Cost-Effectiveness Analysis of Axicabtagene Ciloleucel vs. Standard of Care in Second-Line Treatment for Relapsed/Refractory Large B-Cell Lymphoma in Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
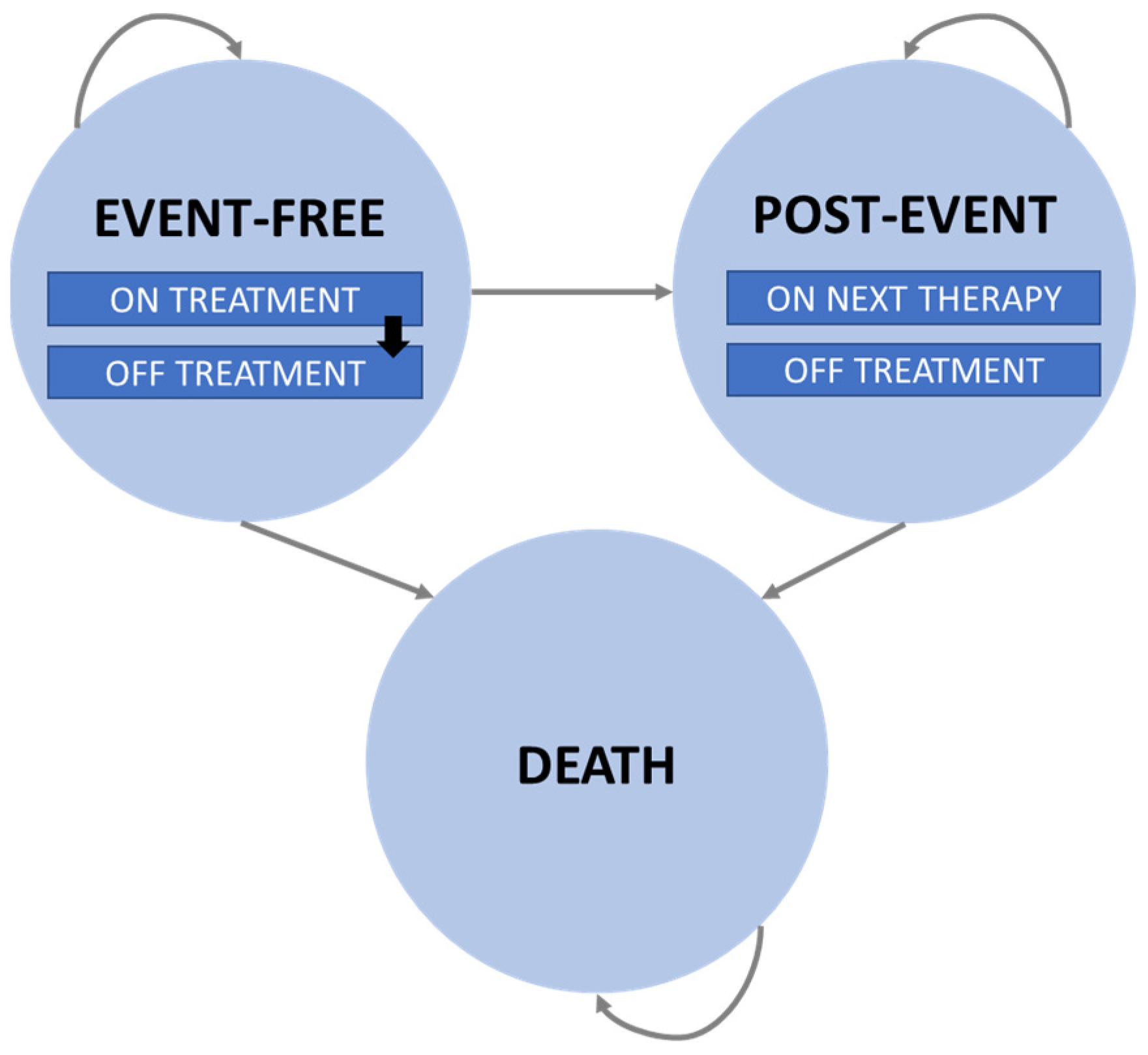
2.1. Model Structure
2.2. Population
2.3. Treatment Strategies
2.4. Clinical Data
2.5. Statistical Methods
2.6. Adverse Events
2.7. Utilities
2.8. Resource Use and Costs
2.9. Sensitivity Analysis
3. Results
3.1. Base Case
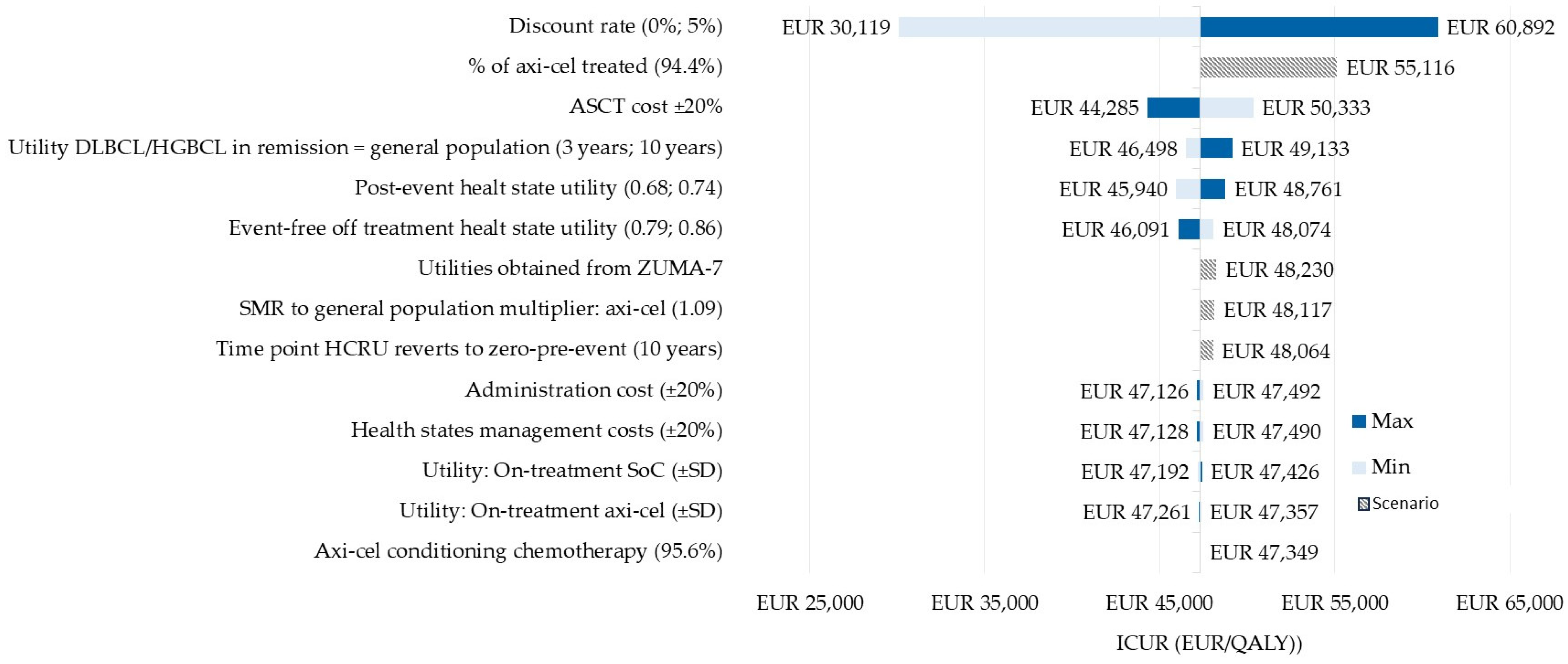
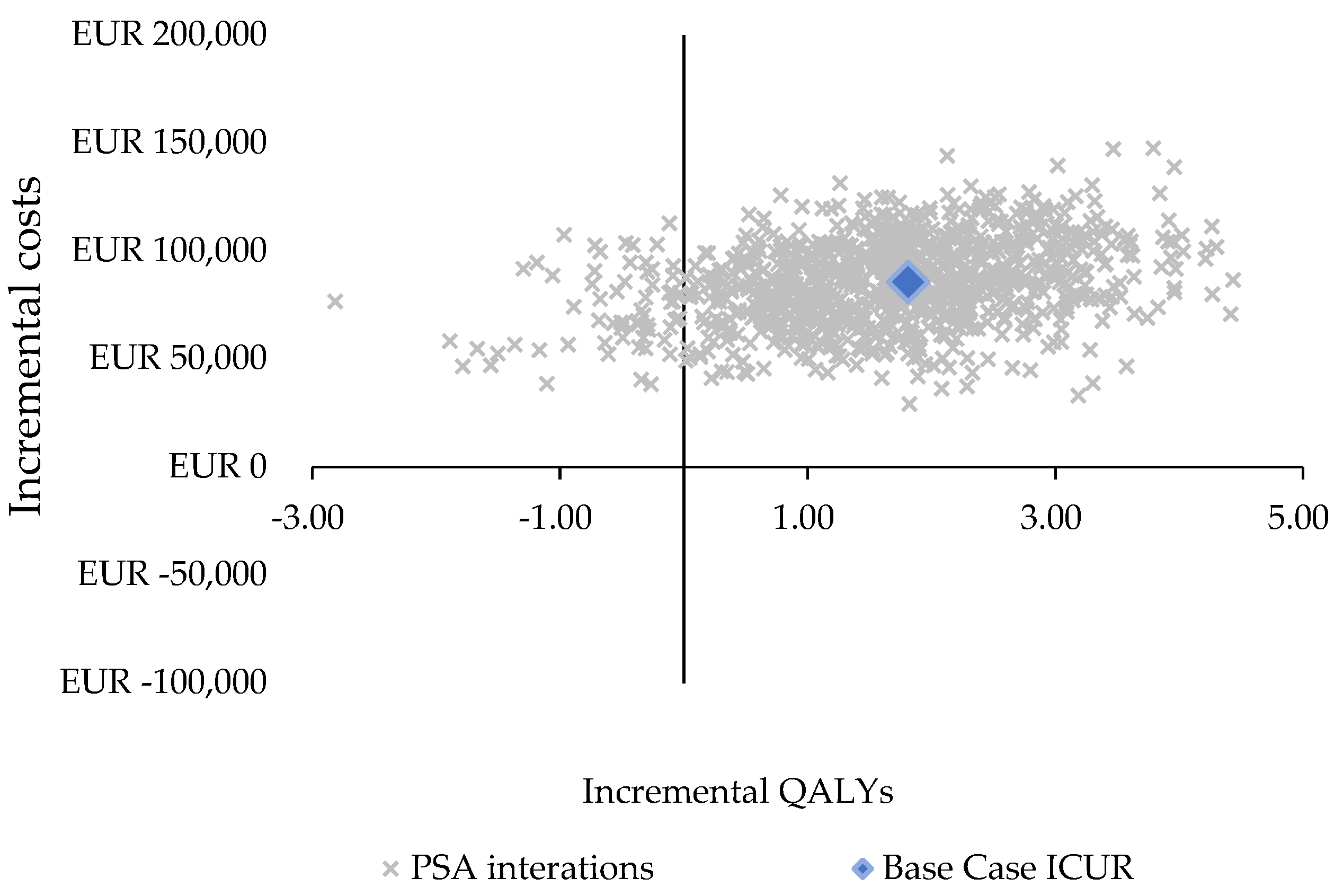
3.2. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, A.; Howell, D.; Patmore, R.; Jack, A.; Roman, E. Incidence of haematological malignancy by sub-type: A report from the Haematological Malignancy Research Network. Br. J. Cancer 2011, 105, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Sociedad Española de Oncología Médica. Las Cifras del Cáncer en España. 2023. Available online: https://seom.org/images/Las_cifras_del_Cancer_en_Espana_2023.pdf (accessed on 6 June 2023).
- Bastos-Oreiro, M.; Muntañola, A.; Panizo, C.; Gonzalez-Barca, E.; de Villambrosia, S.G.; Córdoba, R.; López, J.L.B.; González-Sierra, P.; Terol, M.J.; Gutierrez, A.; et al. RELINF: Prospective epidemiological registry of lymphoid neoplasms in Spain. A project from the GELTAMO group. Ann. Hematol. 2020, 99, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Tilly, H.; da Silva, M.G.; Vitolo, U.; Jack, A.; Meignan, M.; Lopez-Guillermo, A.; Walewski, J.; André, M.; Johnson, P.W.; Pfreundschuh, M.; et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v116–v125. [Google Scholar] [CrossRef] [PubMed]
- Gisselbrecht, C.; Glass, B.; Mounier, N.; Gill, D.S.; Linch, D.C.; Trneny, M.; Bosly, A.; Ketterer, N.; Shpilberg, O.; Hagberg, H.; et al. Salvage Regimens With Autologous Transplantation for Relapsed Large B-Cell Lymphoma in the Rituximab Era. J. Clin. Oncol. 2010, 28, 4184–4190. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S. Anti-CD19 CAR T-Cell Therapy for B-Cell Non-Hodgkin Lymphoma. Transfus. Med. Rev. 2019, 34, 29–33. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Medicines: Yescarta. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/yescarta (accessed on 9 June 2023).
- European Medicines Agency. Summary of Product Characteristics: Yescarta. Available online: https://www.ema.europa.eu/en/documents/product-information/yescarta-epar-product-information_en.pdf (accessed on 9 June 2023).
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Perales, M.-A.; Kuruvilla, J.; Snider, J.T.; Vadgama, S.; Blissett, R.; El-Moustaid, F.; Smith, N.J.; Patel, A.R.; Johnston, P.B. The Cost-Effectiveness of Axicabtagene Ciloleucel as Second-Line Therapy in Patients with Large B-Cell Lymphoma in the United States: An Economic Evaluation of the ZUMA-7 Trial. Transplant. Cell Ther. 2022, 28, 750.e1–750.e6. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.A.; Sullivan, S.D.; Lin, V.W.; Bansal, A.; Purdum, A.G.; Navale, L.; Cheng, P.; Ramsey, S.D. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J. Med. Econ. 2018, 21, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Bastos-Oreiro, M.; Heras, A.d.L.; Presa, M.; Casado, M.A.; Pardo, C.; Martín-Escudero, V.; Sureda, A. Cost-Effectiveness Analysis of Axicabtagene Ciloleucel vs. Tisagenlecleucel for the Management of Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Spain. Cancers 2022, 14, 538. [Google Scholar] [CrossRef] [PubMed]
- NICE. Axicabtagene Ciloleucel for Treating Diffuse Large B-Cell Lymphoma and Primary Mediastinal Large B-Cell Lymphoma after 2 or More Systemic Therapies. Technology Appraisal Guidance [TA872]. Available online: https://www.nice.org.uk/guidance/ta872 (accessed on 15 July 2023).
- NICE. Axicabtagene Ciloleucel for Treating Relapsed or Refractory Diffuse Large B-Cell Lymphoma after First-Line Chemoimmunotherapy. Technology Appraisal Guidance [TA895]. Available online: https://www.nice.org.uk/guidance/ta895 (accessed on 15 July 2023).
- Felizzi, F.; Paracha, N.; Pöhlmann, J.; Ray, J. Mixture Cure Models in Oncology: A Tutorial and Practical Guidance. PharmacoEconomics Open 2021, 5, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, F.A.; Beck, J.R. Markov models in medical decision making: A practical guide. Med. Decis. Mak. 1993, 13, 322–338. [Google Scholar] [CrossRef] [PubMed]
- López Bastida, J.; Oliva, J.; Antoñanzas, F.; García-Altés, A.; Gisbert, R.; Mar, J.; Puig-Junoy, J. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias [Guide proposal for economic evaluation applied to health technologies]. Gac Sanit. 2010, 24, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Marín, R.; Fraga, M.D.; López-Briz, E.; Puigventós, F. Guía de Evaluación Económica e Impacto Presupuestario en los Informes de Evaluación de Medicamentos. Guía Práctica Asociada al Programa MADRE v 4.0 [Economic Evaluation and Budgetary Impact Guide in Drug Evaluation Reports. Practical Guide Associated with the MADRE v 4.0 Program] [Homepage on the Internet]. Madrid: Sociedad Española de Farmacia Hospitalaria. 2017. Available online: http://gruposdetrabajo.sefh.es/genesis (accessed on 23 February 2021).
- Howlader, N.; Mariotto, A.B.; Besson, C.; Suneja, G.; Robien, K.; Younes, N.; Engels, E.A. Cancer-specific mortality, cure fraction, and noncancer causes of death among diffuse large B-cell lymphoma patients in the immunochemotherapy era. Cancer 2017, 123, 3326–3334. [Google Scholar] [CrossRef] [PubMed]
- INE. Population Mortality Tables for SPAIN by Year, Sex, Age and Functions. Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=27153 (accessed on 15 July 2023).
- NICE. Tisagenlecleucel for Treating Relapsed or Refractory Diffuse Large B-Cell Lymphoma after 2 or More Systemic Therapies [TA567]. Available online: https://www.nice.org.uk/guidance/ta567 (accessed on 15 July 2023).
- Spanish National Health Survey 2011–2012. Health-Related Quality of Life in Adults: EQ-5D. Monographic Reports Series nº3. Available online: https://www.sanidad.gob.es/fr/estadEstudios/estadisticas/encuestaNacional/encuestaNac2011/informesMonograficos/CVRS_adultos_EQ_5D_5L.pdf (accessed on 15 July 2023).
- Botplus Web 2.0. General Council of Pharmacists Official College. Available online: www.portalfarma.com (accessed on 20 October 2022).
- Real Decreto-Ley 8/2010, de 20 de Mayo, Por el Que se Adoptan Medidas Extraordinarias Para la Reducción del Déficit Público [Royal Decree-Law]. Available online: https://www.sanidad.gob.es/profesionales/farmacia/pdf/Deducciones_Octubre_22.pdf (accessed on 20 October 2022).
- Oblikue Consulting. Base de Datos de Costes Sanitarios eSalud. Oblikue Consulting. 2020. Available online: http://www.oblikue.com/bddcostes/ (accessed on 10 October 2022).
- Ortega, M. Análisis de Costes Sanitarios y no Sanitarios Asociados al Tratamiento Oncológico en Pacientes con Neoplasia Hematológica Desde una Perspectiva Económica. Granada. 2015. Available online: http://digibug.ugr.es/handle/10481/40272 (accessed on 10 October 2022).
- Instituto Nacional de Estadística. Cálculo de Variaciones Del Índice de Precios de Consumo; Instituto Nacional de Estadística: Madrid, Spain, 2020; Available online: https://www.ine.es/calcula/calcula.do (accessed on 15 October 2022).
- Maurer, M.J.; Ghesquières, H.; Jais, J.-P.; Witzig, T.E.; Haioun, C.; Thompson, C.A.; Delarue, R.; Micallef, I.N.; Peyrade, F.; Macon, W.R.; et al. Event-Free Survival at 24 Months Is a Robust End Point for Disease-Related Outcome in Diffuse Large B-Cell Lymphoma Treated With Immunochemotherapy. J. Clin. Oncol. 2014, 32, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Sacristán, J.A.; Oliva, J.; Campillo-Artero, C.; Puig-Junoy, J.; Pinto-Prades, J.L.; Dilla, T.; Rubio-Terrés, C.; Ortún, V. ¿Qué es una intervención sanitaria eficiente en España en 2020? [What is an efficient health intervention in Spain in 2020?]. Gac Sanit. 2020, 34, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad. Informe de Posicionamiento Terapéutico de Nivolumab (Opdivo®) en Carcinoma de Células Escamosas de Esófago Avanzado, Recurrente o Metastásico Irresecable Tras una Quimioterapia Previa de Combinación Basada en Fluoropirimidina y Platino; REvalMed SNS: Madrid, Spain, 2022; Available online: https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/2021/IPT_52-2021-Opdivo.pdf (accessed on 16 February 2024).
- Loftager, A.S.L.; Danø, A.; Eklund, O.; Vadgama, S.; Kanje, V.H.; Munk, E. Axicabtagene ciloleucel compared to standard of care in Swedish patients with large B-cell lymphoma: A cost-effectiveness analysis of the ZUMA-7 trial. J. Med. Econ. 2023, 26, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Westin, J.R.; Oluwole, O.O.; Kersten, M.J.; Miklos, D.B.; Perales, M.-A.; Ghobadi, A.; Rapoport, A.P.; Sureda, A.; Jacobson, C.A.; Farooq, U.; et al. Survival with Axicabtagene Ciloleucel in Large B-Cell Lymphoma. N. Engl. J. Med. 2023, 389, 148–157. [Google Scholar] [CrossRef] [PubMed]

| Health States | Utility Value | Reference |
|---|---|---|
| Event-free: on treatment with axi-cel (one cycle) | 0.74 | Roth et al., [22] |
| Event-free: on treatment with SoC (three cycles) | 0.67 | Roth et al., [22] |
| Event-free: off treatment | 0.82 | Roth et al., [22] |
| Post-event | 0.71 | TA567 [23] |
| Axi-Cel-Related Costs | |
| Acquisition cost | EUR 313,920 * |
| Leukapheresis | EUR 1025 |
| Bridging therapy | EUR 2599 |
| Conditioning chemotherapy | EUR 1249 |
| Administration and monitoring | EUR 9794 |
| SoC-related costs | |
| Salvage immunochemotherapy | EUR 4063 |
| Administration | EUR 2443 |
| Stem cell harvest | EUR 1025 |
| High-dose chemotherapy | EUR 9205 |
| ASCT (procedure and annual monitoring) | EUR 79,358 |
| Subsequent treatment total cost | |
| After axi-cel | EUR 32,754 |
| After SoC | EUR 233,412 |
| Health states management costs | |
| Event-free with axi-cel (EUR/month) | EUR 305 |
| Event-free with SoC (EUR/month) | EUR 527 |
| Post-event with axi-cel (EUR/month) | EUR 537 |
| Post-event with SoC (EUR/month) | EUR 352 |
| Adverse event grade ≥ 3 management costs | |
| Cytokine release syndrome (per-event cost) | EUR 2077 |
| Neurological events (per-event cost) | EUR 24 |
| End-of-life care costs (one-off cost) | EUR 6267 |
| Axi-cel | SoC | Incremental (axi-cel vs. SoC) | |
|---|---|---|---|
| Total LYG | 10.00 | 8.28 | 1.72 |
| LYG in event-free state | 7.10 | 3.12 | 3.98 |
| LYG in post-event state | 2.90 | 5.15 | −2.25 |
| Total QALY | 7.85 | 6.04 | 1.81 |
| QALY in event-free state | 5.90 | 2.57 | 3.33 |
| QALY in post-event state | 1.95 | 3.47 | −1.52 |
| Total costs per patient | EUR 343,581 | EUR 257,994 | EUR 85,587 |
| Axi-cel-related costs | EUR 294,326 | EUR 0 | EUR 294,326 |
| SoC-related costs | EUR 0 | EUR 40,889 | EUR –40,889 |
| Subsequent treatment | EUR 18,598 | EUR 184,632 | EUR –166,034 |
| Health state management | EUR 26,112 | EUR 27,748 | EUR −1636 |
| AEs management | EUR 140 | EUR 0 | EUR 140 |
| Palliative care | EUR 4406 | EUR 4726 | EUR −319 |
| ICER (axi-cel vs. SoC) | 49,626 EUR/LYG | ||
| ICUR (axi-cel vs. SoC) | 47,308 EUR/QALY |
| Axi-cel vs. SoC | |
| Incremental QALY | 1.61 |
| Incremental costs | EUR 85,433 |
| ICUR (mean) | 52,953 EUR/QALY |
| ICUR (median, IQR) | 46,740 EUR/QALY (33,454–72,146 EUR/QALY) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín García-Sancho, A.; Presa, M.; Pardo, C.; Martín-Escudero, V.; Oyagüez, I.; Ortiz-Maldonado, V. Cost-Effectiveness Analysis of Axicabtagene Ciloleucel vs. Standard of Care in Second-Line Treatment for Relapsed/Refractory Large B-Cell Lymphoma in Spain. Cancers 2024, 16, 2301. https://doi.org/10.3390/cancers16132301
Martín García-Sancho A, Presa M, Pardo C, Martín-Escudero V, Oyagüez I, Ortiz-Maldonado V. Cost-Effectiveness Analysis of Axicabtagene Ciloleucel vs. Standard of Care in Second-Line Treatment for Relapsed/Refractory Large B-Cell Lymphoma in Spain. Cancers. 2024; 16(13):2301. https://doi.org/10.3390/cancers16132301
Chicago/Turabian StyleMartín García-Sancho, Alejandro, María Presa, Carlos Pardo, Victoria Martín-Escudero, Itziar Oyagüez, and Valentín Ortiz-Maldonado. 2024. "Cost-Effectiveness Analysis of Axicabtagene Ciloleucel vs. Standard of Care in Second-Line Treatment for Relapsed/Refractory Large B-Cell Lymphoma in Spain" Cancers 16, no. 13: 2301. https://doi.org/10.3390/cancers16132301
APA StyleMartín García-Sancho, A., Presa, M., Pardo, C., Martín-Escudero, V., Oyagüez, I., & Ortiz-Maldonado, V. (2024). Cost-Effectiveness Analysis of Axicabtagene Ciloleucel vs. Standard of Care in Second-Line Treatment for Relapsed/Refractory Large B-Cell Lymphoma in Spain. Cancers, 16(13), 2301. https://doi.org/10.3390/cancers16132301






