Eosinophilic Cells in Ovarian Borderline Serous Tumors as a Predictor of BRAF Mutation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
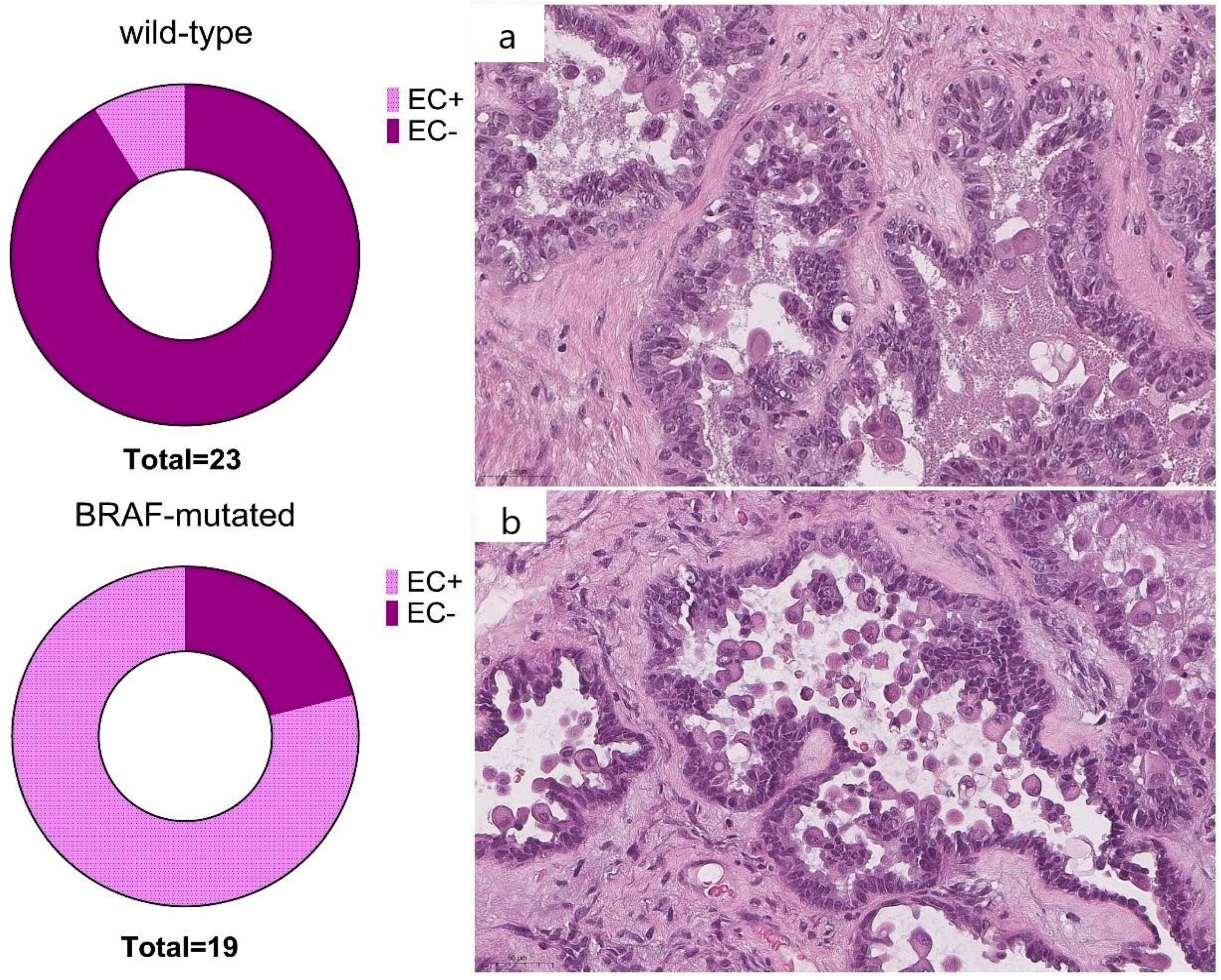
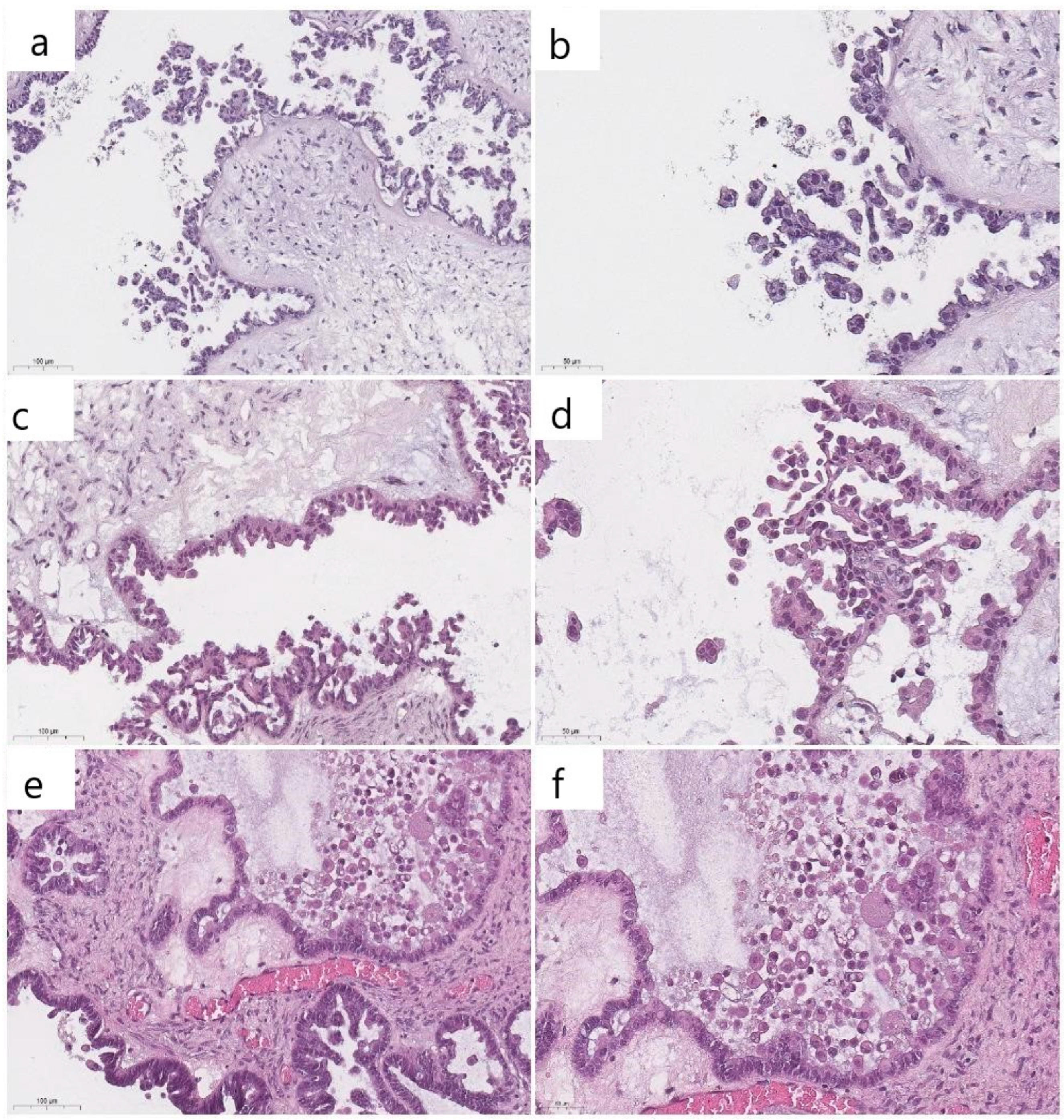
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vang, R.; Davidson, B.; Kong, C.S.; Longacre, T.A.; Malpica, A. WHO Classification of Female Genital Tumors; International Agency for Research on Cancer: Lyon, France, 2021; pp. 38–42. [Google Scholar]
- Schweizer, L.; Krishnan, R.; Shimizu, A.; Metousis, A.; Kenny, H.; Mendoza, R.; Nordmann, T.M.; Rauch, S.; Kelliher, L.; Heide, J.; et al. Spatial proteo-transcriptomic profiling reveals the molecular landscape of borderline ovarian tumors and their invasive progression. medRxiv 2023. [Google Scholar] [CrossRef]
- Longacre, T.A.; McKenney, J.K.; Tazelaar, H.D.; Kempson, R.L.; Hendrickson, M.R. Ovarian serous tumors of low malignant potential (borderline tumors): Outcome-based study of 276 patients with long-term (> or =5-year) follow-up. Am. J. Surg. Pathol. 2005, 29, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Chui, M.H.; Murali, R.; Soslow, R.A.; Matrai, C.; Xing, D.; Vang, R. Interobserver Reproducibility in Assessing Eosinophilic Cells in Ovarian Serous Borderline Tumors to Predict BRAF Mutational Status. Int. J. Gynecol. Pathol. 2023, 42, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Chui, M.H.; Kjaer, S.K.; Frederiksen, K.; Hannibal, C.G.; Wang, T.L.; Vang, R.; Shih, I.M. BRAFV600E-mutated ovarian serous borderline tumors are at relatively low risk for progression to serous carcinoma. Oncotarget 2019, 10, 6870–6878. [Google Scholar] [CrossRef]
- McHenry, A.; Rottmann, D.A.; Buza, N.; Hui, P. KRAS mutation in primary ovarian serous borderline tumors correlates with tumor recurrence. Virchows Arch. 2023, 483, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Turashvili, G.; Grisham, R.N.; Chiang, S.; DeLair, D.F.; Park, K.J.; Soslow, R.A.; Murali, R. BRAFV600E mutations and immunohistochemical expression of VE1 protein in low-grade serous neoplasms of the ovary. Histopathology 2018, 73, 438–443. [Google Scholar] [CrossRef]
- Aird, K.M.; Zhang, R. Metabolic alterations accompanying oncogene-induced senescence. Mol. Cell Oncol. 2014, 1, e963481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wajapeyee, N.; Serra, R.W.; Zhu, X.; Mahalingam, M.; Green, M.R. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 2008, 132, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Zeppernick, F.; Ardighieri, L.; Hannibal, C.G.; Vang, R.; Junge, J.; Kjaer, S.K.; Zhang, R.; Kurman, R.J.; Shih, I.M. BRAF mutation is associated with a specific cell type with features suggestive of senescence in ovarian serous borderline (atypical proliferative) tumors. Am. J. Surg. Pathol. 2014, 38, 1603–1611. [Google Scholar] [CrossRef]
- Malpica, A.; Wong, K.K. The molecular pathology of ovarian serous borderline tumors. Ann. Oncol. 2016, 27 (Suppl. S1), i16–i19. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, J.; Zhou, J.; Liu, H.; Xu, C. Olaparib induced senescence under P16 or P53 dependent manner in ovarian cancer. J. Gynecol. Oncol. 2019, 30, e26. [Google Scholar] [CrossRef] [PubMed]
- Günakan, E.; Tohma, Y.A.; Karakaş, L.A.; Akilli, H.; Haberal, A.N.; Ayhan, A. Prognostic impact of p16 and p53 gene expressions in stage 1a epithelial ovarian cancer. Obstet. Gynecol. Sci. 2020, 63, 464–469. [Google Scholar] [CrossRef]
- Gopas, J.; Stern, E.; Zurgil, U.; Ozer, J.; Ben-Ari, A.; Shubinsky, G.; Braiman, A.; Sinay, R.; Ezratty, J.; Dronov, V.; et al. Reed-Sternberg cells in Hodgkin’s lymphoma present features of cellular senescence. Cell Death Dis. 2016, 7, e2457. [Google Scholar] [CrossRef] [PubMed]
- Prieto, L.I.; Sturmlechner, I.; Graves, S.I.; Zhang, C.; Goplen, N.P.; Yi, E.S.; Sun, J.; Li, H.; Baker, D.J. Senescent alveolar macrophages promote early-stage lung tumorigenesis. Cancer Cell. 2023, 41, 1261–1275.e6. [Google Scholar] [CrossRef] [PubMed]
- Preusser, M.; Capper, D.; Berghoff, A.S.; Horvat, R.; Wrba, F.; Schindl, M.; Schoppmann, S.F.; von Deimling, A.; Birner, P. Expression of BRAF V600E mutant protein in epithelial ovarian tumors. Appl. Immunohistochem. Mol. Morphol. 2013, 21, 159–164. [Google Scholar] [CrossRef]
- Ho, C.-L.; Kurman, R.J.; Dehari, R.; Wang, T.-L.; Shih, I.-M. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res. 2004, 64, 6915–6918. [Google Scholar] [CrossRef] [PubMed]
- Vang, R.; Shih, I.M.; Kurman, R.J. Ovarian low-grade and high-grade serous carcinoma: Pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv. Anat. Pathol. 2009, 16, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Tone, A.A.; McConechy, M.K.; Yang, W.; Ding, J.; Yip, S.; Kong, E.; Wong, K.K.; Gershenson, D.M.; Mackay, H.; Shah, S.; et al. Intratumoral heterogeneity in a minority of ovarian low-grade serous carcinomas. BMC Cancer 2014, 14, 982. [Google Scholar] [CrossRef]
- Tsang, Y.T.; Deavers, M.T.; Sun, C.C.; Kwan, S.Y.; Kuo, E.; Malpica, A.; Mok, S.C.; Gershenson, D.M.; Wong, K.K. KRAS (but not BRAF) mutations in ovarian serous borderline tumour are associated with recurrent low-grade serous carcinoma. J. Pathol. 2013, 231, 449–456. [Google Scholar] [CrossRef]
- Grisham, R.N.; Slomovitz, B.M.; Andrews, N.; Banerjee, S.; Brown, J.; Carey, M.S.; Chui, H.; Coleman, R.L.; Fader, A.N.; Gaillard, S.; et al. Low-grade serous ovarian cancer: Expert consensus report on the state of the science. Int. J. Gynecol. Cancer 2023, 33, 1331–1344. [Google Scholar] [CrossRef]
- Xing, D.; SuryoRahmanto, Y.; Zeppernick, F.; Hannibal, C.G.; Kjaer, S.K.; Vang, R.; Shih, I.M.; Wang, T.L. Mutation of NRAS is a rare genetic event in ovarian low-grade serous carcinoma. Hum. Pathol. 2017, 68, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Wong, S.; Buza, N.; Hui, P. KRAS mutation of extraovarian implants of serous borderline tumor: Prognostic indicator for adverse clinical outcome. Mod. Pathol. 2018, 31, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Piton, N.; Borrini, F.; Bolognese, A.; Lamy, A.; Sabourin, J.C. KRAS and BRAF Mutation Detection: Is Immunohistochemistry a Possible Alternative to Molecular Biology in Colorectal Cancer? Gastroenterol. Res. Pract. 2015, 2015, 753903. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Sasaki, H.; Takeshita, S.; Nishikawa, R.; Nishikawa, H.; Arakawa, A.; Yamashita, Y.; Takahashi, S.; Sugiura-Ogasawara, M. Usefulness of immunohistochemistry for the detection of the BRAF V600E mutation in ovarian serous borderline tumors. Oncol. Rep. 2014, 32, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Bösmüller, H.; Fischer, A.; Pham, D.L.; Fehm, T.; Capper, D.; von Deimling, A.; Bonzheim, I.; Staebler, A.; Fend, F. Detection of the BRAF V600E mutation in serous ovarian tumors: A comparative analysis of immunohistochemistry with a mutation-specific monoclonal antibody and allele-specific PCR. Hum. Pathol. 2013, 44, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Grisham, R.N.; Iyer, G.; Garg, K.; Delair, D.; Hyman, D.M.; Zhou, Q.; Iasonos, A.; Berger, M.F.; Dao, F.; Spriggs, D.R.; et al. BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer 2013, 119, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Moujaber, T.; Etemadmoghadam, D.; Kennedy, C.J.; Chiew, Y.E.; Balleine, R.L.; Saunders, C.; Wain, G.V.; Gao, B.; Hogg, R.; Srirangan, S.; et al. BRAF Mutations in Low-Grade Serous Ovarian Cancer and Response to BRAF Inhibition. JCO Precis. Oncol. 2018, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.M.; Anglesio, M.S.; Ryland, G.L.; Sharma, R.; Chiew, Y.E.; Rowley, S.M.; Doyle, M.A.; Li, J.; Gilks, C.B.; Moss, P.; et al. Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget 2015, 6, 37663–37677. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.-H.; Savarese, T.M. Codeletion of the genes for p16INK4, methylthioadenosine phosphorylase, interferon- alpha1, interferon-beta1, and other 9p21 markers in human malignant cell lines. Cancer Genet. Cytogenet. 1996, 86, 22–28. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Yuen, M.L.; Rath, E.M.; Johnson, B.; Zhuang, L.; Yu, T.K.; Aleksova, V.; Linton, A.; Kao, S.; Clarke, C.J.; et al. CDKN2A and MTAP Are Useful Biomarkers Detectable by Droplet Digital PCR in Malignant Pleural Mesothelioma: A Potential Alternative Method in Diagnosis Compared to Fluorescence In Situ Hybridisation. Front. Oncol. 2020, 10, 579327. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Yin, F.; Deng, X.; Feng, L.; Wei, L.; Huang, Y.; Liang, Z.; Huang, Y.; Wei; Zhang; et al. MTAP deficiency is associated with an unfavourable prognosis and platinum resistance in ovarian cancer. Int. J. Clin. Exp. Pathol. 2017, 10, 1122–1130. [Google Scholar]
- Nilforoushan, N.; Moatamed, N.A. Evaluation of MTAP immunohistochemistry loss of expression in ovarian serous borderline tumors as a potential marker for prognosis and progression. Ann. Diagn. Pathol. 2020, 48, 151582. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Luo, F.; Lu, J.J.; Baltayan, A.; Press, M.F.; Zhang, Z.F.; Pike, M.C. Reduction of BRCA1 expression in sporadic ovarian cancer. Gynecol. Oncol. 2000, 76, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.M.; Kurman, R.J. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Giurgea, L.N.; Ungureanu, C.; Mihailovici, M.S. The immunohistochemical expression of p53 and Ki67 in ovarian epithelial borderline tumors. Correlation with clinicopathological factors. Rom. J. Morphol. Embryol. 2012, 53, 967–973. [Google Scholar]
- Wong, K.K.; Lu, K.H.; Malpica, A.; Bodurka, D.C.; Shvartsman, H.S.; Schmandt, R.E.; Thornton, A.D.; Deavers, M.T.; Silva, E.G.; Gershenson, D.M. Significantly greater expression of ER, PR, and ECAD in advanced-stage low-grade ovarian serous carcinoma as revealed by immunohistochemical analysis. Int. J. Gynecol. Pathol. 2007, 26, 404–409. [Google Scholar] [CrossRef]
- Escobar, J.; Klimowicz, A.C.; Dean, M.; Chu, P.; Nation, J.G.; Nelson, G.S.; Ghatage, P.; Kalloger, S.E.; Köbel, M. Quantification of ER/PR expression in ovarian low-grade serous carcinoma. Gynecol. Oncol. 2013, 128, 371–376. [Google Scholar] [CrossRef]
- Llaurado Fernandez, M.; Dawson, A.; Kim, H.; Lam, N.; Russell, H.; Bruce, M.; Bittner, M.; Hoenisch, J.; Scott, S.A.; Talhouk, A.; et al. Hormone receptor expression and outcomes in low-grade serous ovarian carcinoma. Gynecol. Oncol. 2020, 157, 12–20. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. A systematic review and meta-analysis of hormone receptor expression in low-grade serous ovarian carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 172–178. [Google Scholar] [CrossRef]
- Arias-Pulido, H.; Smith, H.O.; Joste, N.E.; Bocklage, T.; Qualls, C.R.; Chavez, A.; Prossnitz, E.R.; Verschraegen, C.F. Estrogen and progesterone receptor status and outcome in epithelial ovarian cancers and low malignant potential tumors. Gynecol. Oncol. 2009, 114, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Sallum, L.F.; Sarian, L.O.; Lucci De Angelo Andrade, L.; Vassallo, J.; Soares, F.A.; Pinto, G.A.; Ferreira, P.A.; Derchain, S. Survival of women with ovarian carcinomas and borderline tumors is not affected by estrogen and progesterone receptor status. J. Gynecol. Oncol. 2013, 24, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Němejcová, K.; Šafanda, A.; Bártů, M.K.; Michálková, R.; Drozenová, J.; Fabian, P.; Hausnerová, J.; Laco, J.; Matěj, R.; Méhes, G.; et al. A comprehensive immunohistochemical analysis of 26 markers in 250 cases of serous ovarian tumors. Diagn. Pathol. 2023, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, P.; Patro, M.K.; Dash, A.; Pradhan, S.P.; Senapati, S.R.; Mohanty, S.R. Evaluation of estrogen receptor, progesterone receptor, and Ki 67 immunoexpression in epithelial ovarian tumors with histopathological correlation. MGM J. Med. Sci. 2023, 10, 602–609. [Google Scholar] [CrossRef]



| Primers | Primer Sequence | Annealing Temperature (°C) |
|---|---|---|
| Forward primer (F) | 5′-AATGCTTGCTCTGATAGGAA | 58 |
| Reverse primer (R) | 5′-TCAGTGGAAAATAGCCTCAAT | 58 |
| Feature | Wild-Type | (n) | Mutant-Type | (n) | Total (n) | p-Value | |
|---|---|---|---|---|---|---|---|
| Age (median, Q1–Q3) | 43.9 (34–57) | 23 | 33.6 (23–38) | 19 | 42 | 0.049 | |
| FIGO stage I | IA | 7 (58.3%) | 12 | 15 (100%) | 15 | 27 | 0.128 |
| IB | 5 (41.7%) | 0 | |||||
| IC | 0 | 0 | |||||
| FIGO stage > I | IIA | 1 (16.7%) | 6 | 1 (50%) | 2 | 8 | |
| IIB | 2 (33.3%) | 0 | |||||
| IIIA | 0 | 0 | |||||
| IIIB | 3 (50%) | 1 (50%) | |||||
| IIIC | 0 | 0 | |||||
| IV | 0 | 0 | |||||
| Short-term follow-up | 1 (14.3%) | 7 | 0 | 7 | 14 | 0.299 | |
| Mid-term follow-up | 1 (9%) | 11 | 1 (12.5%) | 9 | 19 | 0.811 | |
| Pathologist 1 | Pathologist 2 | Pathologist 3 | |
|---|---|---|---|
| Pathologist 1 | 0.793 | 0.652 | |
| Pathologist 2 | 0.652 | ||
| Results of genetic test | 0.608 | 0.608 | 0.759 |
| Case No. | BRAF Mutation | Pathologist 1 | Pathologist 2 | Pathologist 3 | % of Agreement |
|---|---|---|---|---|---|
| 1 | Wild-type | 0 | 0 | 0 | 100 |
| 2 | Wild-type | 0 | 0 | 0 | 100 |
| 3 | p.V600E (c.1799T > A) | 1 | 1 | 1 | 100 |
| 4 | p.V600E (c.1799T > A) | 0 | 0 | 0 | 100 |
| 5 | p.V600E (c.1799T > A) | 1 | 1 | 1 | 100 |
| 6 | p.V600E (c.1799T > A) | 0 | 1 | 1 | 66.7 |
| 7 | Wild-type | 0 | 0 | 0 | 100 |
| 8 | p.V600E (c.1799T > A) | 1 | 1 | 1 | 100 |
| 9 | Wild-type | 0 | 0 | 0 | 100 |
| 10 | Wild-type | 0 | 0 | 0 | 100 |
| 11 | p.V600E (c.1799T > A) | 1 | 1 | 1 | 100 |
| 12 | Wild-type | 0 | 0 | 0 | 100 |
| 13 | Wild-type | 1 | 1 | 0 | 66.7 |
| 14 | Wild-type | 0 | 0 | 0 | 100 |
| 15 | Wild-type | 0 | 0 | 0 | 100 |
| 16 | p.V600E (c.1799T > A) | 0 | 1 | 1 | 100 |
| 17 | Wild-type | 0 | 0 | 0 | 100 |
| 18 | Wild-type | 0 | 0 | 0 | 100 |
| 19 | p.V600E (c.1799T > A) | 1 | 1 | 1 | 100 |
| 20 | Wild-type | 0 | 0 | 0 | 100 |
| 21 | p.V600E (c.1799T > A) | 0 | 0 | 0 | 100 |
| 22 | p.V600E (c.1799T > A) | 1 | 1 | 1 | 100 |
| 23 | p.V600E (c.1799T > A) | 1 | 1 | 1 | 100 |
| 24 | p.V600E (c.1799T > A) | 1 | 1 | 1 | 100 |
| 25 | Wild-type | 0 | 0 | 0 | 100 |
| 26 | Wild-type | 0 | 0 | 1 | 33.3 |
| 27 | Wild-type | 0 | 0 | 0 | 100 |
| 28 | Wild-type | 0 | 0 | 0 | 100 |
| 29 | Wild-type | 0 | 0 | 0 | 100 |
| 30 | p.V600E (c.1799T > A) | 1 | 1 | 0 | 66.7 |
| 31 | p.V600E (c.1799T > A) | 0 | 0 | 1 | 33.3 |
| 32 | p.V600E (c.1799T > A) | 1 | 1 | 1 | 100 |
| 33 | Wild-type | 1 | 1 | 1 | 100 |
| 34 | p.V600E (c.1799T > A) | 1 | 0 | 1 | 66.7 |
| 35 | Wild-type | 0 | 0 | 0 | 100 |
| 36 | Wild-type | 0 | 0 | 0 | 100 |
| 37 | p.V600E (c.1799T > A) | 1 | 0 | 1 | 66.7 |
| 38 | Wild-type | 0 | 0 | 0 | 100 |
| 39 | p.V600E (c.1799T > A) | 0 | 0 | 1 | 33.3 |
| 40 | p.V600E (c.1799T > A) | 1 | 1 | 1 | 100 |
| 41 | Wild-type | 0 | 0 | 0 | 100 |
| 42 | Wild-type | 0 | 0 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badlaeva, A.; Tregubova, A.; Palicelli, A.; Asaturova, A. Eosinophilic Cells in Ovarian Borderline Serous Tumors as a Predictor of BRAF Mutation. Cancers 2024, 16, 2322. https://doi.org/10.3390/cancers16132322
Badlaeva A, Tregubova A, Palicelli A, Asaturova A. Eosinophilic Cells in Ovarian Borderline Serous Tumors as a Predictor of BRAF Mutation. Cancers. 2024; 16(13):2322. https://doi.org/10.3390/cancers16132322
Chicago/Turabian StyleBadlaeva, Alina, Anna Tregubova, Andrea Palicelli, and Aleksandra Asaturova. 2024. "Eosinophilic Cells in Ovarian Borderline Serous Tumors as a Predictor of BRAF Mutation" Cancers 16, no. 13: 2322. https://doi.org/10.3390/cancers16132322
APA StyleBadlaeva, A., Tregubova, A., Palicelli, A., & Asaturova, A. (2024). Eosinophilic Cells in Ovarian Borderline Serous Tumors as a Predictor of BRAF Mutation. Cancers, 16(13), 2322. https://doi.org/10.3390/cancers16132322







