Antibody-Drug Conjugates in Urothelial Cancer: From Scientific Rationale to Clinical Development
Abstract
Simple Summary
Abstract
1. Introduction
2. Antibody-Drug Conjugates
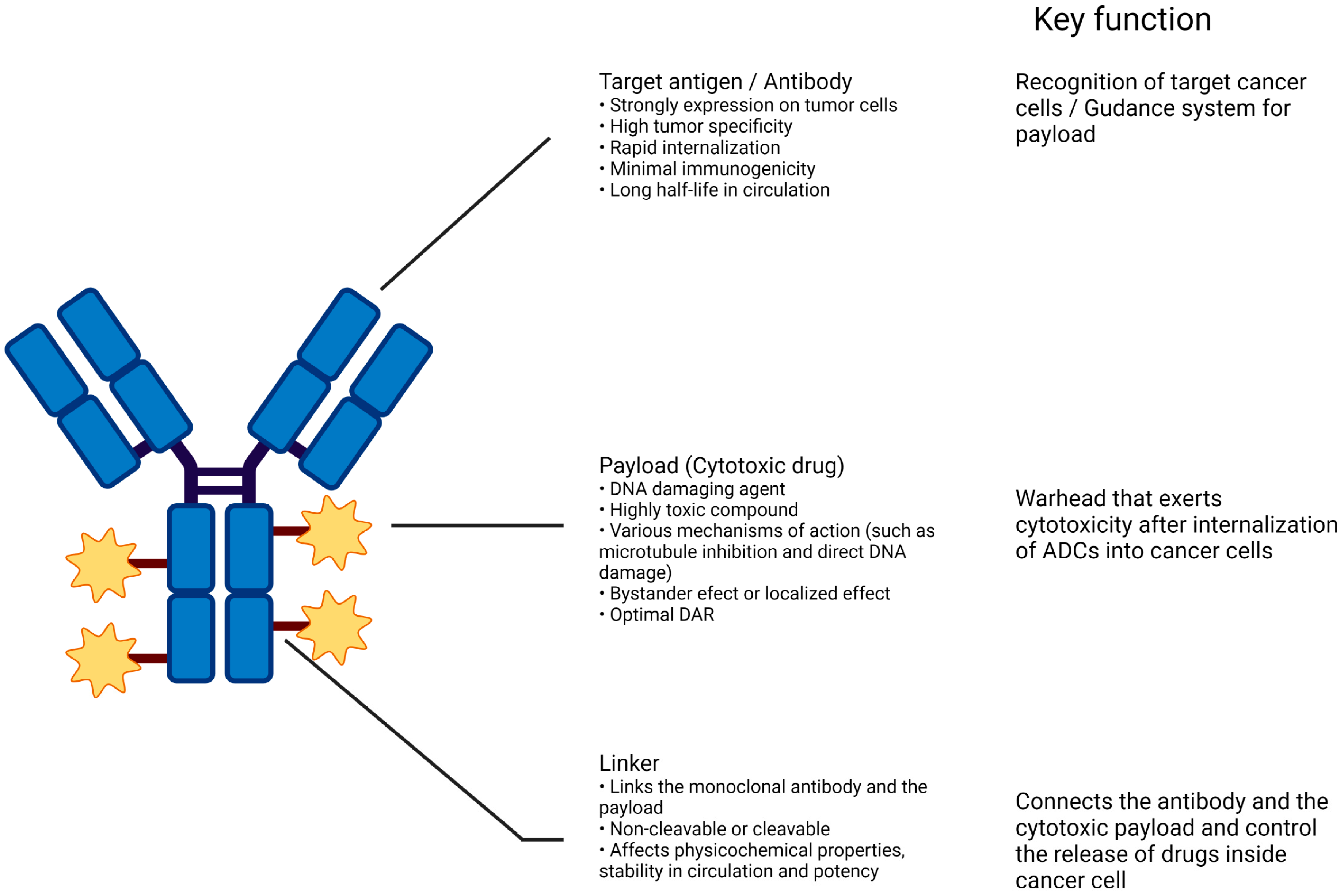
2.1. Structures of ADC
2.1.1. Antibody
2.1.2. Linker
2.1.3. Payloads
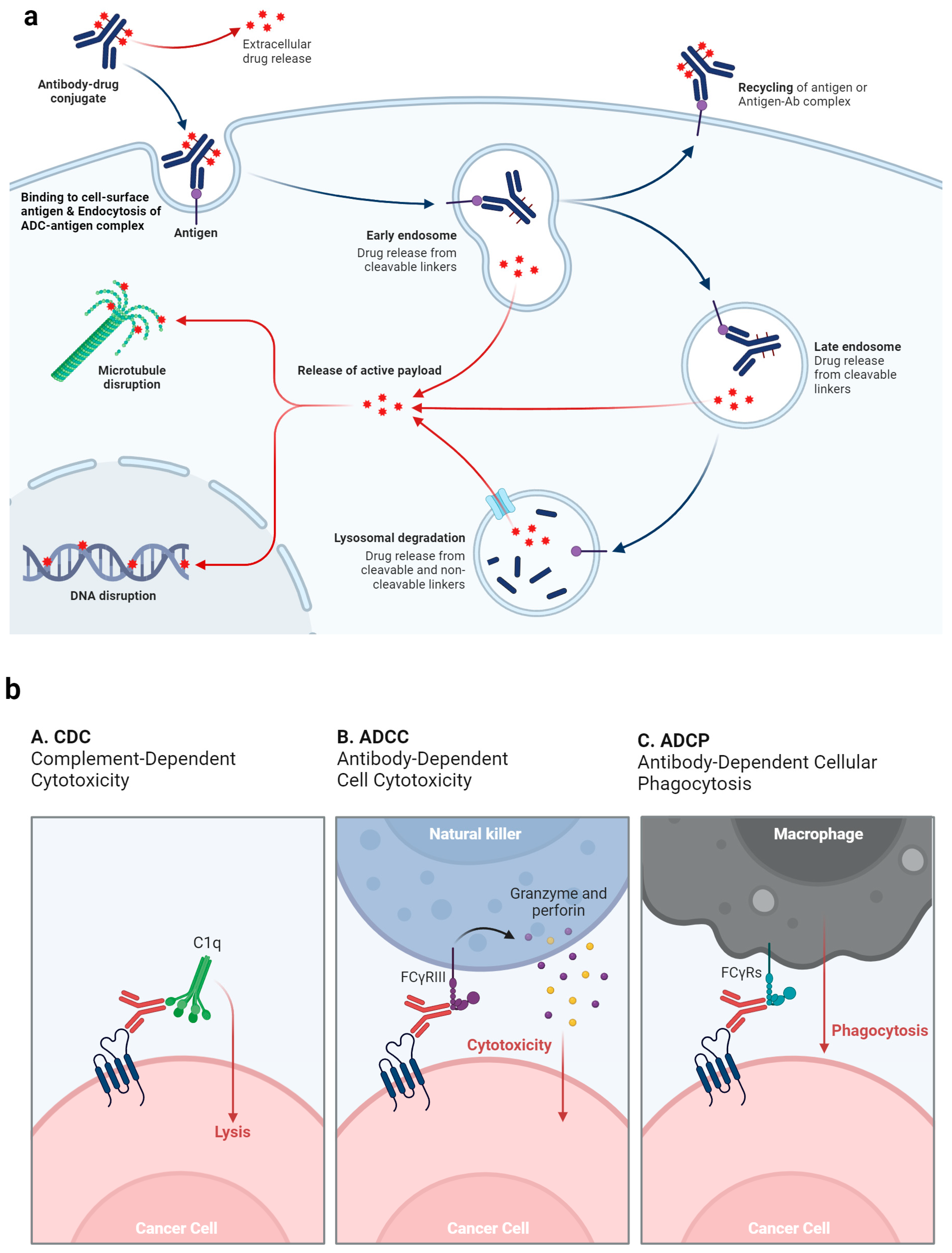
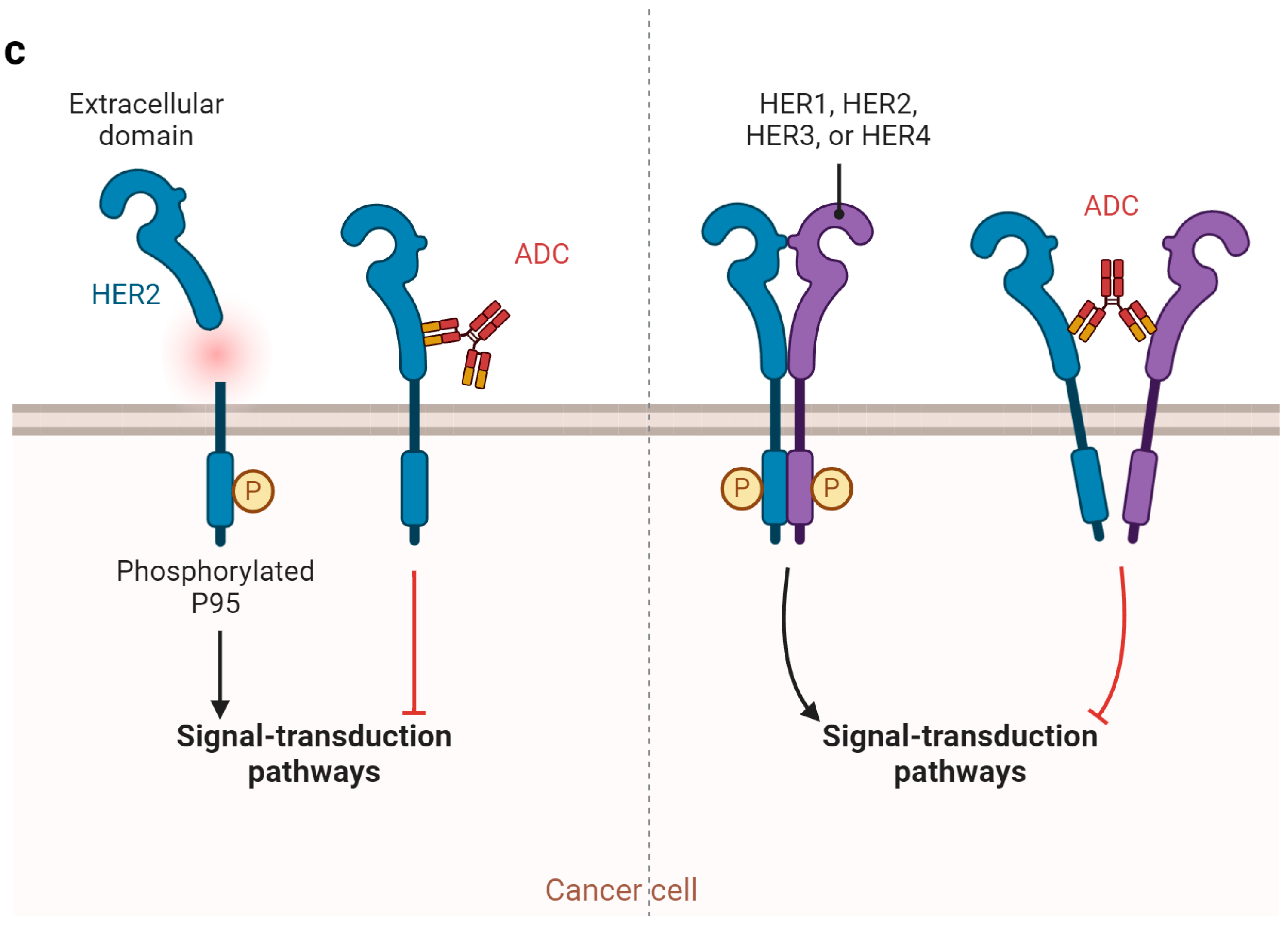
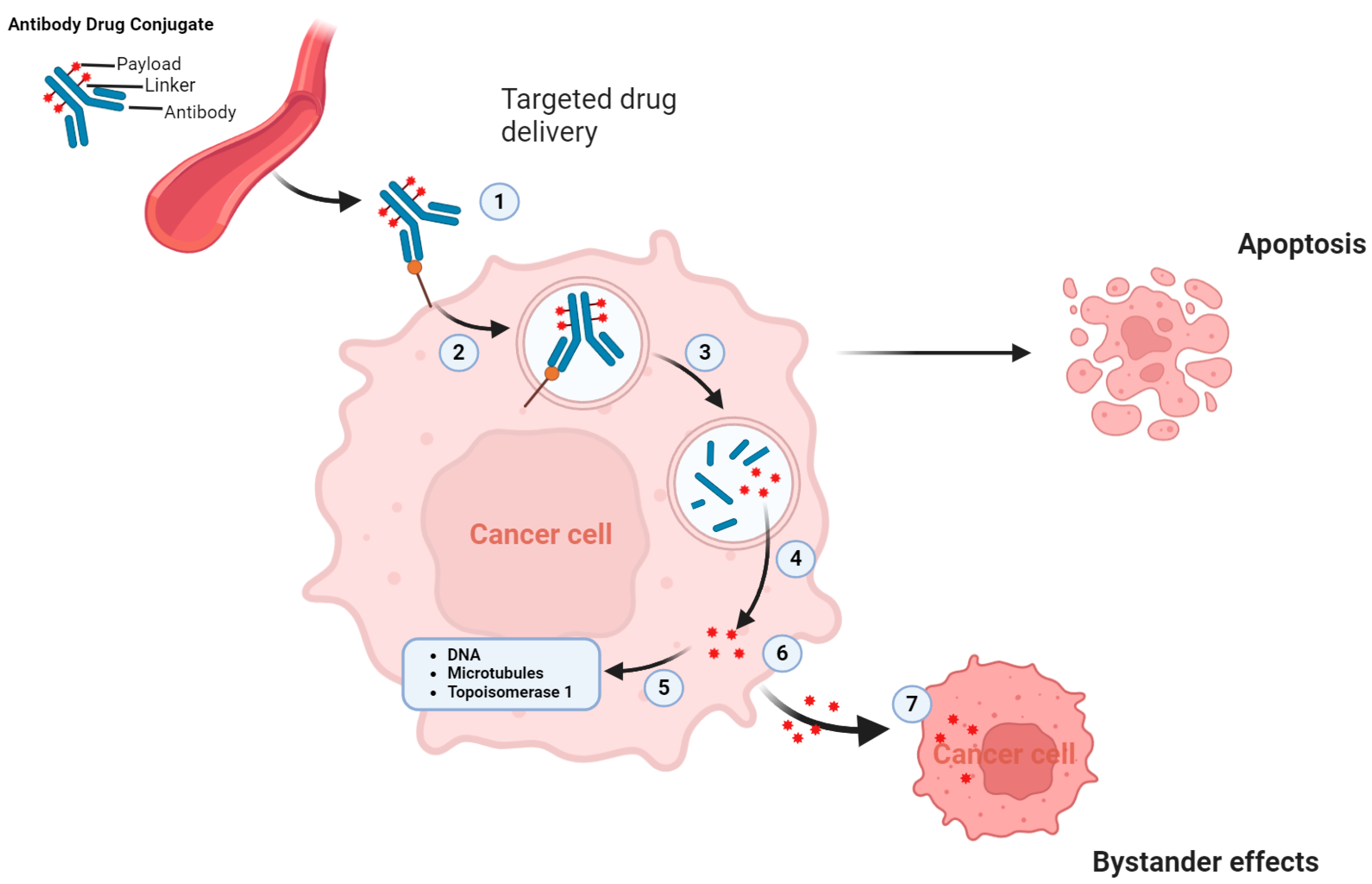
2.2. Mechanism of Action of ADC and Why ADCs Are Particularly Beneficial for UC
3. Clinical Development of ADCs in UC
3.1. Enfortumab Vedotin (EV) Targeting Nectin-4
3.1.1. Monotherapy
3.1.2. Combinations
3.2. SG Targeting Trophoblast Cell-Surface Antigen 2 (TROP-2)
3.3. Other ADCs
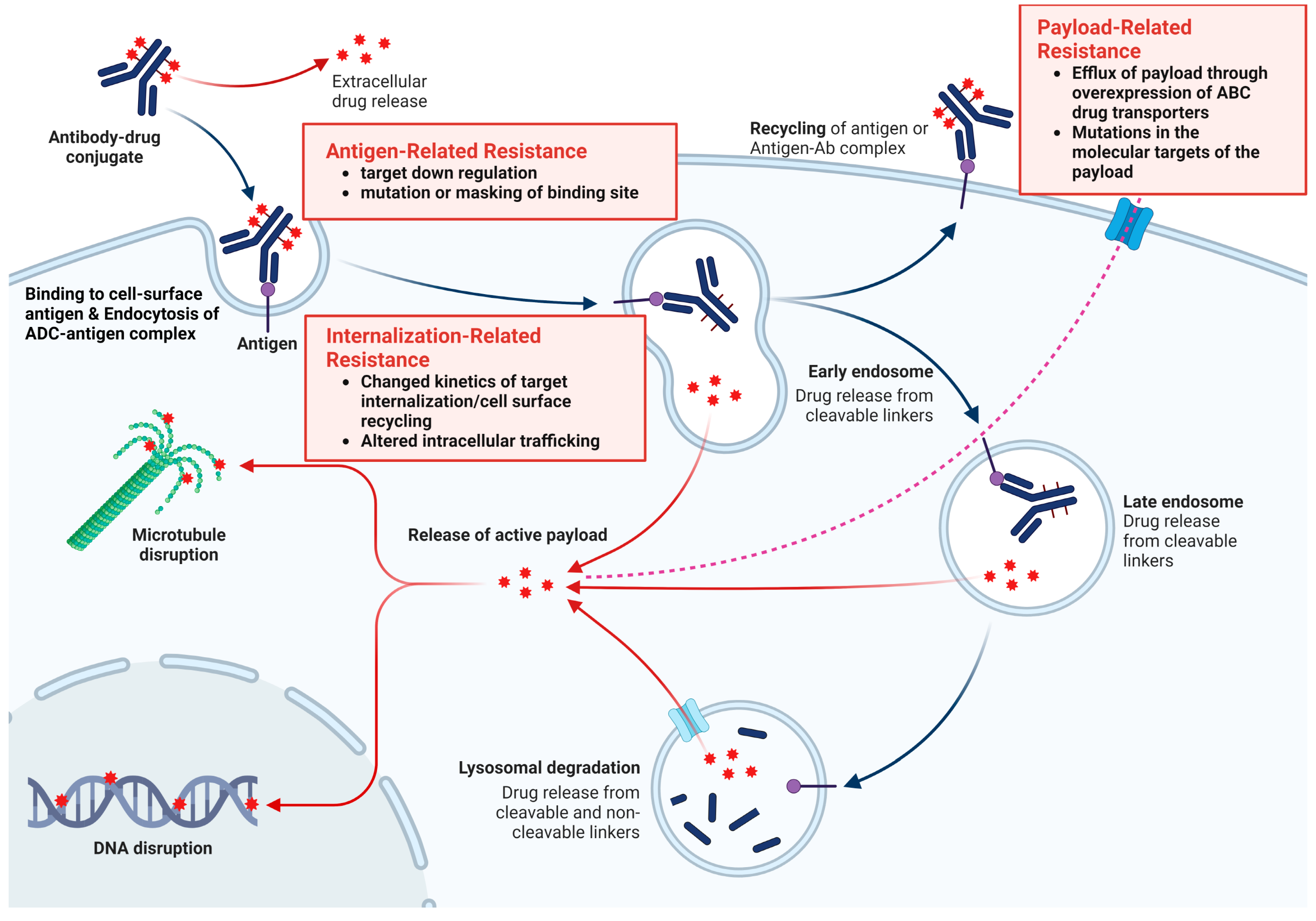
4. Resistance to ADCs
4.1. Antigen-Related Resistance
4.2. Payload-Related Resistance
4.3. Internalization and Trafficking Pathways-Related Resistance
4.4. Other Types of ADC Resistance
5. Strategies to Overcome ADC Resistance
6. Future Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Bladder Cancer. Available online: https://seer.cancer.gov/statfacts/html/urinb.html (accessed on 27 April 2023).
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2020 guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Proietti, F.; Flammia, R.S.; Licari, L.C.; Bologna, E.; Bove, A.M.; Brassetti, A.; Tuderti, G.; Mastroianni, R.; Tu-fano, A.; Simone, G.; et al. Impacts of Neoadjuvant Chemotherapy on Perioperative Outcomes in Patients with Bladder Cancer Treated with Radical Cystectomy: A Single High-Volume Center Experience. J. Pers. Med. 2024, 14, 212. [Google Scholar] [CrossRef]
- Lee, H.W.; Kwon, W.A.; Nguyen, N.T.; Phan, D.T.T.; Seo, H.K. Approaches to Clinical Complete Response after Neoadjuvant Chemotherapy in Muscle-Invasive Bladder Cancer: Possibilities and Limitations. Cancers 2023, 15, 1323. [Google Scholar] [CrossRef]
- Zargar, H.; Espiritu, P.N.; Fairey, A.S.; Mertens, L.S.; Dinney, C.P.; Mir, M.C.; Krabbe, L.-M.; Cookson, M.S.; Jacobsen, N.-E.; Gandhi, N.M. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur. Urol. 2015, 67, 241–249. [Google Scholar] [CrossRef]
- Patel, V.G.; Oh, W.K.; Galsky, M.D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J. Clin. 2020, 70, 404–423. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Network, C.G.A.R. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315. [Google Scholar]
- Alley, S.C.; Okeley, N.M.; Senter, P.D. Antibody–drug conjugates: Targeted drug delivery for cancer. Curr. Opin. Chem. Biol. 2010, 14, 529–537. [Google Scholar] [CrossRef]
- Valent, P.; Groner, B.; Schumacher, U.; Superti-Furga, G.; Busslinger, M.; Kralovics, R.; Zielinski, C.; Pen-ninger, J.M.; Kerjaschki, D.; Stingl, G. Paul Ehrlich (1854–1915) and his contributions to the foundation and birth of translational medicine. J. Innate Immun. 2016, 8, 111–120. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Lindorfer, M.A.; Wiestner, A.; Zent, C.S.; Taylor, R.P. Monoclonal antibody (mAb)-based cancer therapy: Is it time to reevaluate dosing strategies? Oncoimmunology 2012, 1, 959–961. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Liu, K.; Li, M.; Li, Y.; Li, Y.; Chen, Z.; Tang, Y.; Yang, M.; Deng, G.; Liu, H. A review of the clinical efficacy of FDA-approved antibody–drug conjugates in human cancers. Mol. Cancer 2024, 23, 62. [Google Scholar] [CrossRef]
- Diamantis, N.; Banerji, U. Antibody-drug conjugates—An emerging class of cancer treatment. Br. J. Cancer 2016, 114, 362–367. [Google Scholar] [CrossRef]
- Metrangolo, V.; Engelholm, L.H. Antibody-Drug Conjugates: The Dynamic Evolution from Conventional to Next-Generation Constructs. Cancers 2024, 16, 447. [Google Scholar] [CrossRef]
- Tong, J.T.W.; Harris, P.W.R.; Brimble, M.A.; Kavianinia, I. An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules 2021, 26, 5847. [Google Scholar] [CrossRef]
- Criscitiello, C.; Morganti, S.; Curigliano, G. Antibody-drug conjugates in solid tumors: A look into novel targets. J. Hematol. Oncol. 2021, 14, 20. [Google Scholar] [CrossRef]
- Esapa, B.; Jiang, J.; Cheung, A.; Chenoweth, A.; Thurston, D.E.; Karagiannis, S.N. Target Antigen Attributes and Their Contributions to Clinically Approved Antibody-Drug Conjugates (ADCs) in Haematopoietic and Solid Cancers. Cancers 2023, 15, 1845. [Google Scholar] [CrossRef]
- Lucas, A.T.; Price, L.S.L.; Schorzman, A.N.; Storrie, M.; Piscitelli, J.A.; Razo, J.; Zamboni, W.C. Factors Af-fecting the Pharmacology of Antibody-Drug Conjugates. Antibodies 2018, 7, 10. [Google Scholar] [CrossRef]
- Sheyi, R.; de la Torre, B.G.; Albericio, F. Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate. Pharmaceutics 2022, 14, 396. [Google Scholar] [CrossRef]
- Ponziani, S.; Di Vittorio, G.; Pitari, G.; Cimini, A.M.; Ardini, M.; Gentile, R.; Iacobelli, S.; Sala, G.; Capone, E.; Flavell, D.J.; et al. Antibody-Drug Conjugates: The New Frontier of Chemotherapy. Int. J. Mol. Sci. 2020, 21, 5510. [Google Scholar] [CrossRef]
- Balamkundu, S.; Liu, C.F. Lysosomal-Cleavable Peptide Linkers in Antibody-Drug Conjugates. Biomedicines 2023, 11, 3080. [Google Scholar] [CrossRef]
- Kumari, S.; Raj, S.; Babu, M.A.; Bhatti, G.K.; Bhatti, J.S. Antibody-drug conjugates in cancer therapy: Innovations, challenges, and future directions. Arch. Pharmacal Res. 2024, 47, 40–65. [Google Scholar] [CrossRef]
- Fujii, T.; Matsuda, Y. Novel formats of antibody conjugates: Recent advances in payload diversity, conjugation, and linker chemistry. Expert Opin. Biol. Ther. 2023, 23, 1053–1065. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Gou, L.; Li, W.; Wang, Y. Antibody-drug conjugates: Recent advances in payloads. Acta Pharm. Sin. B 2023, 13, 4025–4059. [Google Scholar] [CrossRef]
- Jin, S.; Sun, Y.; Liang, X.; Gu, X.; Ning, J.; Xu, Y.; Chen, S.; Pan, L. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 39. [Google Scholar] [CrossRef]
- Riccardi, F.; Dal Bo, M.; Macor, P.; Toffoli, G. A comprehensive overview on antibody-drug conjugates: From the conceptualization to cancer therapy. Front. Pharmacol. 2023, 14, 1274088. [Google Scholar] [CrossRef]
- Samantasinghar, A.; Sunildutt, N.P.; Ahmed, F.; Soomro, A.M.; Salih, A.R.C.; Parihar, P.; Memon, F.H.; Kim, K.H.; Kang, I.S.; Choi, K.H. A comprehensive review of key factors affecting the efficacy of antibody drug conjugate. Biomed. Pharmacother. 2023, 161, 114408. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.I.; Rosenberg, J.E. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat. Rev. Urol. 2021, 18, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, M.V.; Mahendran, R. The Bladder Tumor Microenvironment Components That Modulate the Tumor and Impact Therapy. Int. J. Mol. Sci. 2023, 24, 12311. [Google Scholar] [CrossRef] [PubMed]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.; Yang, J.; Salam, M.A.; Sengupta, S.; Al-Amin, M.Y.; Mustafa, S.; Khan, M.A.; Huang, X.; Pawar, J.S. Antibody-drug conjugates: The paradigm shifts in the targeted cancer therapy. Front. Immunol. 2023, 14, 1203073. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Zhu, C.; Yu, P.; Wang, X.; Wang, Y.; Wang, J.; Yu, J.; Wang, K. Emerging strategy for the treatment of urothelial carcinoma: Advances in antibody-drug conjugates combination therapy. Biomed. Pharmacother. 2024, 171, 116152. [Google Scholar] [CrossRef] [PubMed]
- Váradi, M.; Horváth, O.; Módos, O.; Fazekas, T.; Grunewald, C.M.; Niegisch, G.; Krafft, U.; Grünwald, V.; Hadaschik, B.; Olah, C.; et al. Efficacy of immune checkpoint inhibitor therapy for advanced urothelial carcinoma in real-life clinical practice: Results of a multicentric, retrospective study. Sci. Rep. 2023, 13, 17378. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.; Saleh, K.; Khalife, N.; Saleh, M.; Chahine, C.; Ibrahim, R.; Lecesne, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Int. J. Mol. Sci. 2023, 24, 2825–2834. [Google Scholar] [CrossRef]
- Moussa, M.J.; Campbell, M.T.; Alhalabi, O. Revisiting Treatment of Metastatic Urothelial Cancer: Where Do Cisplatin and Platinum Ineligibility Criteria Stand? Biomedicines 2024, 12, 519. [Google Scholar] [CrossRef]
- Ungaro, A.; Tucci, M.; Audisio, A.; Di Prima, L.; Pisano, C.; Turco, F.; Delcuratolo, M.D.; Di Maio, M.; Scagliotti, G.V.; Buttigliero, C. Antibody-Drug Conjugates in Urothelial Carcinoma: A New Therapeutic Opportunity Moves from Bench to Bedside. Cells 2022, 11, 803. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.; Rosenberg, J.E. Targeting nectin-4 by antibody-drug conjugates for the treatment of urothelial carcinoma. Expert Opin. Biol. Ther. 2021, 21, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Censits, J.H.; Lombardo, K.A.; Parimi, V.; Kamanda, S.; Choi, W.; Hahn, N.M.; McConkey, D.J.; McGuire, B.M.; Bivalacqua, T.J.; Kates, M.; et al. Expression of Nectin-4 in Bladder Urothelial Carcinoma, in Morphologic Variants, and Nonurothelial Histotypes. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Goodson, D.A.; Friedlander, T.W. The Future of Antibody–Drug Conjugates in Urothelial Cancer: New Indications and Novel Targets. Adv. Oncol. 2023, 3, 137–159. [Google Scholar] [CrossRef]
- Yaghoubi, S.; Karimi, M.H.; Lotfinia, M.; Gharibi, T.; Mahi-Birjand, M.; Kavi, E.; Hosseini, F.; Sineh Sepehr, K.; Khatami, M.; Bagheri, N. Potential drugs used in the antibody–drug conjugate (ADC) architecture for cancer therapy. J. Cell. Physiol. 2020, 235, 31–64. [Google Scholar] [CrossRef] [PubMed]
- de Claro, R.A.; McGinn, K.; Kwitkowski, V.; Bullock, J.; Khandelwal, A.; Habtemariam, B.; Ouyang, Y.; Saber, H.; Lee, K.; Koti, K.; et al. U.S. Food and Drug Administration Approval Summary: Brentuximab Vedotin for the Treatment of Relapsed Hodgkin Lymphoma or Relapsed Systemic Anaplastic Large-Cell Lymphoma. Clin. Cancer Res. 2012, 18, 5845–5849. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.; Sridhar, S.S.; Zhang, J.; Smith, D.; Ruether, D.; Flaig, T.W.; Baranda, J.; Lang, J.; Plimack, E.R.; Sangha, R.; et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients with Nectin-4-Positive Solid Tumors, Including Metastatic Urothelial Carcinoma. J. Clin. Oncol. 2020, 38, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rosenberg, J.E.; McKay, R.R.; Flaig, T.W.; Petrylak, D.P.; Hoimes, C.J.; Friedlander, T.W.; Bilen, M.A.; Srinivas, S.; Burgess, E.F.; et al. Study EV-103 dose escalation/cohort A: Long-term outcome of enfortumab vedotin + pembrolizumab in first-line (1L) cisplatin-ineligible locally advanced or metastatic urothelial carcinoma (la/mUC) with nearly 4 years of follow-up. J. Clin. Oncol. 2023, 41, 4505. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bu-pathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients with Metastatic Urothelial Carcinoma Progressing after Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma after Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Cas-tellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Maguire, W.F.; Lee, D.; Weinstock, C.; Gao, X.; Bulik, C.C.; Agrawal, S.; Chang, E.; Hamed, S.S.; Bloomquist, E.W.; Tang, S.; et al. FDA Approval Summary: Enfortumab Vedotin Plus Pembrolizumab for Cisplatin-Ineligible Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2024, 30, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Banna, G.L.; Buti, S.; Isella, L.; Stellato, M.; Roberto, M.; Iacovelli, R. Navigating the Rapidly Evolving Advanced Urothelial Carcinoma Treatment Landscape: Insights from Italian Experts. Curr. Oncol. Rep. 2023, 25, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.H.; Milowsky, M.I.; Petrylak, D.P.; Hoimes, C.J.; Flaig, T.W.; Mar, N.; Moon, H.H.; Friedlander, T.W.; McKay, R.R.; Bilen, M.A.; et al. Enfortumab Vedotin with or without Pembrolizumab in Cisplatin-Ineligible Patients with Previously Untreated Locally Advanced or Metastatic Urothelial Cancer. J. Clin. Oncol. 2023, 41, 4107–4117. [Google Scholar] [CrossRef] [PubMed]
- Nadal, R.; Valderrama, B.P.; Bellmunt, J. Progress in systemic therapy for advanced-stage urothelial carcinoma. Nat. Rev. Clin. Oncol. 2024, 21, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zuo, Y.; Chen, S.; Li, Y.; Xing, Y.; Yang, L.; Wang, H.; Guo, R. Antibody-drug conjugates in urothelial carcinoma: Scientometric analysis and clinical trials analysis. Front. Oncol. 2024, 14, 1323366. [Google Scholar] [CrossRef] [PubMed]
- NCCN Gudelines. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1417 (accessed on 23 April 2024).
- Drakaki, A.; Powles, T.; Bamias, A.; Martin-Liberal, J.; Shin, S.J.; Friedlander, T.; Tosi, D.; Park, C.; Gomez-Roca, C.; Joly Lobbedez, F.; et al. Atezolizumab plus Magrolimab, Niraparib, or Tocilizumab versus Atezolizumab Monotherapy in Platinum-Refractory Metastatic Urothelial Carcinoma: A Phase Ib/II Open-Label, Multicenter, Randomized Umbrella Study (MORPHEUS Urothelial Carcinoma). Clin. Cancer Res. 2023, 29, 4373–4384. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wu, S.; Li, R.; Jiang, Y.; Zheng, J.; Li, Z.; Li, M.; Xin, K.; Guan, X.; Li, S.; et al. Novel ADCs and combination therapy in urothelial carcinoma: Latest updates from the 2023 ASCO-GU Cancers Symposium. J. Hematol. Oncol. 2023, 16, 85. [Google Scholar] [CrossRef]
- Liu, X.; Deng, J.; Yuan, Y.; Chen, W.; Sun, W.; Wang, Y.; Huang, H.; Liang, B.; Ming, T.; Wen, J.; et al. Advances in Trop2-targeted therapy: Novel agents and opportunities beyond breast cancer. Pharmacol. Ther. 2022, 239, 108296. [Google Scholar] [CrossRef]
- Shastry, M.; Jacob, S.; Rugo, H.S.; Hamilton, E. Antibody-drug conjugates targeting TROP-2: Clinical development in metastatic breast cancer. Breast 2022, 66, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Fontes, M.S.; Vargas Pivato de Almeida, D.; Cavalin, C.; Tagawa, S.T. Targeted Therapy for Locally Advanced or Metastatic Urothelial Cancer (mUC): Therapeutic Potential of Sacituzumab Govitecan. Onco Targets Ther. 2022, 15, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.; Pouessel, D.; Park, C.H.; Barthelemy, P.; Bupathi, M.; Petrylak, D.P.; Agarwal, N.; Gupta, S.; Fléchon, A.; Ramamurthy, C.; et al. Sacituzumab Govitecan in Combination with Pembrolizumab for Patients with Metastatic Urothelial Cancer That Progressed after Platinum-Based Chemotherapy: TROPHY-U-01 Cohort 3. J. Clin. Oncol. 2024, 42, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.M.; Sharkey, R.M. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin. Biol. Ther. 2020, 20, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer 2015, 6, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.H.; Lin, Y.H.; Luo, H.L.; Sung, W.W. Antibody-drug conjugates targeting HER2 for the treatment of urothelial carcinoma: Potential therapies for HER2-positive urothelial carcinoma. Front. Pharmacol. 2024, 15, 1326296. [Google Scholar] [CrossRef] [PubMed]
- Vlachostergios, P.J.; Jakubowski, C.D.; Niaz, M.J.; Lee, A.; Thomas, C.; Hackett, A.L.; Patel, P.; Rashid, N.; Tagawa, S.T. Antibody-drug conjugates in bladder cancer. Bladder Cancer 2018, 4, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, E.; Drago, J.Z.; Modi, S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: State of the art and future directions. Breast Cancer Res. 2021, 23, 84. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, F.; Zanelli, M.; Palicelli, A.; Bisagni, A.; Zizzo, M.; Ascani, S.; Pedicillo, M.C.; Cormio, A.; Fala-gario, U.G.; Carrieri, G.; et al. HER2 Expression in Bladder Cancer: A Focused View on Its Diagnostic, Prognostic, and Predictive Role. Int. J. Mol. Sci. 2023, 24, 3720. [Google Scholar] [CrossRef]
- Albarrán, V.; Rosero, D.I.; Chamorro, J.; Pozas, J.; San Román, M.; Barrill, A.M.; Alía, V.; Sotoca, P.; Guerrero, P.; Calvo, J.C.; et al. Her-2 Targeted Therapy in Advanced Urothelial Cancer: From Monoclonal Antibodies to Antibody-Drug Conjugates. Int. J. Mol. Sci. 2022, 23, 12659. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, L.; He, Z.; Shi, Y.; Luo, H.; Han, W.; Yao, X.; Shi, B.; Liu, J.; Hu, C.; et al. Efficacy and Safety of Disitamab Vedotin in Patients with Human Epidermal Growth Factor Receptor 2–Positive Locally Advanced or Metastatic Urothelial Carcinoma: A Combined Analysis of Two Phase II Clinical Trials. J. Clin. Oncol. 2024, 42, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Rigby, M.; Bennett, G.; Chen, L.; Mudd, G.E.; Harrison, H.; Beswick, P.J.; Van Rietschoten, K.; Watcham, S.M.; Scott, H.S.; Brown, A.N.; et al. BT8009; A Nectin-4 Targeting Bicycle Toxin Conjugate for Treatment of Solid Tumors. Mol. Cancer Ther. 2022, 21, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Ka-linsky, K.; Zelnak, A.B. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Loganzo, F.; Tan, X.; Sung, M.; Jin, G.; Myers, J.S.; Melamud, E.; Wang, F.; Diesl, V.; Follettie, M.T.; Musto, S. Tumor cells chronically treated with a trastuzumab–maytansinoid antibody–drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol. Cancer Ther. 2015, 14, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Filho, O.M.; Viale, G.; Stein, S.; Trippa, L.; Yardley, D.A.; Mayer, I.A.; Abramson, V.G.; Arteaga, C.L.; Spring, L.M.; Waks, A.G. Impact of HER2 heterogeneity on treatment response of early-stage HER2-positive breast cancer: Phase II neoadjuvant clinical trial of T-DM1 combined with pertuzumab. Cancer Discov. 2021, 11, 2474–2487. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, G.; Lamberts, L.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Afti-mos, P.; Tol, J. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef]
- Hunter, F.W.; Barker, H.R.; Lipert, B.; Rothé, F.; Gebhart, G.; Piccart-Gebhart, M.J.; Sotiriou, C.; Jamieson, S.M. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br. J. Cancer 2020, 122, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.D.L.; Fields, C.T.; Li, G.; Dowbenko, D.; Schaefer, G.; Miller, K.; Andre, F.; Burris III, H.A.; Albain, K.S.; Harbeck, N. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: Critical role for neuregulin blockade in antitumor response to combination therapy. Clin. Cancer Res. 2014, 20, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Klümper, N.; Ralser, D.J.; Ellinger, J.; Roghmann, F.; Albrecht, J.; Below, E.; Alajati, A.; Sikic, D.; Breyer, J.; Bolenz, C.; et al. Membranous NECTIN-4 Expression Frequently Decreases during Metastatic Spread of Urothelial Carcinoma and Is Associated with Enfortumab Vedotin Resistance. Clin. Cancer Res. 2023, 29, 1496–1505. [Google Scholar] [CrossRef]
- Lan, H.R.; Chen, M.; Yao, S.Y.; Chen, J.X.; Jin, K.T. Bispecific antibodies revolutionizing breast cancer treatment: A comprehensive overview. Front. Immunol. 2023, 14, 1266450. [Google Scholar] [CrossRef]
- Lei, Z.N.; Tian, Q.; Teng, Q.X.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z.S. Understanding and targeting resistance mechanisms in cancer. MedComm 2023, 4, e265. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sherman, M.Y. Resistance to TOP-1 inhibitors: Good old drugs still can surprise us. Int. J. Mol. Sci. 2023, 24, 7233. [Google Scholar] [CrossRef] [PubMed]
- Abelman, R.O.; Wu, B.; Spring, L.M.; Ellisen, L.W.; Bardia, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Cancers 2023, 15, 2825–2834. [Google Scholar] [CrossRef] [PubMed]
- Takegawa, N.; Nonagase, Y.; Yonesaka, K.; Sakai, K.; Maenishi, O.; Ogitani, Y.; Tamura, T.; Nishio, K.; Nak-agawa, K.; Tsurutani, J. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int. J. Cancer 2017, 141, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, E.; Gandullo-Sánchez, L.; Ocaña, A.; Pandiella, A. Novel ADCs and strategies to overcome resistance to anti-HER2 ADCs. Cancers 2021, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Conilh, L.; Sadilkova, L.; Viricel, W.; Dumontet, C. Payload diversification: A key step in the development of antibody–drug conjugates. J. Hematol. Oncol. 2023, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.Q.; Luo, S.; Twomey, J.D.; Zhang, B. Targeting cancer with antibody-drug conjugates: Promises and challenges. MAbs Taylor Fr. 2021, 13, 1951427. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.; Patel, B.; Patel, N.; Patel, M.; Patel, A.; Dhanesha, N. Antibody drug conjugates as targeted cancer therapy: Past development, present challenges and future opportunities. Arch. Pharmacal Res. 2023, 46, 361–388. [Google Scholar] [CrossRef]
- Sun, X.; Ponte, J.F.; Yoder, N.C.; Laleau, R.; Coccia, J.; Lanieri, L.; Qiu, Q.; Wu, R.; Hong, E.; Bogalhas, M.; et al. Effects of Drug–Antibody Ratio on Pharmacokinetics, Biodistribution, Efficacy, and Tolerability of Antibody–Maytansinoid Conjugates. Bioconjugate Chem. 2017, 28, 1371–1381. [Google Scholar] [CrossRef]
- Li, S.; Ghosh, C.; Xing, Y.; Sun, Y. Phosphatidylinositol 4, 5-bisphosphate in the Control of Membrane Trafficking. Int. J. Biol. Sci. 2020, 16, 2761. [Google Scholar] [CrossRef]
- Sung, M.; Tan, X.; Lu, B.; Golas, J.; Hosselet, C.; Wang, F.; Tylaska, L.; King, L.; Zhou, D.; Dushin, R. Caveolae-mediated endocytosis as a novel mechanism of resistance to trastuzumab emtansine (T-DM1). Mol. Cancer Ther. 2018, 17, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef][Green Version]
- Kovtun, Y.V.; Audette, C.A.; Mayo, M.F.; Jones, G.E.; Doherty, H.; Maloney, E.K.; Erickson, H.K.; Sun, X.; Wilhelm, S.; Ab, O.; et al. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010, 70, 2528–2537. [Google Scholar] [CrossRef] [PubMed]
- Sabbaghi, M.; Gil-Gómez, G.; Guardia, C.; Servitja, S.; Arpí, O.; García-Alonso, S.; Menendez, S.; Arumi-Uria, M.; Serrano, L.; Salido, M.; et al. Defective Cyclin B1 Induction in Trastuzumab-emtansine (T-DM1) Acquired Resistance in HER2-positive Breast Cancer. Clin. Cancer Res. 2017, 23, 7006–7019. [Google Scholar] [CrossRef]
- Omarini, C.; Piacentini, F.; Sperduti, I.; Cerma, K.; Barbolini, M.; Canino, F.; Nasso, C.; Isca, C.; Caggia, F.; Dominici, M.; et al. T-DM1 efficacy in trastuzumab-pertuzumab pre-treated HER2 positive metastatic breast cancer patients: A meta-analysis. BMC Cancer 2022, 22, 623. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- André, F.; Hee Park, Y.; Kim, S.B.; Takano, T.; Im, S.A.; Borges, G.; Lima, J.P.; Aksoy, S.; Gavila Gregori, J.; De Laurentiis, M.; et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 401, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Golfier, S.; Kopitz, C.; Kahnert, A.; Heisler, I.; Schatz, C.A.; Stelte-Ludwig, B.; Mayer-Bartschmid, A.; Unter-schemmann, K.; Bruder, S.; Linden, L.; et al. Anetumab ravtansine: A novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol. Cancer Ther. 2014, 13, 1537–1548. [Google Scholar] [CrossRef]
- Li, F.; Emmerton, K.K.; Jonas, M.; Zhang, X.; Miyamoto, J.B.; Setter, J.R.; Nicholas, N.D.; Okeley, N.M.; Lyon, R.P.; Benjamin, D.R.; et al. Intracellular Released Payload Influences Potency and Bystander-Killing Effects of Antibody-Drug Conjugates in Preclinical Models. Cancer Res. 2016, 76, 2710–2719. [Google Scholar] [CrossRef]
- Andreev, J.; Thambi, N.; Perez Bay, A.E.; Delfino, F.; Martin, J.; Kelly, M.P.; Kirshner, J.R.; Rafique, A.; Kunz, A.; Nittoli, T.; et al. Bispecific Antibodies and Antibody-Drug Conjugates (ADCs) Bridging HER2 and Prolactin Receptor Improve Efficacy of HER2 ADCs. Mol. Cancer Ther. 2017, 16, 681–693. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Bedard, P.L.; Lee, K.-W.; Han, H.S.; Kang, Y.-K.; Miller, W.H.; Rha, S.Y.; Kim, J.H.; Dotan, E.; Liao, C.-Y. Abstract B130: Phase 1 study of Zanidatamab Zovodotin (ZW49): Safety Profile and Recommended Dose (RD) in patients with Human Epidermal Growth Factor 2 (HER2)-positive solid cancers. Am. Assoc. Cancer Res. (AACR) 2023, 22 (Suppl. S12), B130. [Google Scholar] [CrossRef]
- Qu, F.; Lu, R.; Liu, Q.; Wu, X.; Huang, X.; Yin, Y.; Li, W. Antibody–drug conjugates transform the outcome of individuals with low-HER2-expression advanced breast cancer. Cancer 2024, 130, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikama, K.; Anami, Y.; Ha, S.Y.Y.; Yamazaki, C.M. Exploring the next generation of antibody-drug conjugates. Nat. Rev. Clin. Oncol. 2024, 21, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, C.M.; Yamaguchi, A.; Anami, Y.; Xiong, W.; Otani, Y.; Lee, J.; Ueno, N.T.; Zhang, N.; An, Z.; Tsuchikama, K. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat. Commun. 2021, 12, 3528. [Google Scholar] [CrossRef] [PubMed]
- Dan, N.; Setua, S.; Kashyap, V.K.; Khan, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Antibody-Drug Conjugates for Cancer Therapy: Chemistry to Clinical Implications. Pharmaceuticals 2018, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J.M.; Tenchov, R.; Bird, R.; Iyer, K.A.; Ralhan, K.; Rodriguez, Y.; Zhou, Q.A. The Evolving Landscape of Antibody–Drug Conjugates: In Depth Analysis of Recent Research Progress. Bioconjugate Chem. 2023, 34, 1951–2000. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Keenan, T.E.; Li, T.; Tayob, N.; Wulf, G.M.; Richardson, E.T., 3rd; Attaya, V.; Anderson, L.; Mittendorf, E.A.; Overmoyer, B.; et al. Phase Ib study of pembrolizumab in combination with trastuzumab emtansine for metastatic HER2-positive breast cancer. J. Immunother. Cancer 2022, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Li, P.; Yang, T.; Zhu, J.; Sun, L.; Zhang, Z.; Wang, L.; Tian, X.; Chen, J.; Hu, C.; et al. The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors. J. Hematol. Oncol. 2024, 17, 1. [Google Scholar] [CrossRef]
- Majumder, A. HER3: Toward the Prognostic Significance, Therapeutic Potential, Current Challenges, and Future Therapeutics in Different Types of Cancer. Cells 2023, 12, 2517. [Google Scholar] [CrossRef]
- Zhou, Q. Site-specific antibody conjugation with payloads beyond cytotoxins. Molecules 2023, 28, 917. [Google Scholar] [CrossRef]
- Sadiki, A.; Vaidya, S.R.; Abdollahi, M.; Bhardwaj, G.; Dolan, M.E.; Turna, H.; Arora, V.; Sanjeev, A.; Robinson, T.D.; Koid, A. Site-specific conjugation of native antibody. Antib. Ther. 2020, 3, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Ma, P.; Jiang, Y.; Cheng, K.; Yu, Y.; Jiang, N.; Miao, H.; Tang, Q.; Liu, F.; et al. Drug conjugate-based anticancer therapy—Current status and perspectives. Cancer Lett. 2023, 552, 215969. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.; Wurst, J.M.; Liu, T.; Martinez, R.M.; Datta-Mannan, A.; Feng, Y. Antibody Conjugates-Recent Advances and Future Innovations. Antibodies 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Matsuda, Y. Tag-Free Enzymatic Modification for Antibody—Drug Conjugate Production. ChemistrySelect 2022, 7, e202203753. [Google Scholar] [CrossRef]
- Fujii, T.; Matsuda, Y.; Seki, T.; Shikida, N.; Iwai, Y.; Ooba, Y.; Takahashi, K.; Isokawa, M.; Kawaguchi, S.; Hatada, N. AJICAP second generation: Improved chemical site-specific conjugation technology for antibody–drug conjugate production. Bioconjugate Chem. 2023, 34, 728–738. [Google Scholar] [CrossRef]
| ADC | Brand Name | Year Approved | Target Receptor | Linker | Payload | Payload Action | DAR |
|---|---|---|---|---|---|---|---|
| Enfortumab vedotin | Padcev | 2019 | Nectin-4 | Enzyme-cleavable | MMAE | Microtubule inhibitor | 3.8 |
| Sacituzumab govitecan | Trodelvy | 2020 | Trop2 | Acid-cleavable | SN-38 | Topoisomerase-DNA complex Inhibitor | 7.6 |
| Disitamab vedotin | Aidexi | 2021 | HER2 | Protease cleavable | MMAE | Microtubule inhibitor | 4 |
| Trastuzumab deruxtecan | Enhertu | 2019 | HER2 | Enzyme-cleavable | Deruxtecan | Topoisomerase-DNA complex Inhibitor | 8 |
| Drugs | Trial | NCT Number | Study Phase | Regimen | No. of Pts. | Study Population | Primary End Points | ORR,% (95% CI) | mDOR, Months (95% CI) | mPFS, Months (95% CI) | mOS, Months (95% CI) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enfortumab Vedotin (EV) | EV-101 | NCT02091999 | I | EV | 155 | la/mUC | safety/tolerability and pharmacokinetics | 43 | 12.3 | [48] | ||
| EV-201 | NCT03219333 | II | EV | 125 (cohort 1) | la/mUC, previously treated with ICIs | ORR | 44% (35.1 to 53.2) | 7.6 (6.34 to NA) | 5.8 (4.93 to 7.46) | 12.4 (9.46 to 15.57) | ||
| EV-103 | NCT03288545 | Ib/II | EV and pembrolizumab | 45 (cohort k) | la/mUC, 1L cisplatin-ineligible | safety/tolerability | 73.3% (95% CI, 58.1 to 85.4%) | 22.1 months (8.38, -) | 12.7 months (6.11, -) | 26.1 months (15.51, -) | [49] | |
| EV-302 | NCT04223856 | III | EV and pembrolizumab | 442 | la/mUC, 1L | PFS, OS | 67.7% (95% CI, 63.1 to 72.1) | NA | 12.5 months (10.4 to 16.6) | 31.5 (25.4 to NE) | [50] | |
| Sacituzumab Govitecan (SG) | TROPHY-U-01 | NCT03547973 | II | SG | 113 (cohort 1) | la/mUC, previously treated with Plat and ICIs | ORR | 27% (19.5 to 36.6) | 7.2 (4.7 to 8.6 months) | 5.4 months (3.5 to 7.2 months | 10.9 months (9.0 to 13.8 months) | [51] |
| A. Monotherapy. | ||||||
| Drugs | Trial | NCT number | Study Population | Study Phase | No. of Pts. | Primary or Co-Primary End Points |
| EV | EV-103, cohort H | NCT03288545 | Neoadjuvant in cisplatin-ineligible MIBC naive to systemic therapy | I/II b | 457 | pCRR |
| EV | EV-103, cohort L | NCT03288545 | Perioperative, previously untreated, cis-ineligible MIBC | I/II | 50 | pCRR |
| SG | SURE-01 | NCT05226117 | Neoadjuvant in cisplatin-ineligible MIBC naive to systemic therapy | II | 56 | pCRR |
| SG vs. CTX | TROPiCS-04/Immu-132–13 | NCT04527991 | La/mUC refractory to platinum and anti-PD-1/PD-L1 Therapies (in China) | III a | 696 | OS |
| T-DXd | DestinyPanTumor02, Arm2 | NCT04482309 | HER2-positive solid tumor including bladder cancer | II b | 268 | ORR |
| T-DXd | DestinyPanTumor01 | NCT04639219 | HER2-positive, unresectable or metastatic solid tumors refractory to prior therapy with limited options. | II b | 102 | ORR |
| Abbreviations: AE, adverse event rate or incidence; BCG, bacillus calmette guerin; CTX, standard chemotherapy, often physician’s choice; EV, enfortumab vedotin; Gem, gemcitabine; ICI, immune checkpoint inhibitor; LA, laboratory abnormalities; la/mUC, locally advanced or metastatic urothelial cancer; MIBC, muscle-invasive bladder cancer; MTD, max tolerated dose; NCT, national clinical trial; ORR, objective response rate; OS, overall survival; P, pembrolizumab; pCRR, pathologic complete response rate; PFS, progression-free survival; Plat (platinum), cisplatin or carboplatin; SG, sacituzumab vedotin; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan; Taxane, paclitaxel or docetaxel.—a Randomized; b Represents total aggregated in multicohort or basket trial. | ||||||
| B. Combination Therapy. | ||||||
| Drugs | Trial | NCT number | Study Population | Study Phase | No. of Pts. | Primary or Co-Primary End Points |
| EV + P | EV-103, Cohort A | NCT03288545 | First line in platinum ineligible la/mUC refractory to prior therapies. | I/II | 45 | AE, ORR |
| EV + P | EV-103, Cohort B | NCT03288545 | Second line in la/mUC refractory to platinum therapies. | I/II b | 457 | AE, ORR |
| EV + Cis | EV-103, Cohort D | NCT03288545 | First line in platinum-eligible la/mUC | I/II b | 457 | AE, ORR |
| EV + Carbo | EV-103, Cohort E | NCT03288545 | First line in cisplatin-ineligible, carboplatin-eligible la/mUC | I/II b | 457 | AE, ORR |
| EV + Gem | EV-103, Cohort F | NCT03288545 | First and second line in platinumineligible la/mUC refractory to prior therapies | I/II b | 457 | AE, ORR |
| EV + Plt + P | EV-103, Cohort G | NCT03288545 | First line in platinum-eligible la/mUC | I/II b | 457 | AE, ORR |
| EV + P | EV-103, Cohort J | NCT03288545 | Neoadjuvant in cisplatin-ineligible MIBC naive to systemic therapy | I/II b | 457 | AE, ORR, pCRR |
| EV + P vs. Gem + Plat | EV-302 | NCT04223856 | First line in cisplatin-eligible la/mUC; arm A [3-week cycles of EV 1.25 mg/kg IV on days 1 and 8 (no maximum cycles) + P 200 mg IV (maximum 6 cycles)] or arm B [Gem + Cis or Carbo (maximum 6 cycles)] | III a | 990 | PFS, OS |
| EV + P + Si | 516–003, Cohort 9 | NCT03606174 | La/mUC refractory to platinum and ICI therapies | I/II b | 425 | ORR |
| EV + At | MORPHEUS-UC | NCT03869190 | PD1-expressing la/mUC refractory to platinum therapy | IB/II b | 735 | ORR, pCRR |
| EV + P vs. P vs. Cystectomy | KEYNOTE-905/EV-303 | NCT03924895 | Neoadjuvant therapy or surgery alone in cisplatin-ineligible MIBC; arm A (neoadjuvant P 200 mg IV Q3W up to 3 cycles followed by RC + PLND and adjuvant P 200 mg IV Q3W up to 14 cycles), arm B (RC + PLND followed by observation), or arm C (neoadjuvant EV 1.25 mg/kg + P 200 mg IV Q3W up to 3 cycles followed by RC + PLND and adjuvant EV + P up to 6 cycles and adjuvant P 200 mg IV Q3W up to 8 cycles) | III a | 857 | pCRR, PFS |
| EV + P vs. CTX | Keynote-B15/EV-304 | NCT04700124 | Neoadjuvant in cisplatin-eligible MIBC; arm A (4 cycles of neoadjuvant EV 1.25 mg/kg + P 200 mg IV Q3W followed by 5 cycles of adjuvant EV 1.25 mg/kg + 13 cycles of adjuvant P 200 mg IV Q3W after RC + PLND) or arm B (4 cycles of neoadjuvant Gem 1000 mg/m2 chemot + Cis 70 mg/m2 IV Q3W followed by observation after RC + PLND) | III a | 784 | pCRR, PFS |
| EV + Du + Tr vs. EV + Du | VOLGA | NCT04960709 | Neoadjuvant in cisplatin-ineligible systemic therapy-naive MIBC; arm A (Du 1500 mg day 1 + T 75 mg day 1 + EV 1.25 mg/kg Days 1 & 8); arm B (Du 1500 mg day 1 + EV 1.25 mg/kg Days 1 & 8; or arm C no neoadjuvant treatment (SoC) | III a | 830 | pCRR, EFS |
| SG + Cis + Av | Trophy-U-01, Cohort 4 | NCT03547973 | Platinum naive and cisplatin-eligible la/mUC with responders receiving Avelumab maintenance | II b | 643 | ORR, PFS |
| SG + Cis + Zi | Trophy-U-01, Cohort 4 | NCT03547973 | Platinum naive and cisplatin-eligible la/mUC with responders receiving Avelumab maintenance | II b | 643 | ORR, PFS |
| SG 1 Zi or Av or Zi | Trophy-U-01, Cohort 5 | NCT03547973 | la/mUC maintenance therapy following Gem-Cis | II b | 643 | ORR, PFS |
| SG or SG + Zi or SG + Zi + Do or GC | Trophy-U-01, Cohort 6 | NCT03547973 | Cisplatin-ineligible, treatment-naive la/mUC | II b | 643 | ORR, PFS |
| SG + At | MORPHEUS-UC | NCT03869190 | PD1-expressing la/mUC refractory to platinum therapy | IB/II b | 735 | ORR, pCRR |
| DV | Keynote-D78/RC48G001, Cohort A | NCT04879329 | HER2-positive, platinum-refractory la/mUC | II b | 270 | ORR |
| DV | Keynote-D78/RC48G001, Cohort B | NCT04879329 | HER2 low expressing, platinum refractory la/mUC | II b | 270 | ORR |
| DV vs. DV + P | Keynote-D78/RC48G001, Cohort C | NCT04879329 | HER2-positive, platinum eligible, treatment-naive la/mUC | II b | 270 | ORR |
| DV 1 To vs. Gem + Plat | RC48-C016 | NCT05302284 | HER2-positive platinum-eligible treatment-naive la/mUC; arm A [2-week cycles of DV 2.0 mg/kg IV + 2-week cycles of To 3.0 mg/kg IV (until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal)] or arm B [Gem + Cis or Carbo Q3W (maximum 6 weeks or until investigator assessed loss of clinical benefit, unacceptable toxicity, investigator or participant decision to withdraw from therapy, or death)] | III a | 452 | PFS, OS |
| T-DM11At or Taxane | TDM4529 g | NCT00781612 | Extension at close of parent study in eligible patients | II b | 720 | AE, DLT |
| Abbreviations: AE, adverse event rate or incidence; At, atezolizumab; Av, avelumab; BCG, bacillus calmette guerin; Ca, cabozantinib; Carbo, carboplatin; Cis, cisplatin; CTX, standard chemotherapy, often physician’s choice; DCR, disease control rate; DLT, dose-limiting toxicity; Do, domvanalimab; Du, durvalumab; DV, disitamab vedotin; EFS, event-free survival; EpCAM, epithelial cell adhesion molecule; Er, erdafitinib; EV, enfortumab vedotin; every 3 weeks, Q3W; Gem, gemcitabine; ICI, immune checkpoint inhibitor; Ip, ipilizumab; Intravenously, IV; La/mUC, locally advanced or metastatic urothelial cancer; MIBC, muscle-invasive bladder cancer; MTD, maximum tolerated dose; Ni, nivolumab; NMIBC, non-muscle invasive bladder cancer; OM, oportuzumab monatox; ORR, objective response rate; OS, overall survival; P, pembrolizumab; pCRR, pathologic complete response rate; PFS, progression-free survival; Plat (platinum), cisplatin or carboplatin; SG, sacituzumab vedotin; Si, sitravatinib; SoC, standard of care; T, trastuzumab; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan; Taxane, paclitaxel or docetaxel; TD, trastuzumab duocarmine; Tis, tislelizumab; To, toripalimab; Tr, tremelimumab; Tu, tucatinib; TV, tisotumab vedotin; Zi, zimberelimab.—a Randomized; b Represents total aggregated in multicohort or basket trial. | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, W.-A.; Lee, S.-Y.; Jeong, T.Y.; Kim, H.H.; Lee, M.-K. Antibody-Drug Conjugates in Urothelial Cancer: From Scientific Rationale to Clinical Development. Cancers 2024, 16, 2420. https://doi.org/10.3390/cancers16132420
Kwon W-A, Lee S-Y, Jeong TY, Kim HH, Lee M-K. Antibody-Drug Conjugates in Urothelial Cancer: From Scientific Rationale to Clinical Development. Cancers. 2024; 16(13):2420. https://doi.org/10.3390/cancers16132420
Chicago/Turabian StyleKwon, Whi-An, Seo-Yeon Lee, Tae Yoong Jeong, Hyeon Hoe Kim, and Min-Kyung Lee. 2024. "Antibody-Drug Conjugates in Urothelial Cancer: From Scientific Rationale to Clinical Development" Cancers 16, no. 13: 2420. https://doi.org/10.3390/cancers16132420
APA StyleKwon, W.-A., Lee, S.-Y., Jeong, T. Y., Kim, H. H., & Lee, M.-K. (2024). Antibody-Drug Conjugates in Urothelial Cancer: From Scientific Rationale to Clinical Development. Cancers, 16(13), 2420. https://doi.org/10.3390/cancers16132420







