Does a “Western Lifestyle” Confer a Higher Burden of Colorectal Cancer? A Comparison of EU15+ Countries versus Global Trends between 1990 and 2019
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Data Source
2.2. Handling of the GBD Data
2.3. Statistical Analysis
3. Results:
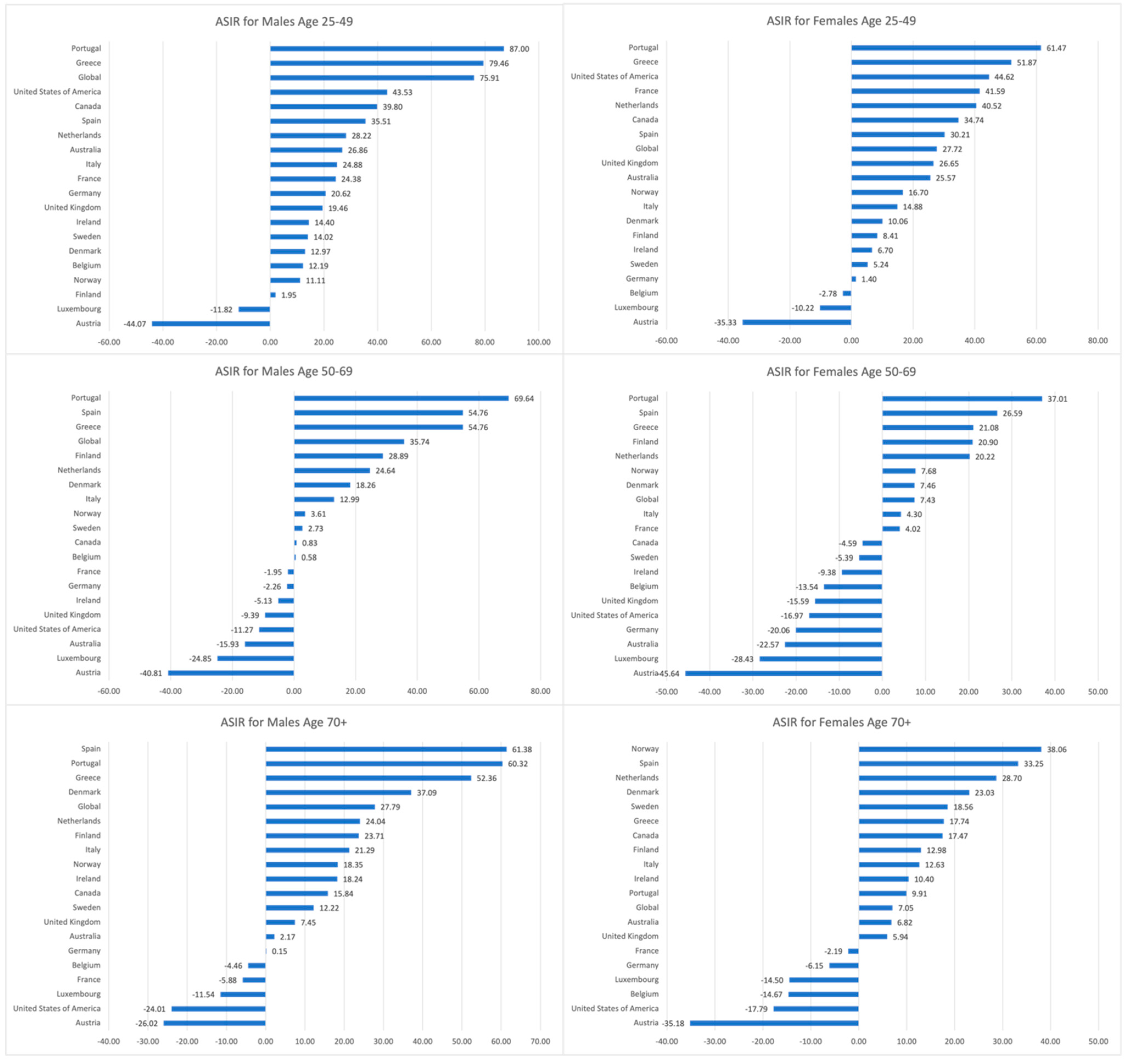
3.1. Trends in CRC ASIRs, 1990–2019
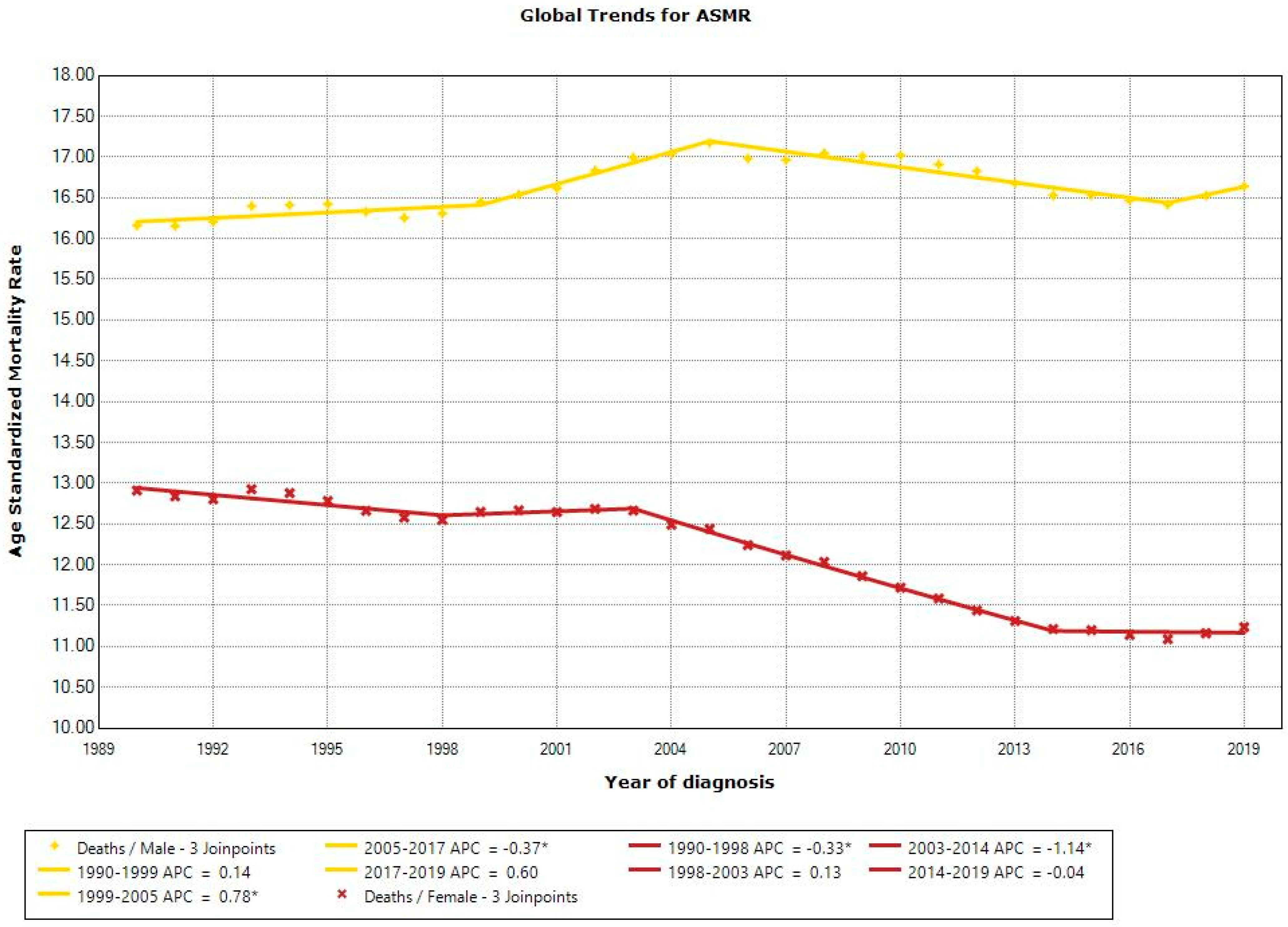
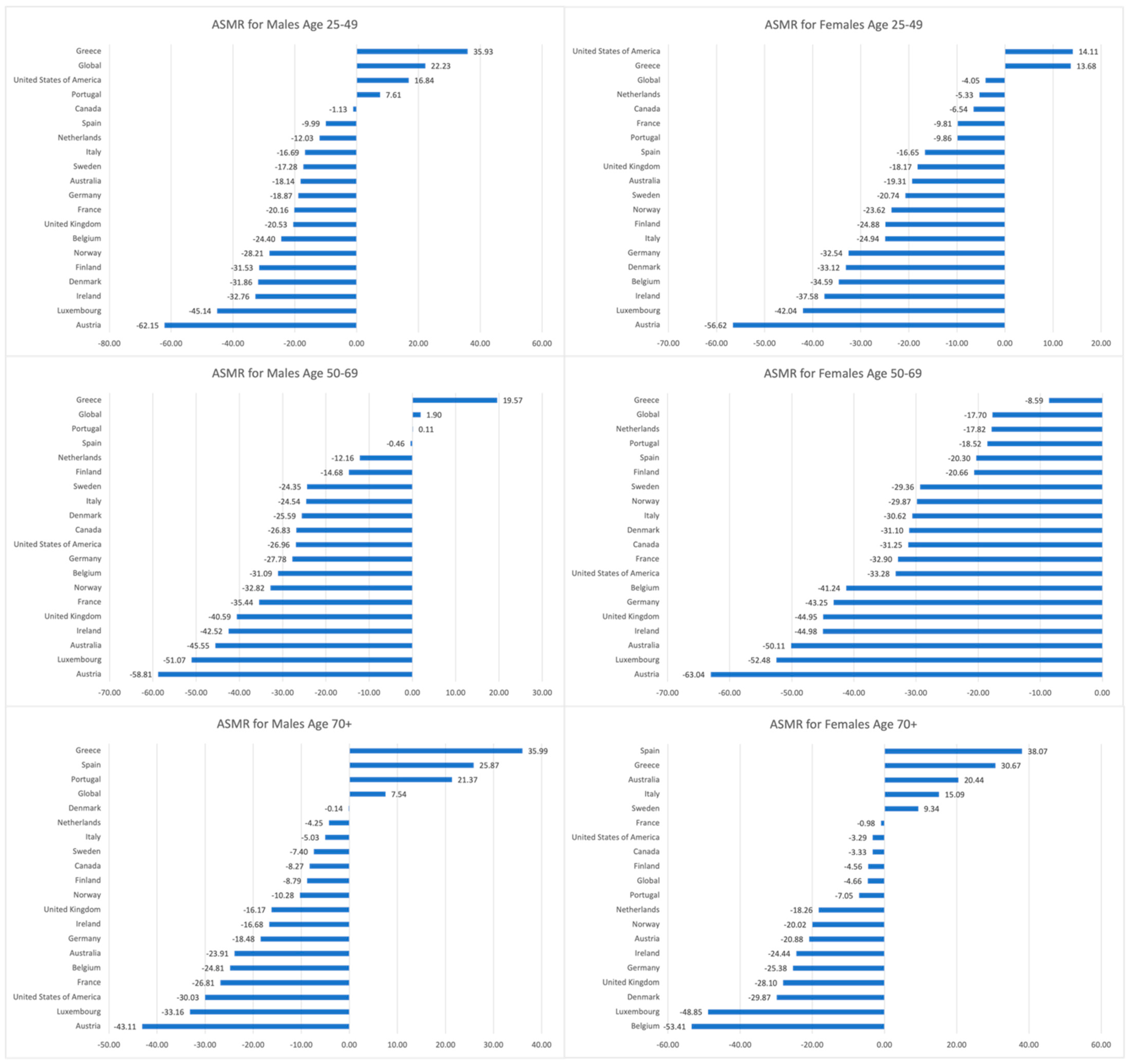
3.2. Trends in CRC ASMRs, 1990–2019
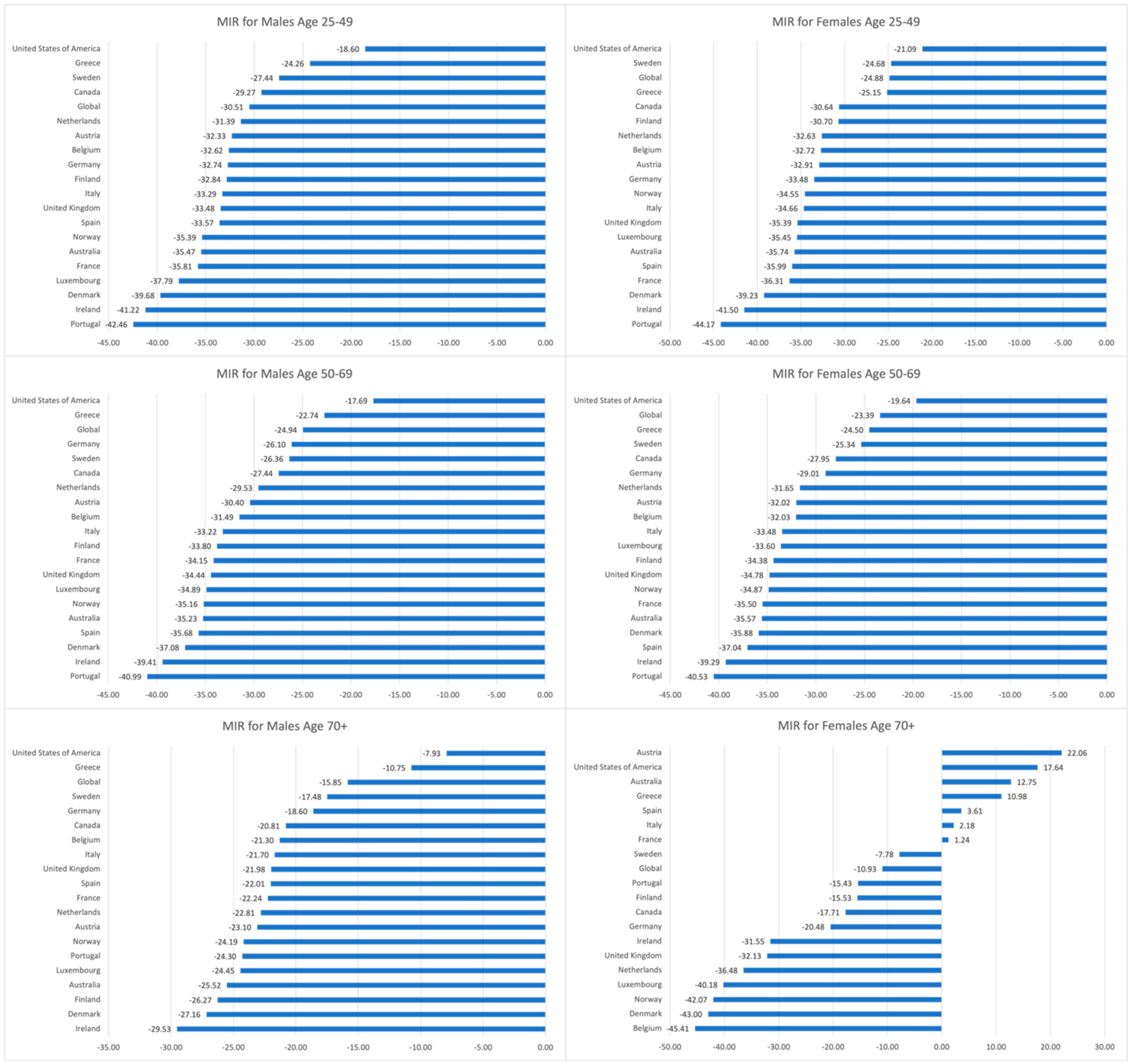
3.3. Trends in CRC MIRs, 1990–2019
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal carcinoma: Pathologic aspects. J. Gastrointest. Oncol. 2012, 3, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; De Schutter, H.; Van Damme, N.; Valerianova, Z.; Atanasov, T.; Majek, O.; et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: An international population-based study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, A.B.; Rutter, C.M.; Peterse, E.F.P.; Lietz, A.P.; Seguin, C.L.; Meester, R.G.S.; Perdue, L.A.; Lin, J.S.; Siegel, R.L.; Doria-Rose, V.P.; et al. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. JAMA 2021, 325, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Wei, E.K.; Giovannucci, E.; Wu, K.; Rosner, B.; Fuchs, C.S.; Willett, W.C.; Colditz, G.A. Comparison of risk factors for colon and rectal cancer. Int. J. Cancer 2004, 108, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Rajesh Sharma, M.A.-K.; Abd-Rabu, R.; Abidi, H.; Abu-Gharbieh, E.; Acuna, J.M.; Adhikari, S.; Advani, S.M.; Afzal, M.S.; Meybodi, M.A.; Ahinkorah, B.O.; et al. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef]
- Goodall, R.; Salciccioli, J.D.; Davies, A.H.; Marshall, D.; Shalhoub, J. Trends in peripheral arterial disease incidence and mortality in EU15+ countries 1990–2017. Eur. J. Prev. Cardiol. 2021, 28, 1201–1213. [Google Scholar] [CrossRef]
- Salciccioli, J.D.; Marshall, D.C.; Shalhoub, J.; Maruthappu, M.; De Carlo, G.; Chung, K.F. Respiratory disease mortality in the United Kingdom compared with EU15+ countries in 1985–2015: Observational study. BMJ 2018, 363, k4680. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Study 2019 (GBD 2019) Data Resources. Available online: https://ghdx.healthdata.org/gbd-2019 (accessed on 9 June 2024).
- Reford, S.; Alexander, L. What Data Sources Go into the GBD? Available online: https://www.healthdata.org/news-events/insights-blog/acting-data/what-data-sources-go-gbd#:~:text=The%20GBD%20estimates%20published%20in%202017%2C%20covering%20the,story%20of%20illness%20and%20health%20around%20the%20world (accessed on 9 June 2024).
- Jani, C.; Abdallah, N.; Mouchati, C.; Jani, R.; Sharma, R.; Bhatt, P.; Hanbury, G.; Salciccioli, J.; Singh, H.; Shalhoub, J.; et al. Trends of kidney cancer burden from 1990 to 2019 in European Union 15+ countries and World Health Organization regions. Sci. Rep. 2022, 12, 22368. [Google Scholar] [CrossRef] [PubMed]
- Jani, C.; Marshall, D.C.; Singh, H.; Goodall, R.; Shalhoub, J.; Al Omari, O.; Salciccioli, J.D.; Thomson, C.C. Lung cancer mortality in Europe and the USA between 2000 and 2017: An observational analysis. ERJ Open Res. 2021, 7. [Google Scholar] [CrossRef]
- Jani, C.; Salcicciol, I.; Rupal, A.; Al Omari, O.; Goodall, R.; Salciccioli, J.D.; Marshall, D.C.; Hanbury, G.; Singh, H.; Weissmann, L.; et al. Trends in Breast Cancer Mortality Between 2001 and 2017: An Observational Study in the European Union and the United Kingdom. JCO Glob. Oncol. 2021, 7, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.V. Five insights from the Global Burden of Disease Study 2019. Lancet 2020, 396, 1135–1159. [Google Scholar] [CrossRef]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Sninsky, J.A.; Shore, B.M.; Lupu, G.V.; Crockett, S.D. Risk Factors for Colorectal Polyps and Cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 195–213. [Google Scholar] [CrossRef]
- Levin, B.; Lieberman, D.A.; McFarland, B.; Smith, R.A.; Brooks, D.; Andrews, K.S.; Dash, C.; Giardiello, F.M.; Glick, S.; Levin, T.R.; et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J. Clin. 2008, 58, 130–160. [Google Scholar] [CrossRef] [PubMed]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Haidinger, G.; Waldhoer, T.; Vutuc, C. Self-reported colonoscopy screening in Austria. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. (ECP) 2008, 17, 354–357. [Google Scholar] [CrossRef]
- Cardoso, R.; Guo, F.; Heisser, T.; Hoffmeister, M.; Brenner, H. Utilisation of Colorectal Cancer Screening Tests in European Countries by Type of Screening Offer: Results from the European Health Interview Survey. Cancers 2020, 12, 1409. [Google Scholar] [CrossRef] [PubMed]
- Senore, C.; Basu, P.; Anttila, A.; Ponti, A.; Tomatis, M.; Vale, D.B.; Ronco, G.; Soerjomataram, I.; Primic-Zakelj, M.; Riggi, E.; et al. Performance of colorectal cancer screening in the European Union Member States: Data from the second European screening report. Gut 2019, 68, 1232–1244. [Google Scholar] [CrossRef]
- Ola, I.; Cardoso, R.; Hoffmeister, M.; Brenner, H. Utilization of colorectal cancer screening tests across European countries: A cross-sectional analysis of the European health interview survey 2018–2020. Lancet Reg. Health Eur. 2024, 41, 100920. [Google Scholar] [CrossRef]
- Quintero, E.; Castells, A.; Bujanda, L.; Cubiella, J.; Salas, D.; Lanas, A.; Andreu, M.; Carballo, F.; Morillas, J.D.; Hernandez, C.; et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N. Engl. J. Med. 2012, 366, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Zauber, A.G. The impact of screening on colorectal cancer mortality and incidence: Has it really made a difference? Dig. Dis. Sci. 2015, 60, 681–691. [Google Scholar] [CrossRef]
- Gini, A.; Jansen, E.E.L.; Zielonke, N.; Meester, R.G.S.; Senore, C.; Anttila, A.; Segnan, N.; Mlakar, D.N.; de Koning, H.J.; Lansdorp-Vogelaar, I.; et al. Impact of colorectal cancer screening on cancer-specific mortality in Europe: A systematic review. Eur. J. Cancer 2020, 127, 224–235. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Hendifar, A.; Yang, D.; Lenz, F.; Lurje, G.; Pohl, A.; Lenz, C.; Ning, Y.; Zhang, W.; Lenz, H.J. Gender disparities in metastatic colorectal cancer survival. Clin. Cancer Res. 2009, 15, 6391–6397. [Google Scholar] [CrossRef] [PubMed]
- Silla, I.O.; Rueda, D.; Rodriguez, Y.; Garcia, J.L.; de la Cruz Vigo, F.; Perea, J. Early-onset colorectal cancer: A separate subset of colorectal cancer. World J. Gastroenterol. 2014, 20, 17288–17296. [Google Scholar] [CrossRef]
- Mauri, G.; Sartore-Bianchi, A.; Russo, A.G.; Marsoni, S.; Bardelli, A.; Siena, S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2019, 13, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Jakubowski, C.D.; Fedewa, S.A.; Davis, A.; Azad, N.S. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. In American Society of Clinical Oncology Educational Book; ASCO: Alexandria, VA, USA, 2020; Volume 40, pp. 1–14. [Google Scholar] [CrossRef]
- Kim, S.E.; Paik, H.Y.; Yoon, H.; Lee, J.E.; Kim, N.; Sung, M.K. Sex- and gender-specific disparities in colorectal cancer risk. World J. Gastroenterol. 2015, 21, 5167–5175. [Google Scholar] [CrossRef] [PubMed]
- Overview of the SEER Program. Available online: https://seer.cancer.gov/about/overview.html#:~:text=SEER%20coverage%20includes%2042.0%20percent,percent%20of%20Hawaiian%2FPacific%20Islanders (accessed on 9 June 2024).
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 1990–2019 (GBD 1990–2019) Results; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2020; Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 4 May 2024).
- Burger, E.H.; van der Merwe, L.; Volmink, J. Errors in the completion of the death notification form. S. Afr. Med. J. 2007, 97, 1077–1081. [Google Scholar] [PubMed]
- Lu, T.H.; Shau, W.Y.; Shih, T.P.; Lee, M.C.; Chou, M.C.; Lin, C.K. Factors associated with errors in death certificate completion. A national study in Taiwan. J. Clin. Epidemiol. 2001, 54, 232–238. [Google Scholar] [CrossRef]
- Katsakiori, P.F.; Panagiotopoulou, E.C.; Sakellaropoulos, G.C.; Papazafiropoulou, A.; Kardara, M. Errors in death certificates in a rural area of Greece. Rural Remote Health 2007, 7, 822. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, B.; Jani, C.T.; Liu, W.; Punjwani, S.; Kareff, S.; Ceglowski, P.; Singh, H.; Mariano, M.; Salciccioli, J.D.; Borges, L.; et al. Does a “Western Lifestyle” Confer a Higher Burden of Colorectal Cancer? A Comparison of EU15+ Countries versus Global Trends between 1990 and 2019. Cancers 2024, 16, 2277. https://doi.org/10.3390/cancers16122277
Walker B, Jani CT, Liu W, Punjwani S, Kareff S, Ceglowski P, Singh H, Mariano M, Salciccioli JD, Borges L, et al. Does a “Western Lifestyle” Confer a Higher Burden of Colorectal Cancer? A Comparison of EU15+ Countries versus Global Trends between 1990 and 2019. Cancers. 2024; 16(12):2277. https://doi.org/10.3390/cancers16122277
Chicago/Turabian StyleWalker, Bradley, Chinmay T. Jani, Weitao Liu, Shoheera Punjwani, Samuel Kareff, Peter Ceglowski, Harpreet Singh, Melissa Mariano, Justin D. Salciccioli, Lawrence Borges, and et al. 2024. "Does a “Western Lifestyle” Confer a Higher Burden of Colorectal Cancer? A Comparison of EU15+ Countries versus Global Trends between 1990 and 2019" Cancers 16, no. 12: 2277. https://doi.org/10.3390/cancers16122277
APA StyleWalker, B., Jani, C. T., Liu, W., Punjwani, S., Kareff, S., Ceglowski, P., Singh, H., Mariano, M., Salciccioli, J. D., Borges, L., & Lopes, G. (2024). Does a “Western Lifestyle” Confer a Higher Burden of Colorectal Cancer? A Comparison of EU15+ Countries versus Global Trends between 1990 and 2019. Cancers, 16(12), 2277. https://doi.org/10.3390/cancers16122277





