The Impact of Urine-Sample HPV Testing on the Effectiveness of Screening for Cervical Cancer: An Umbrella Review
Abstract
Simple Summary
Abstract
1. Introduction
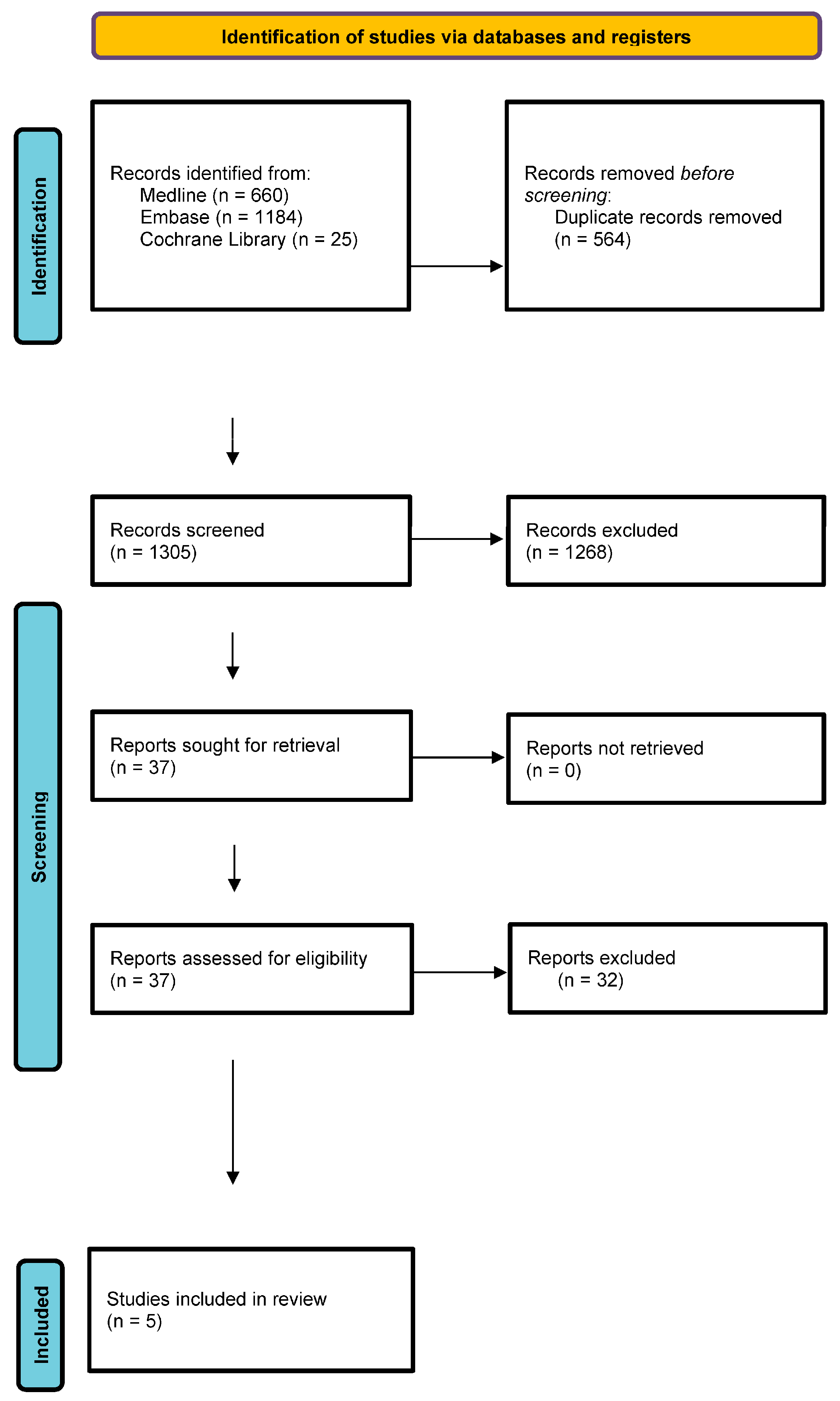
2. Materials and Methods
- determination of the criteria for the inclusion of studies in the review;
- development/verification of search strategies;
- the query/re-query of medical information sources;
- identification of full-text reports potentially useful in the clinical analysis;
- selection of studies based on the inclusion criteria;
- result processing;
- qualitative synthesis involving the analysis of the statistical and clinical significance of the results of the studies included in the review.
Limitations of the Review
3. Results
- Jordaens, et al. 2023: a systematic review of 924 publications describing the detection of biomarkers in urine for oncological purposes [8];
- Cho, et al. 2022: a meta-analysis of 21 observational studies that evaluated the diagnostic accuracy of the urinary HPV screening test for cervical intraepithelial neoplasia (CIN2) compared with the cervical HPV screening test [9];
- Bober, et al. 2021: a meta-analysis of 15 observational studies assessing the diagnostic accuracy of HPV screening in urine compared with cervical screening for HPV [10];
- Nishimura, et al. 2021: a systematic review of 72 cross-sectional studies that examined the values and preferences for the self-collection of HPV samples [11];
- Pathak, et al. 2014: a meta-analysis of 14 observational studies that assessed the diagnostic accuracy of the urinary HPV screening test compared with the cervical HPV screening test [12].
3.1. The Use of Urine Samples as Liquid Biopsies in Non-Invasive HPV Testing
- the use of first-void urine collected with appropriately designed devices;
- the use of a preservative to prevent degradation during extraction and storage;
- the use of polymerase chain reaction (PCR)-based assays;
- the collection of a sufficient volume of urine.
3.2. Diagnostic Precision of the Detection of HPV Infection from Urine Samples
3.3. Diagnostic Precision of the Detection of CIN2+ Using Urine-Sample HPV Tests
3.4. The Acceptability of Self-Collection of Urine Samples for HPV Screening Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.who.int/today (accessed on 7 March 2024).
- Meites, E.; Gee, J.; Unger, E.; Markowitz, L. Human Papillomavirus. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/hpv.pdf (accessed on 7 March 2024).
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Lyon, France, 2020; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- National Cancer Institute 2023. Cervical Cancer Screening. Available online: https://www.cancer.gov/types/cervical/screening (accessed on 7 March 2024).
- Tatara, T.; Wnuk, K.; Miazga, W.; Świtalski, J.; Karauda, D.; Mularczyk-Tomczewska, P.; Religioni, U.; Gujski, M. The Influence of Vaginal HPV Self-Sampling on the Efficacy of Populational Screening for Cervical Cancer—An Umbrella Review. Cancers 2022, 14, 5913. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration in London: London, UK, 2021; Version 6.2. [Google Scholar]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include andomized or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Jordaens, S.; Zwaenepoel, K.; Tjalma, W.; Deben, C.; Beyers, K.; Vankerckhoven, V.; Pauwels, P.; Vorsters, A. Urine biomarkers in cancer detection: A systematic review of preanalytical parameters and applied methods. Int. J. Cancer 2023, 152, 2186–2205. [Google Scholar] [CrossRef]
- Cho, H.W.; Shim, R.S.; Lee, J.K.; Hong, J.H. Accuracy of human papillomavirus tests on self-collected urine versus clinician-collected samples for the detection of cervical precancer: A systematic review and meta-analysis. J. Gynecol. Oncol. 2022, 33, e4. [Google Scholar] [CrossRef] [PubMed]
- Bober, P.; Firment, P.; Sabo, J. Diagnostic Test Accuracy of First-Void Urine Human Papillomaviruses for Presence Cervical HPV in Women: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 13314. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Yeh, P.T.; Oguntade, H.; Kennedy, C.E.; Narasimhan, M. HPV self-sampling for cervical cancer screening: A systematic review of values and preferences. BMJ Glob. Health 2021, 6, e003743. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Dodds, J.; Zamora, J.; Khan, K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: Systematic review and meta-analysis. BMJ 2014, 349, g5264. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. The American Cancer Society Guidelines for the Prevention and Early Detection of Cervical Cancer. 2021. Available online: https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/cervical-cancer-screeningguidelines.html (accessed on 4 March 2024).
- World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention. 2021. Available online: https://www.who.int/publications/i/item/9789240030824 (accessed on 4 March 2024).
- United States Preventive Services Task Force. Screening for Cervical Cancer US Preventive Services Task Force Recommendation Statement. 2018. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancerscreening (accessed on 4 March 2024).
- Zigras, T.; Mayrand, M.H.; Bouchard, C.; Salvador, S.; Eiriksson, L.; Almadin, C.; Kean, S.; Dean, E.; Malhotra, U.; Todd, N.; et al. Canadian Guideline on the Management of a Positive Human Papillomavirus Test and Guidance for Specific Populations. Curr. Oncol. 2023, 30, 5652–5679. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Ibáñez, R.; Robles, C.; Peremiquel-Trillas, P.; De Sanjosé, S.; Bruni, L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev. Med. 2022, 154, 106900. [Google Scholar] [CrossRef]
- Daponte, A.; Michail, G.; Daponte, A.I.; Daponte, N.; Valasoulis, G. Urine HPV in the Context of Genital and Cervical Cancer Screening—An Update of Current Literature. Cancers 2021, 13, 1640. [Google Scholar] [CrossRef] [PubMed]
| Author/Year | Sample Collection Method for HPV Test | Endpoint | Endpoint Detection Precision | |
|---|---|---|---|---|
| Sensitivity (95% CI) [No. of Studies] | Specificity (95% CI) [No. of Studies] | |||
| Cho 2022 (MA) [9] | Total urine sample | Cervical intraepithelial neoplasia grade two or worse (CIN) (2+) | 79% (0.72; 0.85) [19 OS]. | 55% (0.46; 0.63) [19 OS]. |
| Bober 2021 (MA) [10] | First-void urine | Infection with any HPV genotype | 87% (0.74; 0.94)] [10 OS] | 89% (0.81; 0.93) [10 OS]. |
| Infection with a high-risk HPV genotype | 78% (0.70; 0.84) [12 OS] | 89% (0.81; 0.94) [12 OS] | ||
| Infection with HPV genotype 16 or 18 | 77% (0.76; 0.77) [7 OS] | 98% (0.98; 0.98) [7 OS] | ||
| Pathak 2014 (MA) [12] | Total urine sample | Infection with any HPV genotype | 87% (0.78; 0.92) [14 OS]. | 94% (0.82; 0.98) [14 OS]. |
| Infection with a high-risk HPV genotype (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, or 82) | 77% (0.68; 0.84) [11 OS]. | 88% (0.58; 0.97) [11 OS]. | ||
| Infection with HPV genotype 16 or 18 | 73% (0.56; 0.86) [11 OS] | 98% (0.91; 1.00) [11 OS] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miazga, W.; Tatara, T.; Wnuk, K.; Gujski, M.; Pinkas, J.; Religioni, U. The Impact of Urine-Sample HPV Testing on the Effectiveness of Screening for Cervical Cancer: An Umbrella Review. Cancers 2024, 16, 2244. https://doi.org/10.3390/cancers16122244
Miazga W, Tatara T, Wnuk K, Gujski M, Pinkas J, Religioni U. The Impact of Urine-Sample HPV Testing on the Effectiveness of Screening for Cervical Cancer: An Umbrella Review. Cancers. 2024; 16(12):2244. https://doi.org/10.3390/cancers16122244
Chicago/Turabian StyleMiazga, Wojciech, Tomasz Tatara, Katarzyna Wnuk, Mariusz Gujski, Jarosław Pinkas, and Urszula Religioni. 2024. "The Impact of Urine-Sample HPV Testing on the Effectiveness of Screening for Cervical Cancer: An Umbrella Review" Cancers 16, no. 12: 2244. https://doi.org/10.3390/cancers16122244
APA StyleMiazga, W., Tatara, T., Wnuk, K., Gujski, M., Pinkas, J., & Religioni, U. (2024). The Impact of Urine-Sample HPV Testing on the Effectiveness of Screening for Cervical Cancer: An Umbrella Review. Cancers, 16(12), 2244. https://doi.org/10.3390/cancers16122244









