1.0–5.6) | |||
| IIIC | 12 (38.7) | 3.8 (1.5–9.8) | |||
| Pathology subtype | 0.003 | ||||
| Ductal | 92 (20) | Ref | Ref | ||
| Lobular | 8 (57.1) | 3.6 (1.7–7.5) | 4.4 (1.9–10.1) | 0.000 | |
| Others | 3 (33.3) | 1.8 (0.5–5.6) | 1.1 (0.3–3.8) | ||
| Grade | 0.175 | ||||
| I | 3 (15) | Ref | |||
| II | 49 (25.3) | 1.7 (0.5–5.6) | |||
| III | 47 (18.3) | 1.3 (0.4–4.1) | |||
| Ki 67 | 0.125 | ||||
| ≤30% | 49 (24.1) | Ref | |||
| >30% | 54 (19.4) | 0.8 (0.5–1.2) | |||
| Molecular surrogate subtype | 0.074 | ||||
| Luminal A-like | 10 (21.7) | Ref | Ref | ||
| Luminal B-like | 37 (25.7) | 1.2 (0.6–2.5) | 1.4 (0.6–3.2) | ||
| LuminalBHEr2 | 14 (15.4) | 0.7 (0.3–1.6) | 2.1 (0.8–5.6) | ||
| HER2 | 10 (12.8) | 0.6 (0.2–1.4) | 2.5 (0.9–7.3) | ||
| TNBC | 32 (26) | 1.4 (0.7–2.8) | 4.0 (1.3–11.8) | 0.012 | |
| TILs | 0.023 | ||||
| ≤20% | 73 (24.7) | 1.6 (1–2.4) | |||
| >20% | 30 (16) | Ref | |||
| Breast surgery | 0.000 | ||||
| Conservative | 51 (16) | Ref | Ref | ||
| Mastectomy | 52 (31.7) | 2.1 (1.4–3.2) | 2.2 (1.4–3.4) | 0.000 | |
| pCR | 0.000 | a | |||
| Yes | 5 (4.1) | Ref | |||
| No | 98 (27.2) | 7.8 (3.1–19.3) | |||
| RCB | 0.000 | ||||
| 0 | 5 (4.1) | Ref | Ref | ||
| I | 6 (10.5) | 2.8 (0.8–9.2) | 2.2 (0.6–7.3) | ||
| II | 17 (19.8) | 5.4 (2.0–14.8) | 4.4 (1.5–12.8) | 0.006 | |
| III | 75 (34.6) | 10.3 (4.1–25.6) | 8.0 (2.9–21.7) | 0.000 | |
| Vascular invasion | 0.000 | ||||
| No | 61 (16.2) | Ref | Ref | ||
| Yes | 41 (40.6) | 3.1 (2.0–4.6) | 2.4 (1.5–3.7) | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falo, C.; Azcarate, J.; Fernandez-Gonzalez, S.; Perez, X.; Petit, A.; Perez, H.; Vethencourt, A.; Vazquez, S.; Laplana, M.; Ales, M.; et al. Breast Cancer Patient’s Outcomes after Neoadjuvant Chemotherapy and Surgery at 5 and 10 Years for Stage II–III Disease. Cancers 2024, 16, 2421. https://doi.org/10.3390/cancers16132421
Falo C, Azcarate J, Fernandez-Gonzalez S, Perez X, Petit A, Perez H, Vethencourt A, Vazquez S, Laplana M, Ales M, et al. Breast Cancer Patient’s Outcomes after Neoadjuvant Chemotherapy and Surgery at 5 and 10 Years for Stage II–III Disease. Cancers. 2024; 16(13):2421. https://doi.org/10.3390/cancers16132421
Chicago/Turabian StyleFalo, Catalina, Juan Azcarate, Sergi Fernandez-Gonzalez, Xavier Perez, Ana Petit, Héctor Perez, Andrea Vethencourt, Silvia Vazquez, Maria Laplana, Miriam Ales, and et al. 2024. "Breast Cancer Patient’s Outcomes after Neoadjuvant Chemotherapy and Surgery at 5 and 10 Years for Stage II–III Disease" Cancers 16, no. 13: 2421. https://doi.org/10.3390/cancers16132421
APA StyleFalo, C., Azcarate, J., Fernandez-Gonzalez, S., Perez, X., Petit, A., Perez, H., Vethencourt, A., Vazquez, S., Laplana, M., Ales, M., Stradella, A., Fullana, B., Pla, M. J., Gumà, A., Ortega, R., Varela, M., Pérez, D., Ponton, J. L., Cobo, S., ... Garcia-Tejedor, A. (2024). Breast Cancer Patient’s Outcomes after Neoadjuvant Chemotherapy and Surgery at 5 and 10 Years for Stage II–III Disease. Cancers, 16(13), 2421. https://doi.org/10.3390/cancers16132421





