A Systematic Review of Cost-Effectiveness Studies on Gastric Cancer Screening
Abstract
Simple Summary
Abstract
1. Introduction
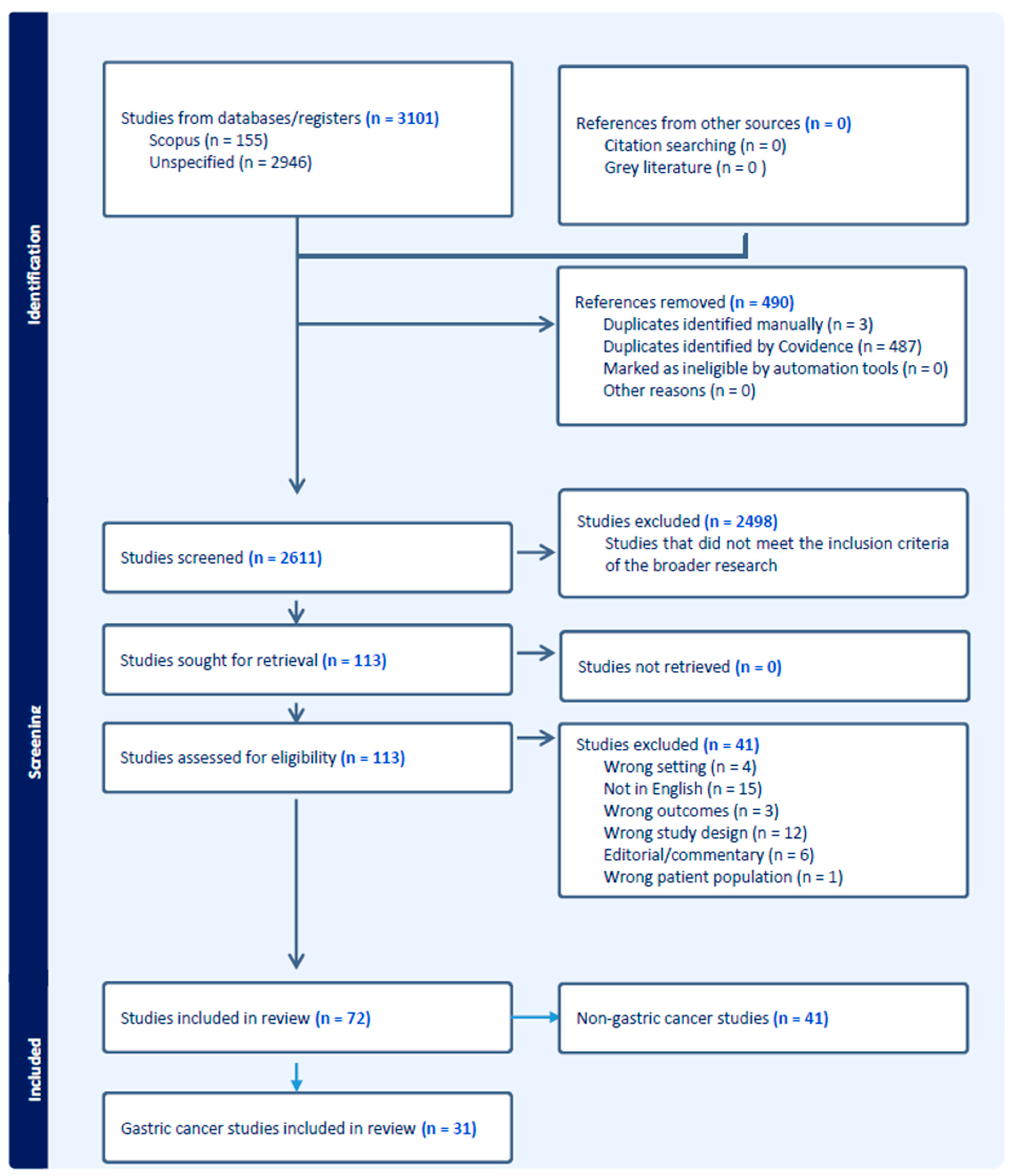
2. Materials and Methods
- Studies that focused on the screening of stomach cancers through primary and/or secondary methods compared to no screening or screening using alternative methods;
- Studies that focused on populations at average risk or above average risk for gastric cancer;
- Studies that reported on patient outcomes measured in terms of quality-adjusted life-years (QALY) or life-years gained (LYG);
- Studies based on decision-analytic modeling assessing both the long-term effectiveness and the cost-effectiveness of different early detection;
- Studies that reported the incremental cost-effectiveness ratio (ICER) or provided data for ICER calculation;
- Studies that outlined cost per quality-adjusted life-year (QALY) or cost per life-year gained, or cost per utility gained;
- Studies that had full economic evaluations;
- Studies published in English.
- Non-original studies;
- Studies not published in English;
- Grey literature;
- Systematic reviews, editorials, letters, abstracts, and studies that are not full health economic evaluations or evaluated only follow-up or treatment strategies.
3. Results
| References | Country/Region | Cancer Risk | Intervention Strategy | Reference Strategy | Study Objective |
|---|---|---|---|---|---|
| Zhou et al., 2011 [30] | Zhuanghe County, China | High risk | Epidemiological survey, serum PG + endoscopy, pathological examination | No screening | To evaluate the economic cost-effectiveness of the screening program for gastric cancer in a high-risk population |
| Zheng & Liu, 2023 [2] | China | High risk | Hp screening, NGCS | No screening | To evaluate the cost-effectiveness of Hp and new gastric cancer screening scoring system in areas with a high incidence of gastric cancer |
| Yeh et al., 2016 [12] | USA | Current smokers, former smokers, non-smokers | Serum PG, endoscopic-based screening, Hp screening | No screening | To estimate the cost-effectiveness of non-cardia gastric adenocarcinoma (NCGA) screening strategies based on new biomarker and endoscopic technologies |
| Xia et al., 2021 [13] | China | High risk | Endoscopic screening: once per lifetime, and every 10 years, 5 years, 3 years, 2 years | No screening | To evaluate the cost-effectiveness of endoscopic screening for esophageal and gastric cancers among people aged 40–69 years in areas of China where risk of these cancers is high |
| Wu et al., 2016 [31] | Singapore | Intermediate risk | Annual EGD surveillance; 2-yearly EGD screenings | No EGD follow-up | To identify the optimal strategy in the prevention of GC in Singapore based on cost-effectiveness ratios estimated by Markov models |
| Wei et al., 2011 [28] | Linzhou, China | High risk | Endoscopy with mucosal iodine staining in combination with index biopsies | Not reported | To evaluate the cost–benefit of the Early Detection and Early Treatment of Esophageal and Cardiac Cancer (EDETEC) program |
| Wang et al., 2022 [32] | China | Average and high risk | Hp eradication; electronic endoscopy (one-time, annual, biennial, or triennial) | Status quo | To compare the effects, affordability, and cost-effectiveness of different GC prevention approaches |
| Tsuji et al., 1991 [27] | Japan | Not reported | Gastro-fluorography screening | No screening | To compare the CE ratio of colorectal cancer screening with other cancer screening program in Japan (gastric cancer) |
| Suh et al., 2020 [25] | Republic of Korea | High risk | Screening: endoscopy or UGI | No screening | To evaluate the treatment benefit and cost of the National Cancer Screening Program for gastric cancer |
| Shah et al., 2020 [15] | USA | Average risk and met colorectal cancer screening requirement | 1-time EGD during colonoscopy + EDG every 3 years if IM is identified; 1-time EGD during colonoscopy + biennial EGD regardless of finding | No screening | To compare the CE of two endoscopic strategies for gastric cancer screening with no screening (by sex and ethnic group) |
| Saumoy et al., 2018 [33] | USA | High risk | 1-time EGD during colonoscopy + EDG every 3 years if IM is identified; 1-time EGD during colonoscopy + biennial EGD regardless of finding | No screening | To determine whether selected non-cardia gastric cancer screening for high-risk races and ethnicities within the US is cost-effective |
| Saito et al., 2018 [34] | Japan | High risk | ABC method + endoscopy if needed | Annual endoscopic screening | To evaluate the cost-effectiveness of gastric cancer screening by the ABC method in Japanese individuals from the perspective of the Japanese healthcare payer |
| Qin et al., 2022 [35] | China | High risk | 7 GCRSS strategies with different start ages: 40, 45, 50, 55, 60, 65, and 70 years | No screening | To assess the clinical benefits, harm, cost, and cost-effectiveness of the gastric cancer risk score scale (GCRSS) screening strategy from a Chinese healthcare system perspective |
| Qin et al., 2022 [36] | China | High risk | 30 alternative screening strategies with varying starting ages, including NGCS, modified NGCS, and endoscopy with varying screening intervals | No screening | To assess the clinical benefits and the cost-effectiveness of the NGCS strategy in GC high-risk areas of China from a societal perspective |
| Oh et al., 2022 [37] | USA | Average risk | 1. Single-population screening for H. pylori using 13C-UBT and treating those who tested positive with eradication therapy, and 2. single-population screening for H. pylori with upper endoscopy and PCR of gastric biopsies and treating those who tested positive with eradication therapy | No screening with opportunistic eradication | To evaluate the cost-effectiveness of C-UBT and PCR population screening strategies of H. pylori for the prevention of PUD and gastric cancer in the United States |
| Ma et al., 2022 [38] | China | High risk | 1. FBCM; 2. screen and treat | No screening | To investigate the cost-effectiveness of family-based Hp infection control and management (FBCM) |
| Li et al., 2014 [39] | Zhuanghe County, China | High risk | Gastric cancer screening program: epidemiology survey, serum PG, health education, gastroscope, gastric mucosa biopsy | Not reported | To assess the screening program on gastric cancer in Zhuanghe, China, using the health economics methodology |
| Kowada, 2023 [40] | Japan | Average risk | Annual, biennial, and triennial endoscopic screening and Hp eradication strategy | No screening | To evaluate which strategy was the most optimal and cost-effective among the Hp eradication strategy; annual, biennial, and triennial endoscopic screening; and no screening as a national gastric cancer prevention program |
| Kowada, 2021 [41] | Japan | Not reported | Annual and biennial endoscopic screening after Hp eradication strategy | No screening | To assess the cost-effectiveness of annual endoscopy versus biennial endoscopy versus no screening for gastric cancer screening in patients after successful Helicobacter pylori eradication |
| Kowada, 2019 [42] | Japan | High risk | 1. UGI; 2. endoscopy | Hp screening | To assess the cost-effectiveness of H. pylori screening compared to UGI and endoscopy to evaluate the optimal gastric cancer screening method in high-prevalence countries |
| Kapoor et al., 2020 [43] | Singapore | Intermediate risk | Initial screening using miRNA test followed by endoscopy for test-positive individuals and a 3-yearly follow-up screening for test-negative individuals | No screening | To evaluate the cost-effectiveness of a novel screening strategy using a microRNA (miRNA) blood test as a screen, followed by endoscopy for diagnosis confirmation in a 3-yearly population screening program for gastric cancer |
| Huang et al., 2020 [44] | Japan | Average risk | 14 endoscopic screening scenarios with various starting ages, stopping ages, and screening intervals | No screening | To estimate the cost-effectiveness of current screening guidelines and alternative screening strategies in Japan |
| Gupta et al., 2011 [45] | USA | Average risk | Endoscopy with colonoscopy | No endoscopy during colonoscopy | To evaluate the cost-effectiveness of screening the general population for upper gastrointestinal cancer by performing an upper endoscopy at the time of screening colonoscopy |
| Enriquez-Sanchez et al., 2022 [46] | Mexico | Intermediate risk | EGD± follow-up, EGD every 2 years, serum PG detection | No screening | To determine the cost and benefit of esophagogastroduodenoscopy, serum pepsinogen detection, and no screening |
| Di Giulio et al., 2009 [26] | USA | Not reported | Endoscopy | No screening | To assess the clinical and economic impact of ASGE and EPAGE guidelines in selecting patients referred for upper endoscopy (EDG) relative to the direction of gastro-oesophageal cancer |
| Dan et al., 2006 [47] | Singapore | Intermediate risk and various high-risk groups | 2 yearly endoscopies | No screening | To conduct a cost–utility analysis to determine whether endoscopic screening for stomach cancer in an intermediate-risk population would be cost-effective and to better define the high-risk groups in the population who would benefit from such a strategy |
| Cho et al., 2013 [29] | Republic of Korea | Not reported | 1. Upper-gastrointestinal X-ray; 2. endoscopy | No screening | To evaluate the cost-effectiveness outcomes of the national cancer screening program for gastric cancer (UGI and endoscopy) |
| Chang et al., 2012 [48] | Republic of Korea | Not reported | 1. Endoscopy; 2. UGI series | No screening | To explore the cost-effectiveness of various gastric cancer screening programs using endoscopy or UGI series in Korea relative to no screening and determine the most favorable screening alternative for gastric cancer with regard to starting age and screening interval |
| Babazono & Hillman, 1995 [14] | Japan | High risk | 1. Yearly indirect X-ray, followed by endoscopy if positive; 2. yearly direct X-ray, followed by endoscopy if positive; and 3. yearly endoscopy alone | No screening | The specific aims of this case study of gastric cancer screening in Japan were to (a) determine the most cost-effective strategy; (b) decide on the optimal target ages stratified by sex; and (c) identify the change of cost-effectiveness between the 1980s and 1990s |
| Ascherman & Hur, 2021 [49] | Brazil, France, Japan, Nigeria, and the United States | High risk | Endoscopic screening: once per lifetime, and every 10 years, 5 years, 2 years | No screening | To performance a cost-effectiveness analysis to compare screening and surveillance strategies for gastric cancer in Brazil, France, Japan, Nigeria, and the United States |
| Areia et al., 2018 [50] | Portugal | Intermediate risk | 1. Stand-alone upper endoscopy, 2. endoscopy combined with a colorectal cancer screening colonoscopy after a positive fecal occult blood test or pepsinogen serologic screening | No Screening | To determine the cost–utility of screening strategies for gastric cancer in a European country |
| References | Age Range | Analytical Model | Cycle Length | Time Horizon | Compliance | Perspective | Discount Rate | Source of Clinical Input |
|---|---|---|---|---|---|---|---|---|
| Zhou et al., 2011 [30] | >35 years | Observational | Not applicable | 2 years | Full | Direct cost | 5% | Prospective data from study population |
| Zheng & Liu, 2023 [2] | 40–77 years | Markov | 1 year | 37 years | Full | Not reported | 3% | Literature |
| Yeh et al., 2016 [12] | 20–death | Markov | Monthly | Lifetime | Full | Societal | 3% | Literature, SEER |
| Xia et al., 2021 [13] | 40–69 years | Markov | 1 year | Lifetime | 49% | Healthcare system | 5% | Clinical trial, literature |
| Wu et al., 2016 [31] | 50–69 years | Markov | 1 year | Lifetime | Full | Healthcare payer | 3% | Database, literature |
| Wei et al., 2011 [28] | 40–69 years | Observational | Not applicable | 3 years | 67% | 3rd-party payer | Not reported | Prospective data from study population |
| Wang et al., 2022 [32] | 40–65 years | Markov | 1 year | 15 years | 89% | Not reported | 3% | Database, literature |
| Tsuji et al., 1991 [27] | 40–79 years | Simulation Model | Not reported | Not reported | Not reported | Not reported | Not reported | Literature, databases |
| Suh et al., 2020 [25] | ≥40 years | Observational | Not reported | 10 years | Not reported | Ministry of Health: National Health Insurance | Not reported | National Health Insurance Service, Korean National Health Insurance Big Data Base |
| Shah et al., 2020 [15] | 50–80 years | Markov | 1 year | 30 years | Not reported | Healthcare system | 3% | SEER, registries, literature |
| Saumoy et al., 2018 [33] | 50–80 years | Markov | 1 year | 30 years | Not reported | Healthcare system | 3% | Literature, CDC |
| Saito et al., 2018 [34] | 50–80 years | Markov | 1 year | 30 years | 60% | Healthcare payer | 2% | Literature, registry |
| Qin et al., 2022 [35] | 40–70 years | Markov | 1 year | Lifetime | Full | Healthcare system | 5% | Literature, registries |
| Qin et al., 2022 [36] | 40–70 | Markov | 1 year | Lifetime | Full | Societal | 5% | Literature, registries |
| Oh et al., 2022 [37] | 40–100 years | Markov | 1 year | Lifetime | Full | 3rd- party payer | 3% | Literature, registries |
| Ma et al., 2022 [38] | 18–78 years | Markov | 1 year | Lifetime | Not reported | Healthcare provider | 3% | Database, literature |
| Li et al., 2014 [39] | 40–69 years | Observational | Not reported | 2008–2012 | Full | Not reported | 3% | Program data |
| Kowada, 2023 [40] | 20–80 years | Markov | 1 year | Lifetime | 49.50% | Healthcare payer | 3% | Literature, registries |
| Kowada, 2021 [41] | 50–lifetime | Markov | 1 year | Lifetime | Not reported | Healthcare payer | 3% | Literature, registries |
| Kowada, 2019 [42] | 40–80 years | Markov | 1 year | Lifetime | Not reported | Healthcare payer | 3% | Registries, literature |
| Kapoor et al., 2020 [43] | 50–75 years | Markov | 1 year | 25 years | 45% | Healthcare | 3% | Literature, registries |
| Huang et al., 2020 [44] | 40–80 years | Markov | 1 year | Lifetime | Not reported | Societal | 3% | Literature, registries |
| Gupta et al., 2011 [45] | 50-year-olds | Markov | 1 year | 30 years | Full | 3rd-party payer | 3% | Registries |
| Enriquez-Sanchez et al., 2022 [46] | 50–80 years | Markov | 1 year | 30 years | Not reported | Public healthcare | 3% | Literature, registries |
| Di Giulio et al., 2009 [26] | 60-year-olds | Decision Tree | Not applicable | Lifetime | Not reported | Societal | Not reported | Registries |
| Dan et al., 2006 [47] | 50–70 years | Markov | 1 year | Lifetime | Full | Societal | 3% | Literature, registries |
| Cho et al., 2013 [29] | 40 years+ | Observational | Not reported | 9 years | Varying | Direct + indirect + productivity loss | Not reported | Registries |
| Chang et al., 2012 [48] | 50–80 years | Markov | 1 year | Lifetime | Male:18.7%; Female: 24.7% | Societal | 3% | Literature, registries |
| Babazono & Hillman, 1995 [14] | 40–80 years | Markov | 1 year | 10 years | 13% | Government payer | 5% | Literature, registries |
| Ascherman & Hur, 2021 [49] | 40–75 years | Markov | 1 year | 35 years | Full | Not reported | 3% | Literature, SEER, registries |
| Areia et al., 2018 [50] | 50–75 years | Markov | 1 year | 25 years | Not reported | Not reported | 3% | Literature, SEER, registries |
| References | Intervention Strategy | Reference Strategy | Currency/ Year | Incremental Cost-Effectiveness Ratio (ICER) | Willingness to Pay Threshold | Sensitivity Analysis | Sensitive Variables—1-Way SA | Probability Sensitivity Analysis | CHEERS Checklist |
|---|---|---|---|---|---|---|---|---|---|
| Zhou et al., 2011 [30] | Epidemiological survey, serum PG + endoscopy, pathological examination | No screening | 2001 | USD 459/QALY | Not reported | Not reported | Not reported | Not reported | Not reported |
| Zheng & Liu, 2023 [2] | Hp screening, NGCS | No screening | CNY | No screening: dominated to NGCS; NGCS to Hp screening: CNY 538,680/QALY; Hp screening to no screening: CNY 5536/QALY | CNY 80,976/QALY | 1-way SA and PSA | NGCS: probability of transitioning from IM to dysplasia; Hp screening: probability of transitioning from Hp gastritis to AG | NGCS CE 95%; Hp CE 99% | Not reported |
| Yeh et al., 2016 [12] | Serum PG, endoscopic-based screening, Hp screening | No screening | 2012 USD | Hp and endoscopic screening dominated. Serum PG: USD 105,400/QALY | USD 100,000/QALY | 1-way SA & PSA | Serum PG: Hp prevalence, screen age, serum PG test sensitivity, endoscopic follow-up costs | Serum PG CE 47% for general population; 97% for current smokers; 85% former smokers | Not reported |
| Xia et al., 2021 [13] | Endoscopic screening: once per lifetime, and every 10 years, 5 years, 3 years, 2 years | No screening | 2019 USD | (Screen age 40–45 years) vs. no screening—ICER: once: USD 3035, 10 years: USD 1566; 5 yrs: USD 1781; 3 years: USD 2720; 2 yrs: USD 4511 | WHO definition: not CE if ICER > 3times GDP per capita (USD 10,276) | 1-way SA & PSA | Varying utility scores for GC and EC | Screening every 2 years CE 98% compared to other strategies | Reported but not included |
| Wu et al., 2016 [31] | Annual EGD surveillance; 2-yearly EGD screening | No EGD follow-up | 2011 SGD | SGD 23,470–SGD 39,761/QALY | SGD 44,000/QALY | 1-way SA & PSA | Discount rate, surveillance start age, cost of program, cost of EGD/biopsy, odds of precancerous legions, utility of GC stage 1 | Surveillance CE 96.5% | Not reported |
| Wei et al., 2011 [28] | Endoscopy with mucosal iodine staining in combination with index biopsies | Not reported | CNY | Benefit–cost ratio: 4.49–10.37 | Not reported | Not reported | Not reported | Not reported | Not reported |
| Wang et al., 2022 [32] | Hp eradication; electronic endoscopy (one-time, annual, biennial, or triennial) | Status quo | 2020 CNY | Hp dominant | CNY 70,000/ QALY | 1-way SA | GC prevalence, annual progress rates between the states AG, IN, ESC, and ASC; the compliance rate with and success rate of Hp treatment as well as eradication-related impact on state transition; and the prevalence of risk factor(s) used to define the target population were the parameters with the most marked impact on cost-effectiveness among the strategies | Not reported | Reported but not included |
| Tsuji et al., 1991 [27] | Gastro-fluorography screening | No screening | JPY | Male: JPY 606.3K/LYS; female: JPY 1542.5K/LYS | Not reported | Not reported | Not reported | Not reported | Not reported |
| Suh et al., 2020 [25] | Screening: endoscopy or UGI | No screening | USD/ KRW | Average cost/LYS: USD 20,309 | WHO definition: not CE if ICER > 3 times GDP per capita (USD 20,565) | Not reported | Not reported | Not reported | Not reported |
| Shah et al., 2020 [15] | 1-time EGD during colonoscopy + EDG every 3 years if IM is identified; 1-time EGD during colonoscopy + biennial EGD regardless of finding | No screening | 2015 USD | 1-time: USD 74,329/QALY; biennial: absolutely dominated | USD 100,000/ QALY | 1-way SA and PSA | Transition probability: local–regional NCGA, dysplasia–local, regional–distant; probability that dysplastic lesion undergo endoscopy, cost of endoscopy | 1-time endoscopy is preferred at the WTP | Not reported |
| Saumoy et al., 2018 [33] | 1-time EGD during colonoscopy + EDG every 3 years if IM is identified; 1-time EGD during colonoscopy + biennial EGD regardless of finding | No screening | 2015 USD | 1-time: USD 71,451–USD 80,278/QALY; for non-Hispanic whites, 1-time is not CE; biennial: dominated in all scenarios | USD 100,000/QALY | 1-way SA and PSA | Transition probability: IM–dysplasia, dysplasia–local, local–regional; probability of IM, cost of upper endoscopy, EGD, gastrectomy | No screening is CE for non-Hispanic whites 56.2%; 1-time endoscopy CE 55.2% (Hispanic), 52.9% (non-Hispanic blacks), 58.9% (Asians) | Not reported |
| Saito et al., 2018 [34] | ABC method + endoscopy if needed | Annual endoscopic screening | 2014 USD | ABC dominant: more effective and less costly | USD 50,000/ QALY | 1-way SA and PSA | ABC remained CE for all range of values | ABC CE 99.7% at WTP USD 10,000/QALY | Not reported |
| Qin et al., 2022 [35] | 7 GCRSS strategies with different start ages: 40, 45, 50, 55, 60, 65, and 70 years | No screening | 2021 USD | USD 10,315–USD 27,446/QALY | WHO definition: not CE if ICER > 3 times GDP per capita (USD 37,655 per QALY) | 1-way SA and PSA | Risk of progressing dysplasia after surgery; cost of surgery | 40-GCRSS CE 85.6% | Not reported |
| Qin et al., 2022 [36] | 30 alternative screening strategies with varying starting ages, including NGCS, modified NGCS and endoscopy with varying screening intervals | No screening | 2021 USD | NGCS strategies: USD 12,514–USD 118,852/QALY. 40-NGCS: USD 15,668/QALY. 40-NGCS is the most optimal screening strategy. | 1.45 times GDP per capita: USD 17,922/QALY | 1-way SA and PSA | Relative risk of progressing to preclinical stage I after surgery, transition probability from gastritis to atrophy, and cost of surgery | 40-NGCS CE 86.3% | Not reported |
| Oh et al., 2022 [37] | 1. Single population screening for H. pylori using 13C-UBT and treating those who tested positive with eradication therapy, and 2. single population screening for H. pylori with upper endoscopy and PCR of gastric biopsies and treating those who tested positive with eradication therapy | No screening with opportunistic eradication | 2020 USD | Versus no screening: C-UBT: USD 116.46; PCR + biopsy: USD 2329.69. C-UBT vs. PCR: USD 38,591.89 | USD 100,000/ QALY | 1-way SA and PSA | Risk of gastric cancer if H. pylori-positive, the risk of gastric cancer after Hp eradication, H. pylori prevalence, and costs of screening | PCR CE 65% | Reported |
| Ma et al., 2022 [38] | 1. FBCM; 2. screen and treat | No screening | 2020 USD | Versus no screening: FBCM: USD 9.18/QALY; screen and treat: USD 12.08/QALY; screen and treat vs. FBCM: USD 27.44/QALY | USD 31,315/QALY; 3 times GDP per capita | 1-way SA and PSA | Variables not sensitive | Screen and treat CE > 99% | Not reported |
| Li et al., 2014 [39] | Gastric cancer screening program: epidemiology survey, serum PG, health education, gastroscope, gastric mucosa biopsy | Not reported | Not provided | CNY 1370/QALY | Not reported | 1-way SA on discount rate | CNY 1413/QALY for discount rate of 5% | Not reported | Not reported |
| Kowada, 2023 [40] | Annual, biennial, and triennial endoscopic screening and Hp eradication strategy | No screening | 2021 USD | USD 24.4/QALY | USD 50,000/ QALY | 1-way SA and PSA | ICER not sensitive to selected variables | Hp eradication CE 100% | Not reported |
| Kowada, 2021 [41] | Annual and biennial endoscopic screening after Hp eradication strategy | No screening | 2018 USD | Biennial: USD 135,566/QALY | USD 100,000/ QALY | 1-way SA and PSA | Incidence of gastric cancer and the proportion of stage I | Biennial CE 100% | Not reported |
| Kowada, 2019 [42] | 1. UGI; 2. endoscopy | Hp screening | 2018 USD | Hp screening dominated all other options | USD 50,000/ QALY | 1-way SA, multiway sensitivity analysis, and PSA | Results robust | Hp screening CE 100% | Not reported |
| Kapoor et al., 2020 [43] | Initial screening using miRNA test followed by endoscopy for test-positive individuals and a 3-yearly follow-up screening for test negative individuals | No screening | 2018 USD | Intervention: USD 40,971/QALY | USD 70,000/ QALY | 1-way SA and PSA | Incidence of gastric cancer, cost of screening tests, sensitivity and specificity of the miRNA test, and endoscopy and utility values of cancer-free individuals | Intervention CE 95% | Not reported |
| Huang et al., 2020 [44] | 14 endoscopic screening scenarios with various starting ages, stopping ages, and screening intervals | No screening | 2015 USD | Triennial screening of 50–75 yr olds: ICER: USD 45,665/QALY | USD 50,000/ QALY | 1-way SA and PSA | Direct cost of endoscopy, direct cost of EGD, sensitivity of endoscopy, complete resection rate of EGD, specificity of endoscopy, first-year medical cost for local cancer, and first-year medical cost for distal cancer and regional cancer | Intervention CE 92.6% | Reported |
| Gupta et al., 2011 [45] | Endoscopy with colonoscopy | No endoscopy during colonoscopy | 2009 USD | USD 115,664/QALY | USD 50,000/ QALY | 1-way SA | Prevalence rate of gastric cancer | Not reported | Not reported |
| Enriquez-Sanchez et al., 2022 [46] | EGD± follow-up, EGD every 2 years, serum PG detection | No screening | 2019 USD | EGD ± follow-up: USD 129/QALY; serum PG: USD 1590/QALY | USD 9000/ QALY | PSA | Not reported | EDG± follow-up CE 100% | Not reported |
| Di Giulio et al., 2009 [26] | Endoscopy | No screening | 2007 USD | Appropriate endoscopy: USD 16,577/LYG; inappropriate endoscopy: USD 301,203/LYG | USD 150,000/ LYG | Systematic analysis | Cancer prevalence | Not reported | Not reported |
| Dan et al., 2006 [47] | 2 yearly endoscopies | No screening | 2003 USD | USD 26,836/QALY | USD 28,000 | 1- and 2-way SA | Cost of screening endoscopy and the distribution of cancer stage at screening | Not reported | Not reported |
| Cho et al., 2013 [29] | 1. Upper-gastrointestinal X-ray; 2. endoscopy | No screening | 2009 KRW | UGI: KRW 260,201–371,011,000 KW/survival; endoscopy: KRW 119,099,000–17,870,000 KW/survival | Not reported | Scenario analysis | Upper screening age limit | Not reported | Not reported |
| Chang et al., 2012 [48] | 1. Endoscopy; 2. UGI series | No screening | 2008 USD | Male: USD 4820/QALY, female: USD 6073/QALY | WHO definition: not CE if ICER > 3 times GDP per capita (USD 19,162) | 1-way SA | Screening cost, distribution of cancer stages at screening | Not reported | Not reported |
| Babazono & Hillman, 1995 [14] | 1. Yearly indirect X-ray, followed by endoscopy if positive; 2. yearly direct X-ray, followed by endoscopy if positive; and 3. yearly endoscopy alone | No screening | 1990 USD | Male: USD 50,888–USD 6376; female: USD 40,568–USD 19,424 | Not reported | 1-way SA and scenario analysis | No change in conclusion | Not reported | Not reported |
| Ascherman & Hur, 2021 [49] | Endoscopic screening: once per lifetime, and every 10 years, 5 years, 2 years | No screening | 2020 USD | Screening every 10 years most CE but does not meet WTP threshold. Japan: USD $76,221/QALY | 2 times GNI per capita. Brazil: USD 18,822; France: USD 84,596; Japan: USD 85,069; Nigeria: USD 4036; US: USD 129,900 | 1-way SA | Starting age of screening, cost of endoscopy, and baseline probability of local gastric cancer at time of diagnosis | Not reported | No reported |
| Areia et al., 2018 [50] | 1. Stand-alone upper endoscopy, 2. endoscopy combined with a colorectal cancer screening colonoscopy after a positive fecal occult blood test or pepsinogen serologic screening | No Screening | 2016 EUR | Endoscopy: EUR 15,407/QALY; endoscopy + colonoscopy: EUR 30,908/QALY; serum PG: EUR 143,344/QALY | 2-times 2016 GNI per capita. EUR 37,000/QALY | 1-way SA and PSA | Endoscopy costs, the number of endoscopies per patient over the screening age range and the ASR of gastric cancer | Intervention CE 86% | Reported but not included |
3.1. Interventions Identified from the Systematic Review
3.2. Cost-Effectiveness Analysis of Asian-Based Versus Non-Asian-Based Studies
3.3. Study Type and Model Parameters
3.4. Willingness-to-Pay Thresholds and Sensitive Variables
3.5. Results on Quality of Reporting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I. Bray Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.who.int/today (accessed on 17 February 2024).
- Zheng, P.; Liu, J. Cost-Effectiveness Analysis of Hp and New Gastric Cancer Screening Scoring System for Screening and Prevention of Gastric Cancer. Curr. Oncol. 2023, 30, 1132–1145. [Google Scholar] [CrossRef] [PubMed]
- Katai, H.; Ishikawa, T.; Akazawa, K.; Isobe, Y.; Miyashiro, I.; Oda, I.; Tsujitani, S.; Ono, H.; Tanabe, S.; Fukagawa, T.; et al. Five-Year Survival Analysis of Surgically Resected Gastric Cancer Cases in Japan: A Retrospective Analysis of More than 100,000 Patients from the Nationwide Registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer 2018, 21, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Sumiyama, K. Past and Current Trends in Endoscopic Diagnosis for Early Stage Gastric Cancer in Japan. Gastric Cancer 2017, 20, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Mahar, A.L.; Zagorski, B.; Coburn, N. Patient-Level Costs of Treating Metastatic Gastric Cancer by Treatment Strategy. J. Clin. Oncol. 2018, 36, 167. [Google Scholar] [CrossRef]
- Parkin, D.M. The Global Health Burden of Infection-associated Cancers in the Year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P. Kyoto Global Consensus Report on Helicobacter Pylori Gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter Pylori Infection—The Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Choi, K.S.; Jun, J.K.; Suh, M.; Park, B.; Noh, D.K.; Song, S.H.; Jung, K.W.; Lee, H.-Y.; Choi, I.J.; Park, E.-C. Effect of Endoscopy Screening on Stage at Gastric Cancer Diagnosis: Results of the National Cancer Screening Programme in Korea. Br. J. Cancer 2015, 112, 608–612. [Google Scholar] [CrossRef]
- Lee, S.; Jun, J.K.; Suh, M.; Park, B.; Noh, D.K.; Jung, K.-W.; Choi, K.S. Gastric Cancer Screening Uptake Trends in Korea. Medicine 2015, 94, e533. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef]
- Yeh, J.M.; Hur, C.; Ward, Z.; Schrag, D.; Goldie, S.J. Gastric Adenocarcinoma Screening and Prevention in the Era of New Biomarker and Endoscopic Technologies: A Cost-Effectiveness Analysis. Gut 2016, 65, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Zeng, H.; Liu, W.; Xie, L.; Shen, M.; Li, P.; Li, H.; Wei, W.; Chen, W.; Zhuang, G. Estimated Cost-Effectiveness of Endoscopic Screening for Upper Gastrointestinal Tract Cancer in High-Risk Areas in China. JAMA Netw. Open 2021, 4, e2121403. [Google Scholar] [CrossRef] [PubMed]
- Babazono, A.; Hillman, A.L. Declining Cost Effectiveness of Screening for Disease: The Case of Gastric Cancer in Japan. Int. J. Technol. Assess. Health Care 1995, 11, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Canakis, A.; Peek, R.M.; Saumoy, M. Endoscopy for Gastric Cancer Screening Is Cost-Effective for Asian Americans in the United States. Clin. Gastroenterol. Hepatol. 2020, 18, 3026–3039. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chen, W.; Zheng, R.; Zhang, S.; Ji, J.S.; Zou, X.; Xia, C.; Sun, K.; Yang, Z.; Li, H.; et al. Changing Cancer Survival in China during 2003–15: A Pooled Analysis of 17 Population-Based Cancer Registries. Lancet Glob. Health 2018, 6, e555–e567. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans Biological Agents. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 1–441.
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global Burden of Cancers Attributable to Infections in 2008: A Review and Synthetic Analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology Guidelines on the Diagnosis and Management of Patients at Risk of Gastric Adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Piazuelo, M.B. The Gastric Precancerous Cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef]
- Yao, X.; Smolka, A.J. Gastric Parietal Cell Physiology and Helicobacter Pylori-Induced Disease. Gastroenterology 2019, 156, 2158–2173. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Helicobacter pylori Working Group Helicobacter Pylori Eradication as a Strategy for Preventing Gastric Cancer. In IARC Working Group Report Volume 8; World Health Organization: Geneva, Switzerland, 2014; Volume 8.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakun-apruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 Explanation and Elaboration: A Report of the ISPOR CHEERS II Good Practices Task Force. Value Health 2022, 25, 10–31. [Google Scholar] [CrossRef]
- Suh, Y.; Lee, J.; Woo, H.; Shin, D.; Kong, S.; Lee, H.; Shin, A.; Yang, H. National Cancer Screening Program for Gastric Cancer in Korea: Nationwide Treatment Benefit and Cost. Cancer 2020, 126, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Giulio, E.D.; Hassan, C.; Pickhardt, P.J.; Zullo, A.; Laghi, A.; Kim, D.H.; Iafrate, F. Cost-Effectiveness of Upper Gastrointestinal Endoscopy According to the Appropriateness of the Indication. Scand. J. Gastroenterol. 2009, 44, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, I.; Fukao, A.; Sugawara, N.; Shoji, T.; Kuwajima, I.; Hisamichi, S. Cost-Effectiveness Analysis of Screening for Gastric Cancer in Japan. Tohoku J. Exp. Med. 1991, 164, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.-Q.; Yang, C.-X.; Lu, S.-H.; Yang, J.; Li, B.-Y.; Lian, S.-Y.; Qiao, Y.-L. Cost-Benefit Analysis of Screening for Esophageal and Gastric Cardiac Cancer. Chin. J. Cancer 2011, 30, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Kang, M.H.; Choi, K.S.; Suh, M.; Jun, J.K.; Park, E.-C. Cost-Effectiveness Outcomes of the National Gastric Cancer Screening Program in South Korea. Asian Pac. J. Cancer Prev. 2013, 14, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Wang, X.; Li, S.; Tan, C.; Zeng, X.; Luo, X.; Yi, L.; Peng, L.; Wu, M.; Peng, Y.; et al. Clinical Benefit and Cost Effectiveness of Risk-Stratified Gastric Cancer Screening Strategies in China: A Modeling Study. Pharmacoeconomics 2022, 40, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Wang, X.; Li, S.; Tan, C.; Zeng, X.; Wu, M.; Peng, Y.; Wang, L.; Wan, X. Benefit-to-Harm Ratio and Cost-Effectiveness of Government-Recommended Gastric Cancer Screening in China: A Modeling Study. Front. Public Health 2022, 10, 955120. [Google Scholar] [CrossRef]
- Saito, S.; Azumi, M.; Muneoka, Y.; Nishino, K.; Ishikawa, T.; Sato, Y.; Terai, S.; Akazawa, K. Cost-Effectiveness of Combined Serum Anti-Helicobacter Pylori IgG Antibody and Serum Pepsinogen Concentrations for Screening for Gastric Cancer Risk in Japan. Eur. J. Health Econ. 2018, 19, 545–555. [Google Scholar] [CrossRef]
- Kowada, A. Cost-Effectiveness of Helicobacter Pylori Test and Eradication versus Upper Gastrointestinal Series versus Endoscopy for Gastric Cancer Mortality and Outcomes in High Prevalence Countries. Scand. J. Gastroenterol. 2019, 54, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-S.; Park, E.-C.; Chung, W.; Nam, C.M.; Choi, K.S.; Cho, E.; Cho, W.-H. Comparing Endoscopy and Upper Gastrointestinal X-Ray for Gastric Cancer Screening in South Korea: A Cost-Utility Analysis. Asian Pac. J. Cancer Prev. 2012, 13, 2721–2728. [Google Scholar] [CrossRef] [PubMed]
- Enríquez-Sánchez, L.B.; Gallegos-Portillo, L.G.; Camarillo-Cisneros, J.; Cisneros-Castolo, M.; Montelongo-Santiesteban, J.J.; Aguirre-Baca, D.A.; Pérez-Echavarría, A.I.; Contreras-Pacheco, A.E. Cost-Benefit of Serum Pepsinogen Screening for Gastric Adenocarcinoma in the Mexican Population. Rev. De Gastroenterol. México (Engl. Ed.) 2022, 87, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yu, M.; Shao, Q.; Yu, X.; Zhang, C.; Zhao, J.; Yuan, L.; Qi, Y.; Hu, R.; Wei, P.; et al. Both Family-based Helicobacter Pylori Infection Control and Management Strategy and Screen-and-treat Strategy Are Cost-effective for Gastric Cancer Prevention. Helicobacter 2022, 27, e12911. [Google Scholar] [CrossRef] [PubMed]
- Kowada, A. A Population-Based Helicobacter Pylori Eradication Strategy Is More Cost-Effective than Endoscopic Screening. Dig. Dis. Sci. 2023, 68, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Kowada, A. Endoscopy Is Cost-Effective for Gastric Cancer Screening after Successful Helicobacter Pylori Eradication. Dig. Dis. Sci. 2021, 66, 4220–4226. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Leung, C.Y.; Saito, E.; Katanoda, K.; Hur, C.; Kong, C.Y.; Nomura, S.; Shibuya, K. Effect and Cost-Effectiveness of National Gastric Cancer Screening in Japan: A Microsimulation Modeling Study. BMC Med. 2020, 18, 257. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; So, J.B.Y.; Zhu, F.; Too, H.-P.; Yeoh, K.-G.; Yoong, J.S.-Y. Evaluating the Use of MicroRNA Blood Tests for Gastric Cancer Screening in a Stratified Population-Level Screening Program: An Early Model-Based Cost-Effectiveness Analysis. Value Health 2020, 23, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Zhou, J.; Naidoo, N.; Yang, W.Y.; Lin, X.C.; Wang, P.; Ding, J.Q.; Wu, C.B.; Zhou, H.J. Determining the Cost-Effectiveness of Endoscopic Surveillance for Gastric Cancer in Patients with Precancerous Lesions. Asia-Pac. J. Clin. Oncol. 2016, 12, 359–368. [Google Scholar] [CrossRef]
- Saumoy, M.; Schneider, Y.; Shen, N.; Kahaleh, M.; Sharaiha, R.Z.; Shah, S.C. Cost-Effectiveness of Gastric Cancer Screening According to Race and Ethnicity. Gastroenterology 2018, 155, 648–660. [Google Scholar] [CrossRef]
- Areia, M.; Spaander, M.C.; Kuipers, E.J.; Dinis-Ribeiro, M. Endoscopic Screening for Gastric Cancer: A Cost-utility Analysis for Countries with an Intermediate Gastric Cancer Risk. United Eur. Gastroenterol. J. 2018, 6, 192–202. [Google Scholar] [CrossRef]
- Wang, Z.; Han, W.; Xue, F.; Zhao, Y.; Wu, P.; Chen, Y.; Yang, C.; Gu, W.; Jiang, J. Nationwide Gastric Cancer Prevention in China, 2021–2035: A Decision Analysis on Effect, Affordability and Cost-Effectiveness Optimisation. Gut 2022, 71, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.Y.; So, J.B.Y.; Yeoh, K.G. Endoscopic Screening for Gastric Cancer. Clin. Gastroenterol. Hepatol. 2006, 4, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Bansal, A.; Wani, S.B.; Gaddam, S.; Rastogi, A.; Sharma, P. Endoscopy for Upper GI Cancer Screening in the General Population: A Cost-Utility Analysis. Gastrointest. Endosc. 2011, 74, 610–624.e2. [Google Scholar] [CrossRef]
- Ascherman, B.; Oh, A.; Hur, C. International Cost-Effectiveness Analysis Evaluating Endoscopic Screening for Gastric Cancer for Populations with Low and High Risk. Gastric Cancer 2021, 24, 878–887. [Google Scholar] [CrossRef]
- Oh, A.; Truong, H.; Kim, J.; Rustgi, S.D.; Abrams, J.A.; Hur, C. Cost-Effectiveness of Screening with Polymerase Chain Reaction for Helicobacter Pylori to Prevent Gastric Cancer and Peptic Ulcers. J. Gastrointest. Oncol. 2022, 13, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Guan, P.; Sun, L.-P.; He, Q.-C.; Yuan, Y.; Zhou, B.-S. Health Economic Assessment for Screening of Gastric Cancer in a High Risk Population in Northeastern China. Chin. J. Cancer Res. 2011, 23, 21–24. [Google Scholar] [CrossRef][Green Version]
- Li, D.; Yuan, Y.; Sun, L.-P.; Fang, X.; Zhou, B.-S. Health Economics Evaluation of a Gastric Cancer Early Detection and Treatment Program in China. Asian Pac. J. Cancer Prev. 2014, 15, 5133–5136. [Google Scholar] [CrossRef]
- World Health Organization. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis; Tan-Torres Edejer, T., Baltussen, R., Adam, T., Hutubessy, R., Acharya, A., Evans, D., Murray, D., Murray, C., Eds.; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Bae, Y.H.J.; Mullins, C.D. Do Value Thresholds for Oncology Drugs Differ from Non-Oncology Drugs? J. Manag. Care Pharm. 2014, 20, 1086–1092. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement. BMJ 2013, 346, f1049. [Google Scholar] [CrossRef]
- Lansdorp-Vogelaar, I.; Meester, R.G.S.; Laszkowska, M.; Escudero, F.A.; Ward, Z.J.; Yeh, J.M. Cost-Effectiveness of Prevention and Early Detection of Gastric Cancer in Western Countries. Best Pract. Res. Clin. Gastroenterol. 2021, 50–51, 101735. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.K.; Lin, S.-R.; Ching, J.Y.L.; To, K.-F.; Ng, E.K.W.; Chan, F.K.L.; Lau, J.Y.W.; Sung, J.J.Y. Factors Predicting Progression of Gastric Intestinal Metaplasia: Results of a Randomised Trial on Helicobacter Pylori Eradication. Gut 2004, 53, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.C.; van Grieken, N.C.T.; Looman, C.W.N.; Casparie, M.K.; de Vries, E.; Meijer, G.A.; Kuipers, E.J. Gastric Cancer Risk in Patients with Premalignant Gastric Lesions: A Nationwide Cohort Study in the Netherlands. Gastroenterology 2008, 134, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.-M.; Malfertheiner, P.; Lee, Y.-C.; Sheu, B.-S.; Sugano, K.; Cheng, H.-C.; Yeoh, K.-G.; Hsu, P.-I.; Goh, K.-L.; Mahachai, V.; et al. Screening and Eradication of Helicobacter Pylori for Gastric Cancer Prevention: The Taipei Global Consensus. Gut 2020, 69, 2093–2112. [Google Scholar] [CrossRef] [PubMed]
- Fock, K.M.; Talley, N.; Moayyedi, P.; Hunt, R.; Azuma, T.; Sugano, K.; Xiao, S.D.; Lam, S.K.; Goh, K.L.; Chiba, T.; et al. Asia–Pacific Consensus Guidelines on Gastric Cancer Prevention. J. Gastroenterol. Hepatol. 2008, 23, 351–365. [Google Scholar] [CrossRef]
- Talley, N.J.; Fock, K.M.; Moayyedi, P. Gastric Cancer Consensus Conference Recommends Helicobacter Pylori Screening and Treatment in Asymptomatic Persons from High-Risk Populations to Prevent Gastric Cancer. Am. J. Gastroenterol. 2008, 103, 510–514. [Google Scholar] [CrossRef]
- Mera, R.M.; Bravo, L.E.; Camargo, M.C.; Bravo, J.C.; Delgado, A.G.; Romero-Gallo, J.; Yepez, M.C.; Realpe, J.L.; Schneider, B.G.; Morgan, D.R.; et al. Dynamics of Helicobacter Pylori Infection as a Determinant of Progression of Gastric Precancerous Lesions: 16-Year Follow-up of an Eradication Trial. Gut 2018, 67, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Chen, S.; Hu, J.; Guo, Q.; Liu, R.; Zheng, H.; Jin, Z.; Yuan, Y.; Xi, Y.; et al. Endoscopic Screening in Asian Countries Is Associated with Reduced Gastric Cancer Mortality: A Meta-Analysis and Systematic Review. Gastroenterology 2018, 155, 347–354.e9. [Google Scholar] [CrossRef] [PubMed]
- Hirota, W.K.; Zuckerman, M.J.; Adler, D.G.; Davila, R.E.; Egan, J.; Leighton, J.A.; Qureshi, W.A.; Rajan, E.; Fanelli, R.; Wheeler-Harbaugh, J.; et al. ASGE Guideline: The Role of Endoscopy in the Surveillance of Premalignant Conditions of the Upper GI Tract. Gastrointest. Endosc. 2006, 63, 570–580. [Google Scholar] [CrossRef]
- Yeh, J.M.; Hur, C.; Schrag, D.; Kuntz, K.M.; Ezzati, M.; Stout, N.; Ward, Z.; Goldie, S.J. Contribution of H. Pylori and Smoking Trends to US Incidence of Intestinal-Type Noncardia Gastric Adenocarcinoma: A Microsimulation Model. PLoS Med. 2013, 10, e1001451. [Google Scholar] [CrossRef]
- Maskarinec, G.; Noh, J.J. The Effect of Migration on Cancer Incidence among Japanese in Hawaii. Ethn. Dis. 2004, 14, 431–439. [Google Scholar] [PubMed]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Zhu, C.; Yuan, Y.; Feng, Q.; Feng, Y.; Hao, Y.; Li, J.; Zhang, K.; Ye, G.; Ye, L.; et al. Development and Validation of a Prediction Rule for Estimating Gastric Cancer Risk in the Chinese High-Risk Population: A Nationwide Multicentre Study. Gut 2019, 68, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yu, J.; Zhang, Z.-M.; Song, Y.-K.; Li, Y.-H.; Wang, L. Missed Diagnosis of Early Gastric Cancer or High-Grade Intraepithelial Neoplasia. World J. Gastroenterol. 2013, 19, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Ribeiro, M.; Areia, M.; de Vries, A.C.; Marcos-Pinto, R.; Monteiro-Soares, M.; O’Connor, A.; Pereira, C.; Pimentel-Nunes, P.; Correia, R.; Ensari, A.; et al. Management of Precancerous Conditions and Lesions in the Stomach (MAPS): Guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012, 44, 74–94. [Google Scholar] [CrossRef] [PubMed]
- Gerson, L.B.; Groeneveld, P.W.; Triadafilopoulos, G. Cost-Effectiveness Model of Endoscopic Screening and Surveillance in Patients with Gastroesophageal Reflux Disease. Clin. Gastroenterol. Hepatol. 2004, 2, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.M.; Hur, C.; Kuntz, K.M.; Ezzati, M.; Goldie, S.J. Cost-Effectiveness of Treatment and Endoscopic Surveillance of Precancerous Lesions to Prevent Gastric Cancer. Cancer 2010, 116, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Pabla, B.S.; Shah, S.C.; Corral, J.E.; Morgan, D.R. Increased Incidence and Mortality of Gastric Cancer in Immigrant Populations from High to Low Regions of Incidence: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 347–359.e5. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, C. Helicobacter Pylori: The African Enigma. Gut 1992, 33, 429–431. [Google Scholar] [CrossRef]
- Smith, S.; Fowora, M.; Pellicano, R. Infections with Helicobacter Pylori and Challenges Encountered in Africa. World J. Gastroenterol. 2019, 25, 3183–3195. [Google Scholar] [CrossRef]
- Smith, S.I.; Ajayi, A.; Jolaiya, T.; Onyekwere, C.; Setshedi, M.; Schulz, C.; Otegbayo, J.A.; Ndip, R.; Dieye, Y.; Alboraie, M.; et al. Helicobacter Pylori Infection in Africa: Update of the Current Situation and Challenges. Dig. Dis. 2022, 40, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.J.; Meijer, G.A. Helicobacter Pylori Gastritis in Africa. Eur. J. Gastroenterol. Hepatol. 2000, 12, 601–603. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jaka, H.; Smith, S.I. Forty Years of Helicobacter Pylori: The African Perspective. Dig. Dis. 2023, 42, 161–165. [Google Scholar] [CrossRef]
- Graham, D.Y.; Lu, H.; Yamaoka, Y. African, Asian or Indian Enigma, the East Asian Helicobacter Pylori: Facts or Medical Myths. J. Dig. Dis. 2009, 10, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G. The African Enigma: The Parasite’s Perspective. Gut 2001, 49, 156–157. [Google Scholar] [CrossRef][Green Version]
- Ghoshal, U.C.; Chaturvedi, R.; Correa, P. The Enigma of Helicobacter Pylori Infection and Gastric Cancer. Indian J. Gastroenterol. 2010, 29, 95–100. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, D.; Jimenez, L.; Mansour, M.H.; Horton, S.; Wong, W.W.L. A Systematic Review of Cost-Effectiveness Studies on Gastric Cancer Screening. Cancers 2024, 16, 2353. https://doi.org/10.3390/cancers16132353
Lewis D, Jimenez L, Mansour MH, Horton S, Wong WWL. A Systematic Review of Cost-Effectiveness Studies on Gastric Cancer Screening. Cancers. 2024; 16(13):2353. https://doi.org/10.3390/cancers16132353
Chicago/Turabian StyleLewis, Diedron, Laura Jimenez, Manel Haj Mansour, Susan Horton, and William W. L. Wong. 2024. "A Systematic Review of Cost-Effectiveness Studies on Gastric Cancer Screening" Cancers 16, no. 13: 2353. https://doi.org/10.3390/cancers16132353
APA StyleLewis, D., Jimenez, L., Mansour, M. H., Horton, S., & Wong, W. W. L. (2024). A Systematic Review of Cost-Effectiveness Studies on Gastric Cancer Screening. Cancers, 16(13), 2353. https://doi.org/10.3390/cancers16132353





