The Anticancer Application of Delivery Systems for Honokiol and Magnolol
Abstract
Simple Summary
Abstract
1. Introduction
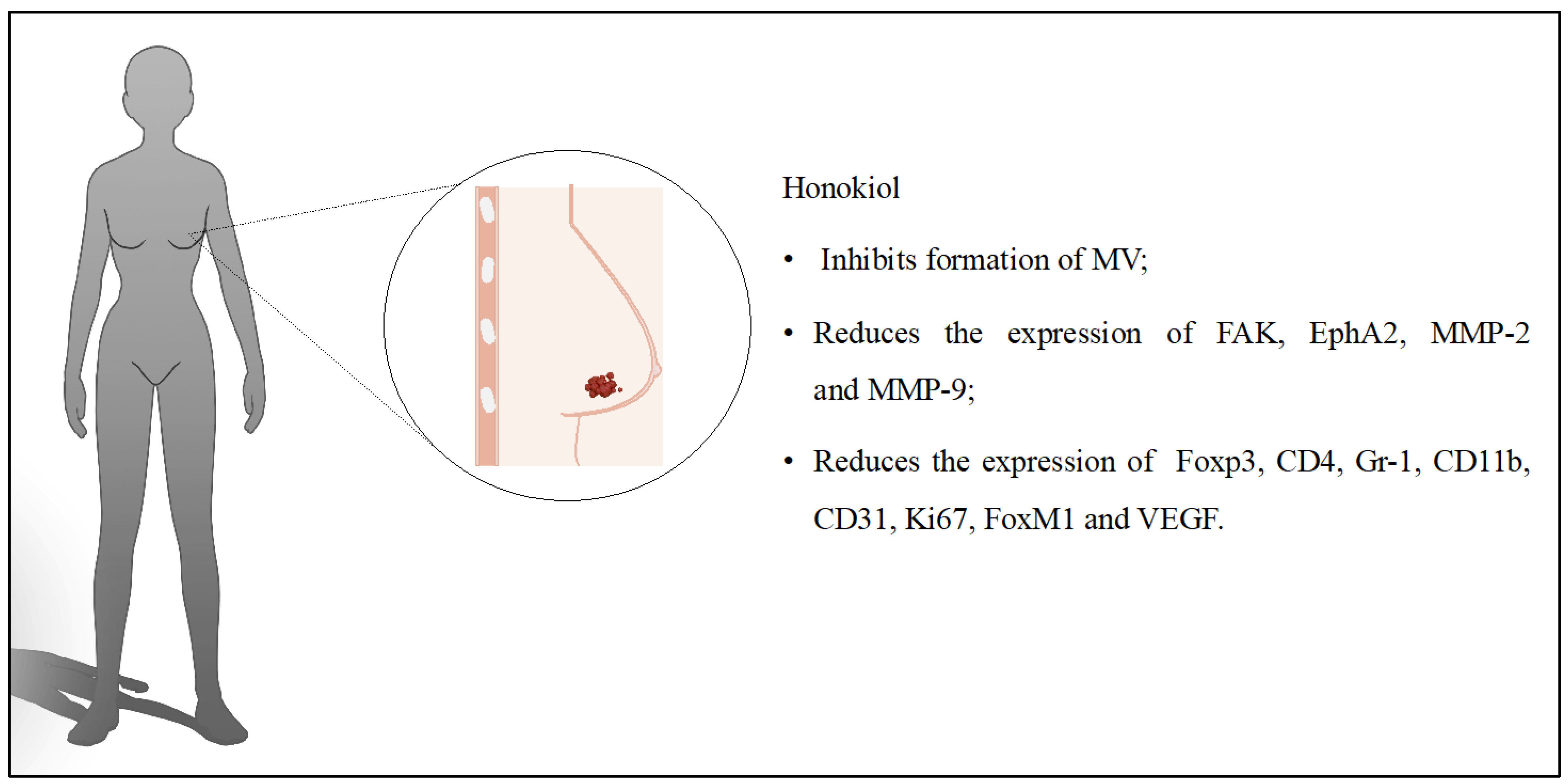
2. Breast Cancer
3. Glioblastoma
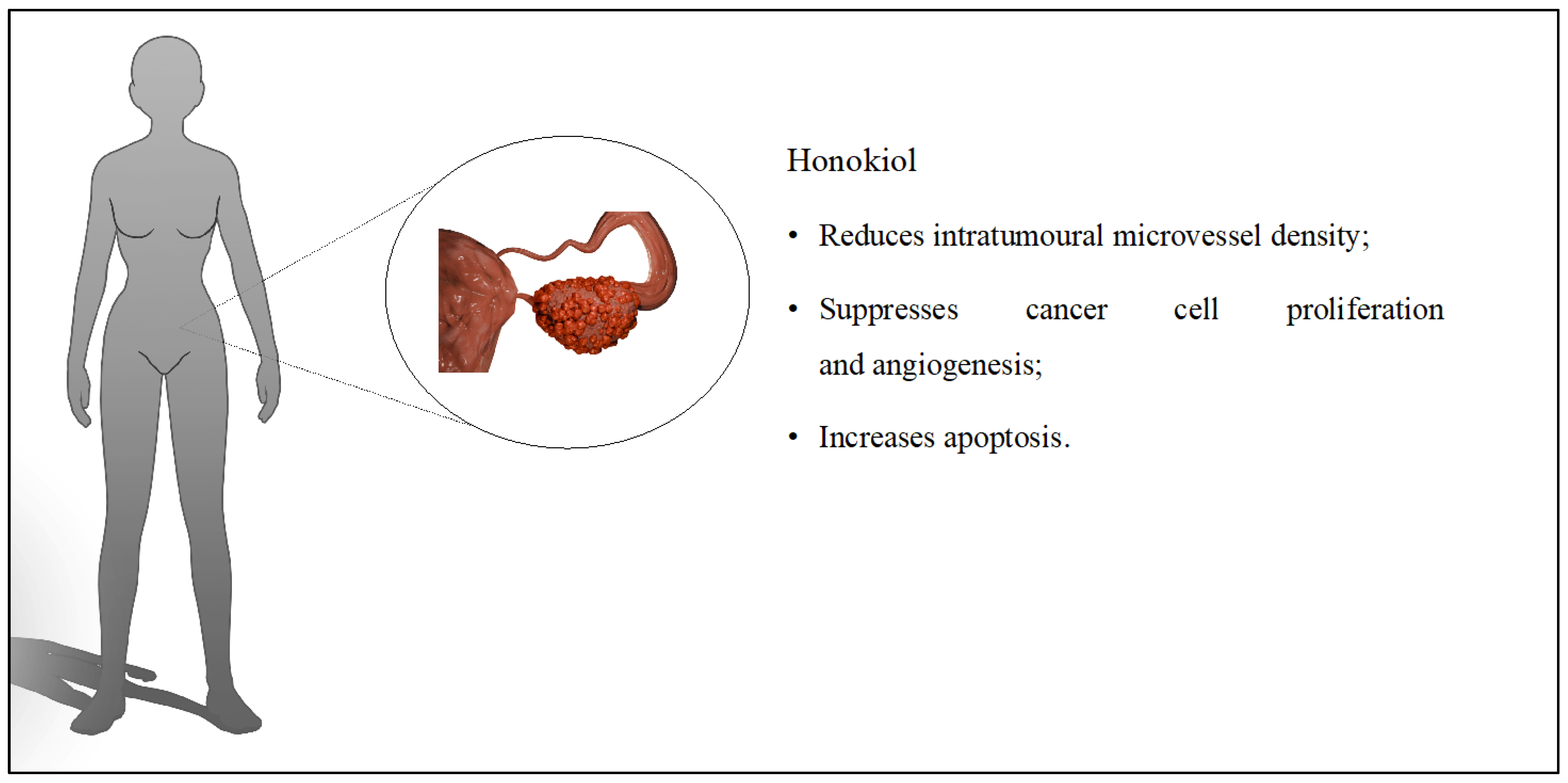
4. Ovarian Cancer
5. Lung Cancer
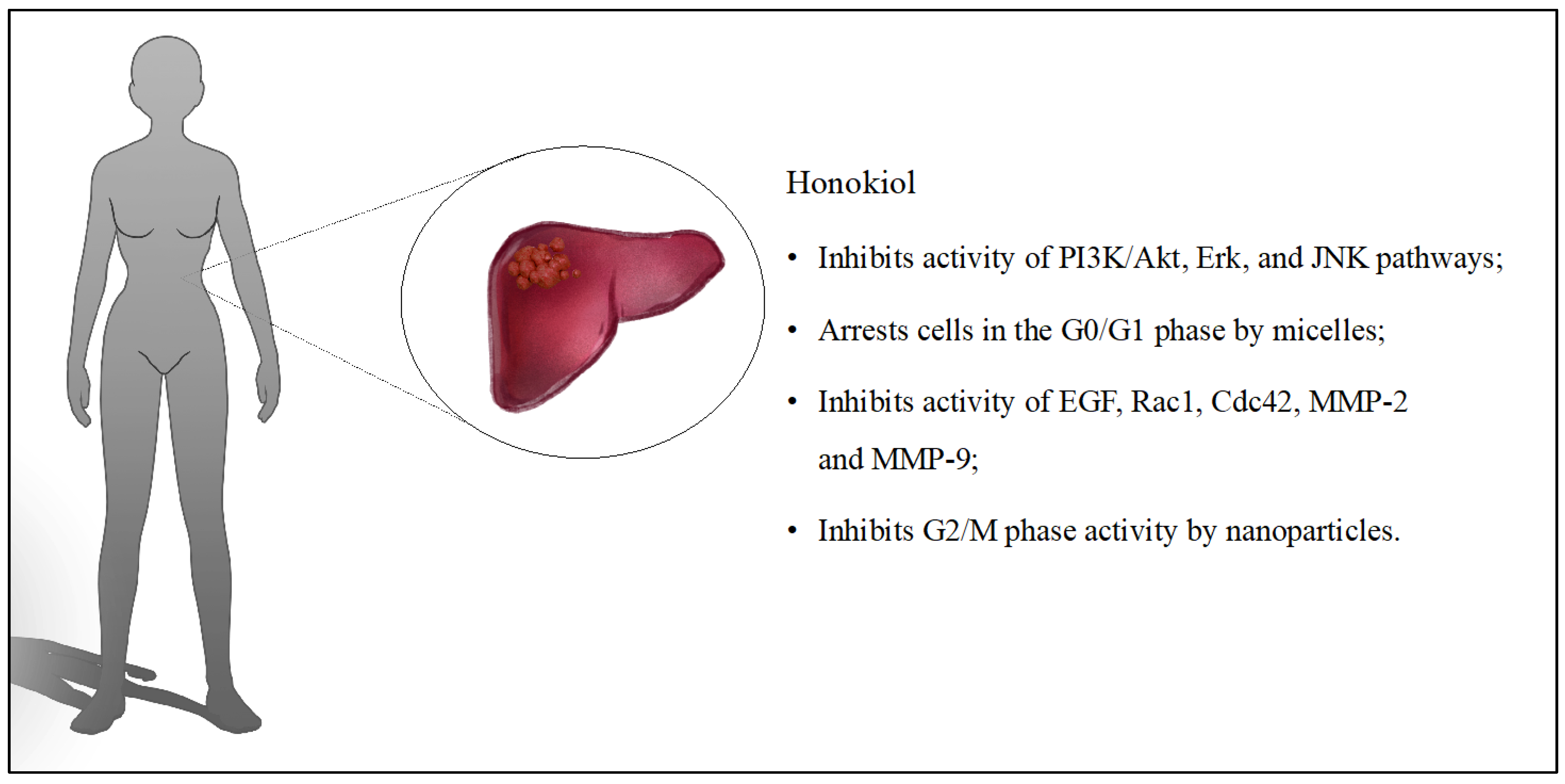
6. Liver Cancer
7. Other Cancers
8. Other Cancer-Related Effects
9. Anticancer Effect of Magnolol—Positional Isomer of Honokiol
10. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and Toxicology of Magnolol and Honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, J.; Zhang, W.; An, Q.; Wen, J.; Wang, A.; Jin, H.; Chen, S. Acute and sub-chronic toxicity studies of honokiol microemulsion. Regul. Toxicol. Pharmacol. 2015, 71, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Usach, I.; Alaimo, A.; Fernández, J.; Ambrosini, A.; Mocini, S.; Ochiuz, L.; Peris, J.E. Magnolol and Honokiol: Two Natural Compounds with Similar Chemical Structure but Different Physicochemical and Stability Properties. Pharmaceutics 2021, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- Faysal, M.; Khan, J.; Zehravi, M.; Nath, N.; Singh, L.P.; Kakkar, S.; Perusomula, R.; Khan, P.A.; Nainu, F.; Asiri, M.; et al. Neuropharmacological Potential of Honokiol and Its Derivatives from Chinese Herb Magnolia Species: Understandings from Therapeutic Viewpoint. Chin. Med. 2023, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Duan, X.; Yang, G.; Zhang, X.; Deng, L.; Zheng, H.; Deng, C.; Wen, J.; Wang, N.; Peng, C.; et al. Honokiol Crosses BBB and BCSFB, and Inhibits Brain Tumor Growth in Rat 9l Intracerebral Gliosarcoma Model and Human U251 Xenograft Glioma Model. PLoS ONE 2011, 6, e18490. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.U.; Kim, J.H.; Kong, T.Y.; Choi, W.G.; Lee, H.S. Comparative Metabolism of Honokiol in Mouse, Rat, Dog, Monkey, and Human Hepatocytes. Arch. Pharm. Res. 2016, 39, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Gong, H.; Xu, J.; Huang, Y.; Wu, F.; He, Z. Nanomedicines for Overcoming Cancer Drug Resistance. Pharmaceutics 2022, 14, 1606. [Google Scholar] [CrossRef]
- The Lifetime Risk (Ages 0–74) of Developing Breast Cancer Most Common Cancer Causes of Death for Women in the eu Breast Cancer in the EU. 2023, Volume 800. Available online: https://ecis.jrc.ec.europa.eu/pdf/factsheets/Breast_cancer_2022-Oct_2023.pdf (accessed on 20 April 2024).
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast Cancer: Biology, Biomarkers, and Treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef]
- Li, X.; Guan, S.; Li, H.; Li, D.; Liu, D.; Wang, J.; Zhu, W.; Xing, G.; Yue, L.; Cai, D.; et al. Polysialic Acid-Functionalized Liposomes for Efficient Honokiol Delivery to Inhibit Breast Cancer Growth and Metastasis. Drug Deliv. 2023, 30, 2181746. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Guan, S.; Zhu, W.; Fan, L.; Zhang, Q.; Cai, D. Hyaluronic Acid-Modified Liposomal Honokiol Nanocarrier: Enhance Anti-Metastasis and Antitumor Efficacy against Breast Cancer. Carbohydr. Polym. 2020, 235, 115981. [Google Scholar] [CrossRef]
- Hou, W.; Chen, L.; Yang, G.; Zhou, H.; Jiang, Q.; Zhong, Z.; Hu, J.; Chen, X.; Wang, X.; Yuan, Y.; et al. Synergistic Antitumor Effects of Liposomal Honokiol Combined with Adriamycin in Breast Cancer Models. Phytother. Res. 2008, 22, 1125–1132. [Google Scholar] [CrossRef]
- Ju, R.J.; Cheng, L.; Qiu, X.; Liu, S.; Song, X.L.; Peng, X.M.; Wang, T.; Li, C.Q.; Li, X.T. Hyaluronic Acid Modified Daunorubicin plus Honokiol Cationic Liposomes for the Treatment of Breast Cancer along with the Elimination Vasculogenic Mimicry Channels. J. Drug Target. 2018, 26, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Z.; Nie, S.; Song, L.; He, T.; Yang, S.; Yang, X.; Yi, C.; Wu, Q.; Gong, C. Biodegradable Polymeric Micelles Coencapsulating Paclitaxel and Honokiol: A Strategy for Breast Cancer Therapy in Vitro and in Vivo. Int. J. Nanomed. 2017, 12, 1499–1514. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Wang, D.; Zou, Y.; Qu, X.; He, C.; Deng, Y.; Jin, Y.; Zhou, Y.; Zhou, Y.; et al. Concurrently Suppressing Multidrug Resistance and Metastasis of Breast Cancer by Co-Delivery of Paclitaxel and Honokiol with PH-Sensitive Polymeric Micelles. Acta Biomater. 2017, 62, 144–156. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, Y.; Jin, Y.; He, C.; Deng, Y.; Han, S.; Zhou, C.; Li, X.; Zhou, Y.; Liu, Y. Synergistically Enhanced Antimetastasis Effects by Honokiol-Loaded PH-Sensitive Polymer-Doxorubicin Conjugate Micelles. ACS Appl. Mater. Interfaces 2018, 10, 18585–18600. [Google Scholar] [CrossRef]
- Godugu, C.; Doddapaneni, R.; Singh, M. Honokiol Nanomicellar Formulation Produced Increased Oral Bioavailability and Anticancer Effects in Triple Negative Breast Cancer (TNBC). Colloids Surf. B Biointerfaces 2017, 153, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Liu, Q.; Jiang, M.; Yang, M.; Zhan, S.; Qiu, T.; He, K.; Zhang, X. Tuning MPEG-PLA/Vitamin E-TPGS-Based Mixed Micelles for Combined Celecoxib/Honokiol Therapy for Breast Cancer. Eur. J. Pharm. Sci. 2020, 146, 105277. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y.A.; Ibrahim, R.R.; Hafiz, A.A. Design, Formulation and in Vivo Evaluation of Novel Honokiol-Loaded PEGylated PLGA Nanocapsules for Treatment of Breast Cancer. Int. J. Nanomed. 2020, 15, 1625–1642. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, D.; Guan, S.; Liu, D.; Wang, J.; Xing, G.; Yue, L.; Cai, D. Tumor-Targeted Delivery of Honokiol via Polysialic Acid Modified Zein Nanoparticles Prevents Breast Cancer Progression and Metastasis. Int. J. Biol. Macromol. 2022, 203, 280–291. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Liu, D.; Zhu, W.; Guan, S.; Fan, L.; Cai, D. Targeted Delivery of Honokiol by Zein/Hyaluronic Acid Core-Shell Nanoparticles to Suppress Breast Cancer Growth and Metastasis. Carbohydr. Polym. 2020, 240, 116325. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.Q.; Zou, Y.; Liu, B.; Guo, Y.; Wang, X.; Han, M. Surface Modification of PH-Sensitive Honokiol Nanoparticles Based on Dopamine Coating for Targeted Therapy of Breast Cancer. Colloids Surf. B Biointerfaces 2019, 177, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.; Andrews, N.G.; Raghavan, V. Honokiol-Camptothecin Loaded Graphene Oxide Nanoparticle towards Combinatorial Anticancer Drug Delivery. IET Nanobiotechnol. 2020, 14, 796–802. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Yuan, R.; Li, Y.; Zhang, Y.; Hu, X.; Qu, J.; Chen, Y.; Wang, Z.; Xia, M.; et al. Augment the Efficacy of Eradicating Metastatic Lesions and Tumor Proliferation in Breast Cancer by Honokiol-Loaded PH-Sensitive Targeted Lipid Nanoparticles. Colloids Surf. B Biointerfaces 2021, 207, 112008. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, Y.; Wang, T.; Zhao, S.; Qiu, H.; Han, M.; Wang, X. Honokiol Nanoparticles Stabilized by Oligoethylene Glycols Codendrimer: In Vitro and in Vivo Investigations. J. Mater. Chem. B 2017, 5, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Lu, X.; Yang, P.; Zhang, Z.; Lv, H. Honokiol Nanosuspensions Loaded Thermosensitive Hydrogels as the Local Delivery System in Combination with Systemic Paclitaxel for Synergistic Therapy of Breast Cancer. Eur. J. Pharm. Sci. 2022, 175, 106212. [Google Scholar] [CrossRef]
- Won, K.A.; Spruck, C. Triple-negative Breast Cancer Therapy: Current and Future Perspectives. Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhang, J.; Jiang, J.; He, Y.; Zhang, W.; Mo, X.; Kang, X.; Xu, Q.; Wang, B.; Huang, Y. Remodeling Tumor Immune Microenvironment (TIME) for Glioma Therapy Using Multi-Targeting Liposomal Codelivery. J. Immunother. Cancer 2020, 8, e000207. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Chen, J.; Fan, Y.; Wang, C.; Du, Y.; Guo, C.; Chen, F.; Li, W. Liposomal Honokiol Inhibits Glioblastoma Growth through Regulating Macrophage Polarization. Ann. Transl. Med. 2021, 9, 1644. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.M.; Ju, R.J.; Xiao, Y.; Wang, X.; Song, X.L.; Gu, L.Y.; Cheng, L.; Li, X.T.; Chen, G.R. Antitumor Efficacy of Lf Modified Daunorubicin plus Honokiol Liposomes in Treatment of Brain Glioma. Eur. J. Pharm. Sci. 2017, 106, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yu, T.; Xu, G.; Guo, G.; Liu, X.; Hu, X.; Wang, X.; Liu, Y.; Mao, Q.; You, C.; et al. Enhancing the Anti-Glioma Therapy of Doxorubicin by Honokiol with Biodegradable Self-Assembling Micelles through Multiple Evaluations. Sci. Rep. 2017, 7, 43501. [Google Scholar] [CrossRef]
- Wang, C.; Cai, Z.; Huang, Y.; Liu, X.; Liu, X.; Chen, F.; Li, W. Honokiol in Glioblastoma Recurrence: A Case Report. Front. Neurol. 2023, 14, 1172860. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, W.; Chen, T.; Yang, Q.; Huang, T.; Fu, Y.; Gong, T.; Zhang, Z. Hyaluronic Acid-Modified Micelles Encapsulating Gem-C 12 and HNK for Glioblastoma Multiforme Chemotherapy. Mol. Pharm. 2018, 15, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.H.; Hsu, Y.C.; Chang, K.C.; Shyong, Y.J. Porous Hydroxyapatite Carrier Enables Localized and Sustained Delivery of Honokiol for Glioma Treatment. Eur. J. Pharm. Biopharm. 2023, 189, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Rauh-Hain, J.A.; Krivak, T.C.; Del Carmen, M.G.; Olawaiye, A.B. Ovarian Cancer Screening and Early Detection in the General Population. Rev. Obstet. Gynecol. 2011, 4, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhong, Q.; Chen, L.J.; Qi, X.R.; Fu, A.F.; Yang, H.S.; Yang, F.; Lin, H.G.; Wei, Y.Q.; Zhao, X. Liposomal Honokiol, a Promising Agent for Treatment of Cisplatin-Resistant Human Ovarian Cancer. J. Cancer Res. Clin. Oncol. 2008, 134, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, L.; He, X.; Fan, L.; Yang, G.; Chen, X.; Lin, X.; Du, L.; Li, Z.; Ye, H.; et al. Enhancement of Therapeutic Effectiveness by Combining Liposomal Honokiol with Cisplatin in Ovarian Carcinoma. Int. J. Gynecol. Cancer 2008, 18, 652–659. [Google Scholar] [CrossRef]
- Wang, B.; Gou, M.; Zheng, X.; Wei, X.; Gong, C.; Wang, X.; Zhao, Y.; Luo, F.; Chen, L.; Qian, Z.; et al. Co-Delivery Honokiol and Doxorubicin in MPEG-PLA Nanoparticles. J. Nanosci. Nanotechnol. 2010, 10, 4166–4172. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung Cancer: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef]
- Min Song, J.; Anandharaj, A.; Upadhyaya, P.; Kirtane, A.R.; Kim, J.-H.; Ho Hong, K.; Panyam, J.; Kassie, F. Honokiol suppresses lung tumorigenesis by targeting EGFR and its downstream effectors. Oncotarget 2016, 7, 57752–57769. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Ju, R.J.; Xiao, Y.; Wang, X.; Liu, S.; Fu, M.; Liu, J.J.; Gu, L.Y.; Li, X.T.; Cheng, L. Application of Multifunctional Targeting Epirubicin Liposomes in the Treatment of Non-Small-Cell Lung Cancer. Int. J. Nanomed. 2017, 12, 7433–7451. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.Q.; Fan, L.Y.; Yang, G.L.; Guo, W.H.; Hou, W.L.; Chen, L.J.; Wei, Y.Q. Improved Therapeutic Effectiveness by Combining Liposomal Honokiol with Cisplatin in Lung Cancer Model. BMC Cancer 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, L.J.; Liu, L.; Chen, X.; Chen, P.; Yang, G.L.; Hou, W.L.; Tang, M.H.; Zhang, F.; Wang, X.H.; et al. Liposomal Honokiol, a Potent Anti-Angiogenesis Agent, in Combination with Radiotherapy Produces a Synergistic Antitumor Efficacy without Increasing Toxicity. Exp. Mol. Med. 2008, 40, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, W.; Wen, J.; Ye, H.; Luo, H.; Bai, P.; Tang, M.; Wang, F.; Zheng, L.; Yang, S.; et al. Liposomal Honokiol Induced Lysosomal Degradation of Hsp90 Client Proteins and Protective Autophagy in Both Gefitinib-Sensitive and Gefitinib-Resistant NSCLC Cells. Biomaterials 2017, 141, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yang, Q.; Cai, N.; Zhang, Z. A Cocktail of Betulinic Acid, Parthenolide, Honokiol and Ginsenoside Rh2 in Liposome Systems for Lung Cancer Treatment. Nanomedicine 2019, 15, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, L.; Xie, H.J.; Ju, R.J.; Xiao, Y.; Fu, M.; Liu, J.J.; Li, X.T. Functional Paclitaxel plus Honokiol Micelles Destroying Tumour Metastasis in Treatment of Non-Small-Cell Lung Cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 2), 1154–1169. [Google Scholar] [CrossRef]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Yang, J.; Pei, H.; Luo, H.; Fu, A.; Yang, H.; Hu, J.; Zhao, C.; Chai, L.; Chen, X.; Shao, X.; et al. Non-toxic dose of liposomal honokiol suppresses metastasis of hepatocellular carcinoma through destabilizing EGFR and inhibiting the downstream pathways. Oncotarget 2017, 8, 915–932, Correction in Oncotarget 2020, 11, 3350–3351. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Li, Q.; Wu, X.; Di, G.; Fan, J.; Wei, D.; Guo, C. Novel Nanomicelles Based on Rebaudioside A: A Potential Nanoplatform for Oral Delivery of Honokiol with Enhanced Oral Bioavailability and Antitumor Activity. Int. J. Pharm. 2020, 590, 119899. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Sun, Q.; Yang, H.; Tang, B.; Pu, H.; Li, H. Honokiol Nanoparticles Based on Epigallocatechin Gallate Functionalized Chitin to Enhance Therapeutic Effects against Liver Cancer. Int. J. Pharm. 2018, 545, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Xia, T.; Han, Y.; He, Q.J.; Zhao, R.; Ma, J.R. Synergistic Antitumor Effects of Liposomal Honokiol Combined with Cisplatin in Colon Cancer Models. Oncol. Lett. 2011, 2, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Su, H.; Qu, Z. Preparation of Honokiol-Loaded Titanium Dioxide Nanotube Drug Delivery System and Its Effect on CAL-27 Cells. Front. Bioeng. Biotechnol. 2023, 11, 1249349. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ni, X.; Chen, L.; Zhang, H.; Ren, P.; Feng, Y.; Chen, Y.; Fu, S.; Wu, J. Honokiol-Loaded Polymeric Nanoparticles: An Active Targeting Drug Delivery System for the Treatment of Nasopharyngeal Carcinoma. Drug Deliv. 2017, 24, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, H.; Zhang, Y.; Huang, Q.; Feng, J.; Xing, H.; Fu, X.; Yan, X.; Zhang, Y.; Xu, Q.; et al. HA-DOPE-Modified Honokiol-Loaded Liposomes Targeted Therapy for Osteosarcoma. Int. J. Nanomed. 2022, 17, 5137–5151. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.Y.; Shi, S.; Wang, X.H.; Wang, Y.J.; Fu, S.Z.; Dong, P.W.; Chen, L.J.; Zhao, X.; Wei, Y.Q.; Qian, Z.Y. Novel Composite Drug Delivery System for Honokiol Delivery: Self-Assembled Poly(Ethylene Glycol)-Poly(ε-Caprolactone)-Poly(Ethylene Glycol) Micelles in Thermosensitive Poly(Ethylene Glycol)-Poly(ε-Caprolactone)-Poly(Ethylene Glycol) Hydrogel. J. Phys. Chem. B 2009, 113, 10183–10188. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Wang, T.E.; Hsu, Y.T.; Chou, C.C.; Huang, K.H.; Hsu, C.C.; Liang, H.J.; Chang, H.W.; Lee, T.H.; Tsai, P.S. Nanoparticulated Honokiol Mitigates Cisplatin-Induced Chronic Kidney Injury by Maintaining Mitochondria Antioxidant Capacity and Reducing Caspase 3-Associated Cellular Apoptosis. Antioxidants 2019, 8, 466. [Google Scholar] [CrossRef]
- Fang, F.; Gong, C.Y.; Qian, Z.Y.; Zhang, X.N.; Gou, M.L.; You, C.; Zhou, L.X.; Liu, J.G.; Zhang, Y.; Guo, G.; et al. Honokiol Nanoparticles in Thermosensitive Hydrogel: Therapeutic Effects on Malignant Pleural Effusion. ACS Nano 2009, 3, 4080–4088. [Google Scholar] [CrossRef]
- Wen, J.; Fu, A.F.; Chen, L.J.; Xie, X.J.; Yang, G.L.; Chen, X.G.; Wang, Y.S.; Li, J.; Chen, P.; Tang, M.H.; et al. Liposomal Honokiol Inhibits VEGF-D-Induced Lymphangiogenesis and Metastasis in Xenograft Tumor Model. Int. J. Cancer 2009, 124, 2709–2718. [Google Scholar] [CrossRef]
- Elhabak, M.; Osman, R.; Mohamed, M.; El-Borady, O.M.; Awad, G.A.S.; Mortada, N. Near IR Responsive Targeted Integrated Lipid Polymer Nanoconstruct for Enhanced Magnolol Cytotoxicity in Breast Cancer. Sci. Rep. 2020, 10, 8771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, C.; Huang, L.; Liu, M.; Li, L.; Wang, X.; Wang, L.; Sun, S.; Xu, H.; Ma, G.; et al. Magnolol-loaded cholesteryl biguanide conjugate hydrochloride nanoparticles for triple-negative breast cancer therapy. Int. J. Pharm. 2022, 615, 121509. [Google Scholar] [CrossRef] [PubMed]
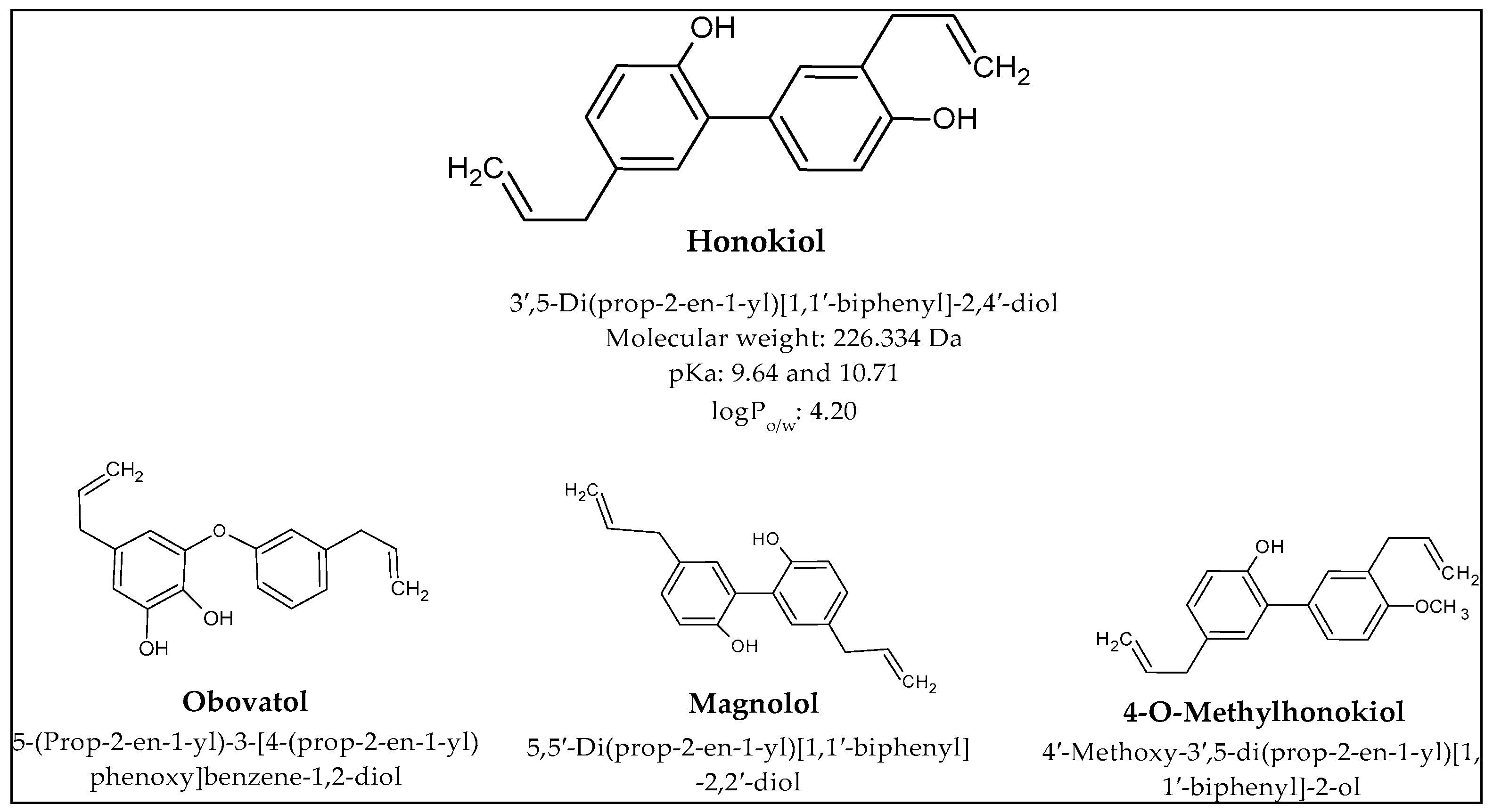


| Main Compounds | Carrier | Aim of the Study | Disease; In Vitro/In Vivo | Conclusion |
|---|---|---|---|---|
| HON + polysialic acid [11] | Liposomes | Preparation of HON liposomes modified with polysialic acid and evaluation of their anticancer activity. | BC; in vitro (4T1) and in vivo (BALB/c mice) | The IC50 value was 4.84 μg/mL; liposomes reduced the concentration of HON required to achieve the same antitumor effect. The tumor volume in mice was reduced by 52% with liposomes. This effect was probably due to the accumulation of HON and the presence of polysialic acid. |
| HON + hyaluronic acid [12] | Liposomes | Preparation of modified liposomes with HON and preliminary evaluation of their anticancer activity in vitro and in vivo studies. | BC; in vitro (4T1) and in vivo (BALB/c mice) | Cytotoxicity was greater with hyaluronic acid-modified micelles. The liposomes had greater accumulation in tumor cells and 59.5% inhibition of tumor growth. There was no systemic toxicity manifested by weight loss in the test subjects. |
| HON + adriamycin [13] | Liposomes | Determination of the anticancer effect of adriamycin with HON on cancer cells in vitro and in vivo studies. | BC; in vitro (4T1) and in vivo (BALB/c mice) | HON reduced the IC50 value for adriamycin by up to 15 times in vitro. The combined compounds induced apoptosis of cancer cells. The survival time of the test organisms was extended to 51 days (compared to 43 days for adriamycin). |
| HON + daunorubicin + hyaluronic acid [14] | Liposomes | Designing modified liposomes with HON to determine their effect on BC. | BC; in vitro (MCF-7 and MDA-MB-435S) and in vivo (BALB/c mice) | In vitro as well as in vivo studies confirmed the inhibitory effect of liposomes on cancer cells. Liposomes had higher cytotoxicity and provided prolonged release of substances, showing limited systemic toxicity in mice. |
| HON + paclitaxel [15] | Micelles | Designing micelles to improve anticancer activity of selected compounds. | BC; in vitro (4T1 and HEK293) and in vivo (BALB/c mice) | HON and paclitaxel in micelles had high cytotoxicity against cancer cells compared to other tested samples. The prepared system had also a higher percentage of apoptosis processes (34.02 ± 0.05%), inhibited angiogenesis, and suppressed the proliferation of tumor cells more effectively. |
| HON + paclitaxel [16] | Micelles | Use of micelles with HON and paclitaxel to prevent metastasis of BC and suppress multidrug resistance. | BC; in vitro (MCF-7/ADR and MDA-MB-231) and in vivo (BALB/c mice) | The micelles inhibited cell invasion regardless of the concentration of compounds in the system. Cell migration dropped to 66.6% with simultaneous paclitaxel and HON in the micelles. Bioluminescence imaging system proved that the administered system inhibited metastasis formation. |
| HON + doxorubicin [17] | Micelles | Prevention of metastasis during BC through the use of HON combined with the anticancer drug doxorubicin. | BC; in vitro (MDA-MB-231) and in vivo (BALB/c mice) | Migration and invasion of cancer cells were suppressed by created micelles. Adverse effects of doxorubicin were limited due to the reduction in premature drug release. |
| HON [18] | Micelles | Determination of antitumor effect of HON on triple-negative BC cells. | Triple-negative BC; in vitro (MDA-MB-231, MDA-MB-453, and MDA-MB-468) and in vivo (BALB/c mice) | Conducted studies demonstrated an increase in Cmax and AUC following oral administration of micelle-encapsulated HON. In addition, in vivo studies showed a reduction in tumor weight. In some individuals, complete recovery was observed. |
| HON + celecoxib [19] | Micelle solution | Developing and evaluating a mixed micelle solution to treat BC. | BC; in vitro (4T1) and in vivo (BALB/c mice) | The use of micelles resulted in a 45.71% apoptosis of cancer cells. In addition, there was a reduction in the expression of tumor growth biomarkers and a reduction in the number of collagen fibers in tumor tissue. |
| HON [20] | Nanocapsules | Systemic use of HON in the treatment of BC. | BC; in vitro (MCF-7 and EAC) and in vivo (BALB/c mice) | Conducted tests showed that using HON-loaded nanocapsules resulted in an 80% inhibition of tumor growth and an 85% reduction in tumor weight. As a result, the prepared system had very good results both in the in vitro and in the in vivo studies. |
| HON + zein + polysialic acid [21] | Polymeric nanoparticles | Prevention of BC growth and inhibition of metastasis by using HON encapsulated in nanoparticles. | BC; in vitro (4T1) and in vivo (BALB/c mice) | Polysialic acid nanoparticles inhibited tumor growth more strongly, increasing the penetration of the system into cells. Tumor growth was inhibited by 52%. The applied system reduced metastasis to other organs. |
| HON + zein + hyaluronic acid [22] | Polymeric nanoparticles | Development of core–shell nanoparticles to improve cellular uptake and distribution to enhance the antitumor effects of HON and inhibit metastasis. | BC; in vitro (4T1) and in vivo (BALB/c mice) | Nanoparticles were more effective in inhibiting tumor growth (77%) compared to free HON (25.8%). Mitochondrial pathway was involved in the apoptosis process. The encapsulation of HON in nanoparticles increased the efficacy of the compound in vivo, where antitumor activity was observed even at a dose of 15 mg/kg. |
| HON + polydopamine + folic acid [23] | Polymeric nanoparticles | Preparation of nanoparticles containing HON, polydopamine, and folic acid for the targeted treatment of cancer. | BC; in vitro (4T1) and in vivo (BALB/c mice) | The IC50 value was 2.25 after 48 h of incubation for the prepared system with polydopamine and folic acid. The particles were mainly captured by tumor cells. In vivo studies indicated a tumor growth inhibition of 79%. |
| HON + camptothecin + chitosan + folic acid [24] | Polymeric nanoparticles | Determination of the effect of HON nanoparticles on BC cells. | BC; in vitro (MCF-7) | In vitro studies confirmed the inhibition of cancer cell growth by the nanoparticles. |
| HON [25] | Polymeric nanoparticles | Increasing the use of poorly soluble HON in the treatment of BC and prevention of lung metastasis. | BC and lung metastases; in vitro (4T1) and in vivo (BALB/c mice) | The percentage of apoptotic cells was 64% with the designed nanoparticles. The same parameter was only 5% with free HON. The nanoparticles also reduced tumor volume and tumor growth at a dose of 15 mg/kg HON. |
| HON + amphiphilic codendrimer + oligoethylene glycol [26] | Polymeric nanoparticles | Design of dendrimer-based nanoparticles for precise delivery of HON. | BC; in vitro (4T1) and in vivo (BALB/c mice) | In vitro, after 48 h, the IC50 value for the prepared system was 2.2 μg/mL. The same parameter for free HON was 5.7 μg/mL. The system had a higher cytotoxicity on cancer cells, probably due to the greater transport of the compound into the cells. In vivo studies showed that HON nanoparticles (at a dose of 40 mg/kg) inhibited tumor growth by 72% compared to a saline-treated control group. |
| HON + paclitaxel [27] | Nanosuspensions/hydrogel | Combined anticancer activity of HON hydrogel suspension and paclitaxel for the treatment of BC. | BC; in vitro (4T1) and in vivo (BALB/c mice) | The combination of HON and paclitaxel enhanced the cytotoxic effect of both compounds. The apoptosis rate was more than 22% for HON in hydrogel, and when administered with paclitaxel, the rate increased to 65%. In vivo studies showed that paclitaxel with HON in hydrogel had the strongest antitumor effect at a dose of 40 mg/kg. |
| Main Compounds | Carrier | Aim of the Study | Disease; In Vitro/In Vivo | Conclusion |
|---|---|---|---|---|
| HON + disulfiram + copper + binding peptide (DCDX) [30] | Liposomes | DCDX peptide-modified HON liposomes for the treatment of glioblastoma. | Glioblastoma; in vitro (U87, C6, and BEND3) and in vivo (BALB/c mice) | The prepared liposomes showed a higher antitumor effect compared to free drugs. Liposomes induced autophagy and apoptosis by inhibiting Akt/mTOR phosphorylation. The average survival time of the tested mice was 27 days, of which 30% were still alive at the end of the study. |
| HON [31] | Liposomes | Determining the mechanism of action of liposomes in the treatment of glioblastoma. | Glioblastoma; in vitro (U87, LN229, RAW264.7, and BV2) and in vivo (ICR mice) | Studies have shown that HON liposomes act on tumor-associated macrophages. The antitumor lignan modulates their activity by increasing M1 polarization and inhibiting M2 polarization. |
| HON + daunorubicin + lactoferrin [32] | Liposomes | Designing liposomes to cross the blood–brain barrier and use them in brain glioma. | Glioblastoma; in vitro (C6 and BMVECs) and in vivo (ICR mice) | In vitro studies showed that the survival rate of tumor cells was 3% after use of the prepared system. The liposomes were better at inhibiting the formation of VM channels. The used liposomes prolonged the survival time of the mice in the experiment. This ranged from 23 to 51 days. |
| HON + doxorubicin [33] | Micelles | Utilizing the antitumor effects of HON and doxyrubicin for the treatment of malignant glioma. | Malignant gliomas; in vitro (C6) and in vivo (zebrafish model embryos and BALB/c mice) | HON in combination with doxorubicin more effectively inhibited cell proliferation and angiogenesis and induced apoptosis. Moreover, both substances showed synergistic effects when used together. |
| HON [34] | Liposomes | Evaluation of safety and efficacy of HON-loaded liposomes in a patient with glioblastoma. | Glioblastoma; case study | The patient did not experience any serious side effects. There were no elevated liver enzymes or blood counts. The HON administered supported the patient’s treatment with anticancer drugs. |
| HON + lauroyl-gemcitabine + hyaluronic acid [35] | Micelles | Combining HON with gemcitabine to exploit their properties in the treatment of glioblastoma multiforme. | Glioblastoma multiforme; in vitro (U87, B16F10, and HK2) and in vivo (SD rats and BALB/c mice) | The compounds showed a synergistic effect by inhibiting glioma progression. The micelles produced led to uptake by the tumor cells, greater accumulation of the drug at the site of action, and prolonged life of the mice due to greater induction of apoptosis and inhibition of cell proliferation. |
| HON + hydroxyapatite + stearic acid [36] | Microparticles | Use of hydroxyapatite to deliver HON to target cancer cells. | Glioblastoma; in vitro (ALTS1C1) and in vivo (C57BL/6 mice) | In vitro, as well as in vivo studies, indicated that the designed system provided controlled release of HON. Compound promoted tumor cell apoptosis and significantly reduced tumor size compared to control groups. |
| Main Compounds | Carrier | Aim of the Study | Disease; In Vitro/In Vivo | Conclusion |
|---|---|---|---|---|
| HON [38] | Liposomes | Evaluation of the effect of HON on ovarian cancer cells. | Ovarian cancer; in vitro (A2780s and A2780cp) and in vivo (BALB/c mice) | Liposomal HON promoted apoptosis in cell lines (A2780s and A2780cp) in vitro. The system reduced tumor volume in mice during in vivo studies. |
| HON + cisplatin [39] | Liposomes | Use of PEG liposomal HON with cisplatin for the treatment of ovarian cancer. | Ovarian cancer; in vitro (SKOV3) and in vivo (BALB/c mice) | The prepared liposomes induced apoptosis in vitro at a dose of 1 μg/mL. The mean tumor volume was 403 mm3 after application of the liposomes. Compared to the control group, tumor volumes were 90% lower. |
| HON + doxorubicin [40] | Polymeric nanoparticles | Determination of the anticancer potential of manufactured nanoparticles on ovarian cancer cells. | Ovarian cancer; in vitro (A2780s) | Compared to free doxorubicin and HON at the same doses, nanoparticles containing both compounds were more effective at inhibiting tumor cell proliferation. |
| Main Compounds | Carrier | Aim of the Study | Disease; In Vitro/In Vivo | Conclusion |
|---|---|---|---|---|
| HON [42] | Liposomes | Evaluation of HON-loaded liposomes against lung cancer. | Lung cancer; in vitro (BEAS-2B, 1179, 1198, and 1170) and in vivo (A/J mice) | HON inhibited phospho-extracellular signal-regulated kinases (ERK), Akt, and STAT3, while it promoted CD2 or CD4. Decrease in epidermal growth factor receptor (EGFR) phosphorylation was registered 6 h after administration. The compound showed an antitumor effect, by reducing proliferation (93%) and inducing apoptosis (61%). Similar results were obtained during in vivo studies in A/J mice. The test organisms showed a reduction in the size of lung tumors regardless of their size. Liposomes reduced EGFR, Akt, and STAT3 activity. Concomitant use of HON and erlotinib showed a stronger antitumor effect compared to the compounds separately. |
| HON + epirubicin + octreotide [43] | Liposomes | Preparation and evaluation of multifunctional liposomes for the treatment of non-small cell lung cancer. | Non-small cell lung cancer; in vitro (LLT) and in vivo (C57BL/6 mice) | The study found that multifunctional epirubicin liposomes exhibited the strongest cytotoxicity compared to other groups. The system provided increased penetration into tumor cells. The liposomes reduced tumor volume in test mice and prolonged survival to 48 days. |
| HON + cisplatin [44] | Liposomes | Determination of the antitumor activity of HON and evaluation of cisplatin potentiation by lignan. | Lung cancer; in vivo (nude mice inoculated with A549 cells) | The concentration of HON was above 30 μg/mL 24 h after liposome administration. In contrast, free HON concentrations were below 5 μg/mL after 12 h. The combination of HON and ciplastin inhibited tumor growth to a greater extent than the control groups. |
| HON [45] | Liposomes | Evaluation of radiotherapy enhancement by HON encapsulated in liposomes. | Lung cancer; in vitro (SPC-A1, A549, and LL/2) and in vivo (C57BL/6 mice) | Use of liposomes and radiotherapy together reduced tumor volume by 78% compared to untreated tumors. The therapies used separately resulted in a 42% reduction in tumor volume. Tumor growth was also delayed by more than 8 days. The combined therapies also reduced angiogenesis, and the average number of blood vessels was 16.2. |
| HON [46] | Liposomes | Liposomal HON for the treatment of non-small cell lung cancer of both gefitinib-sensitive and gefitinib-resistant cells. | Non-small cell lung cancer; in vitro (H1975, HCC827, H460, SPC-A1, H441, H1650, H226, H522, and H1993) | HON caused the degradation of HSP90 client proteins. This occurred via the lysosomal pathway. In a further step, misfolded proteins appeared, leading to ER stress and cell autophagy. |
| HON + betulinic acid + parthenolide + ginsenoside Rh2 [47] | Liposomes | Harnessing the anticancer effects of natural compounds in lung cancer. | Lung cancer; in vivo (A549) and in vivo (nude mice injected with A549 cells) | Liposomes inhibited the cell cycle in the G2/M phase, as the reference drug cisplatin. The rate of apoptosis was half that of cisplatin. The difference between the two groups was 4.40% for tumor growth in in vivo studies. |
| HON + paclitaxel + dequalinium [48] | Micelles | Development of a delivery system targeting VM cannel inhibition and tumor cell suppression. | Non-small cell lung cancer; in vitro (LLT) and in vivo (C57BL/6 mice) | Prepared micelles decreased regulation of FAK, phosphatidylinositol-3-kinase (PI3K), MMP-2, and MMP-9. Micelles had the strongest cytotoxic effects, reduced the formation of VM channels, and induced apoptosis of LLT cells. During in vivo test, delivery system increased the accumulation of chemotherapeutic drugs. |
| Main Compounds | Carrier | Aim of the Study | Disease; In Vitro/In Vivo | Conclusion |
|---|---|---|---|---|
| HON [51] | Liposomes | Using HON’s inhibitory effect on EGFR to suppress metastasis in hepatocellular carcinoma. | Hepatocellular carcinoma; in vitro (HepG2) and in vivo (zebrafish embryos and nude mice injected with HepG2/B16F10 cells) | HON inhibited and led to EGFR degradation and had inhibitory effects on the PI3K/Akt, Erk, c-Jun N-terminal kinases (JNK) pathways. A dose of 20 mg/kg reduced metastasis formation in the lungs of the mice tested. In addition, HON caused inhibition of tumor growth by 50% compared to control groups. |
| HON + rebaudioside A [52] | Micelles | Evaluation of prepared HON micelles with rebaudioside A on cancer cells. | Hepatocellular carcinoma; in vitro (HuH-7 and H22) and in vivo (BALB/c mice) | The survival rate of cancer cells treated with micelles containing HON was 15.69% during in vitro study. HON micelles inhibited the G0/G1 cell cycle by almost 82%. Depending on the dose administered, in vivo studies showed an inhibition of tumor growth of between 43% and 72%. |
| HON + epigallocatechin-3-gallate + chitin [53] | Polymeric nanoparticles | Determining the properties of HON nanoparticles on liver cancer. | Liver and lung cancer; in vitro (HepG2 and A549) and in vivo (BALB/c mice—liver cancer) | Nanoparticles inhibited tumor growth by almost 84%, compared to free HON, where the value was 30%. The nanoparticles had low systemic toxicity and stopped the cycle of tumor cells in the G2/M phase. |
| Main Compounds | Carrier | Aim of the Study | Disease; In Vitro/In Vivo | Conclusion |
|---|---|---|---|---|
| HON + cisplatin [54] | Liposomes | Enhancement of the antitumor effect of cisplatin on colon cancer cells by HON. | Colon cancer; in vitro (CT26) and in vivo (BALB/c mice) | The combination of cisplatin and HON promoted tumor cell apoptosis (just under 62%), compared to 21% with cisplatin and 20% with HON. Analysis at 32 days showed that tumor volume was 501 mm3, demonstrating greater suppression with the drug combination. |
| HON + titanium dioxide [55] | Nanotubes | Evaluation of the anticancer effect of HON in nanotubes on tongue cancer cells. | Tongue cancer; in vitro (CAL-27) | The prepared nanotubes with HON inhibited proliferation and promoted apoptosis of cancer cells due to increased expression of apoptotic proteins. |
| HON [56] | Polymeric nanoparticles | Development of a novel drug delivery with HON for the treatment of nasopharyngeal carcinoma. | Nasopharyngeal carcinoma; in vitro (HNE-1) and in vivo (BALB/c mice) | The nanoparticles inhibited HNE-1 cell growth more effectively than free HON and extended the life span of the mice in the study to almost 56 days. |
| HON + hyaluronic acid-phospholipid conjugates [57] | Liposomes | Determining the effect of HON liposomes in bone cancer in in vitro and in vivo studies. | Osteosarcoma; in vitro (143B) and in vivo (nude mice injected with 143B cells) | Liposomes enhanced the anticancer effects of HON. Scientists observed increased apoptosis and inhibition of cancer cell proliferation. |
| HON [58] | Micelles/hydrogel | Development of HON micelles in a thermosensitive gel to increase the solubility of the compound and enhance its potential use in medicine. | Melanoma; in vitro (B16) | The prepared system inhibited cell growth dose-dependently and provided prolonged HON release. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominiak, K.; Gostyńska, A.; Szulc, M.; Stawny, M. The Anticancer Application of Delivery Systems for Honokiol and Magnolol. Cancers 2024, 16, 2257. https://doi.org/10.3390/cancers16122257
Dominiak K, Gostyńska A, Szulc M, Stawny M. The Anticancer Application of Delivery Systems for Honokiol and Magnolol. Cancers. 2024; 16(12):2257. https://doi.org/10.3390/cancers16122257
Chicago/Turabian StyleDominiak, Katarzyna, Aleksandra Gostyńska, Michał Szulc, and Maciej Stawny. 2024. "The Anticancer Application of Delivery Systems for Honokiol and Magnolol" Cancers 16, no. 12: 2257. https://doi.org/10.3390/cancers16122257
APA StyleDominiak, K., Gostyńska, A., Szulc, M., & Stawny, M. (2024). The Anticancer Application of Delivery Systems for Honokiol and Magnolol. Cancers, 16(12), 2257. https://doi.org/10.3390/cancers16122257








