Overcoming Treatment Resistance in Medulloblastoma: Underlying Mechanisms and Potential Strategies
Abstract
Simple Summary
Abstract
1. Introduction
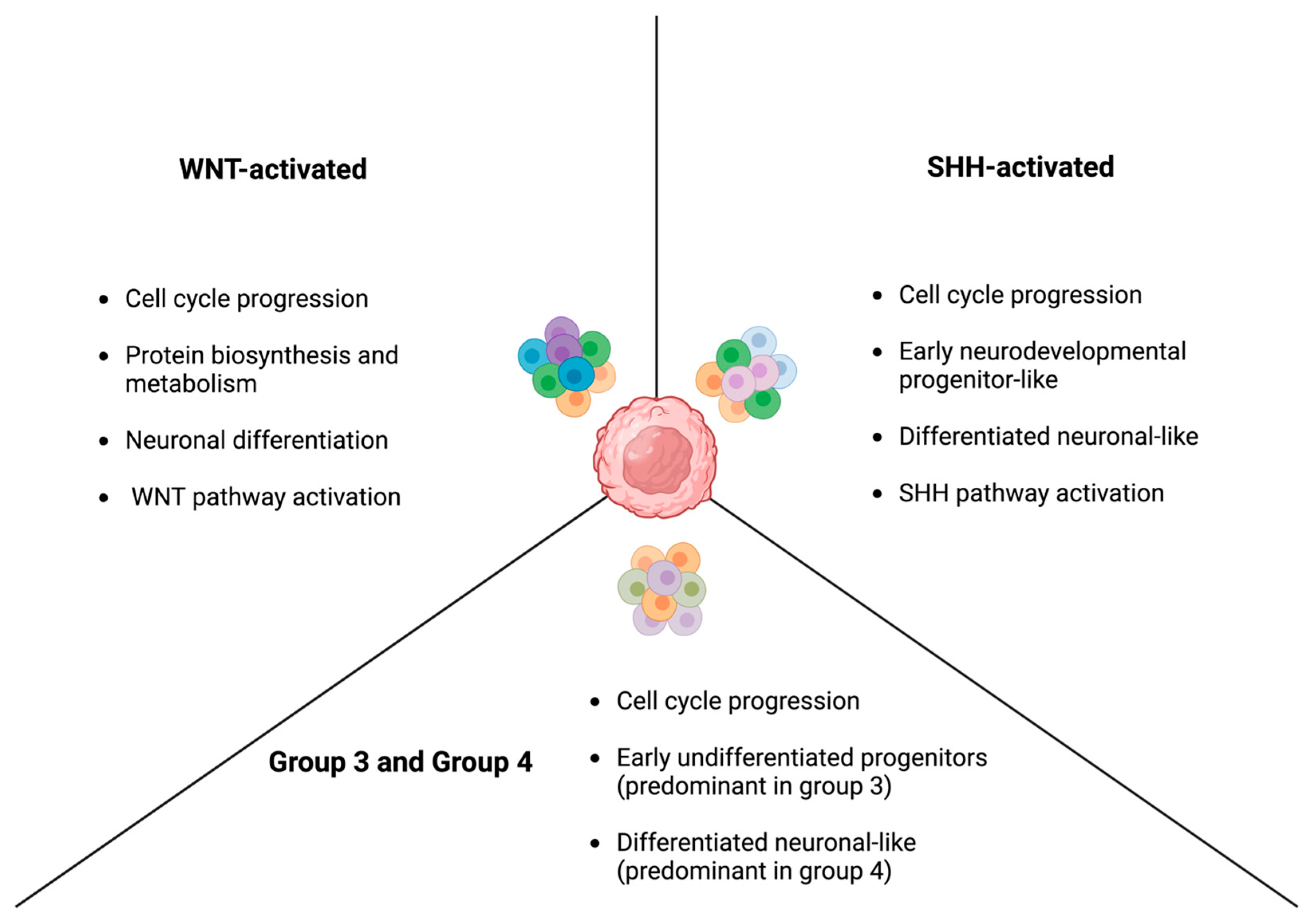
2. The Molecular Subgroups of Medulloblastoma
2.1. WNT-Activated Medulloblastoma
2.2. SHH-Activated Medulloblastoma
2.3. Non-WNT/Non-SHH: Group 3 and Group 4
2.3.1. Group 3 Medulloblastoma
2.3.2. Group 4 Medulloblastoma
3. Main Drivers of Treatment Resistance
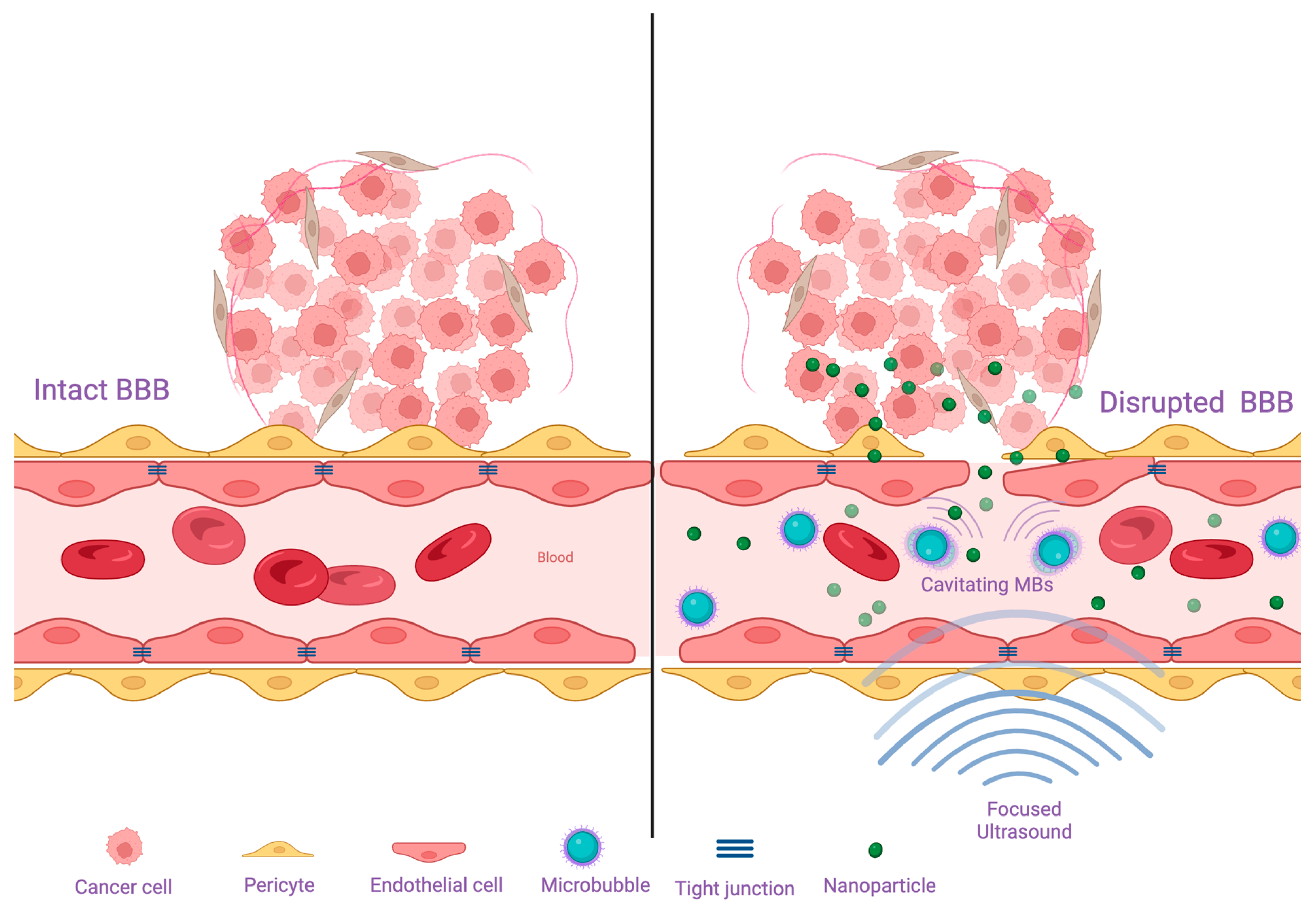
3.1. Blood-Brain Barrier and Blood-Brain-Tumor Barrier
3.2. Genetic, Epigenetic, and Molecular Drivers of Resistance
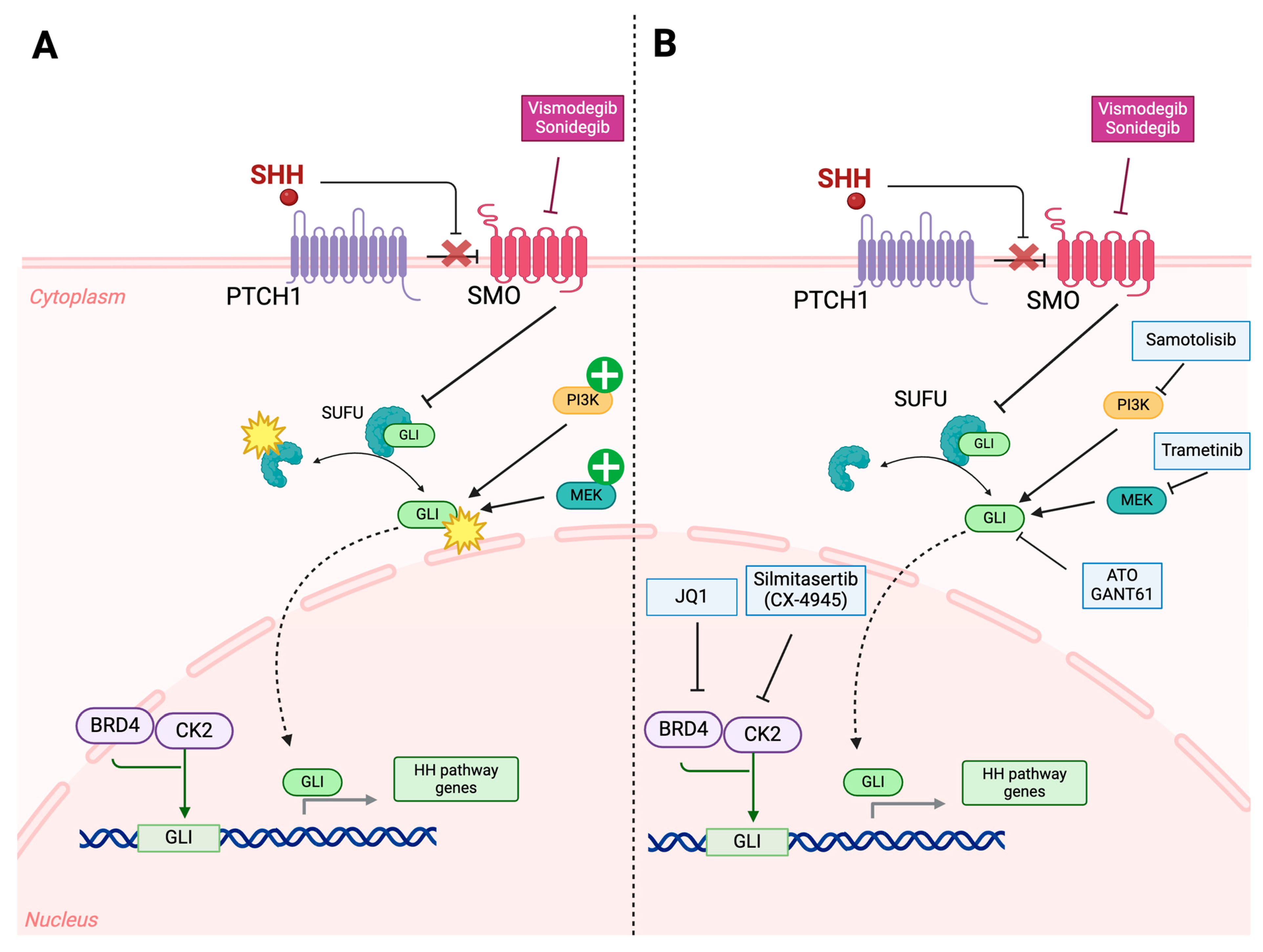
3.3. Overexpression of Alternative Pathways/Downstream Effectors
3.4. Cancer Stem Cells
3.5. Intratumoral and Intertumoral Heterogeneity
3.6. Metabolic Plasticity
4. Therapeutic Modalities to Overcome Resistance
4.1. Disrupting the Blood Brain Barrier
4.2. Targeting Genetic, Epigenetic, and Molecular Drivers of Resistance
| Non-Coding RNA | Target | Effect | Reference |
|---|---|---|---|
| shRNA | YB1 | Suppress invasion and increase sensitivity to vincristine, Panobinostat, and JQ1 | [35] |
| miR-29c-3p | - | Augment drug sensitivity, promote apoptosis, and inhibit metastasis | [118] |
| siRNA | SMO | Induce apoptosis in SHH-activated medulloblastoma cells | [100] |
| miR-23a-3p | GLS | Reverse metabolic changes and increase sensitivity to cisplatin | [124] |
4.3. Targeting Cancer Stem Cells
4.4. Disrupting Metabolic Patterns
4.5. Harvesting the Potential of the Immune System
4.5.1. Chimeric Antigen Receptor T-Cells (CAR T-Cells)
4.5.2. Oncolytic Virotherapy
4.5.3. Cancer Vaccines
4.5.4. Immune Checkpoint Inhibition
4.5.5. Adoptive Natural Killer Cell Therapy
5. Future Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, A.R. Brain Tumors in Children. N. Engl. J. Med. 2022, 386, 1922–1931. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro-Oncology 2023, 25 (Suppl. S4), iv1–iv99. [Google Scholar] [CrossRef] [PubMed]
- Juraschka, K.; Taylor, M.D. Medulloblastoma in the age of molecular subgroups: A review. J. Neurosurg. Pediatr. 2019, 24, 353–363. [Google Scholar] [CrossRef] [PubMed]
- BAILEY, P.; Cushing, H. Medulloblastoma cerebelli: A common type of midcerebellar glioma of childhood. Arch. Neurol. Psychiatry 1925, 14, 192–224. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Northcott, P.A.; Robinson, G.W.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Primers 2019, 5, 11. [Google Scholar] [CrossRef]
- Suk, Y.; Gwynne, W.D.; Burns, I.; Venugopal, C.; Singh, S.K. Childhood Medulloblastoma: An Overview. Methods Mol. Biol. 2022, 2423, 1–12. [Google Scholar] [PubMed]
- Cotter, J.A.; Hawkins, C. Medulloblastoma: WHO 2021 and Beyond. Pediatr. Dev. Pathol. 2022, 25, 23–33. [Google Scholar] [CrossRef]
- Phoenix, T.N. The origins of medulloblastoma tumours in humans. Nature 2022, 609, 901–903. [Google Scholar] [CrossRef]
- Orr, B.A. Pathology, diagnostics, and classification of medulloblastoma. Brain Pathol. 2020, 30, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Patay, Z.; DeSain, L.A.; Hwang, S.N.; Coan, A.; Li, Y.; Ellison, D.W. MR Imaging Characteristics of Wingless-Type-Subgroup Pediatric Medulloblastoma. AJNR Am. J. Neuroradiol. 2015, 36, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, A.; Sahm, F.; Zheludkova, O.; Golanov, A.; Stichel, D.; Schrimpf, D.; Ryzhova, M.; Potapov, A.; Habel, A.; Meyer, J.; et al. DNA methylation profiling is a method of choice for molecular verification of pediatric WNT-activated medulloblastomas. Neuro-Oncology 2019, 21, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Doussouki, M.E.; Gajjar, A.; Chamdine, O. Molecular genetics of medulloblastoma in children: Diagnostic, therapeutic and prognostic implications. Future Neurol. 2019, 14, FNL8. [Google Scholar] [CrossRef]
- Phoenix, T.N.; Patmore, D.M.; Boop, S.; Boulos, N.; Jacus, M.O.; Patel, Y.T.; Roussel, M.F.; Finkelstein, D.; Goumnerova, L.; Perreault, S.; et al. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell 2016, 29, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Coltin, H.; Sundaresan, L.; Smith, K.S.; Skowron, P.; Massimi, L.; Eberhart, C.G.; Schreck, K.C.; Gupta, N.; Weiss, W.A.; Tirapelli, D.; et al. Subgroup and subtype-specific outcomes in adult medulloblastoma. Acta Neuropathol. 2021, 142, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, F.M.G.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.H.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2017, 31, 737–754.e6. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Chaturvedi, N.K.; Bhakat, K.K.; Rizzino, A.; Mahapatra, S. Subgroup-Specific Diagnostic, Prognostic, and Predictive Markers Influencing Pediatric Medulloblastoma Treatment. Diagnostics 2021, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Northcott, P.A.; Korshunov, A.; Witt, H.; Hielscher, T.; Eberhart, C.G.; Mack, S.; Bouffet, E.; Clifford, S.C.; Hawkins, C.E.; French, P.; et al. Medulloblastoma comprises four distinct molecular variants. J. Clin. Oncol. 2011, 29, 1408–1414. [Google Scholar] [CrossRef]
- Cho, Y.J.; Tsherniak, A.; Tamayo, P.; Santagata, S.; Ligon, A.; Greulich, H.; Berhoukim, R.; Amani, V.; Goumnerova, L.; Eberhart, C.G.; et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J. Clin. Oncol. 2011, 29, 1424–1430. [Google Scholar] [CrossRef]
- Yoon, J.W.; Gilbertson, R.; Iannaccone, S.; Iannaccone, P.; Walterhouse, D. Defining a role for Sonic hedgehog pathway activation in desmoplastic medulloblastoma by identifying GLI1 target genes. Int. J. Cancer 2009, 124, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.J.; Martini, T.E.I.; Armandari, I.; Bockaj, I.; Zomerman, W.W.; de Camargo Magalhaes, E.S.; Siragna, Z.; Meeuwsen, T.G.J.; Scherpen, F.J.G.; Schoots, M.H.; et al. The developmental stage of the medulloblastoma cell-of-origin restricts Sonic hedgehog pathway usage and drug sensitivity. J. Cell Sci. 2022, 135, jeb258608. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, L.J.; Northfield, D.W.C. The medulloblastoma and the so–called “arachnoidal cerebellar sarcoma”: A critical re–examination of a nosological problem. Brain 1964, 87, 379–412. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, C.G.; Kepner, J.L.; Goldthwaite, P.T.; Kun, L.E.; Duffner, P.K.; Friedman, H.S.; Strother, D.R.; Burger, P.C. Histopathologic grading of medulloblastomas: A Pediatric Oncology Group study. Cancer 2002, 94, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.W.; Rudneva, V.A.; Buchhalter, I.; Billups, C.A.; Waszak, S.M.; Smith, K.S.; Bowers, D.C.; Bendel, A.; Fisher, P.G.; Partap, S.; et al. Risk-adapted therapy for young children with medulloblastoma (SJYC07): Therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol. 2018, 19, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.; Schwalbe, E.C.; Hicks, D.; Aldinger, K.A.; Lindsey, J.C.; Crosier, S.; Richardson, S.; Goddard, J.; Hill, R.M.; Castle, J.; et al. Medulloblastoma group 3 and 4 tumors comprise a clinically and biologically significant expression continuum reflecting human cerebellar development. Cell Rep. 2022, 40, 111162. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Alimonti, P.; Raj, D.; Caraway, C.; Alomari, S.; Jackson, E.M.; Tyler, B. The Neurodevelopmental and Molecular Landscape of Medulloblastoma Subgroups: Current Targets and the Potential for Combined Therapies. Cancers 2023, 15, 3889. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, L.A.; Puttick, S.; Millar, A.; Kojic, M.; Ji, P.; Lagendijk, A.K.; Brighi, C.; Bonder, C.S.; Adolphe, C.; Wainwright, B.J. Patient-derived orthotopic xenograft models of medulloblastoma lack a functional blood-brain barrier. Neuro-Oncology 2021, 23, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Haumann, R.; Videira, J.C.; Kaspers, G.J.L.; van Vuurden, D.G.; Hulleman, E. Overview of Current Drug Delivery Methods Across the Blood-Brain Barrier for the Treatment of Primary Brain Tumors. CNS Drugs 2020, 34, 1121–1131. [Google Scholar] [CrossRef]
- Gorelick, N.; Jackson, E.; Tyler, B.; Brem, H. Chapter 11—Interstitial Chemotherapy and Polymer Drug Delivery, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 155–165. [Google Scholar]
- Chen, X.; Momin, A.; Wanggou, S.; Wang, X.; Min, H.K.; Dou, W.; Gong, Z.; Chan, J.; Dong, W.; Fan, J.J.; et al. Mechanosensitive brain tumor cells construct blood-tumor barrier to mask chemosensitivity. Neuron 2023, 111, 30–48 e14. [Google Scholar] [CrossRef]
- Morris, E.K.; Daignault-Mill, S.; Stehbens, S.J.; Genovesi, L.A.; Lagendijk, A.K. Addressing blood-brain-tumor-barrier heterogeneity in pediatric brain tumors with innovative preclinical models. Front. Oncol. 2023, 13, 1101522. [Google Scholar] [CrossRef] [PubMed]
- Ingram, W.J.; Crowther, L.M.; Little, E.B.; Freeman, R.; Harliwong, I.; Veleva, D.; Hassall, T.E.; Remke, M.; Taylor, M.D.; Hallahan, A.R. ABC transporter activity linked to radiation resistance and molecular subtype in pediatric medulloblastoma. Exp. Hematol. Oncol. 2013, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Othman, R.T.; Kimishi, I.; Bradshaw, T.D.; Storer, L.C.; Korshunov, A.; Pfister, S.M.; Grundy, R.G.; Kerr, I.D.; Coyle, B. Overcoming multiple drug resistance mechanisms in medulloblastoma. Acta Neuropathol. Commun. 2014, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Wade, P.K.; Johnson, J.E.C.; Aldighieri, M.; Morlando, S.; Di Leva, G.; Kerr, I.D.; Coyle, B. Drug Resistance in Medulloblastoma Is Driven by YB-1, ABCB1 and a Seven-Gene Drug Signature. Cancers 2023, 15, 1086. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, A.S.; Fattet, S.; Kulesza, D.W.; Atamer, A.; Elsing, A.N.; Shalaby, T.; Jackson, S.P.; Schoenwaelder, S.M.; Grotzer, M.A.; Delattre, O.; et al. A sensitized RNA interference screen identifies a novel role for the PI3K p110gamma isoform in medulloblastoma cell proliferation and chemoresistance. Mol. Cancer Res. 2011, 9, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Bacolod, M.D.; Fehdrau, R.; Johnson, S.P.; Bullock, N.S.; Bigner, D.D.; Colvin, M.; Friedman, H.S. BCNU-sequestration by metallothioneins may contribute to resistance in a medulloblastoma cell line. Cancer Chemother. Pharmacol. 2009, 63, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Ocasio, J.K.; Babcock, B.; Malawsky, D.; Weir, S.J.; Loo, L.; Simon, J.M.; Zylka, M.J.; Hwang, D.; Dismuke, T.; Sokolsky, M.; et al. scRNA-seq in medulloblastoma shows cellular heterogeneity and lineage expansion support resistance to SHH inhibitor therapy. Nat. Commun. 2019, 10, 5829. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.; Ramaswamy, V.; Remke, M.; Martin, D.C.; Castelo-Branco, P.; Zhang, C.H.; Fraser, M.; Tse, K.; Poon, R.; Shih, D.J.; et al. WNT activation by lithium abrogates TP53 mutation associated radiation resistance in medulloblastoma. Acta Neuropathol. Commun. 2014, 2, 174. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Harish, V.; Coste, S.; Parasido, E.M.; Choudhry, M.U.; Kromer, L.F.; Ihemelandu, C.; Petricoin, E.F.; Pierobon, M.; Noon, M.S.; et al. Regulation of Chemosensitivity in Human Medulloblastoma Cells by p53 and the PI3 Kinase Signaling Pathway. Mol. Cancer Res. 2022, 20, 114–126. [Google Scholar] [CrossRef]
- Kasuga, C.; Nakahara, Y.; Ueda, S.; Hawkins, C.; Taylor, M.D.; Smith, C.A.; Rutka, J.T. Expression of MAGE and GAGE genes in medulloblastoma and modulation of resistance to chemotherapy. Laboratory investigation. J. Neurosurg. Pediatr. 2008, 1, 305–313. [Google Scholar] [CrossRef]
- Gjerstorff, M.F.; Harkness, L.; Kassem, M.; Frandsen, U.; Nielsen, O.; Lutterodt, M.; Mollgard, K.; Ditzel, H.J. Distinct GAGE and MAGE-A expression during early human development indicate specific roles in lineage differentiation. Hum. Reprod. 2008, 23, 2194–2201. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; McGuire, T.; Coulter, D.W.; Sharp, J.G.; Mahato, R.I. Challenges and Recent Advances in Medulloblastoma Therapy. Trends Pharmacol. Sci. 2017, 38, 1061–1084. [Google Scholar] [CrossRef]
- Gabriel, N.; Balaji, K.; Jayachandran, K.; Inkman, M.; Zhang, J.; Dahiya, S.; Goldstein, M. Loss of H3K27 Trimethylation Promotes Radiotherapy Resistance in Medulloblastoma and Induces an Actionable Vulnerability to BET Inhibition. Cancer Res. 2022, 82, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Shen, Y.; Chen, B.; Wu, Y.; Jia, L.; Li, Y.; Zhu, Y.; Yan, Y.; Li, M.; Chen, R.; et al. H3K27me3 induces multidrug resistance in small cell lung cancer by affecting HOXA1 DNA methylation via regulation of the lncRNA HOTAIR. Ann. Transl. Med. 2018, 6, 440. [Google Scholar] [CrossRef]
- Fu, Y.S.; Wang, Q.; Ma, J.X.; Yang, X.H.; Wu, M.L.; Zhang, K.L.; Kong, Q.Y.; Chen, X.Y.; Sun, Y.; Chen, N.N.; et al. CRABP-II methylation: A critical determinant of retinoic acid resistance of medulloblastoma cells. Mol. Oncol. 2012, 6, 48–61. [Google Scholar] [CrossRef]
- Fitzgerald, M.C.; O’Halloran, P.J.; Kerrane, S.A.; Ni Chonghaile, T.; Connolly, N.M.C.; Murphy, B.M. The identification of BCL-XL and MCL-1 as key anti-apoptotic proteins in medulloblastoma that mediate distinct roles in chemotherapy resistance. Cell Death Dis. 2023, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Yauch, R.L.; Dijkgraaf, G.J.; Alicke, B.; Januario, T.; Ahn, C.P.; Holcomb, T.; Pujara, K.; Stinson, J.; Callahan, C.A.; Tang, T.; et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science 2009, 326, 572–574. [Google Scholar] [CrossRef]
- Wilk, A.; Waligorska, A.; Waligorski, P.; Ochoa, A.; Reiss, K. Inhibition of ERbeta induces resistance to cisplatin by enhancing Rad51-mediated DNA repair in human medulloblastoma cell lines. PLoS ONE 2012, 7, e33867. [Google Scholar] [CrossRef] [PubMed]
- Bakhshinyan, D.; Adile, A.A.; Liu, J.; Gwynne, W.D.; Suk, Y.; Custers, S.; Burns, I.; Singh, M.; McFarlane, N.; Subapanditha, M.K.; et al. Temporal profiling of therapy resistance in human medulloblastoma identifies novel targetable drivers of recurrence. Sci. Adv. 2021, 7, eabi5568. [Google Scholar] [CrossRef]
- Pambid, M.R.; Berns, R.; Adomat, H.H.; Hu, K.; Triscott, J.; Maurer, N.; Zisman, N.; Ramaswamy, V.; Hawkins, C.E.; Taylor, M.D.; et al. Overcoming resistance to Sonic Hedgehog inhibition by targeting p90 ribosomal S6 kinase in pediatric medulloblastoma. Pediatr. Blood Cancer 2014, 61, 107–115. [Google Scholar] [CrossRef]
- Sreenivasan, L.; Wang, H.; Yap, S.Q.; Leclair, P.; Tam, A.; Lim, C.J. Autocrine IL-6/STAT3 signaling aids development of acquired drug resistance in Group 3 medulloblastoma. Cell Death Dis. 2020, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Giotta Lucifero, A.; Brambilla, I.; Semeria Mantelli, S.; Mosconi, M.; Foiadelli, T.; Savasta, S. Targeting the medulloblastoma: A molecular-based approach. Acta Biomed. 2020, 91, 79–100. [Google Scholar]
- Pan, W.; Song, X.Y.; Hu, Q.B.; Zhang, M.; Xu, X.H. TSP2 acts as a suppresser of cell invasion, migration and angiogenesis in medulloblastoma by inhibiting the Notch signaling pathway. Brain Res. 2019, 1718, 223–230. [Google Scholar] [CrossRef]
- Folgiero, V.; Miele, E.; Carai, A.; Ferretti, E.; Alfano, V.; Po, A.; Bertaina, V.; Goffredo, B.M.; Benedetti, M.C.; Camassei, F.D.; et al. IDO1 involvement in mTOR pathway: A molecular mechanism of resistance to mTOR targeting in medulloblastoma. Oncotarget 2016, 7, 52900–52911. [Google Scholar] [CrossRef]
- Kumar, V.; Wang, Q.; Sethi, B.; Lin, F.; Kumar, V.; Coulter, D.W.; Dong, Y.; Mahato, R.I. Polymeric nanomedicine for overcoming resistance mechanisms in hedgehog and Myc-amplified medulloblastoma. Biomaterials 2021, 278, 121138. [Google Scholar] [CrossRef] [PubMed]
- Alammar, H.; Nassani, R.; Alshehri, M.M.; Aljohani, A.A.; Alrfaei, B.M. Deficiency in the Treatment Description of mTOR Inhibitor Resistance in Medulloblastoma, a Systematic Review. Int. J. Mol. Sci. 2021, 23, 464. [Google Scholar] [CrossRef]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Peitzsch, C.; Kurth, I.; Kunz-Schughart, L.; Baumann, M.; Dubrovska, A. Discovery of the cancer stem cell related determinants of radioresistance. Radiother. Oncol. 2013, 108, 378–387. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Hersh, A.M.; Gaitsch, H.; Alomari, S.; Lubelski, D.; Tyler, B.M. Molecular Pathways and Genomic Landscape of Glioblastoma Stem Cells: Opportunities for Targeted Therapy. Cancers 2022, 14, 3743. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells—Perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Cojoc, M.; Mabert, K.; Muders, M.H.; Dubrovska, A. A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Semin. Cancer Biol. 2015, 31, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Raso, A.; Mascelli, S.; Biassoni, R.; Nozza, P.; Kool, M.; Pistorio, A.; Ugolotti, E.; Milanaccio, C.; Pignatelli, S.; Ferraro, M.; et al. High levels of PROM1 (CD133) transcript are a potential predictor of poor prognosis in medulloblastoma. Neuro-Oncology 2011, 13, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Moritake, T.; Zheng, Y.W.; Suzuki, K.; Gerelchuluun, A.; Hong, Z.; Zenkoh, J.; Taniguchi, H.; Tsuboi, K. In vitro stemness characterization of radio-resistant clones isolated from a medulloblastoma cell line ONS-76. J. Radiat. Res. 2013, 54, 61–69. [Google Scholar] [CrossRef]
- Yu, C.C.; Chiou, G.Y.; Lee, Y.Y.; Chang, Y.L.; Huang, P.I.; Cheng, Y.W.; Tai, L.K.; Ku, H.H.; Chiou, S.H.; Wong, T.T. Medulloblastoma-derived tumor stem-like cells acquired resistance to TRAIL-induced apoptosis and radiosensitivity. Childs Nerv. Syst. 2010, 26, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Read, T.A.; Fogarty, M.P.; Markant, S.L.; McLendon, R.E.; Wei, Z.; Ellison, D.W.; Febbo, P.G.; Wechsler-Reya, R.J. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell 2009, 15, 135–147. [Google Scholar] [CrossRef]
- Selvadurai, H.J.; Luis, E.; Desai, K.; Lan, X.; Vladoiu, M.C.; Whitley, O.; Galvin, C.; Vanner, R.J.; Lee, L.; Whetstone, H.; et al. Medulloblastoma Arises from the Persistence of a Rare and Transient Sox2(+) Granule Neuron Precursor. Cell Rep. 2020, 31, 107511. [Google Scholar] [CrossRef]
- Vanner, R.J.; Remke, M.; Gallo, M.; Selvadurai, H.J.; Coutinho, F.; Lee, L.; Kushida, M.; Head, R.; Morrissy, S.; Zhu, X.; et al. Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell 2014, 26, 33–47. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.; Liu, X.; Zhang, F.; Huang, L.F.; Potter, A.S.; Xu, L.; Zhou, W.; Zheng, T.; Luo, Z.; et al. Single-Cell Transcriptomics in Medulloblastoma Reveals Tumor-Initiating Progenitors and Oncogenic Cascades during Tumorigenesis and Relapse. Cancer Cell 2019, 36, 302–318.e7. [Google Scholar] [CrossRef] [PubMed]
- Borgenvik, A.; Holmberg, K.O.; Bolin, S.; Zhao, M.; Savov, V.; Rosen, G.; Hutter, S.; Garancher, A.; Rahmanto, A.S.; Bergstrom, T.; et al. Dormant SOX9-Positive Cells Facilitate MYC-Driven Recurrence of Medulloblastoma. Cancer Res. 2022, 82, 4586–4603. [Google Scholar] [CrossRef]
- Pistollato, F.; Rampazzo, E.; Persano, L.; Abbadi, S.; Frasson, C.; Denaro, L.; D’Avella, D.; Panchision, D.M.; Della Puppa, A.; Scienza, R.; et al. Interaction of hypoxia-inducible factor-1alpha and Notch signaling regulates medulloblastoma precursor proliferation and fate. Stem Cells 2010, 28, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Hambardzumyan, D.; Becher, O.J.; Rosenblum, M.K.; Pandolfi, P.P.; Manova-Todorova, K.; Holland, E.C. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008, 22, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Li, H.; Zeng, H.; Zhang, B.; Lu, Y.; Liu, X.; Xu, Z.; Zhang, J.; Zhang, L. Heterogeneity and tumoral origin of medulloblastoma in the single-cell era. Oncogene 2024, 43, 839–850. [Google Scholar] [CrossRef]
- Hovestadt, V.; Smith, K.S.; Bihannic, L.; Filbin, M.G.; Shaw, M.L.; Baumgartner, A.; DeWitt, J.C.; Groves, A.; Mayr, L.; Weisman, H.R.; et al. Resolving medulloblastoma cellular architecture by single-cell genomics. Nature 2019, 572, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Riemondy, K.A.; Venkataraman, S.; Willard, N.; Nellan, A.; Sanford, B.; Griesinger, A.M.; Amani, V.; Mitra, S.; Hankinson, T.C.; Handler, M.H.; et al. Neoplastic and immune single-cell transcriptomics define subgroup-specific intra-tumoral heterogeneity of childhood medulloblastoma. Neuro-Oncology 2022, 24, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xin, X.; Dai, Q.; Sun, M.; Chen, J.; Mostafavi, E.; Shen, Y.; Li, X. Medulloblastoma targeted therapy: From signaling pathways heterogeneity and current treatment dilemma to the recent advances in development of therapeutic strategies. Pharmacol. Ther. 2023, 250, 108527. [Google Scholar] [CrossRef]
- Pham, C.D.; Flores, C.; Yang, C.; Pinheiro, E.M.; Yearley, J.H.; Sayour, E.J.; Pei, Y.; Moore, C.; McLendon, R.E.; Huang, J.; et al. Differential Immune Microenvironments and Response to Immune Checkpoint Blockade among Molecular Subtypes of Murine Medulloblastoma. Clin. Cancer Res. 2016, 22, 582–595. [Google Scholar] [CrossRef]
- Morrissy, A.S.; Garzia, L.; Shih, D.J.; Zuyderduyn, S.; Huang, X.; Skowron, P.; Remke, M.; Cavalli, F.M.; Ramaswamy, V.; Lindsay, P.E.; et al. Divergent clonal selection dominates medulloblastoma at recurrence. Nature 2016, 529, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.; Remke, M.; Bouffet, E.; Faria, C.C.; Perreault, S.; Cho, Y.J.; Shih, D.J.; Luu, B.; Dubuc, A.M.; Northcott, P.A.; et al. Recurrence patterns across medulloblastoma subgroups: An integrated clinical and molecular analysis. Lancet Oncol. 2013, 14, 1200–1207. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Zaal, E.A.; Berkers, C.R. The Influence of Metabolism on Drug Response in Cancer. Front. Oncol. 2018, 8, 500. [Google Scholar] [CrossRef] [PubMed]
- Marabitti, V.; Giansanti, M.; De Mitri, F.; Gatto, F.; Mastronuzzi, A.; Nazio, F. Pathological implications of metabolic reprogramming and its therapeutic potential in medulloblastoma. Front. Cell Dev. Biol. 2022, 10, 1007641. [Google Scholar] [CrossRef]
- Manfreda, L.; Rampazzo, E.; Persano, L.; Viola, G.; Bortolozzi, R. Surviving the hunger games: Metabolic reprogramming in medulloblastoma. Biochem. Pharmacol. 2023, 215, 115697. [Google Scholar] [CrossRef] [PubMed]
- Park, A.K.; Lee, J.Y.; Cheong, H.; Ramaswamy, V.; Park, S.H.; Kool, M.; Phi, J.H.; Choi, S.A.; Cavalli, F.; Taylor, M.D.; et al. Subgroup-specific prognostic signaling and metabolic pathways in pediatric medulloblastoma. BMC Cancer 2019, 19, 571. [Google Scholar] [CrossRef]
- Sun, L.; Moritake, T.; Ito, K.; Matsumoto, Y.; Yasui, H.; Nakagawa, H.; Hirayama, A.; Inanami, O.; Tsuboi, K. Metabolic analysis of radioresistant medulloblastoma stem-like clones and potential therapeutic targets. PLoS ONE 2017, 12, e0176162. [Google Scholar] [CrossRef]
- Daggubati, V.; Hochstelter, J.; Bommireddy, A.; Choudhury, A.; Krup, A.L.; Kaur, P.; Tong, P.; Li, A.; Xu, L.; Reiter, J.F.; et al. Smoothened-activating lipids drive resistance to CDK4/6 inhibition in Hedgehog-associated medulloblastoma cells and preclinical models. J. Clin. Investig. 2021, 131, e141171. [Google Scholar] [CrossRef] [PubMed]
- Sprowls, S.A.; Saralkar, P.; Arsiwala, T.; Adkins, C.E.; Blethen, K.E.; Pizzuti, V.J.; Shah, N.; Fladeland, R.; Lockman, P.R. A Review of Mathematics Determining Solute Uptake at the Blood-Brain Barrier in Normal and Pathological Conditions. Pharmaceutics 2021, 13, 756. [Google Scholar] [CrossRef] [PubMed]
- Sprowls, S.A.; Lathia, J.D. Breaking down the barrier to medulloblastoma treatment: Piezo2 knockout disrupts the BTB and increases vascular permeability. Neuron 2023, 111, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Tylawsky, D.E.; Kiguchi, H.; Vaynshteyn, J.; Gerwin, J.; Shah, J.; Islam, T.; Boyer, J.A.; Boue, D.R.; Snuderl, M.; Greenblatt, M.B.; et al. P-selectin-targeted nanocarriers induce active crossing of the blood-brain barrier via caveolin-1-dependent transcytosis. Nat. Mater. 2023, 22, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Dismuke, T.; Tikunov, A.; Rosen, E.P.; Kagel, J.R.; Ramsey, J.D.; Lim, C.; Zamboni, W.; Kabanov, A.V.; Gershon, T.R.; et al. Poly(2-oxazoline) nanoparticle delivery enhances the therapeutic potential of vismodegib for medulloblastoma by improving CNS pharmacokinetics and reducing systemic toxicity. Nanomedicine 2021, 32, 102345. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Dismuke, T.; Malawsky, D.; Ramsey, J.D.; Hwang, D.; Godfrey, V.L.; Kabanov, A.V.; Gershon, T.R.; Sokolsky-Papkov, M. Enhancing CDK4/6 inhibitor therapy for medulloblastoma using nanoparticle delivery and scRNA-seq-guided combination with sapanisertib. Sci. Adv. 2022, 8, eabl5838. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Moore, D.; Lee, C.C.; Su, Y.H. Focused ultrasound for brain metastases: An update on global clinical trials. J. Neurooncol. 2023, 165, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Harvey, B.K.; Borden, M.A. State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics 2018, 8, 4393–4408. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lee, H.; Fang, Z.; Velalopoulou, A.; Kim, J.; Thomas, M.B.; Liu, J.; Abramowitz, R.G.; Kim, Y.; Coskun, A.F.; et al. Single-cell analysis reveals effective siRNA delivery in brain tumors with microbubble-enhanced ultrasound and cationic nanoparticles. Sci. Adv. 2021, 7, eabf7390. [Google Scholar] [CrossRef]
- Ding, H.; Wu, F.; Nair, M.P. Image-guided drug delivery to the brain using nanotechnology. Drug Discov. Today 2013, 18, 1074–1080. [Google Scholar] [CrossRef][Green Version]
- Ojha, T.; Rizzo, L.; Storm, G.; Kiessling, F.; Lammers, T. Image-guided drug delivery: Preclinical applications and clinical translation. Expert Opin. Drug Deliv. 2015, 12, 1203–1207. [Google Scholar] [CrossRef]
- Fan, C.H.; Cheng, Y.H.; Ting, C.Y.; Ho, Y.J.; Hsu, P.H.; Liu, H.L.; Yeh, C.K. Ultrasound/Magnetic Targeting with SPIO-DOX-Microbubble Complex for Image-Guided Drug Delivery in Brain Tumors. Theranostics 2016, 6, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Murai, J.; Okada, M.; Takahashi, H.; Findlay, T.H.; Malebranche, K.; Parthasarathy, A.; Miyashita, S.; Gabdulkhaev, R.; Benkimoun, I.; et al. Epigenetic upregulation of Schlafen11 renders WNT- and SHH-activated medulloblastomas sensitive to cisplatin. Neuro-Oncology 2023, 25, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.K.; Zhang, H.; Lyne, A.M.; Cavalli, F.M.G.; Hassen, W.E.; Stevenson, K.; Kornahrens, R.; Yang, Y.; Li, S.; Dell, S.; et al. ABL1 and ABL2 promote medulloblastoma leptomeningeal dissemination. Neurooncol. Adv. 2023, 5, vdad095. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Jain, S.; Coulter, D.W.; Joshi, S.S.; Chaturvedi, N.K. PRMT5 as a Potential Therapeutic Target in MYC-Amplified Medulloblastoma. Cancers 2023, 15, 5855. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sui, Y.; Li, Q.; Zhao, Y.; Dong, X.; Yang, J.; Liang, Z.; Han, Y.; Tang, Y.; Ma, J. Effective inhibition of MYC-amplified group 3 medulloblastoma by FACT-targeted curaxin drug CBL0137. Cell Death Dis. 2020, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Sarantopoulos, J.; Mahalingam, D.; Sharma, N.; Iyer, R.V.; Ma, W.W.; Ahluwalia, M.S.; Johnson, S.; Purmal, A.; Shpigotskaya, P.; Hards, A.; et al. Results of a completed phase I trial of CBL0137 administered intravenously (IV) to patients (Pts) with advanced solid tumors. J. Clin. Oncol. 2020, 38 (Suppl. S15), 3583. [Google Scholar] [CrossRef]
- Metcalfe, C.; de Sauvage, F.J. Hedgehog fights back: Mechanisms of acquired resistance against Smoothened antagonists. Cancer Res. 2011, 71, 5057–5061. [Google Scholar] [CrossRef]
- Liu, X.; Chen, D.; Chen, H.; Wang, W.; Liu, Y.; Wang, Y.; Duan, C.; Ning, Z.; Guo, X.; Otkur, W.; et al. YB1 regulates miR-205/200b-ZEB1 axis by inhibiting microRNA maturation in hepatocellular carcinoma. Cancer Commun. 2021, 41, 576–595. [Google Scholar] [CrossRef]
- Ruan, H.; Li, S.; Bao, L.; Zhang, X. Enhanced YB1/EphA2 axis signaling promotes acquired resistance to sunitinib and metastatic potential in renal cell carcinoma. Oncogene 2020, 39, 6113–6128. [Google Scholar] [CrossRef]
- Niu, W.; Luo, Y.; Zhou, Y.; Li, M.; Wu, C.; Duan, Y.; Wang, H.; Fan, S.; Li, Z.; Xiong, W.; et al. BRD7 suppresses invasion and metastasis in breast cancer by negatively regulating YB1-induced epithelial-mesenchymal transition. J. Exp. Clin. Cancer Res. 2020, 39, 30. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Li, Y.; Wang, J.; Ding, H.; Huang, W.; Ding, C.; Liu, H.; Tan, W.; Zhang, A. Development of hedgehog pathway inhibitors by epigenetically targeting GLI through BET bromodomain for the treatment of medulloblastoma. Acta Pharm. Sin. B 2021, 11, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Borlase, S.; DeCarlo, A.; Coudiere-Morrison, L.; Liang, L.; Porter, C.J.; Ramaswamy, V.; Werbowetski-Ogilvie, T.E. Cross-species analysis of SHH medulloblastoma models reveals significant inhibitory effects of trametinib on tumor progression. Cell Death Discov. 2023, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui-Jain, A.; Drygin, D.; Streiner, N.; Chua, P.; Pierre, F.; O’Brien, S.E.; Bliesath, J.; Omori, M.; Huser, N.; Ho, C.; et al. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 2010, 70, 10288–10298. [Google Scholar] [CrossRef] [PubMed]
- Pierre, F.; Chua, P.C.; O’Brien, S.E.; Siddiqui-Jain, A.; Bourbon, P.; Haddach, M.; Michaux, J.; Nagasawa, J.; Schwaebe, M.K.; Stefan, E.; et al. Discovery and SAR of 5-(3-chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic acid (CX-4945), the first clinical stage inhibitor of protein kinase CK2 for the treatment of cancer. J. Med. Chem. 2011, 54, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.L.; Wang, Y.X.; Yang, F.C.; Wang, C.; Mao, M.; Gai, Q.J.; He, J.; Qin, Y.; Yao, X.X.; Lan, X.; et al. Targeting AKT and CK2 represents a novel therapeutic strategy for SMO constitutive activation-driven medulloblastoma. CNS Neurosci. Ther. 2022, 28, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Fan, W.J.; An, Z.J.; Sun, Y. Inhibition of Long Noncoding RNA CRNDE Increases Chemosensitivity of Medulloblastoma Cells by Targeting miR-29c-3p. Oncol. Res. 2020, 28, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, M.A.; Schuler-Ortoli, M.; Zerrinius, D.; Hadzalic, A.; Schuster, A.; Strobel, H.; Scheuerle, A.; Wong, T.; Wirtz, C.R.; Debatin, K.M.; et al. Bcl-XL but Not Bcl-2 Is a Potential Target in Medulloblastoma Therapy. Pharmaceuticals 2022, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Levesley, J.; Steele, L.; Bruning-Richardson, A.; Davison, A.; Zhou, J.; Ding, C.; Lawler, S.; Short, S.C. Selective BCL-XL inhibition promotes apoptosis in combination with MLN8237 in medulloblastoma and pediatric glioblastoma cells. Neuro-Oncology 2018, 20, 203–214. [Google Scholar] [CrossRef]
- Zeuner, S.; Vollmer, J.; Sigaud, R.; Oppermann, S.; Peterziel, H.; ElHarouni, D.; Oehme, I.; Witt, O.; Milde, T.; Ecker, J. Combination drug screen identifies synergistic drug interaction of BCL-XL and class I histone deacetylase inhibitors in MYC-amplified medulloblastoma cells. J. Neurooncol. 2024, 166, 99–112. [Google Scholar] [CrossRef]
- Meister, M.T.; Boedicker, C.; Linder, B.; Kogel, D.; Klingebiel, T.; Fulda, S. Concomitant targeting of Hedgehog signaling and MCL-1 synergistically induces cell death in Hedgehog-driven cancer cells. Cancer Lett. 2019, 465, 1–11. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, X.; Morsch, M.; Ismail, M.; Liu, Y.; Rehman, F.U.; Zhang, D.; Wang, Y.; Zheng, M.; Chung, R.; et al. Brain-Targeted Codelivery of Bcl-2/Bcl-xl and Mcl-1 Inhibitors by Biomimetic Nanoparticles for Orthotopic Glioblastoma Therapy. ACS Nano 2022, 16, 6293–6308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, S.; Zhao, Y.; Du, F.; Wang, W.; Lv, P.; Qi, L. Long noncoding RNA NEAT1 modulates cell proliferation and apoptosis by regulating miR-23a-3p/SMC1A in acute myeloid leukemia. J. Cell Physiol. 2019, 234, 6161–6172. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.H.; Xu, Q.F.; Cui, Y.H.; Li, N.; Bian, X.W.; Lv, S.Q. Medulloblastoma stem cells: Promising targets in medulloblastoma therapy. Cancer Sci. 2016, 107, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Chiang, C.H.; Song, W.S.; Tsai, S.K.; Woung, L.C.; Chang, C.H.; Jeng, S.Y.; Tsai, C.Y.; Hsu, C.C.; Lee, H.F.; et al. Inhibition of phosphorylated STAT3 by cucurbitacin I enhances chemoradiosensitivity in medulloblastoma-derived cancer stem cells. Childs Nerv. Syst. 2012, 28, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Lee, H.T.; Chen, C.M.; Shen, C.C.; Ma, H.I. Celecoxib suppresses the phosphorylation of STAT3 protein and can enhance the radiosensitivity of medulloblastoma-derived cancer stem-like cells. Int. J. Mol. Sci. 2014, 15, 11013–11029. [Google Scholar] [CrossRef] [PubMed]
- Nor, C.; Sassi, F.A.; de Farias, C.B.; Schwartsmann, G.; Abujamra, A.L.; Lenz, G.; Brunetto, A.L.; Roesler, R. The histone deacetylase inhibitor sodium butyrate promotes cell death and differentiation and reduces neurosphere formation in human medulloblastoma cells. Mol. Neurobiol. 2013, 48, 533–543. [Google Scholar] [CrossRef] [PubMed]
- da Cunha Jaeger, M.; Ghisleni, E.C.; Cardoso, P.S.; Siniglaglia, M.; Falcon, T.; Brunetto, A.T.; Brunetto, A.L.; de Farias, C.B.; Taylor, M.D.; Nor, C.; et al. HDAC and MAPK/ERK Inhibitors Cooperate To Reduce Viability and Stemness in Medulloblastoma. J. Mol. Neurosci. 2020, 70, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Dijkgraaf, G.J.; Alicke, B.; Weinmann, L.; Januario, T.; West, K.; Modrusan, Z.; Burdick, D.; Goldsmith, R.; Robarge, K.; Sutherlin, D.; et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011, 71, 435–444. [Google Scholar] [CrossRef]
- Frasson, C.; Rampazzo, E.; Accordi, B.; Beggio, G.; Pistollato, F.; Basso, G.; Persano, L. Inhibition of PI3K Signalling Selectively Affects Medulloblastoma Cancer Stem Cells. Biomed. Res. Int. 2015, 2015, 973912. [Google Scholar] [CrossRef]
- Rao, Y.; Fang, Y.; Tan, W.; Liu, D.; Pang, Y.; Wu, X.; Zhang, C.; Li, G. Delivery of Long Non-coding RNA NEAT1 by Peripheral Blood Monouclear Cells-Derived Exosomes Promotes the Occurrence of Rheumatoid Arthritis via the MicroRNA-23a/MDM2/SIRT6 Axis. Front. Cell Dev. Biol. 2020, 8, 551681. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Lai, J.; Gao, Y.; Wang, G.; Shang, J.; Zhang, D.; Zheng, S. NEAT1/miR-23a-3p/KLF3: A novel regulatory axis in melanoma cancer progression. Cancer Cell Int. 2019, 19, 217. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, B.; Zhao, S.; Xu, J. Inhibition of lncRNA NEAT1 sensitizes medulloblastoma cells to cisplatin through modulating the miR-23a-3p-glutaminase (GLS) axis. Bioengineered 2022, 13, 7670–7682. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, E.; Rampazzo, E.; Bortolozzi, R.; Rruga, F.; Zeni, I.; Manfreda, L.; Marchioro, C.; Canton, M.; Cani, A.; Magni, R.; et al. Molecular and functional profiling of chemotolerant cells unveils nucleoside metabolism-dependent vulnerabilities in medulloblastoma. Acta Neuropathol. Commun. 2023, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Di Magno, L.; Manni, S.; Di Pastena, F.; Coni, S.; Macone, A.; Cairoli, S.; Sambucci, M.; Infante, P.; Moretti, M.; Petroni, M.; et al. Phenformin Inhibits Hedgehog-Dependent Tumor Growth through a Complex I-Independent Redox/Corepressor Module. Cell Rep. 2020, 30, 1735–1752.e7. [Google Scholar] [CrossRef] [PubMed]
- Contenti, J.; Guo, Y.; Mazzu, A.; Irondelle, M.; Rouleau, M.; Lago, C.; Leva, G.; Tiberi, L.; Ben-Sahra, I.; Bost, F.; et al. The mitochondrial NADH shuttle system is a targetable vulnerability for Group 3 medulloblastoma in a hypoxic microenvironment. Cell Death Dis. 2023, 14, 784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, X.; Wang, Y.; Liu, J.; Li, Z.; Li, S.; Liu, Y.; Gong, X.; Sun, Y.; Wu, W.; et al. Tumor-Associated Macrophages Correlate with Prognosis in Medulloblastoma. Front. Oncol. 2022, 12, 893132. [Google Scholar] [CrossRef] [PubMed]
- Maximov, V.; Chen, Z.; Wei, Y.; Robinson, M.H.; Herting, C.J.; Shanmugam, N.S.; Rudneva, V.A.; Goldsmith, K.C.; MacDonald, T.J.; Northcott, P.A.; et al. Tumour-associated macrophages exhibit anti-tumoural properties in Sonic Hedgehog medulloblastoma. Nat. Commun. 2019, 10, 2410. [Google Scholar] [CrossRef]
- Donovan, L.K.; Delaidelli, A.; Joseph, S.K.; Bielamowicz, K.; Fousek, K.; Holgado, B.L.; Manno, A.; Srikanthan, D.; Gad, A.Z.; Van Ommeren, R.; et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 2020, 26, 720–731. [Google Scholar] [CrossRef]
- McNamara, M.A.; Nair, S.K.; Holl, E.K. RNA-Based Vaccines in Cancer Immunotherapy. J. Immunol. Res. 2015, 2015, 794528. [Google Scholar] [CrossRef] [PubMed]
- Jahanafrooz, Z.; Baradaran, B.; Mosafer, J.; Hashemzaei, M.; Rezaei, T.; Mokhtarzadeh, A.; Hamblin, M.R. Comparison of DNA and mRNA vaccines against cancer. Drug Discov. Today 2020, 25, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu. Rev. Med. 2020, 71, 47–58. [Google Scholar] [CrossRef]
- Moon, Y.W.; Hajjar, J.; Hwu, P.; Naing, A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J. Immunother. Cancer 2015, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Dondero, A.; Negri, F.; Bellora, F.; Nozza, P.; Carnemolla, B.; Raso, A.; Moretta, L.; Moretta, A.; Bottino, C. Both CD133+ and CD133− medulloblastoma cell lines express ligands for triggering NK receptors and are susceptible to NK-mediated cytotoxicity. Eur. J. Immunol. 2007, 37, 3190–3196. [Google Scholar] [CrossRef]
- Khatua, S.; Cooper, L.J.N.; Sandberg, D.I.; Ketonen, L.; Johnson, J.M.; Rytting, M.E.; Liu, D.D.; Meador, H.; Trikha, P.; Nakkula, R.J.; et al. Phase I study of intraventricular infusions of autologous ex vivo expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro-Oncology 2020, 22, 1214–1225. [Google Scholar] [CrossRef]

| WNT | SHH | Group 3 | Group 4 | |
|---|---|---|---|---|
| Prevalence | 10% | 30% | 25% | 35% |
| Peak Incidence (age group) | 10–12 years | <3 years and >17 years | 3–5 years | 5–10 years |
| Prognosis | Good | Good for MBEN | Poor | Intermediate |
| 5-year survival | >90% | 70% | 50% | 75% |
| Predisposing Genes | CTTNB1, APC | SUFU, PTCH1, PALB2, BRCA2, TP53 | PALB2, BRCA2 | |
| Histologic Presentation | Classic | Classic, D/N, LC/A, MBEN | LC/A | |
| Metastasis at diagnosis | Rare (5–10%) | Rare (9–30% depending on subgroup) | Very frequent (around 50%) | Frequent (35–40%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slika, H.; Shahani, A.; Wahi, R.; Miller, J.; Groves, M.; Tyler, B. Overcoming Treatment Resistance in Medulloblastoma: Underlying Mechanisms and Potential Strategies. Cancers 2024, 16, 2249. https://doi.org/10.3390/cancers16122249
Slika H, Shahani A, Wahi R, Miller J, Groves M, Tyler B. Overcoming Treatment Resistance in Medulloblastoma: Underlying Mechanisms and Potential Strategies. Cancers. 2024; 16(12):2249. https://doi.org/10.3390/cancers16122249
Chicago/Turabian StyleSlika, Hasan, Aanya Shahani, Riddhpreet Wahi, Jackson Miller, Mari Groves, and Betty Tyler. 2024. "Overcoming Treatment Resistance in Medulloblastoma: Underlying Mechanisms and Potential Strategies" Cancers 16, no. 12: 2249. https://doi.org/10.3390/cancers16122249
APA StyleSlika, H., Shahani, A., Wahi, R., Miller, J., Groves, M., & Tyler, B. (2024). Overcoming Treatment Resistance in Medulloblastoma: Underlying Mechanisms and Potential Strategies. Cancers, 16(12), 2249. https://doi.org/10.3390/cancers16122249






