Gynotoxic Effects of Chemotherapy and Potential Protective Mechanisms
Abstract
Simple Summary
Abstract
1. Introduction
2. Gynotoxic Properties of Anticancer Drugs
2.1. Alkylating Drugs
2.1.1. Non-Human Studies
2.1.2. Human Tissue Studies
2.1.3. Effects on Cancer Patients
2.2. Antimetabolites
2.2.1. Non-Human Studies
2.2.2. Human Tissue Studies
2.2.3. Effects on Cancer Patients
2.3. Topoisomerase Inhibitors
2.3.1. Non-Human Studies
2.3.2. Human Tissue Studies
2.3.3. Effects on Cancer Patients
2.4. Mitosis Inhibitors
2.4.1. Non-Human Studies
2.4.2. Effects on Cancer Patients
3. Potential Ovarian-Protective Mechanisms
3.1. Hormones
3.1.1. Anti-Müllerian Hormone
3.1.2. Ghrelin
3.1.3. Luteinizing Hormone (Lutropin)
3.1.4. Melatonin
3.2. Modulating Factors
3.2.1. Sphingolipids
3.2.2. MicroRNA
3.3. Natural Compounds
3.3.1. Quercetin
3.3.2. Rapamycin
3.3.3. Resveratrol
3.4. Synthetic Compounds
3.4.1. Bortezomib
3.4.2. Dexrazoxane
3.4.3. Gonadoliberin Analogs
3.4.4. Imatinib
3.4.5. Metformin
3.4.6. Tamoxifen
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Monniaux, D.; Cadoret, V.; Clément, F.; Dalbies-Tran, R.; Elis, S.; Fabre, S.; Maillard, V.; Monget, P.; Uzbekova, S. Folliculogenesis. In Encyclopedia of Endocrine Diseases, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 377–398. [Google Scholar] [CrossRef]
- Zhang, T.; He, M.; Zhang, J.; Tong, Y.; Chen, T.; Wang, C.; Pan, W.; Xiao, Z. Mechanisms of primordial follicle activation and new pregnancy opportunity for premature ovarian failure patients. Front. Physiol. 2023, 14, 1113684. [Google Scholar] [CrossRef] [PubMed]
- Kalous, J.; Aleshkina, D.; Anger, M. A role of PI3K/Akt signaling in oocyte maturation and early embryo development. Cells 2023, 12, 1830. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A. Premature ovarian insufficiency: Pathogenesis and management. J. Midlife Health 2015, 6, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.H.B.; Kelsey, T.W. Human ovarian reserve from conception to the menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Sosulski, A.E.; Zhang, L.; Saatcioglu, H.D.; Wang, D.; Nagykery, N.; Sabatini, M.E.; Gao, G.; Donahoe, P.K.; Pépin, D. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc. Natl. Acad. Sci. USA 2017, 114, E1688–E1697. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.; Odle, A.K.; Aykin-Burns, N.; Allen, A.R. Chemotherapy induced oxidative stress in the ovary: Drug-dependent mechanisms and potential interventions. Biol. Reprod. 2023, 108, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.C.; McKenzie, L.J. Cancer treatment-related ovarian dysfunction in women of childbearing potential: Management and fertility preservation options. J. Clin. Oncol. 2023, 41, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Spears, N.; Lopes, F.; Stefansdottir, A.; Rossi, V.; De Felici, M.; Anderson, R.A.; Klinger, F.G. Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update 2019, 25, 673–693. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D. Reproduction post-chemotherapy in young cancer patients. Mol. Cell. Endocrinol. 2000, 169, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Al-Kawlani, B.; Murrieta-Coxca, J.M.; Chaiwangyen, W.; Fröhlich, K.; Fritzsche, A.; Winkler, S.; Markert, U.R.; Morales-Prieto, D.M. Doxorubicin induces cytotoxicity and miR-132 expression in granulosa cells. Reprod. Toxicol. 2020, 96, 95–101. [Google Scholar] [CrossRef]
- Rosendahl, M.; Andersen, C.Y.; la Cour Freiesleben, N.; Juul, A.; Løssl, K.; Andersen, A.N. Dynamics and mechanisms of chemotherapy-induced ovarian follicular depletion in women of fertile age. Fertil. Steril. 2010, 94, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Decanter, C.; Morschhauser, F.; Pigny, P.; Lefebvre, C.; Gallo, C.; Dewailly, D. Anti-Müllerian hormone follow-up in young women treated by chemotherapy for lymphoma: Preliminary results. Reprod. Biomed. Online 2010, 20, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Themmen, A.P.N.; Al-Qahtani, A.A.; Groome, N.P.; Cameron, D.A. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum. Reprod. 2006, 21, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, N.D.; Marsh, E.E. Ovarian reserve testing: A review of the options, their applications, and their limitations. Clin. Obstet. Gynecol. 2019, 62, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Abdelazim, I.A.; Abu-Faza, M.; Svetlana, S.; Karimova, B.; Zhurabekova, G.; Maghrabi, M.M. Methods of evaluation of the ovarian reserve. J. Obstet. Gynecol. Investig. 2018, 1, e62–e66. [Google Scholar] [CrossRef]
- Anders, C.; Marcom, P.K.; Peterson, B.; Gu, L.; Unruhe, S.; Welch, R.; Lyons, P.; Kimmick, G.; Shaw, H.; Snyder, S.; et al. A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer. Cancer Investig. 2008, 26, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Rosendahl, M.; Kelsey, T.W.; Cameron, D.A. Pretreatment anti-Müllerian hormone predicts for loss of ovarian function after chemotherapy for early breast cancer. Eur. J. Cancer 2013, 49, 3404–3411. [Google Scholar] [CrossRef]
- Xie, Q.; Liao, Q.; Wang, L.; Zhang, Y.; Chen, J.; Bai, H.; Li, K.; Ai, J. The dominant mechanism of cyclophosphamide-induced damage to ovarian reserve: Premature activation or apoptosis of primordial follicles? Reprod. Sci. 2024, 31, 30–44. [Google Scholar] [CrossRef]
- Letourneau, J.M.; Ebbel, E.E.; Katz, P.P.; Oktay, K.H.; McCulloch, C.E.; Ai, W.Z.; Chien, A.J.; Melisko, M.E.; Cedars, M.I.; Rosen, M.P. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer 2012, 118, 1933–1939. [Google Scholar] [CrossRef]
- Petrek, J.A.; Naughton, M.J.; Case, L.D.; Paskett, E.D.; Naftalis, E.Z.; Singletary, S.E.; Sukumvanich, P. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: A prospective study. J. Clin. Oncol. 2006, 24, 1045–1051. [Google Scholar] [CrossRef]
- Meirow, D.; Nugent, D. The effects of radiotherapy and chemotherapy on female reproduction. Hum. Reprod. Update 2001, 7, 535–543. [Google Scholar] [CrossRef]
- Meirow, D.; Biederman, H.; Anderson, R.A.; Wallace, W.H.B. Toxicity of chemotherapy and radiation on female reproduction. Clin. Obstet. Gynecol. 2010, 53, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.N.; Zerafa, N.; Liew, S.H.; Morgan, F.H.; Strasser, A.; Scott, C.L.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 2018, 9, 618. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, J.; Lin, G. Advances in the study of DNA damage and repair in mammalian oocytes. Yi Chuan 2023, 45, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Xue, L.; Li, Y.; Tang, W.; Chen, D.; Zhang, J.; Dai, J.; Zhou, S.; Lu, Z.; Wu, M.; et al. Therapy of endocrine disease: Novel protection and treatment strategies for chemotherapy-associated ovarian damage. Eur. J. Endocrinol. 2021, 184, R177–R192. [Google Scholar] [CrossRef]
- Byrne, J.; Fears, T.R.; Gail, M.H.; Pee, D.; Connelly, R.R.; Austin, D.F.; Holmes, G.F.; Holmes, F.F.; Latourette, H.B.; Meigs, J.W.; et al. Early menopause in long-term survivors of cancer during adolescence. Am. J. Obstet. Gynecol. 1992, 166, 788–793. [Google Scholar] [CrossRef]
- Morgan, S.; Anderson, R.A.; Gourley, C.; Wallace, W.H.; Spears, N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 2012, 18, 525–535. [Google Scholar] [CrossRef]
- Bines, J.; Oleske, D.M.; Cobleigh, M.A. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J. Clin. Oncol. 1996, 14, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Lower, E.E.; Blau, R.; Gazder, P.; Tummala, R. The risk of premature menopause induced by chemotherapy for early breast cancer. J. Womens Health Gend. Based Med. 1999, 8, 949–954. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Marrett, L.D.; Darlington, G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964–1988 in Ontario, Canada. Am. J. Epidemiol. 1999, 150, 245–254. [Google Scholar] [CrossRef]
- Meirow, D.; Lewis, H.; Nugent, D.; Epstein, M. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: Clinical importance and proposed accurate investigative tool. Hum. Reprod. 1999, 14, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Abir, R.; Ben-Haroush, A.; Felz, C.; Okon, E.; Raanani, H.; Orvieto, R.; Nitke, S.; Fisch, B. Selection of patients before and after anticancer treatment for ovarian cryopreservation. Hum. Reprod. 2008, 23, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Raz, A.; Fisch, B.; Okon, E.; Feldberg, D.; Nitke, S.; Raanani, H.; Abir, R. Possible direct cytoxicity effects of cyclophosphamide on cultured human follicles: An electron microscopy study. J. Assist. Reprod. Genet. 2002, 19, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, Y.; Jin, J.; Ren, P.; Zhou, H.; Xu, S.; Zhang, Y.; Hu, Z.; Rong, Y.; Dai, Y.; et al. Cyclophosphamide exposure causes long-term detrimental effect of oocytes developmental competence through affecting the epigenetic modification and maternal factors’ transcription during oocyte growth. Front. Cell Dev. Biol. 2021, 9, 682060. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, R.; Khan, S.N.; Shaeib, F.; Kohan-Ghadr, H.R.; Aldhaheri, S.R.; Najafi, T.; Thakur, M.; Morris, R.; Abu-Soud, H.M. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic. Biol. Med. 2017, 110, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, Y.; Yu, Y.; Xin, X. GnRH antagonist cetrorelix inhibits mitochondria-dependent apoptosis triggered by chemotherapy in granulosa cells of rats. Gynecol. Oncol. 2010, 118, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Potu, B.K. Ovarian folliculogenesis: Detrimental effect of prenatal exposure to cyclophosphamide: A preliminary study. Bratisl. Lek. Listy 2010, 111, 369–372. [Google Scholar] [PubMed]
- Comish, P.B.; Drumond, A.L.; Kinnell, H.L.; Anderson, R.A.; Matin, A.; Meistrich, M.L.; Shetty, G. Fetal cyclophosphamide exposure induces testicular cancer and reduced spermatogenesis and ovarian follicle numbers in mice. PLoS ONE 2014, 9, e93311. [Google Scholar] [CrossRef]
- Salian, S.R.; Uppangala, S.; Cheredath, A.; D’Souza, F.; Kalthur, G.; Nayak, V.C.; Anderson, R.A.; Adiga, S.K. Early prepubertal cyclophosphamide exposure in mice results in long-term loss of ovarian reserve, and impaired embryonic development and blastocyst quality. PLoS ONE 2020, 15, e0235140. [Google Scholar] [CrossRef]
- Di Emidio, G.; D’Aurora, M.; Placidi, M.; Franchi, S.; Rossi, G.; Stuppia, L.; Artini, P.G.; Tatone, C.; Gatta, V. Pre-conceptional maternal exposure to cyclophosphamide results in modifications of DNA methylation in F1 and F2 mouse oocytes: Evidence for transgenerational effects. Epigenetics 2019, 14, 1057–1064. [Google Scholar] [CrossRef]
- Li, Q.; An, X.; Man, X.; Chu, M.; Zhao, T.; Yu, H.; Li, Z. Transcriptome analysis reveals that cyclophosphamide induces premature ovarian failure by blocking cholesterol biosynthesis pathway. Life Sci. 2019, 239, 116999. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.N.; Zerafa, N.; Liew, S.H.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol. Hum. Reprod. 2019, 25, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; You, S. Extended adverse effects of cyclophosphamide on mouse ovarian function. BMC Pharmacol. Toxicol. 2021, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Turan, V.; Lierman, S.; Cuvelier, C.; De Sutter, P.; Oktay, K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum. Reprod. 2014, 29, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Oktem, O.; Oktay, K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res. 2007, 67, 10159–10162. [Google Scholar] [CrossRef] [PubMed]
- Eldani, M.; Luan, Y.; Xu, P.C.; Bargar, T.; Kim, S. Continuous treatment with cisplatin induces the oocyte death of primordial follicles without activation. FASEB J. 2020, 34, 13885–13899. [Google Scholar] [CrossRef] [PubMed]
- Ayres, L.S.; Berger, M.; Durli, I.C.L.d.O.; Kuhl, C.P.; Terraciano, P.B.; Garcez, T.N.A.; dos Santos, B.G.; Guimarães, J.A.; Passos, E.P.; Cirne-Lima, E.O. Kallikrein-kinin system and oxidative stress in cisplatin-induced ovarian toxicity. Reprod. Toxicol. 2020, 93, 1–9. [Google Scholar] [CrossRef]
- Wang, Q.; Hutt, K.J. Evaluation of mitochondria in mouse oocytes following cisplatin exposure. J. Ovarian Res. 2021, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, Y.; Qi, L.; Ju, X.; Liu, H.; Wang, G. Differentially expressed genes in cisplatin-induced premature ovarian failure in rats. Anim. Reprod. Sci. 2013, 137, 205–213. [Google Scholar] [CrossRef]
- Yucebilgin, M.S.; Terek, M.C.; Ozsaran, A.; Akercan, F.; Zekioglu, O.; Isik, E.; Erhan, Y. Effect of chemotherapy on primordial follicular reserve of rat: An animal model of premature ovarian failure and infertility. Aust. N. Z. J. Obstet. Gynaecol. 2004, 44, 6–9. [Google Scholar] [CrossRef]
- Lambouras, M.; Liew, S.H.; Horvay, K.; Abud, H.E.; Stringer, J.M.; Hutt, K.J. Examination of the ovotoxicity of 5-fluorouracil in mice. J. Assist. Reprod. Genet. 2018, 35, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.Z.; Lima, L.F.; Vieira, L.A.; Maside, C.; Ferreira, A.C.A.; Araújo, V.R.; Duarte, A.B.G.; Raposo, R.S.; Báo, S.N.; Campello, C.C.; et al. 5-Fluorouracil disrupts ovarian preantral follicles in young C57BL6J mice. Cancer Chemother. Pharmacol. 2021, 87, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Stringer, J.M.; Swindells, E.O.K.; Zerafa, N.; Liew, S.H.; Hutt, K.J. Multidose 5-fluorouracil is highly toxic to growing ovarian follicles in mice. Toxicol. Sci. 2018, 166, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Naren, G.; Wang, L.; Zhang, X.; Cheng, L.; Yang, S.; Yang, J.; Guo, J.; Nashun, B. The reversible reproductive toxicity of 5-fluorouracil in mice. Reprod. Toxicol. 2021, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Johnson, S.B.; Yuan, G.; Arriba, A.K.; Zubizarreta, M.E.; Chatterjee, S.; Nagarkatti, M.; Nagarkatti, P.; Xiao, S. Doxorubicin obliterates mouse ovarian reserve through both primordial follicle atresia and overactivation. Toxicol. Appl. Pharmacol. 2019, 381, 114714. [Google Scholar] [CrossRef] [PubMed]
- Bar-Joseph, H.; Ben-Aharon, I.; Rizel, S.; Stemmer, S.M.; Tzabari, M.; Shalgi, R. Doxorubicin-induced apoptosis in germinal vesicle (GV) oocytes. Reprod. Toxicol. 2010, 30, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aharon, I.; Bar-Joseph, H.; Tzarfaty, G.; Kuchinsky, L.; Rizel, S.; Stemmer, S.M.; Shalgi, R. Doxorubicin-induced ovarian toxicity. Reprod. Biol. Endocrinol. 2010, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Byun, H.; Li, Y.; Xiao, S.; Miller, D.M.; Wang, Z.; Viswanathan, S.; Hancock, J.M.; Bromfield, J.; Ye, X. Varied effects of doxorubicin (DOX) on the corpus luteum of C57BL/6 mice during early pregnancy. Biol. Reprod. 2021, 105, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Samare-Najaf, M.; Zal, F.; Safari, S.; Koohpeyma, F.; Jamali, N. Stereological and histopathological evaluation of doxorubicin-induced toxicity in female rats’ ovary and uterus and palliative effects of quercetin and vitamin E. Hum. Exp. Toxicol. 2020, 39, 1710–1724. [Google Scholar] [CrossRef]
- Levi, M.; Ben-Aharon, I.; Shalgi, R. Irinotecan (CPT-11) treatment induces mild gonadotoxicity. Front. Reprod. Health 2022, 4, 812053. [Google Scholar] [CrossRef]
- Utsunomiya, T.; Tanaka, T.; Utsunomiya, H.; Umesaki, N. A novel molecular mechanism for anticancer drug-induced ovarian failure: Irinotecan HCl, an anticancer topoisomerase I inhibitor, induces specific FasL expression in granulosa cells of large ovarian follicles to enhance follicular apoptosis. Int. J. Oncol. 2008, 32, 991–1000. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tarumi, W.; Suzuki, N.; Takahashi, N.; Kobayashi, Y.; Kiguchi, K.; Sato, K.; Ishizuka, B. Ovarian toxicity of paclitaxel and effect on fertility in the rat. J. Obstet. Gynaecol. Res. 2009, 35, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Chen, G.; Chen, J.; Cui, M.; Yin, Y.; Liao, Q.; Tang, M.; Feng, X.; Li, X.; Zhang, S.; et al. Transient impact of paclitaxel on mouse fertility and protective effect of gonadotropin-releasing hormone agonist. Oncol. Rep. 2020, 44, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Borovskaya, T.G.; Pakhomova, A.V.; Perova, A.V.; Timina, E.A.; Gol’dberg, V.E. Effect of paclitaxel on morphology and function of rat ovaries. Bull. Exp. Biol. Med. 2007, 143, 18–20. [Google Scholar] [CrossRef]
- Yoshida, K.; Erdenebayar, O.; Kadota, Y.; Kasai, K.; Kawakita, T.; Shinya, A.; Sasasda, H.; Katayama, S.; Nii, M.; Imaizumi, J.; et al. Effect of intraperitoneal docetaxel on ovarian function in mice. J. Obstet. Gynaecol. 2022, 42, 3672–3678. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Lee, S.; Ryu, K.J.; Min, K.J.; Hong, J.H.; Song, J.Y.; Lee, J.K.; Lee, N.W. A gonadotropin-releasing hormone agonist for the prevention of docetaxel-induced gonadal damage. J. Obstet. Gynaecol. 2017, 37, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, R.; Chatzicharalampous, C.; Kohan-Ghadr, H.R.; Awonuga, A.; Joshi, N.; Morris, R.T.; Abu-Soud, H.M. Acrolein, a commonly found environmental toxin, causes oocyte mitochondrial dysfunction and negatively affects embryo development. Free Radic. Res. 2018, 52, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, W.; Liu, H.; Rosenwaks, Z.; Kim, J.; Ku, S.Y. Effects of paclitaxel and cisplatin on in vitro ovarian follicle development. Arch. Med. Sci. 2019, 15, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Lopes, F.; Gourley, C.; Anderson, R.A.; Spears, N. Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; Imatinib provides selective protection only against cisplatin. PLoS ONE 2013, 8, e70117. [Google Scholar] [CrossRef]
- Allen, C.M.; Lopes, F.; Mitchell, R.T.; Spears, N. Comparative gonadotoxicity of the chemotherapy drugs cisplatin and carboplatin on prepubertal mouse gonads. Mol. Hum. Reprod. 2020, 26, 129–140. [Google Scholar] [CrossRef]
- Yuksel, A.; Bildik, G.; Senbabaoglu, F.; Akin, N.; Arvas, M.; Unal, F.; Kilic, Y.; Karanfil, I.; Eryılmaz, B.; Yilmaz, P.; et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum. Reprod. 2015, 30, 2926–2935. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.; Liu, J.; Morgan, S.; Matthews, R.; Nevin, L.; Anderson, R.A.; Spears, N. Single and combined effects of cisplatin and doxorubicin on the human and mouse ovary in vitro. Reproduction 2020, 159, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.Z.; Vieira, L.A.; Maside, C.; Ferreira, A.C.A.; Sá, N.A.R.; Correia, H.H.V.; Araújo, V.R.; Raposo, R.S.; Smitz, J.; Campello, C.C.; et al. In vitro cytotoxic effects of 5-fluorouracil on isolated murine ovarian preantral follicles. Theriogenology 2022, 178, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, W.H.; Feng, L.L.; Huang, H.G. Effect of doxorubicin-induced ovarian toxicity on mouse ovarian granulosa cells. Regul. Toxicol. Pharmacol. 2017, 86, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, J.; Liu, M.; Iwahata, H.; Rogers, H.B.; Woodruff, T.K. Doxorubicin has dose-dependent toxicity on mouse ovarian follicle development, hormone secretion, and oocyte maturation. Toxicol. Sci. 2017, 157, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.U.R.; Geng, C.; Li, W.; Yu, X.; Qin, K.R.; Wang, H.; Liu, B. Doxorubicin induces ER calcium release via Src in rat ovarian follicles. Toxicol. Sci. 2019, 168, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Maidarti, M.; Tarumi, W.; Takae, S.; Wiweko, B.; Suzuki, N. Paclitaxel is evidence to reduce growing ovarian follicle growth in mice model study. Toxicol. In Vitr. 2022, 83, 105386. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.; Smith, R.; Anderson, R.A.; Spears, N. Docetaxel induces moderate ovarian toxicity in mice, primarily affecting granulosa cells of early growing follicles. Mol. Hum. Reprod. 2014, 20, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Chemaitilly, W.; Li, Z.; Krasin, M.J.; Brooke, R.J.; Wilson, C.L.; Green, D.M.; Klosky, J.L.; Barnes, N.; Clark, K.L.; Farr, J.B.; et al. Premature ovarian insufficiency in childhood cancer survivors: A report from the St. Jude Lifetime Cohort. J. Clin. Endocrinol. Metab. 2017, 102, 2242–2250. [Google Scholar] [CrossRef]
- Oktem, O.; Oktay, K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007, 110, 2222–2229. [Google Scholar] [CrossRef]
- Anderson, R.A.; Brewster, D.H.; Wood, R.; Nowell, S.; Fischbacher, C.; Kelsey, T.W.; Wallace, W.H.B. The impact of cancer on subsequent chance of pregnancy: A population-based analysis. Hum. Reprod. 2018, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Freycon, F.; Trombert-Paviot, B.; Casagranda, L.; Berlier, P.; Bertrand, Y.; Plantaz, D.; Stephan, J.L.; Berger, C. Age at birth of first child and fecundity of women survivors of childhood acute lymphoblastic leukemia (1987–2007): A study of the childhood cancer registry of the Rhône-Alpes region in France (ARCERRA). Pediatr. Hematol. Oncol. 2015, 32, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Brayboy, L.M.; Clark, H.; Knapik, L.O.; Schnirman, R.E.; Wessel, G.M. Nitrogen mustard exposure perturbs oocyte mitochondrial physiology and alters reproductive outcomes. Reprod. Toxicol. 2018, 82, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Clark, H.; Pereira Vera, B.; Zhang, Z.; Brayboy, L.M. MDR-1 function protects oocyte mitochondria against the transgenerational effects of nitrogen mustard exposure. Reprod. Toxicol. 2020, 98, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Shai, D.; Aviel-Ronen, S.; Spector, I.; Raanani, H.; Shapira, M.; Gat, I.; Roness, H.; Meirow, D. Ovaries of patients recently treated with alkylating agent chemotherapy indicate the presence of acute follicle activation, elucidating its role among other proposed mechanisms of follicle loss. Fertil. Steril. 2021, 115, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Plowchalk, D.R.; Mattison, D.R. Phosphoramide mustard is responsible for the ovarian toxicity of cyclophosphamide. Toxicol. Appl. Pharmacol. 1991, 107, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.A.; Keating, A.F. Ovarian xenobiotic biotransformation enzymes are altered during phosphoramide mustard-induced ovotoxicity. Toxicol. Sci. 2014, 141, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Desmeules, P.; Devine, P.J. Characterizing the ovotoxicity of cyclophosphamide metabolites on cultured mouse ovaries. Toxicol. Sci. 2006, 90, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Hahn, K.M.E.; Johnson, P.H.; Gordon, N.; Kuerer, H.; Middleton, L.; Ramirez, M.; Yang, W.; Perkins, G.; Hortobagyi, G.N.; Theriault, R.L. Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer 2006, 107, 1219–1226. [Google Scholar] [CrossRef]
- Brown, A.; Kumar, S.; Tchounwou, P.B. Cisplatin-based chemotherapy of human cancers. J. Cancer Sci. Ther. 2019, 11, 97. [Google Scholar]
- Li, Z.; Qi, H.; Li, Z.; Bao, Y.; Yang, K.; Min, Q. Research progress on the premature ovarian failure caused by cisplatin therapy. Front. Oncol. 2023, 13, 1276310. [Google Scholar] [CrossRef] [PubMed]
- Winship, A.L.; Bakai, M.; Sarma, U.; Liew, S.H.; Hutt, K.J. Dacarbazine depletes the ovarian reserve in mice and depletion is enhanced with age. Sci. Rep. 2018, 8, 6516. [Google Scholar] [CrossRef] [PubMed]
- Teinturier, C.; Hartmann, O.; Valteau-Couanet, D.; Benhamou, E.; Bougneres, P. Ovarian function after autologous bone marrow transplantation in childhood: High-dose busulfan is a major cause of ovarian failure. Bone Marrow Transplant. 1998, 22, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Pelloux, M.C.; Picon, R.; Gangnerau, M.N.; Darmoul, D. Effects of busulfan on ovarian folliculogenesis, steroidogenesis and anti-Müllerian activity of rat neonates. Acta Endocrinol. 1988, 118, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Sellami, I.; Beau, I.; Sonigo, C. Chemotherapy and female fertility. Ann. Endocrinol. 2023, 84, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Kashi, O.; Roness, H.; Spector, I.; Derech-Haim, S.; Meirow, D. Dual suppression of follicle activation pathways completely prevents the cyclophosphamide-induced loss of ovarian reserve. Hum. Reprod. 2023, 38, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Barberino, R.S.; Silva, R.L.S.; Palheta Junior, R.C.; Smitz, J.E.J.; Matos, M.H.T. Protective effects of antioxidants on cyclophosphamide-induced ovarian toxicity. Biopreserv. Biobank. 2023, 21, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Ezoe, K.; Daikoku, T.; Yabuuchi, A.; Murata, N.; Kawano, H.; Abe, T.; Okuno, T.; Kobayashi, T.; Kato, K. Ovarian stimulation using human chorionic gonadotrophin impairs blastocyst implantation and decidualization by altering ovarian hormone levels and downstream signaling in mice. Mol. Hum. Reprod. 2014, 20, 1101–1116. [Google Scholar] [CrossRef]
- Saleh, H.; Omar, E.; Froemming, G.; Said, R. Tocotrienol preserves ovarian function in cyclophosphamide therapy. Hum. Exp. Toxicol. 2015, 34, 946–952. [Google Scholar] [CrossRef]
- Luo, Q.; Yin, N.; Zhang, L.; Yuan, W.; Zhao, W.; Luan, X.; Zhang, H. Role of SDF-1/CXCR4 and cytokines in the development of ovary injury in chemotherapy drug induced premature ovarian failure mice. Life Sci. 2017, 179, 103–109. [Google Scholar] [CrossRef]
- Pascuali, N.; Scotti, L.; Di Pietro, M.; Oubiña, G.; Bas, D.; May, M.; Gómez Muñoz, A.; Cuasnicú, P.S.; Cohen, D.J.; Tesone, M.; et al. Ceramide-1-phosphate has protective properties against cyclophosphamide-induced ovarian damage in a mice model of premature ovarian failure. Hum. Reprod. 2018, 33, 844–859. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, G.; Mattiello, L.; Iannizzotto, V.; Ciccone, S.; Maiani, E.; Villani, V.; Diederich, M.; Gonfloni, S. Kinase-independent inhibition of cyclophosphamide-induced pathways protects the ovarian reserve and prolongs fertility. Cell Death Dis. 2019, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Edmonds, M.E.; Woodruff, T.K.; Kim, S.Y. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J. Endocrinol. 2019, 240, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Titus, S.; Szymanska, K.J.; Musul, B.; Turan, V.; Taylan, E.; Garcia-Milian, R.; Mehta, S.; Oktay, K. Individual-oocyte transcriptomic analysis shows that genotoxic chemotherapy depletes human primordial follicle reserve in vivo by triggering proapoptotic pathways without growth activation. Sci. Rep. 2021, 11, 407. [Google Scholar] [CrossRef]
- Ezoe, K.; Murata, N.; Yabuuchi, A.; Okuno, T.; Kobayashi, T.; Kato, O.; Kato, K. Long-term adverse effects of cyclophosphamide on follicular growth and angiogenesis in mouse ovaries. Reprod. Biol. 2014, 14, 238–242. [Google Scholar] [CrossRef]
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med. 2013, 5, 185ra62. [Google Scholar] [CrossRef]
- Goldman, K.N.; Chenette, D.; Arju, R.; Duncan, F.E.; Keefe, D.L.; Grifo, J.A.; Schneider, R.J. MTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc. Natl. Acad. Sci. USA 2017, 114, 3186–3191. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, Y.; Li, S.; Liang, Y.; Qiu, Q.; Lin, H.; Zhang, Q. Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/MTOR signaling pathway in vivo. J. Ovarian Res. 2017, 10, 56. [Google Scholar] [CrossRef]
- Marcozzi, S.; Rossi, V.; Salvatore, G.; Di Rella, F.; De Felici, M.; Klinger, F.G. Distinct effects of epirubicin, cisplatin and cyclophosphamide on ovarian somatic cells of prepuberal ovaries. Aging 2019, 11, 10532–10556. [Google Scholar] [CrossRef]
- Lande, Y.; Fisch, B.; Tsur, A.; Farhi, J.; Prag-Rosenberg, R.; Ben-Haroush, A.; Kessler-Icekson, G.; Zahalka, M.A.; Ludeman, S.M.; Abir, R. Short-term exposure of human ovarian follicles to cyclophosphamide metabolites seems to promote follicular activation in vitro. Reprod. Biomed. Online 2017, 34, 104–114. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, T.; Wang, S.; Chi, H.; Chen, C.; Zheng, J. Cyclophosphamide promotes the proliferation inhibition of mouse ovarian granulosa cells and premature ovarian failure by activating the LncRNA-Meg3-P53-P66Shc pathway. Gene 2017, 596, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.G.; Luderer, U. Effects of cyclophosphamide and buthionine sulfoximine on ovarian glutathione and apoptosis. Free Radic. Biol. Med. 2004, 36, 1366–1377. [Google Scholar] [CrossRef]
- Tsai-Turton, M.; Luong, B.T.; Tan, Y.; Luderer, U. Cyclophosphamide-induced apoptosis in COV434 human granulosa cells involves oxidative stress and glutathione depletion. Toxicol. Sci. 2007, 98, 216–230. [Google Scholar] [CrossRef]
- Petrillo, S.K.; Desmeules, P.; Truong, T.Q.; Devine, P.J. Detection of DNA damage in oocytes of small ovarian follicles following phosphoramide mustard exposures of cultured rodent ovaries in vitro. Toxicol. Appl. Pharmacol. 2011, 253, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Gonfloni, S. DNA damage stress response in germ cells: Role of c-Abl and clinical implications. Oncogene 2010, 29, 6193–6202. [Google Scholar] [CrossRef]
- Sonigo, C.; Beau, I.; Binart, N.; Grynberg, M. The impact of chemotherapy on the ovaries: Molecular aspects and the prevention of ovarian damage. Int. J. Mol. Sci. 2019, 20, 5342. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Yu, S.Y.; Abazarikia, A.; Dong, R.; Kim, S.Y. TAp63 determines the fate of oocytes against DNA damage. Sci. Adv. 2022, 8, eade1846. [Google Scholar] [CrossRef]
- Gonfloni, S.; Di Tella, L.; Caldarola, S.; Cannata, S.M.; Klinger, F.G.; Di Bartolomeo, C.; Mattei, M.; Candi, E.; De Felici, M.; Melino, G.; et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat. Med. 2009, 15, 1179–1185. [Google Scholar] [CrossRef]
- Mandic, A.; Hansson, J.; Linder, S.; Shoshan, M.C. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J. Biol. Chem. 2003, 278, 9100–9106. [Google Scholar] [CrossRef]
- Chang, E.M.; Lim, E.; Yoon, S.; Jeong, K.; Bae, S.; Lee, D.R.; Yoon, T.K.; Choi, Y.; Lee, W.S. Cisplatin induces overactivation of the dormant primordial follicle through PTEN/AKT/FOXO3a pathway which leads to loss of ovarian reserve in mice. PLoS ONE 2015, 10, e0144245. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, Z.; Wu, F.; Chen, W.; Xie, S.; Liu, J.; Huang, X.; Zhou, Y. Sphingosine-1-phosphate suppresses cyclophosphamide induced follicle apoptosis in human fetal ovarian xenografts in nude mice. Fertil. Steril. 2014, 102, 871–877.e3. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ding, C.; Zou, Q.; Wang, W.; Li, H. Cyclophosphamide regulates N6-methyladenosine and M6A RNA enzyme levels in human granulosa cells and in ovaries of a premature ovarian aging mouse model. Front. Endocrinol. 2019, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Asadi Azarbaijani, B.; Sheikhi, M.; Oskam, I.C.; Nurmio, M.; Laine, T.; Tinkanen, H.; Mäkinen, S.; Tanbo, T.G.; Hovatta, O.; Jahnukainen, K. Effect of previous chemotherapy on the quality of cryopreserved human ovarian tissue in vitro. PLoS ONE 2015, 10, e0133985. [Google Scholar] [CrossRef] [PubMed]
- Bildik, G.; Akin, N.; Senbabaoglu, F.; Sahin, G.N.; Karahuseyinoglu, S.; Ince, U.; Taskiran, C.; Selek, U.; Yakin, K.; Guzel, Y.; et al. GnRH agonist leuprolide acetate does not confer any protection against ovarian damage induced by chemotherapy and radiation in vitro. Hum. Reprod. 2015, 30, 2912–2925. [Google Scholar] [CrossRef] [PubMed]
- Maneschi, F.; Benedetti-Panici, P.; Scambia, G.; Salerno, M.G.; D’Agostino, G.; Mancuso, S. Menstrual and hormone patterns in women treated with high-dose cisplatin and bleomycin. Gynecol. Oncol. 1994, 54, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.; Gershenson, D.M.; Herzog, C.E.; Mitchell, M.F.; Silva, E.G.; Wharton, J.T. Outcome and reproductive function after chemotherapy for ovarian dysgerminoma. J. Clin. Oncol. 1999, 17, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, L.E.; Lurain, J.R.; Singh, D.K.; Schink, J.C. Survival and reproductive outcomes in women treated for malignant ovarian germ cell tumors. Gynecol. Oncol. 2011, 121, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Teramoto, K.; Kawamura, T.; Mori, A.; Imamura, M.; Arii, S. Dihydropyrimidine dehydrogenase and thymidylate synthase activities in hepatocellular carcinomas and in diseased livers. Cancer Chemother. Pharmacol. 2003, 52, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.B.; Cheng, Y.C. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol. Ther. 1990, 48, 381–395. [Google Scholar] [CrossRef]
- Longley, D.B.; Latif, T.; Boyer, J.; Allen, W.L.; Maxwell, P.J.; Johnston, P.G. The interaction of thymidylate synthase expression with p53-regulated signaling pathways in tumor cells. Semin. Oncol. 2003, 30, 3–9. [Google Scholar] [CrossRef]
- Grem, J.L. 5-Fluorouracil: Forty-plus and still ticking. A review of its preclinical and clinical development. Investig. New Drugs 2000, 18, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Hrushesky, W.J.M.; Vyzula, R.; Wood, P.A. Fertility maintenance and 5-fluorouracil timing within the mammalian fertility cycle. Reprod. Toxicol. 1999, 13, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Hirshfield, A.N. Stathmokinetic analysis of granulosa cell proliferation in antral follicles of cyclic rats. Biol. Reprod. 1984, 31, 52–58. [Google Scholar] [CrossRef]
- Ayazoglu Demir, E.; Mentese, A.; Kucuk, H.; Turkmen Alemdar, N.; Demir, S. The therapeutic effect of silibinin against 5-fluorouracil-induced ovarian toxicity in rats. J. Biochem. Mol. Toxicol. 2023, 37, e23408. [Google Scholar] [CrossRef] [PubMed]
- Mentese, A.; Demir, S.; Alemdar, N.T.; Aliyazicioglu, Y.; Deger, O. The effect of chlorogenic acid on 5-fluorouracil-induced oxidative damage in rat ovarian tissue. Farabi Med. J. 2022, 1, 1–7. [Google Scholar]
- Aikawa, N. A novel screening test to predict the developmental toxicity of drugs using human induced pluripotent stem cells. J. Toxicol. Sci. 2020, 45, 187–199. [Google Scholar] [CrossRef]
- Yamada, S.; Yamazaki, D.; Kanda, Y. 5-Fluorouracil inhibits neural differentiation via Mfn1/2 reduction in human induced pluripotent stem cells. J. Toxicol. Sci. 2018, 43, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.B.; Østerlind, K. Successful twin pregnancy outcome after in utero exposure to FOLFOX for metastatic colon cancer: A case report and review of the literature. Clin. Color. Cancer 2011, 10, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, J.N.; Miyazawa, K. Inadvertent 5-fluorouracil treatment in early pregnancy: A report of three cases. Reprod. Toxicol. 1990, 4, 233–235. [Google Scholar] [CrossRef]
- Kciuk, M.; Marciniak, B.; Kontek, R. Irinotecan—Still an important player in cancer chemotherapy: A comprehensive overview. Int. J. Mol. Sci. 2020, 21, 4919. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An agent with multiple mechanisms of anticancer activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Knobf, M.T. The influence of endocrine effects of adjuvant therapy on quality of life outcomes in younger breast cancer survivors. Oncologist 2006, 11, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.I.; Knudson, C.M.; Leykin, L.; Korsmeyer, S.J.; Tilly, J.L. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat. Med. 1997, 3, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Jurisicova, A.; Lee, H.J.; D’Estaing, S.G.; Tilly, J.; Perez, G.I. Molecular requirements for doxorubicin-mediated death in murine oocytes. Cell Death Differ. 2006, 13, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, R.; Heytens, E.; Darzynkiewicz, Z.; Oktay, K. Mechanisms of chemotherapy-induced human ovarian aging: Double strand DNA breaks and microvascular compromise. Aging 2011, 3, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Roti Roti, E.C.; Leisman, S.K.; Abbott, D.H.; Salih, S.M. Acute doxorubicin insult in the mouse ovary is cell- and follicle-type dependent. PLoS ONE 2012, 7, e42293. [Google Scholar] [CrossRef] [PubMed]
- Tuppi, M.; Kehrloesser, S.; Coutandin, D.W.; Rossi, V.; Luh, L.M.; Strubel, A.; Hötte, K.; Hoffmeister, M.; Schäfer, B.; De Oliveira, T.; et al. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat. Struct. Mol. Biol. 2018, 25, 261–269. [Google Scholar] [CrossRef]
- Aziz, A.U.R.; Yu, X.; Jiang, Q.; Zhao, Y.; Deng, S.; Qin, K.; Wang, H.; Liu, B. Doxorubicin-induced toxicity to 3D-cultured rat ovarian follicles on a microfluidic chip. Toxicol. In Vitr. 2020, 62, 104677. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Gunasekaran, V.P.; Arunachalam, J.; Ganeshan, M. Doxorubicin-induced female reproductive toxicity: An assessment of ovarian follicular apoptosis, cyclicity and reproductive tissue histology in Wistar rats. Drug Chem. Toxicol. 2018, 41, 72–81. [Google Scholar] [CrossRef]
- Luu, A.Z.; Chowdhury, B.; Al-Omran, M.; Teoh, H.; Hess, D.A.; Verma, S. Role of endothelium in doxorubicin-induced cardiomyopathy. JACC Basic Transl. Sci. 2018, 3, 861–870. [Google Scholar] [CrossRef]
- Kim, J.M.; Yoon, Y.D.; Tsang, B.K. Involvement of the Fas/Fas ligand system in p53-mediated granulosa cell apoptosis during follicular development and atresia. Endocrinology 1999, 140, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tanaka, T.; Yukawa, K.; Akira, S.; Umesaki, N. Irinotecan-induced ovarian follicular apoptosis is attenuated by deleting the kinase domain of death-associated protein kinase. Int. J. Oncol. 2009, 34, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.; Smith, R.; Nash, S.; Mitchell, R.T.; Spears, N. Irinotecan metabolite SN38 results in germ cell loss in the testis but not in the ovary of prepubertal mice. Mol. Hum. Reprod. 2016, 22, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Machet, A.; Poudou, C.; Tomowiak, C.; Gastinne, T.; Gardembas, M.; Systchenko, T.; Moya, N.; Debiais, C.; Levy, A.; Gruchet, C.; et al. Hodgkin lymphoma and female fertility: A multicenter study in women treated with doxorubicin, bleomycin, vinblastine, and dacarbazine. Blood Adv. 2023, 7, 3978–3983. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Utsunomiya, T.; Utsunomiya, H.; Umesaki, N. Irinotecan HCl, an anticancer topoisomerase I inhibitor, frequently induces ovarian failure in premenopausal and perimenopausal women. Oncol. Rep. 2008, 19, 1123–1133. [Google Scholar] [CrossRef][Green Version]
- Wani, M.C.; Horwitz, S.B. Nature as a remarkable chemist: A personal story of the discovery and development of Taxol. Anticancer Drugs 2014, 25, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I.; Lichtenthal, B.; Lee, S.; Wang, C.; Wang, X. Taxane anticancer agents: A patent perspective. Expert Opin. Ther. Pat. 2016, 26, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Maloney, S.M.; Hoover, C.A.; Morejon-Lasso, L.V.; Prosperi, J.R. Mechanisms of taxane resistance. Cancers 2020, 12, 3323. [Google Scholar] [CrossRef]
- Chaqour, J.; Ozcan, M.C.H.; De La Cruz, P.; Woodman-Sousa, M.F.; McAdams, J.N.; Grive, K.J. Effects of maternal taxane chemotherapy exposure on daughters’ ovarian reserve and fertility potential. FS Sci. 2023, 5, 141–153. [Google Scholar] [CrossRef]
- Gücer, F.; Balkanli-Kaplan, P.; Doganay, L.; Yüce, M.A.; Demiralay, E.; Sayin, N.C.; Yardım, T. Effect of paclitaxel on primordial follicular reserve in mice. Fertil. Steril. 2001, 76, 628–629. [Google Scholar] [CrossRef]
- Ozcelik, B.; Turkyilmaz, C.; Ozgun, M.T.; Serin, I.S.; Batukan, C.; Ozdamar, S.; Ozturk, A. Prevention of paclitaxel and cisplatin induced ovarian damage in rats by a gonadotropin-releasing hormone agonist. Fertil. Steril. 2010, 93, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Ntemou, E.; Vidal, P.D.; Alexandri, C.; Van den Steen, G.; Lambertini, M.; Demeestere, I. Ovarian toxicity of carboplatin and paclitaxel in mouse carriers of mutation in BRIP1 tumor suppressor gene. Sci. Rep. 2022, 12, 1658. [Google Scholar] [CrossRef] [PubMed]
- Long, J.P.; Wan, F.; Zhang, F.; Zhou, J.; Don, L.F. DTC chemotherapy regimen is associated with higher incidence of premature ovarian failure in women of reproductive age with breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1087–1092. [Google Scholar] [PubMed]
- Salama, M.; Nahata, L.; Jayasinghe, Y.; Gomez-Lobo, V.; Laronda, M.M.; Moravek, M.B.; Meacham, L.R.; Christianson, M.S.; Lambertini, M.; Anazodo, A.; et al. Pediatric oncofertility care in limited versus optimum resource settings: Results from 39 surveyed centers in Repro-Can-OPEN Study Part I & II. J. Assist. Reprod. Genet. 2023, 40, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Labrune, E.; Bianchetti, S.; Lepinasse, O.; Soignon, G.; Salle, B.; Lornage, J. When to cryopreserve ovarian tissue: Determining the effects of chemotherapy on the ovarian reserve by studying follicular density and apoptosis. Cytopathology 2023, 34, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.K.; Kristensen, S.G.; Macklon, K.T.; Jeppesen, J.V.; Fedder, J.; Ernst, E.; Andersen, C.Y. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum. Reprod. 2015, 30, 2838–2845. [Google Scholar] [CrossRef] [PubMed]
- Sonigo, C.; Beau, I.; Grynberg, M.; Binart, N. AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide-treated mice. FASEB J. 2019, 33, 1278–1287. [Google Scholar] [CrossRef]
- Roness, H.; Spector, I.; Leichtmann-Bardoogo, Y.; Savino, A.M.; Dereh-Haim, S.; Meirow, D. Pharmacological administration of recombinant human AMH rescues ovarian reserve and preserves fertility in a mouse model of chemotherapy, without interfering with anti-tumoural effects. J. Assist. Reprod. Genet. 2019, 36, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Man, L.; Guahmich, N.L.; Kallinos, E.; Zangi, L.; Pépin, D.; Rosenwaks, Z.; James, D. Anti-Müllerian hormone protects ovarian reserve from cyclophosphamide when administered as recombinant protein or modified RNA. Fertil. Steril. 2021, 116, e92. [Google Scholar] [CrossRef]
- Tutar, E.O.; Gözüküçük, M.; Kaya, M.S.; Macun, A.; Yücel, D.; Hücümenoğlu, S.; Çaydere, M.; Üstün, Y. Dose-dependent effects of ghrelin and aberrant anti-Müllerian hormone levels in the prevention of ovarian damage caused by cisplatin in Wistar-albino rats. Arch. Gynecol. Obstet. 2022, 305, 1003–1009. [Google Scholar] [CrossRef]
- Jang, H.; Na, Y.; Hong, K.; Lee, S.; Moon, S.; Cho, M.; Park, M.; Lee, O.; Chang, E.M.; Lee, D.R.; et al. Synergistic effect of melatonin and ghrelin in preventing cisplatin-induced ovarian damage via regulation of FOXO3a phosphorylation and binding to the p27Kip1 promoter in primordial follicles. J. Pineal Res. 2017, 63, e12432. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Lispi, M.; Longobardi, S.; Mattei, M.; Di Rella, F.; Salustri, A.; De Felici, M.; Klinger, F.G. LH prevents cisplatin-induced apoptosis in oocytes and preserves female fertility in mouse. Cell Death Differ. 2017, 24, 72–82. [Google Scholar] [CrossRef]
- Xing, F.; Wang, M.; Ding, Z.; Zhang, J.; Ding, S.; Shi, L.; Xie, Q.; Ahmad, M.J.; Wei, Z.; Tang, L.; et al. Protective effect and mechanism of melatonin on cisplatin-induced ovarian damage in mice. J. Clin. Med. 2022, 11, 7383. [Google Scholar] [CrossRef]
- Huang, J.; Shan, W.; Li, N.; Zhou, B.; Guo, E.; Xia, M.; Lu, H.; Wu, Y.; Chen, J.; Wang, B.; et al. Melatonin provides protection against cisplatin-induced ovarian damage and loss of fertility in mice. Reprod. Biomed. Online 2021, 42, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahat, A.; Hulail, M.A.E.; Soliman, N.M.M.; Khamis, T.; Fericean, L.M.; Arisha, A.H.; Moawad, R.S. Melatonin mitigates cisplatin-induced ovarian dysfunction via altering steroidogenesis, inflammation, apoptosis, oxidative stress, and PTEN/PI3K/Akt/MTOR/AMPK signaling pathway in female rats. Pharmaceutics 2022, 14, 2769. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Ma, W.W.; Li, H.X.; Pei, X.Y.; Deng, S.L.; Jia, H.; Ma, W.Z. Melatonin prevents cyclophosphamide-induced primordial follicle loss by inhibiting ovarian granulosa cell apoptosis and maintaining AMH expression. Front. Endocrinol. 2022, 13, 895095. [Google Scholar] [CrossRef]
- Barberino, R.S.; Lins, T.L.B.G.; Monte, A.P.O.; Gouveia, B.B.; Campinho, D.S.P.; Palheta, R.C.; Smitz, J.E.J.; Matos, M.H.T. Melatonin attenuates cyclophosphamide-induced primordial follicle loss by interaction with MT1 receptor and modulation of PTEN/Akt/FOXO3a proteins in the mouse ovary. Reprod. Sci. 2022, 29, 2505–2514. [Google Scholar] [CrossRef]
- Xu, H.; Bao, X.; Kong, H.; Yang, J.; Li, Y.; Sun, Z. Melatonin protects against cyclophosphamide-induced premature ovarian failure in rats. Hum. Exp. Toxicol. 2022, 41, 9603271221127430. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.; Lee, L.J.; Tzeng, C.R.; Wang, C.W.; Hsu, M.I.; Chen, C.H. Targeted anti-apoptosis activity for ovarian protection against chemotherapy-induced ovarian gonadotoxicity. Reprod. Biomed. Online 2014, 29, 612–620. [Google Scholar] [CrossRef]
- Hancke, K.; Strauch, O.; Kissel, C.; Göbel, H.; Schäfer, W.; Denschlag, D. Sphingosine 1-phosphate protects ovaries from chemotherapy-induced damage in vivo. Fertil. Steril. 2007, 87, 172–177. [Google Scholar] [CrossRef]
- Xiao, G.Y.; Cheng, C.C.; Chiang, Y.S.; Cheng, W.T.K.; Liu, I.H.; Wu, S.C. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci. Rep. 2016, 6, 23120. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Long, H.; Cong, Y.; Gao, H.; Lyu, Q.; Yu, S.; Kuang, Y. Quercetin prevents primordial follicle loss via suppression of PI3K/Akt/Foxo3a pathway activation in cyclophosphamide-treated mice. Reprod. Biol. Endocrinol. 2021, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Miao, C.; Yang, L.; Wang, R.; Chen, B.; Zhang, Q. Quercetin alleviates cyclophosphamide-induced premature ovarian insufficiency in mice by reducing mitochondrial oxidative stress and pyroptosis in granulosa cells. J. Ovarian Res. 2022, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Ma, M.; Chen, Y.; Li, M. Effects of quercetin on ovarian function and regulation of the ovarian PI3K/Akt/FoxO3a signalling pathway and oxidative stress in a rat model of cyclophosphamide-induced premature ovarian failure. Basic Clin. Pharmacol. Toxicol. 2022, 130, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Eren, C.Y.; Gurer, H.G.; Gursoy, O.O.; Yilmaz, O.; Tunc, E.; Aypak, S.U.; Asici, G.S.E. The effect of quercetin on ovary functions in rats with cyclophosphamide induced ovary damage. Clin. Exp. Obstet. Gynecol. 2024, 51, 67. [Google Scholar] [CrossRef]
- Algandaby, M.M. Quercetin attenuates cisplatin-induced ovarian toxicity in rats: Emphasis on anti-oxidant, anti-inflammatory and anti-apoptotic activities. Arab. J. Chem. 2021, 14, 103191. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Z.; Guan, H.; Xia, H.; Gu, C.; Xu, Y.; Li, B.; Zhang, W. Rapamycin maintains the primordial follicle pool and protects ovarian reserve against cyclophosphamide-induced damage. J. Reprod. Dev. 2022, 68, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, S.; Zhou, L.; Lin, H.; Jiao, X.; Qiu, Q.; Liang, Y.; Zhang, Q. Rapamycin preserves the primordial follicle pool during cisplatin treatment in vitro and in vivo. Mol. Reprod. Dev. 2020, 87, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ma, L.; Xue, L.; Ye, W.; Lu, Z.; Li, X.; Jin, Y.; Qin, X.; Chen, D.; Tang, W.; et al. Resveratrol alleviates chemotherapy-induced oogonial stem cell apoptosis and ovarian aging in mice. Aging 2019, 11, 1030–1044. [Google Scholar] [CrossRef]
- Nie, Z.; Zhang, L.; Chen, W.; Zhang, Y.; Wang, W.; Hua, R.; Zhang, T.; Zhao, C.; Gong, M.; Wu, H. The protective effects of resveratrol pretreatment in cyclophosphamide-induced rat ovarian injury: An vivo study. Gynecol. Endocrinol. 2021, 37, 914–919. [Google Scholar] [CrossRef]
- Herrero, Y.; Velázquez, C.; Pascuali, N.; May, M.; Abramovich, D.; Scotti, L.; Parborell, F. Resveratrol alleviates doxorubicin-induced damage in mice ovary. Chem. Biol. Interact. 2023, 376, 110431. [Google Scholar] [CrossRef] [PubMed]
- Chinwe, G.S.; Azuka, O.I.; Adaeze, N.C. Resveratrol supplementation rescues pool of growing follicles and ovarian stroma from cisplatin-induced toxicity on the ovary in Sprague-Dawley rats: An experimental study. Int. J. Reprod. Biomed. 2018, 16, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Said, R.S.; Mantawy, E.M.; El-Demerdash, E. Mechanistic perspective of protective effects of resveratrol against cisplatin-induced ovarian injury in rats: Emphasis on anti-inflammatory and anti-apoptotic effects. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Albahlol, I.A.; Wani, F.A.; Abd-Eltawab Tammam, A.; Kelleni, M.T.; Sayeed, M.U.; Abd El-Fadeal, N.M.; Mohamed, A.A. Resveratrol protects against cisplatin-induced ovarian and uterine toxicity in female rats by attenuating oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2021, 338, 109402. [Google Scholar] [CrossRef] [PubMed]
- Atli, M.; Engin-Ustun, Y.; Tokmak, A.; Caydere, M.; Hucumenoglu, S.; Topcuoglu, C. Dose dependent effect of resveratrol in preventing cisplatin-induced ovarian damage in rats: An experimental study. Reprod. Biol. 2017, 17, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Roti Roti, E.C.; Ringelstetter, A.K.; Kropp, J.; Abbott, D.H.; Salih, S.M. Bortezomib prevents acute doxorubicin ovarian insult and follicle demise, improving the fertility window and pup birth weight in mice. PLoS ONE 2014, 9, e108174. [Google Scholar] [CrossRef] [PubMed]
- Kropp, J.; Roti Roti, E.C.; Ringelstetter, A.; Khatib, H.; Abbott, D.H.; Salih, S.M. Dexrazoxane diminishes doxorubicin-induced acute ovarian damage and preserves ovarian function and fecundity in mice. PLoS ONE 2015, 10, e0142588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, J.X.; Tao, X.; Lu, Z.Y.; Wang, J.J.; Feng, W.W.; Hua, K.Q. Goserelin can inhibit ovarian cancer proliferation and simultaneously protect ovarian function from cisplatin: An in vitro and in vivo study. J. Chemother. 2013, 25, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kishk, E.A.F.; Mohammed Ali, M.H. Effect of a gonadotropin-releasing hormone analogue on cyclophosphamide-induced ovarian toxicity in adult mice. Arch. Gynecol. Obstet. 2013, 287, 1023–1029. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, X.; Liang, L.; Wang, Y.; Wang, R.; Cheng, X.; Yan, Z.; Chen, Y.; Qi, P. Mechanistic study on triptorelin action in protecting from 5-FU-induced ovarian damage in rats. Oncol. Res. 2014, 22, 283–292. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, M.J.; Chen, S.U.; Ho, H.N.; Yang, Y.S. Metformin: A novel option of fertility preservation during cyclophosphamide-containing chemotherapy. Fertil. Steril. 2019, 112, e84. [Google Scholar] [CrossRef]
- Huang, C.C.; Chou, C.H.; Yang, Y.S.; Ho, H.N.; Shun, C.T.; Wen, W.F.; Chen, S.U.; Chen, M.J. Metformin: A novel promising option for fertility preservation during cyclophosphamide-based chemotherapy. Mol. Hum. Reprod. 2021, 27, gaaa084. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tang, X.; Yao, T.; Zhang, Y.; Zhong, Y.; Wu, S.; Wang, Y.; Pan, Z. Metformin protects ovarian granulosa cells in chemotherapy-induced premature ovarian failure mice through AMPK/PPAR-γ/SIRT1 pathway. Sci. Rep. 2024, 14, 1447. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, S.; Hancerliogullari, N.; Guney, G.; Gozukucuk, M.; Caydere, M.; Guney, S.S.; Tokmak, A.; Ustun, Y. Does the addition of metformin to carboplatin treatment decreases ovarian reserve damage associated with carboplatin usage? J. Ovarian Res. 2023, 16, 184. [Google Scholar] [CrossRef]
- Ting, A.Y.; Petroff, B.K. Tamoxifen decreases ovarian follicular loss from experimental toxicant DMBA and chemotherapy agents cyclophosphamide and doxorubicin in the rat. J. Assist. Reprod. Genet. 2010, 27, 591–597. [Google Scholar] [CrossRef]
- Rosario, R.; Stewart, H.L.; Spears, N.; Telfer, E.E.; Anderson, R.A. Anti-Müllerian hormone attenuates both cyclophosphamide-induced damage and PI3K signalling activation, while rapamycin attenuates only PI3K signalling activation, in human ovarian cortex in vitro. Hum. Reprod. 2024, 39, 382–392. [Google Scholar] [CrossRef]
- Alexandri, C.; Stamatopoulos, B.; Rothé, F.; Bareche, Y.; Devos, M.; Demeestere, I. MicroRNA profiling and identification of let-7a as a target to prevent chemotherapy-induced primordial follicles apoptosis in mouse ovaries. Sci. Rep. 2019, 9, 9636. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Zhang, L.; Chen, W.; Zhang, Y.; Hua, R.; Wang, W.; Zhang, T.; Wu, H. The protective effects of pretreatment with resveratrol in cyclophosphamide-induced rat ovarian granulosa cell injury: In vitro study. Reprod. Toxicol. 2020, 95, 66–74. [Google Scholar] [CrossRef]
- Roti Roti, E.C.; Salih, S.M. Dexrazoxane ameliorates doxorubicin-induced injury in mouse ovarian cells. Biol. Reprod. 2012, 86, 96. [Google Scholar] [CrossRef]
- Salih, S.M.; Ringelstetter, A.K.; Elsarrag, M.Z.; Abbott, D.H.; Roti Roti, E.C. Dexrazoxane abrogates acute doxorubicin toxicity in marmoset ovary. Biol. Reprod. 2015, 92, 73. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cordeiro, M.H.; Serna, V.A.; Ebbert, K.; Butler, L.M.; Sinha, S.; Mills, A.A.; Woodruff, T.K.; Kurita, T. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013, 20, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Kim, S.J.; Kim, S.K.; Lee, S.C.; Jun, J.H.; Jee, B.C.; Kim, S.H. Impact of imatinib or dasatinib coadministration on in vitro preantral follicle development and oocyte acquisition in cyclophosphamide-treated mice. Clin. Exp. Reprod. Med. 2020, 47, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Piasecka-Srader, J.; Blanco, F.F.; Delman, D.H.; Dixon, D.A.; Geiser, J.L.; Ciereszko, R.E.; Petroff, B.K. Tamoxifen prevents apoptosis and follicle loss from cyclophosphamide in cultured rat ovaries. Biol. Reprod. 2015, 92, 132. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Ceppi, M.; Anderson, R.A.; Cameron, D.A.; Bruzzone, M.; Franzoi, M.A.; Massarotti, C.; El-Abed, S.; Wang, Y.; Lecocq, C.; et al. Impact of anti-HER2 therapy alone and with weekly paclitaxel on the ovarian reserve of young women with HER2-positive breast cancer. J. Natl. Compr. Cancer Netw. 2023, 21, 33–41.e16. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, D.T.; Donahoe, P.K. Müllerian inhibiting substance/anti-Müllerian hormone: A potential therapeutic agent for human ovarian and other cancers. Future Oncol. 2010, 6, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Sonigo, C.; Beau, I.; Binart, N.; Grynberg, M. Anti-Müllerian hormone in fertility preservation: Clinical and therapeutic applications. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119854755. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Garcia-Touza, M.; Hijazi, R.A.; Taffet, G.; Epner, D.; Mann, D.; Smith, R.G.; Cunningham, G.R.; Marcelli, M. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J. Clin. Endocrinol. Metab. 2005, 90, 2920–2926. [Google Scholar] [CrossRef]
- Garcia, J.M.; Chen, J.; Guillory, B.; Donehower, L.A.; Smith, R.G.; Lamb, D.J. Ghrelin prevents cisplatin-induced testicular damage by facilitating repair of DNA double strand breaks through activation of p53 in mice. Biol. Reprod. 2015, 93, 24. [Google Scholar] [CrossRef] [PubMed]
- Whirledge, S.D.; Garcia, J.M.; Smith, R.G.; Lamb, D.J. Ghrelin partially protects against cisplatin-induced male murine gonadal toxicity in a GHSR-1a-dependent manner. Biol. Reprod. 2015, 92, 76. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, J.J.; Luo, F.; Zheng, Y.; Feng, Y.; Felix, J.C.; Lauchlan, S.C.; Pike, M.C. Ovarian epithelial tumor growth promotion by follicle-stimulating hormone and inhibition of the effect by luteinizing hormone. Gynecol. Oncol. 2000, 76, 80–88. [Google Scholar] [CrossRef]
- Kurbacher, C.M.; Jäger, W.; Kurbacher, J.A.; Bittl, A.; Wildt, L.; Lang, N. Influence of human luteinizing hormone on cell growth and CA125 secretion of primary epithelial ovarian carcinomas in vitro. Tumor Biol. 1995, 16, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Miyazaki, K.; Okamura, H.; Iwai, A.; Fukumoto, M. C-myc over-expression in human primary ovarian tumours: Its relevance to tumour progression. Int. J. Cancer 1992, 50, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Mandai, M.; Konishi, I.; Yura, Y.; Tsuruta, Y.; Hamid, A.A.; Nanbu, K.; Matsushita, K.; Mori, T. Human chorionic gonadotropin (hCG) inhibits cisplatin-induced apoptosis in ovarian cancer cells: Possible role of up-regulation of insulin-like growth factor-1 by hCG. Int. J. Cancer 1998, 76, 571–578. [Google Scholar] [CrossRef]
- Tourgeman, D.E.; Lu, J.J.; Boostanfar, R.; Amezcua, C.; Felix, J.C.; Paulson, R.J. Human chorionic gonadotropin suppresses ovarian epithelial neoplastic cell proliferation in vitro. Fertil. Steril. 2002, 78, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, Y.; Xia, L.; Tang, J.; Wen, H.; Zhang, M. Luteinizing hormone compromises the in vivo anti-tumor effect of cisplatin on human epithelial ovarian cancer cells. Oncol. Lett. 2018, 15, 3141–3146. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wen, H.; Han, X.; Tang, J.; Huang, Y. Luteinizing hormone inhibits cisplatin-induced apoptosis in human epithelial ovarian cancer cells. Oncol. Lett. 2016, 11, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Haghi-Aminjan, H.; Asghari, M.H.; Farhood, B.; Rahimifard, M.; Hashemi Goradel, N.; Abdollahi, M. The role of melatonin on chemotherapy-induced reproductive toxicity. J. Pharm. Pharmacol. 2018, 70, 291–306. [Google Scholar] [CrossRef]
- Sanchez-Barcelo, E.J.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Reiter, R.J. Melatonin uses in oncology: Breast cancer prevention and reduction of the side effects of chemotherapy and radiation. Expert Opin. Investig. Drugs 2012, 21, 819–831. [Google Scholar] [CrossRef]
- Rodrigues, T.D.; Lima, K.R.; Uggioni, M.L.R.; Ferraz, S.D.; Cardoso, H.S.; Colonetti, T.; da Rosa, M.I. Effectiveness of melatonin adjuvant treatment in cisplatin to prevent depletion of ovarian follicles in mice: Systematic review. Biol. Reprod. 2022, 107, 1386–1394. [Google Scholar] [CrossRef]
- Jang, H.; Hong, K.; Choi, Y. Melatonin and fertoprotective adjuvants: Prevention against premature ovarian failure during chemotherapy. Int. J. Mol. Sci. 2017, 18, 1221. [Google Scholar] [CrossRef]
- Sookprasert, A.; Johns, N.P.; Phunmanee, A.; Pongthai, P.; Cheawchanwattana, A.; Johns, J.; Konsil, J.; Plaimee, P.; Porasuphatana, S.; Jitpimolmard, S. Melatonin in patients with cancer receiving chemotherapy: A randomized, double-blind, placebo-controlled trial. Anticancer Res. 2014, 34, 7327–7337. [Google Scholar] [PubMed]
- Jang, H.; Lee, O.; Lee, Y.; Yoon, H.; Chang, E.M.; Park, M.; Lee, J.; Hong, K.; Kim, J.O.; Kim, N.K.; et al. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J. Pineal Res. 2016, 60, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Barberino, R.S.; Menezes, V.G.; Ribeiro, A.E.A.S.; Palheta, R.C., Jr.; Jiang, X.; Smitz, J.E.J.; Matos, M.H.T. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biol. Reprod. 2017, 96, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Perez, G.I.; Paris, F.; Miranda, S.R.; Ehleiter, D.; Haimovitz-Friedman, A.; Fuks, Z.; Xie, Z.; Reed, J.C.; Schuchman, E.H.; et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat. Med. 2000, 6, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Guzel, Y.; Bildik, G.; Dilege, E.; Oktem, O. Sphingosine-1-phosphate reduces atresia of primordial follicles occurring during slow-freezing and thawing of human ovarian cortical strips. Mol. Reprod. Dev. 2018, 85, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Winship, A.L.; Stringer, J.M.; Liew, S.H.; Hutt, K.J. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum. Reprod. Update 2018, 24, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhu, L.; Shen, H.; Lu, J.; Zou, Q.; Huang, C.; Li, H.; Huang, B. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells 2020, 38, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Anand David, A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, P.; Rai, D.K. Quercetin: A versatile flavonoid. Int. J. Med. Update 2007, 2, 20–35. [Google Scholar] [CrossRef]
- Wang, J.; Qian, X.; Gao, Q.; Lv, C.; Xu, J.; Jin, H.; Zhu, H. Quercetin increases the antioxidant capacity of the ovary in menopausal rats and in ovarian granulosa cell culture in vitro. J. Ovarian Res. 2018, 11, 51. [Google Scholar] [CrossRef]
- Elkady, M.; Shalaby, S.; Fathi, F.; El-Mandouh, S. Effects of quercetin and rosuvastatin each alone or in combination on cyclophosphamide-induced premature ovarian failure in female albino mice. Hum. Exp. Toxicol. 2019, 38, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Khazaei, M.; Rashidi, Z. Synergistic effects of capsaicin and quercetin improved induced premature ovarian failure in rat. Cell J. 2023, 25, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Powers, T. The origin story of rapamycin: Systemic bias in biomedical research and cold war politics. Mol. Biol. Cell 2022, 33, pe7. [Google Scholar] [CrossRef]
- Adhikari, D.; Risal, S.; Liu, K.; Shen, Y. Pharmacological inhibition of mTORC1 prevents over-activation of the primordial follicle pool in response to elevated PI3K signaling. PLoS ONE 2013, 8, e53810. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kawamura, K. Rapamycin treatment maintains developmental potential of oocytes in mice and follicle reserve in human cortical fragments grafted into immune-deficient mice. Mol. Cell. Endocrinol. 2020, 504, 110694. [Google Scholar] [CrossRef] [PubMed]
- Celik, S.; Ozkavukcu, S.; Celik-Ozenci, C. Altered expression of activator proteins that control follicle reserve after ovarian tissue cryopreservation/transplantation and primordial follicle loss prevention by rapamycin. J. Assist. Reprod. Genet. 2020, 37, 2119–2136. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Cucciolla, V.; Borriello, A.; Oliva, A.; Galletti, P.; Zappia, V.; Della Ragione, F. Resveratrol: From basic science to the clinic. Cell Cycle 2007, 6, 2495–2510. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Annaji, M.; Poudel, I.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-loaded nanomedicines for cancer applications. Cancer Rep. 2021, 4, e1353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, Z.; Cha, L.; Li, L.; Zhu, D.; Fang, Z.; He, Z.; Huang, J.; Pan, Z. Resveratrol plays a protective role against premature ovarian failure and prompts female germline stem cell survival. Int. J. Mol. Sci. 2019, 20, 3605. [Google Scholar] [CrossRef] [PubMed]
- Özcan, P.; Fıçıcıoğlu, C.; Yıldırım, Ö.K.; Özkan, F.; Akkaya, H.; Aslan, İ. Protective effect of resveratrol against oxidative damage to ovarian reserve in female Sprague-Dawley rats. Reprod. Biomed. Online 2015, 31, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Mutluay, D.; Tenekeci, G.Y.; Monsef, Y.A. Bortezomib-induced ovarian toxicity in mice. Toxicol. Pathol. 2022, 50, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, R.S.; Scott, L.J. Dexrazoxane: A review of its use for cardioprotection during anthracycline chemotherapy. Drugs 2005, 65, 1005–1024. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, R.; Yeh, E.T.H. Molecular mechanisms of anthracycline-induced cardiotoxicity. In Cardio-Oncology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 55–68. [Google Scholar] [CrossRef]
- Kropp, J.; Abbott, D.H.; Roti Roti, E.C. The possibility of dexrazoxane to prevent ovarian damage caused by toxicity. Expert Rev. Qual. Life Cancer Care 2016, 1, 269–275. [Google Scholar] [CrossRef]
- Blumenfeld, Z. Fertility preservation using GnRH agonists: Rationale, possible mechanisms, and explanation of controversy. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119870163. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Horicks, F.; Del Mastro, L.; Partridge, A.H.; Demeestere, I. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: From biological evidence to clinical application. Cancer Treat. Rev. 2019, 72, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Vitek, W.S.; Shayne, M.; Hoeger, K.; Han, Y.; Messing, S.; Fung, C. Gonadotropin-releasing hormone agonists for the preservation of ovarian function among women with breast cancer who did not use tamoxifen after chemotherapy: A systematic review and meta-analysis. Fertil. Steril. 2014, 102, 808–815.e1. [Google Scholar] [CrossRef]
- Shen, Y.W.; Zhang, X.M.; Lv, M.; Wang, F.; Qin, T.J.; Chen, L.; Liu, P.J.; Yang, J.; Yang, J. Utility of gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage in premenopausal women with breast cancer: A systematic review and meta-analysis. OncoTargets Ther. 2015, 8, 3349–3359. [Google Scholar] [CrossRef]
- Munhoz, R.R.; Pereira, A.A.L.; Sasse, A.D.; Hoff, P.M.; Traina, T.A.; Hudis, C.A.; Marques, R.J. Gonadotropin-releasing hormone agonists for ovarian function preservation in premenopausal women undergoing chemotherapy for early-stage breast cancer. JAMA Oncol. 2016, 2, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Senra, J.C.; Roque, M.; Talim, M.C.T.; Reis, F.M.; Tavares, R.L.C. Gonadotropin-releasing hormone agonists for ovarian protection during cancer chemotherapy: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Del Mastro, L.; Boni, L.; Michelotti, A.; Gamucci, T.; Olmeo, N.; Gori, S.; Giordano, M.; Garrone, O.; Pronzato, P.; Bighin, C.; et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer. JAMA 2011, 306, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Gerber, B.; von Minckwitz, G.; Stehle, H.; Reimer, T.; Felberbaum, R.; Maass, N.; Fischer, D.; Sommer, H.L.; Conrad, B.; Ortmann, O.; et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: The GBG 37 ZORO study. J. Clin. Oncol. 2011, 29, 2334–2341. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Lambertini, M.; Bighin, C.; Conte, B.; Blondeaux, E.; D’Alonzo, A.; Dellepiane, C.; Buzzatti, G.; Molinelli, C.; Boccardo, F.; et al. Potential mechanisms of ovarian protection with gonadotropin-releasing hormone agonist in breast cancer patients: A review. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119864584. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist 2007, 12, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, Y.; Endo, T.; Nagasawa, K.; Manase, K.; Honnma, H.; Baba, T.; Hayashi, T.; Chiba, H.; Sawada, N.; Saito, T. Hyperstimulation and a gonadotropin-releasing hormone agonist modulate ovarian vascular permeability by altering expression of the tight junction protein claudin-5. Endocrinology 2006, 147, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Imai, A.; Sugiyama, M.; Furui, T.; Tamaya, T.; Ohno, T. Direct protection by a gonadotropin-releasing hormone analog from doxorubicin-induced granulosa cell damage. Gynecol. Obstet. Investig. 2007, 63, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Ataya, K.; Tadros, M.; Ramahi, A. Gonadotropin-releasing hormone agonist inhibits physiologic ovarian follicular loss in rats. Acta Endocrinol. 1989, 121, 55–60. [Google Scholar] [CrossRef]
- Valsamakis, G.; Valtetsiotis, K.; Charmandari, E.; Lambrinoudaki, I.; Vlahos, N.F. GnRH analogues as a co-treatment to therapy in women of reproductive age with cancer and fertility preservation. Int. J. Mol. Sci. 2022, 23, 2287. [Google Scholar] [CrossRef]
- Detti, L.; Uhlmann, R.A.; Zhang, J.; Diamond, M.P.; Saed, G.M.; Fletcher, N.M.; Lu, M.; Williams, L.J. Goserelin fosters bone elongation but does not prevent ovarian damage in cyclophosphamide-treated prepubertal mice. Fertil. Steril. 2014, 101, 1157–1164.e1. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Lai, Y.; Li, T. Ovarian protection and safety of gonadotropin-releasing hormone agonist after cervical cancer surgery: Systematic review and meta-analysis. Ann. Transl. Med. 2022, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pei, L.; Hu, T.; Jia, M.; Wang, S. Protective effect of goserelin on ovarian reserve during (neo)adjuvant chemotherapy in young breast cancer patients: A prospective cohort study in China. Hum. Reprod. 2021, 36, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Del Mastro, L.; Catzeddu, T.; Boni, L.; Bell, C.; Sertoli, M.R.; Bighin, C.; Clavarezza, M.; Testa, D.; Venturini, M. Prevention of chemotherapy-induced menopause by temporary ovarian suppression with goserelin in young, early breast cancer patients. Ann. Oncol. 2006, 17, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.C.F.; Unger, J.M.; Phillips, K.A.; Boyle, F.; Hitre, E.; Porter, D.; Francis, P.A.; Goldstein, L.J.; Gomez, H.L.; Vallejos, C.S.; et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N. Engl. J. Med. 2015, 372, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; O’Neill, S.; Walsh, G.; Smith, I.E. Goserelin with chemotherapy to preserve ovarian function in pre-menopausal women with early breast cancer: Menstruation and pregnancy outcomes. Ann. Oncol. 2013, 24, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Urruticoechea, A.; Arnedos, M.; Walsh, G.; Dowsett, M.; Smith, I.E. Ovarian protection with goserelin during adjuvant chemotherapy for pre-menopausal women with early breast cancer (EBC). Breast Cancer Res. Treat. 2008, 110, 411–416. [Google Scholar] [CrossRef]
- Sverrisdottir, A.; Nystedt, M.; Johansson, H.; Fornander, T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: Results from a randomized trial. Breast Cancer Res. Treat. 2009, 117, 561–567. [Google Scholar] [CrossRef]
- Rabie, A.S.I.; Elberry, A.A.; Shaaban, A.H.; Hussein, R.R.S. Prevention of chemotherapy-induced ovarian failure with goserelin in premenopausal lymphoma patients. Bahrain Med. Bull. 2021, 43, 463–470. [Google Scholar]
- Kim, S.E.; Kim, W.J.; Choi, D.; Lee, D.Y. Comparison of goserelin and leuprorelin for ovarian protection during chemotherapy in young patients with breast cancer. Breast Cancer Res. Treat. 2023, 198, 231–237. [Google Scholar] [CrossRef]
- Han, W.; Youn, H.J. Clinical studies investigating the use of leuprorelin in breast cancer patients from Asia. Asian Pac. J. Cancer Prev. 2019, 20, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Martinez, A.; Scheurer, M.E.; Allen-Rhoades, W.; Okcu, M.F.; Horne, V.E. Leuprolide protects ovarian reserve in adolescents undergoing gonadotoxic therapy. J. Adolesc. Young Adult Oncol. 2023, 12, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Gao, H.; Yuan, Z. Effect of leuprolide acetate on ovarian function after cyclophosphamide-doxorubicin-based chemotherapy in premenopausal patients with breast cancer: Results from a phase II randomized trial. Med. Oncol. 2013, 30, 667. [Google Scholar] [CrossRef]
- Maisano, R.; Caristi, N.; Mare, M.; Bottari, M.; Adamo, V.; Mafodda, A.; Calogero, M.G.; Caruso, M.; Nardi, M. Protective effect of leuprolide on ovarian function in young women treated with adjuvant chemotherapy for early breast cancer: A multicenter phase II study. J. Chemother. 2008, 20, 740–743. [Google Scholar] [CrossRef]
- Park, H.J.; Koo, Y.A.; Im, Y.H.; Yoon, B.K.; Choi, D. GnRH agonist therapy to protect ovarian function in young Korean breast cancer patients. J. Korean Med. Sci. 2010, 25, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Boni, L.; Michelotti, A.; Gamucci, T.; Scotto, T.; Gori, S.; Giordano, M.; Garrone, O.; Levaggi, A.; Poggio, F.; et al. Ovarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival. JAMA 2015, 314, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Munster, P.N.; Moore, A.P.; Ismail-Khan, R.; Cox, C.E.; Lacevic, M.; Gross-King, M.; Xu, P.; Carter, W.B.; Minton, S.E. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J. Clin. Oncol. 2012, 30, 533–538. [Google Scholar] [CrossRef]
- Chun, E.K.; Jee, B.C.; Kim, J.Y.; Kim, S.H.; Moon, S.Y. Effect of imatinib coadministration on in vitro oocyte acquisition and subsequent embryo development in cyclophosphamide-treated mice. Reprod. Sci. 2014, 21, 906–914. [Google Scholar] [CrossRef][Green Version]
- Zamah, A.M.; Mauro, M.J.; Druker, B.J.; Oktay, K.; Egorin, M.J.; Cedars, M.I.; Rosen, M.P. Will imatinib compromise reproductive capacity? Oncologist 2011, 16, 1422–1427. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, T.E.; Jee, B.C. Impact of imatinib administration on the mouse ovarian follicle count and levels of intra-ovarian proteins related to follicular quality. Clin. Exp. Reprod. Med. 2022, 49, 93–100. [Google Scholar] [CrossRef]
- Bildik, G.; Acılan, C.; Sahin, G.N.; Karahuseyinoglu, S.; Oktem, O. C-Abl is not activated in DNA damage-induced and Tap63-mediated oocyte apoptosis in human ovary. Cell Death Dis. 2018, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.B.; Hutt, K.J.; Cook, M.; Speed, T.P.; Strasser, A.; Findlay, J.K.; Scott, C.L. Cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nat. Med. 2012, 18, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zheng, Y.; Yao, Y.; Jia, R.; Ge, S.; Zhuang, A. Metformin and cancer hallmarks: Shedding new lights on therapeutic repurposing. J. Transl. Med. 2023, 21, 403. [Google Scholar] [CrossRef] [PubMed]
- Aljofan, M.; Riethmacher, D. Anticancer activity of metformin: A systematic review of the literature. Future Sci. OA 2019, 5, FSO410. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, M.; Serrano, D.; Dunn, B.K.; Heckman-Stoddard, B.M.; Lee, O.; Khan, S.; Decensi, A. Oral low dose and topical tamoxifen for breast cancer prevention: Modern approaches for an old drug. Breast Cancer Res. 2012, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Chang, L.T.; Chen, C.H.; Tam, K.W. Fertility preservation for women with breast cancer before chemotherapy: A systematic review and meta-analysis. Reprod. Biomed. Online 2022, 44, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Shandley, L.M.; Spencer, J.B.; Fothergill, A.; Mertens, A.C.; Manatunga, A.; Paplomata, E.; Howards, P.P. Impact of tamoxifen therapy on fertility in breast cancer survivors. Fertil. Steril. 2017, 107, 243–252.e5. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, S.B.; Turan, V.; Bedoschi, G.; Taylan, E.; Abdo, N.; Cigler, T.; Bang, H.; Patil, S.; Dickler, M.N.; Oktay, K.H. Impact of adjuvant chemotherapy or tamoxifen-alone on the ovarian reserve of young women with breast cancer. Breast Cancer Res. Treat. 2021, 185, 165–173. [Google Scholar] [CrossRef]
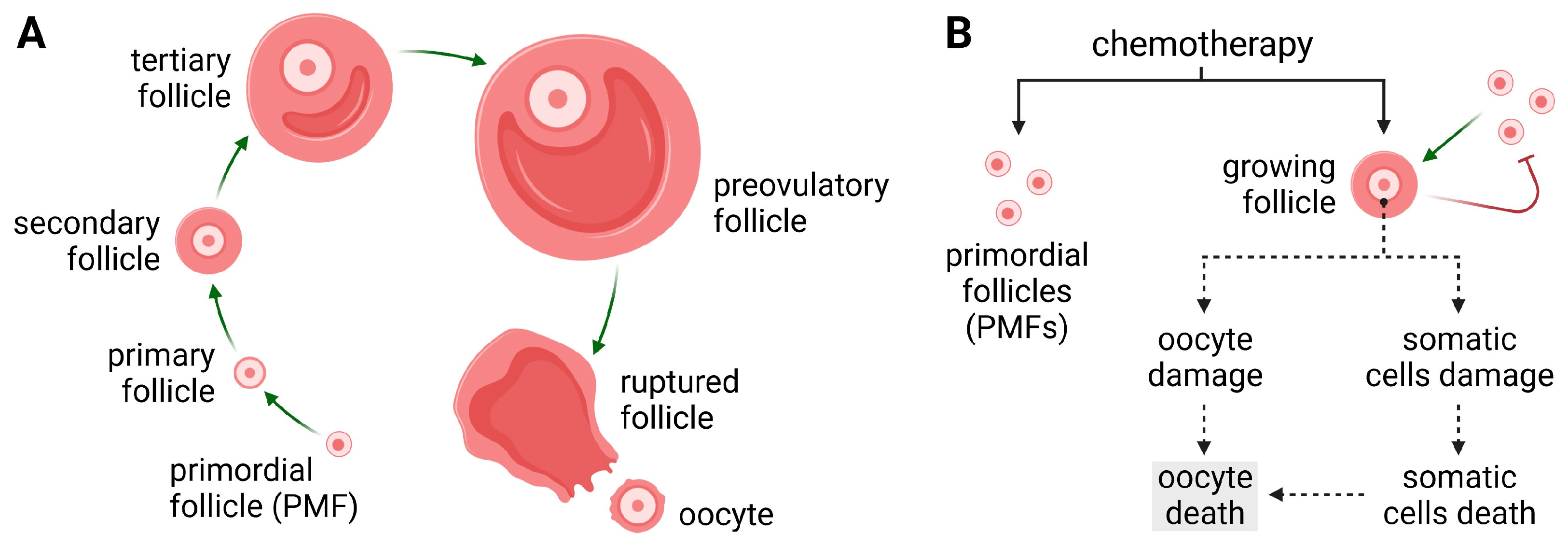
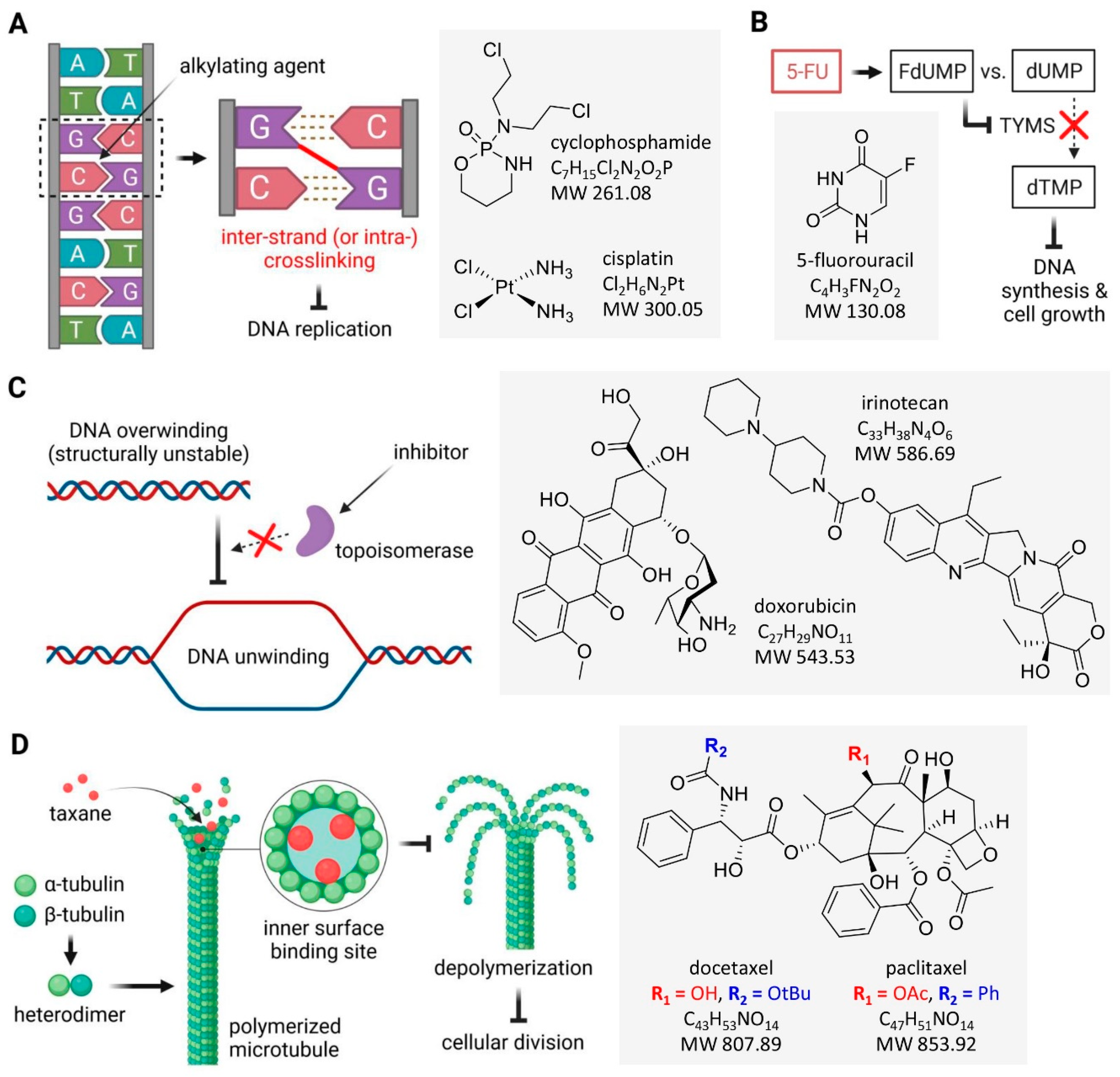
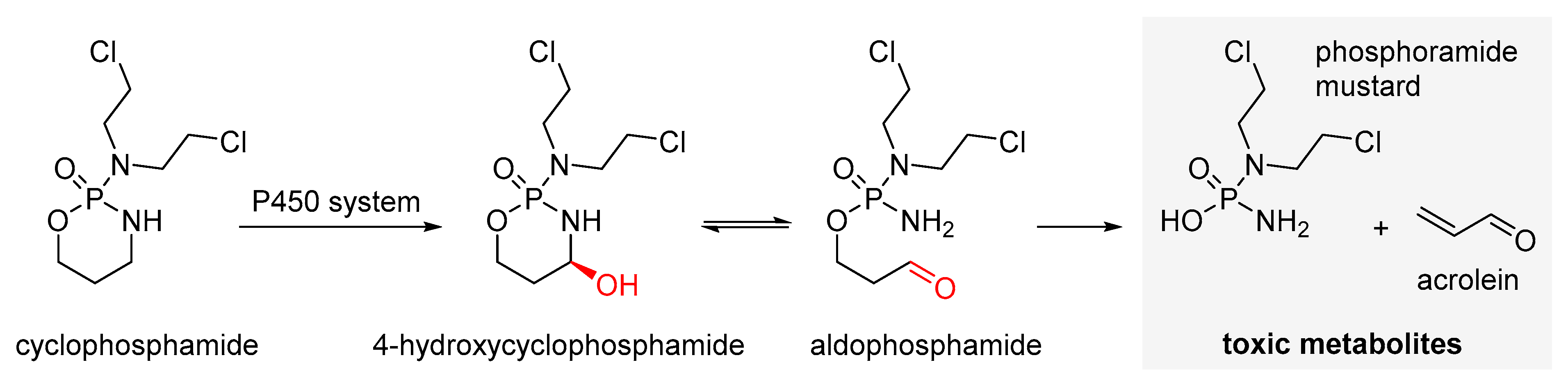
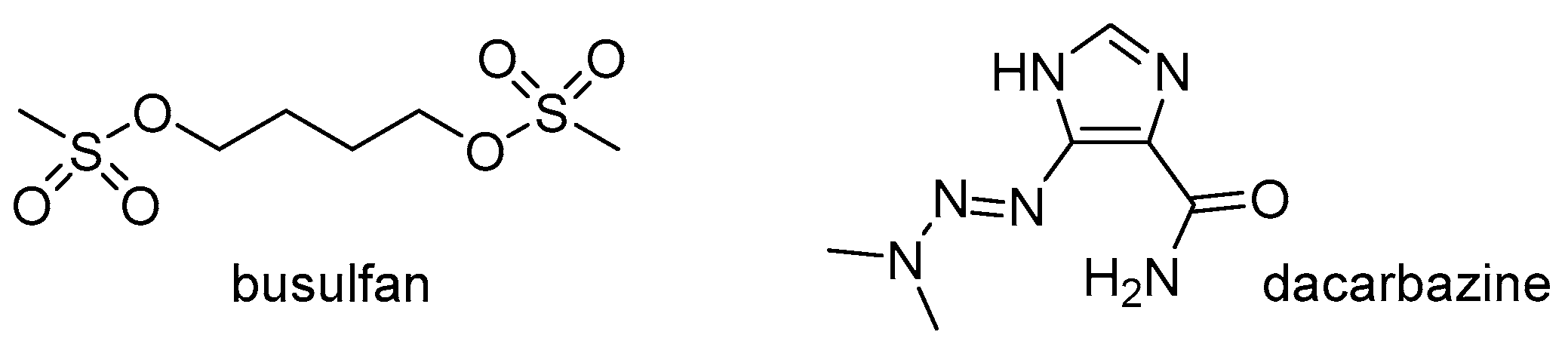
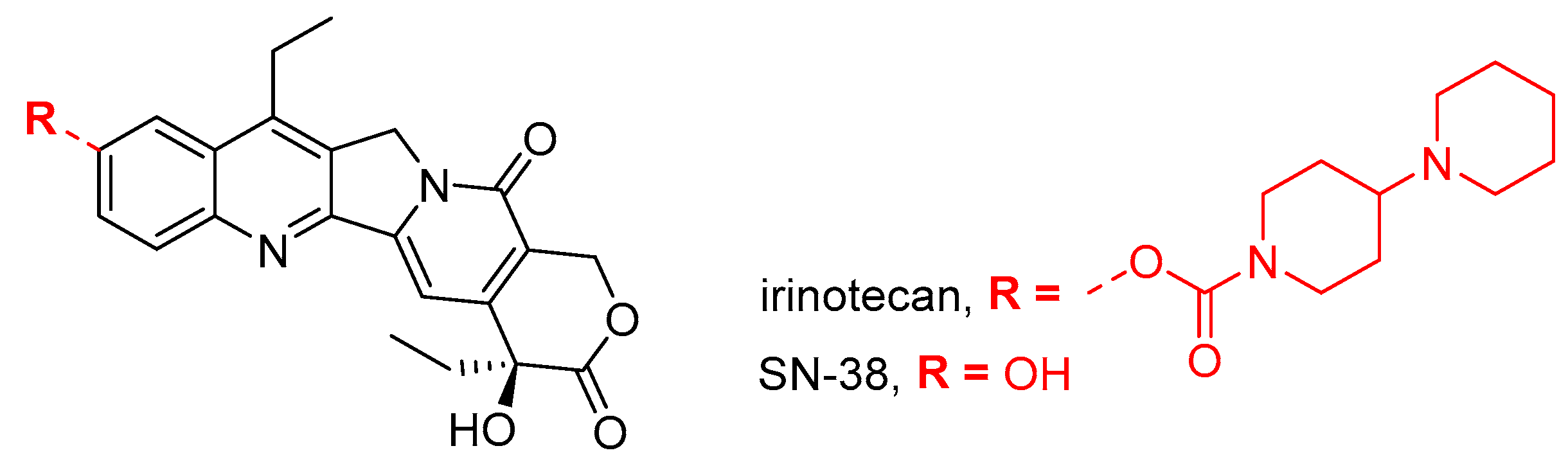


| Drug | Model | Dose | Treatment Duration | Gynotoxic Effects | Ref. |
|---|---|---|---|---|---|
| Cyclophosphamide | 3-week-old ICR mice | 100 mg/kg (i.p.) | Single dose | Decrease in the number of follicles, impaired hemostasis of oocyte quality, reduced ability to develop an embryo | [35] |
| 3-week-old B6C3F1 mice | 120 mg/kg (i.p.) | Single dose | Deterioration of oocytes, reduction and fibrosis of the ovaries | [36] | |
| Sprague–Dawley rats | 20 mg/kg (i.p.) | Once per day for 10 days | Apoptosis of ovarian cells (apoptosis index [AI] = 13.6%) | [37] | |
| Pregnant Charles Foster rats (average age: 120 days) | 2 mg/kg (i.p.) | Single dose | Prevention of folliculogenesis in offspring resulting in anovulation and infertility | [38] | |
| Pregnant 129 or L1 mice | 7.5 mg/kg (i.p.) | 10.5 and 11.5 days of pregnancy | Loss of PMFs and increased follicle growth activation in offspring | [39] | |
| Swiss albino mice | 200 mg/kg (i.p.) | Single dose on the 14th, 21st, or 28th day after birth | Long-term effects on oocyte developmental competence in early but not adult life | [40] | |
| 4–8-week-old CD-1 mice | 100 mg/kg (i.p.) | Single dose | Possible negative effects on the fertility of subsequent generations | [41] | |
| 6–8-week-old C57BL/6 mice | 70 mg/kg (i.p.) | Single dose | POF induction | [42] | |
| C57BL/6 mice | 300 mg/kg (i.p.) | Single dose | PMF depletion | [43] | |
| 8-week-old C57BL/6 mice | 100 mg/kg (i.p.) | 6 doses over 2 weeks | Loss of follicles, irreversible deterioration of oocytes | [44] | |
| Experimental xenografts of human ovaries | 75 mg/kg (i.v.) | Single dose | Apoptotic follicle death | [45] | |
| Experimental xenografts of human ovaries | 200 mg/kg (i.p.) | Single dose | Reduction in PMFs | [46] | |
| Cisplatin | 6-week-old CD-1 mice | 2 mg/kg (i.p.) | Once per day for 15 days | Reduction in the total number of follicles in the ovaries, especially PMFs | [47] |
| 8-week-old C57BL/6 mice | 2.5 mg/kg (i.p.) | Once per day for 5 days → one week break → once per day for 5 days | Increased number of atretic follicles, decreased number of antral follicles | [48] | |
| PN10 and PN50 of C57BL/6J mice | 2 or 4 mg/kg (i.p.) | Single dose | Mitochondrial dysfunction in oocytes | [49] | |
| C57BL/6 mice | 5 mg/kg (i.p.) | Single dose | PMF depletion | [43] | |
| Mature Sprague-Dawley mice | 0.5, 1, 1.5, 2, 3 or 4 mg/kg (i.p.) | Once a day for 10 days | Disorders of the estrous cycle, lack of mature follicles | [50] | |
| 5–6-week-old rats | 5 mg/kg (i.p.) | Single dose | Reduction in PMFs | [51] | |
| 5-Fluorouracil | 6–8-week-old C57BL/6 mice | 150 mg/kg (i.p.) | Single dose | Mild ovotoxic effects | [52] |
| 26–30-day-old C57BL/6J mice | 450 mg/kg (i.p.) | Single dose | Decreased survival of preantral follicles | [53] | |
| 8–9-week-old C57BL/6J mice | 125 mg/kg (i.p.) | 3-fold injection | Progressive atresia of growing follicles, reduction in ovarian volume, no change in number of PMFs | [54] | |
| 8-week-old ICR mice | 50 mg/kg (i.p.) | Once per day for 4 days | Inhibition of oocyte maturation and early embryonic development | [55] | |
| Doxorubicin | 5-day, 21-day or 8-week CD-1 mice | 10 mg/kg (i.p.) | Single dose | Ovarian reserve depletion, PMFs atresia, excessive PMF activation | [56] |
| 7–8-week-old ICR mice | 10 mg/kg (i.v.) | Single dose | Effects on oocytes | [57] | |
| 4-week-old or 7–8-week-old ICR mice | 7.5 or 10 mg/kg (i.p.) | Single dose | Reduction in the size and weight of the ovary, reduction in ovulation, reduction in the population of PMFs and secondary follicles | [58] | |
| 6-week-old or 10–22-week-old C57BL/6 or 129/Sv mice | 10 mg/kg (i.p.) | Single dose | Impaired expression of collagen IV, less differentiated luteal cells (smaller cytoplasm, reduced expression of StAR) | [59] | |
| Sprague-Dawley rats | 15 mg/kg (cumulative dose; i.p.) | 7 injections | Decrease in the number of follicles, decrease in the volume of the ovaries and the uterus | [60] | |
| Irinotecan | 2-month-old ICR mice | 100 mg/kg (i.p.) | Single dose | Mild toxicity to the ovaries | [61] |
| 8-week-old MCH mice | 20, 100 or 500 µg/mouse (i.p.) | Single dose | Increased apoptotic processes in ovarian follicles | [62] | |
| Paclitaxel | 6–8-week-old Wistar rats | 5 mg/kg (i.p.) | 5 doses at 3-day intervals | Reduction in the number of antral follicles | [63] |
| 7-week-old ICR mice | 30 mg/kg (i.p.) | Single dose | Destruction of antral follicles on day 1 after chemotherapy | [64] | |
| 2-month-old Wistar rats | 4.6 mg/kg (MTD, i.v.) | Single dose | Destruction of PMFs and follicles bilaterally and multilaterally, decreased ovarian reserve, increased fetal mortality | [65] | |
| 5–6-week-old rats | 7.5 mg/kg (i.p.) | Single dose | Reduction in the number of PMFs | [51] | |
| Docetaxel | 8-week-old CD-1 mice | 5 or 10 mg/kg (i.p.) | 2 doses (1st and 14th day) | Reduction in ovarian weight, number of secondary follicles, and total number of follicles | [66] |
| 6–8 week B6 mice | 30 mg/kg (i.p.) | Single dose | Reduction in total number of follicles, destruction of ovarian structure | [67] |
| Drug | Model | Dose | Treatment Duration | Gynotoxic Effects | Ref. |
|---|---|---|---|---|---|
| Cyclophosphamide | Metaphase II mouse oocytes | 10.25 µM | 45-min incubation | Mitochondrial membrane damage in oocytes | [68] |
| Human ovary cortex sections taken from premenopausal cancer patients | 0.5–500 µg/mL | 2–48-h incubation | Damage to granular cell nuclei, changes in basement membrane (depending on cyclophosphamide concentration) | [34] | |
| Cisplatin | Follicles from ovaries of 13-day-old C57BL/6 mice | 10−2, 10−3, or 10−4 µM | 13-day incubation | Decrease in survival and growth of follicles | [69] |
| Ovaries of newborn mice | 0.1, 0.5, 1, or 5 µg/mL | 24-h incubation | Ovarian damage, loss of follicles | [70] | |
| Ovaries of young CD-1 mice | 0.5, 1, or 5 μg/mL | 24-h incubation | Reduction in number and condition of follicles, damage to PMFs and granulosa cells | [71] | |
| Human granulosa cells (COV434, HGrC1, HLGC) | 20, 40, or 100 µg/mL | 140-h incubation | Induction of apoptosis in mitotic non-luteinized and non-mitotic luteinized granulosa cells | [72] | |
| Biopsies of human ovaries | 5 or 10 μg/mL | 6-day incubation | Deterioration of follicle health, increased cell apoptosis, reduced proliferation | [73] | |
| 5-Fluorouracil | Isolated preantral mouse ovarian follicles | 0.3, 1, 3, 10, or 30 mM | Maximum 12-day incubation | Negative effects on follicle growth, estradiol production, and oocyte maturation | [74] |
| Doxorubicin | Granulosa cells from ovaries of 21–23-day-old ICR mice | 0.4, 0.8, or 1.6 μg/mL | 24-h incubation | Induced cell apoptosis, increase in ROS levels, decrease in mitochondrial membrane potential | [75] |
| Follicles from ovaries of 7–8-week-old ICR mice | 10 µM | 2-h incubation | Effects on oocytes | [57] | |
| Multilayered secondary ovarian follicles of CD-1 mice | 2, 20, 100, or 200 nM | 24-h incubation | Dose-dependent toxicity for follicle growth and survival, as well as 17β-estradiol secretion | [76] | |
| Multilayered secondary ovarian follicles of rats | 200 nM | Single dose | Ca2+ release from the endoplasmic reticulum through activation of Src kinase | [77] | |
| Human primary granulosa cells | 0.01, 0.05, 0.1, 0.2, or 0.5 μg/mL | 48-h incubation | Induction of cytotoxicity and miR-132 expression in granulosa cells | [11] | |
| Biopsies of human ovaries | 1 or 2 μg/mL | 6-day incubation | Deterioration of follicle health, increased cell apoptosis, reduced proliferation | [73] | |
| Paclitaxel | Early secondary follicles taken from BDF1 mice | 2.5 × 10−4, 2.5 × 10−3, or 2.5 × 10−2 µM | 12-day incubation | Reduced growth of preantral or more mature follicles (depending on paclitaxel concentration) | [78] |
| Follicles from ovaries of 13-day-old C57BL/6 mice | 10−8–10−10 M | 13-day incubation | Reduced survival and growth of ovarian follicles | [69] | |
| Docetaxel | Neonatal ovaries taken from C57Bl/6J mice | 0.1, 1, or 10 µM | 24-h incubation | Negative effects on early growing follicles, reduction of PMFs, induction of somatic cell apoptosis | [79] |
| Protective Factor | Drug | Model | Protective Mechanisms | Ref. |
|---|---|---|---|---|
| Anti-Müllerian hormone | Cyclophosphamide | 6-week-old Swiss mice | Protection against loss of PMFs | [168] |
| Cyclophosphamide | 6-week-old mice | Reducing follicle activation, protecting follicle reserve, improving long-term fertility and reproductive outcomes | [169] | |
| Cyclophosphamide | 8-week-old C57/B6 mice | Protection of ovarian reserve | [170] | |
| Cyclophosphamide, carboplatin, doxorubicin | 6–7-week-old nu/nu mice | Protection of ovarian reserve by blocking activation of PMFs | [6] | |
| Cyclophosphamide | Heterotransplantation of human ovarian tissue from a 12-year-old female donor | Protection of ovarian reserve | [170] | |
| Ghrelin | Cisplatin | Wistar rats | Preservation of the number of PMFs and primary follicles | [171] |
| Cisplatin | 5-week-old ICR mice | Synergistic protective effects of ghrelin and melatonin against follicular damage and ovarian failure | [172] | |
| Luteinizing hormone (lutropin) | Cisplatin | 4- or 5-week-old mice | Protecting PMFs reserve, preserving fertility at reproductive age | [173] |
| Melatonin | Cisplatin | 7-week-old C57BL/6J mice | Mitigating ovarian damage | [174] |
| Cisplatin | 8-week-old C57BL/6 mice | Reducing toxicity to ovaries, preserving long-term fertility | [175] | |
| Cisplatin | 3-month-old Wistar rats | Modulating ovarian dysfunction, preserving fertility | [176] | |
| Cyclophosphamide | 3- or 6-week-old ICR mice | Prevention of ovarian granulosa cell loss | [177] | |
| Cyclophosphamide | 8-week-old Swiss mice | Prevention of PMFs loss, reduction of cell apoptosis and oxidative damage in the ovary | [178] | |
| Cyclophosphamide | Sprague-Dawley rats | Protecting ovaries from damage by activating the Hippo pathway | [179] | |
| Sphingolipids (C1P, S1P) | Cyclophosphamide | 6–8-week-old mice | Reduction of ovarian damage | [102] |
| Busulfan | 8-week-old FVB/NJNarl mice | Protection of PMFs, partial preservation of ovarian function | [180] | |
| Dacarbazine | 6–8-week-old BALB/c mice | Preserving the number of PMFs | [181] | |
| Cyclophosphamide, doxorubicin | Heterotransplantation of human ovarian tissue fragments | Protection of follicles from chemotherapeutic-induced apoptosis | [45] | |
| Cyclophosphamide | Human fetal ovarian xenografts | Prevention of follicle apoptosis, maintenance of the PMF population in transplants | [122] | |
| MicroRNA | Cyclophosphamide, busulfan | 6-week-old ICR mice | Prevention of follicular atresia | [182] |
| Quercetin | Cyclophosphamide | 5–6-week-old C57BL/6 mice | Reducing follicle loss and apoptosis of growing follicles | [183] |
| Cyclophosphamide | 6–8-week-old C57BL/6 mice | Protection of ovarian reserve by reversing dysfunction and activating mitochondrial biogenesis, regulating pyroptosis | [184] | |
| Cyclophosphamide | 12-week-old Sprague-Dawley rats | Restoring ovarian function, inhibiting oxidative stress | [185] | |
| Cyclophosphamide | 10–12-week-old Wistar rats | Protection of early-stage and total follicles | [186] | |
| Cisplatin | Wistar rats | Ovarian protection through antioxidant, anti-inflammatory, and anti-apoptotic activities | [187] | |
| Rapamycin (sirolimus) | Cyclophosphamide | 8-week-old BALB/c mice | Prevention of follicle activation | [109] |
| Cyclophosphamide | 5-week-old C57BL/6 mice | Protection of PMFs, reduction of follicular cell apoptosis | [188] | |
| Cisplatin | 4-week-old BALB/c mice | Protection of PMFs | [189] | |
| Resveratrol | Cyclophosphamide, busulfan | 6-week-old C57BL/6 mice | Prevention of oogonial stem cell loss, reduction of ovarian cell apoptosis | [190] |
| Cyclophosphamide | 7-week-old Sprague-Dawley rats | Prevention of PMF activation, reduction of chemotherapeutic-induced cell apoptosis | [191] | |
| Doxorubicin | 6–8-week-old C57BL/6 mice | Increase in number of primary and antral follicles, decrease in number of atretic follicles, preservation of number of PMFs | [192] | |
| Cisplatin | Sprague-Dawley rats | Protection of ovarian follicles, increasing levels of progesterone, folliculotropic hormone, and LH | [193] | |
| Cisplatin | 3-week-old Sprague-Dawley rats | Mitigation of follicle loss and AMH lowering, inhibition of inflammatory mediator elevation | [194] | |
| Cisplatin | 12–14-week-old Wistar rats | Mitigation of oxidative stress, inflammation, and cell apoptosis | [195] | |
| Cisplatin | Wistar rats | Maintaining the number of PMFs and primary follicles | [196] | |
| Bortezomib | Doxorubicin | 4-week-old CD-1 mice | Preventing accumulation of chemotherapeutics in ovaries, reducing DNA damage, prolonging fertility time | [197] |
| Dexrazoxane | Doxorubicin | 4-week-old CD-1 mice | Protection of ovaries, improvement of reproductive health | [198] |
| Goserelin | Cisplatin | 6-week-old BALB/c nu/nu mice | Shortening of estrous cycles, prolongation of estrous duration, protection of PMF and preantral follicles | [199] |
| Triptorelin | Cyclophosphamide | 10-week-old mice | Increase in the number of PMFs, primary, secondary and antral follicles | [200] |
| 5-Fluorouracil | 50-day-old Sprague-Dawley rats | Protection against ovarian damage by modulating hormones, Bax, Bcl-2, and NF-κB | [201] | |
| Imatinib | Cisplatin | 5-day CD1 mice | Oocyte protection | [119] |
| Metformin | Cyclophosphamide | 6–8-week-old C57BL/6 mice | Preservation of ovarian function and fertility in mice | [202] |
| Cyclophosphamide | 6–8-week-old C57BL/6 mice | Increase in number of antral follicles, AMH levels, and litter size | [203] | |
| Cyclophosphamide together with busulfan | 6-week-old mice | Protection against ovarian damage, reducing oxidative damage and inflammation | [204] | |
| Carboplatin | 3-month-old Wistar rats | Increased AMH levels and number of PMFs | [205] | |
| Tamoxifen | Cyclophosphamide, doxorubicin | 4–6-week-old Sprague-Dawley rats | Reduction in follicle loss, improvement in reproductive function | [206] |
| Protective Factor | Drug | Model | Protective Mechanisms | Ref. |
|---|---|---|---|---|
| Anti-Müllerian hormone | Cyclophosphamide | Human ovarian cortex biopsies | Reduction in follicle damage (by about half) and PI3K activation | [207] |
| MicroRNA | Cyclophosphamide (active metabolite) | Mouse ovaries | Reduction of ovarian cell apoptosis | [208] |
| Rapamycin (sirolimus) | Cisplatin | Rat ovaries | Inhibition of activation and protection of PMFs | [189] |
| Resveratrol | Cyclophosphamide (active metabolite) | Primary cultures of rat granulosa cells | Reduction of oxidative stress levels | [209] |
| Dexrazoxane | Doxorubicin | Mouse cell line KK-15 | Protection against DNA damage, increase in cell viability | [210] |
| Doxorubicin | Marmoset ovarian tissue | Protection from DSBs, increase in number of granulosa cells in antral follicles | [211] | |
| Imatinib | Cisplatin | Ovaries of newborn mice | Protection of ovarian follicles | [70] |
| Cisplatin | In vitro culture and subrenal grafting of mouse ovaries | Oocyte protection | [212] | |
| Cyclophosphamide | Preantral mouse follicles | Protection of AMH production, no effect on metaphase II oocyte retrieval | [213] | |
| Tamoxifen | Cyclophosphamide | Ovaries of newborn rats | Counteracting follicle loss | [214] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Gynotoxic Effects of Chemotherapy and Potential Protective Mechanisms. Cancers 2024, 16, 2288. https://doi.org/10.3390/cancers16122288
Markowska A, Antoszczak M, Markowska J, Huczyński A. Gynotoxic Effects of Chemotherapy and Potential Protective Mechanisms. Cancers. 2024; 16(12):2288. https://doi.org/10.3390/cancers16122288
Chicago/Turabian StyleMarkowska, Anna, Michał Antoszczak, Janina Markowska, and Adam Huczyński. 2024. "Gynotoxic Effects of Chemotherapy and Potential Protective Mechanisms" Cancers 16, no. 12: 2288. https://doi.org/10.3390/cancers16122288
APA StyleMarkowska, A., Antoszczak, M., Markowska, J., & Huczyński, A. (2024). Gynotoxic Effects of Chemotherapy and Potential Protective Mechanisms. Cancers, 16(12), 2288. https://doi.org/10.3390/cancers16122288








