The European Thyroid Imaging and Reporting Data System as a Remedy for the Overdiagnosis and Overtreatment of Thyroid Cancer: Results from the EUROCRINE Surgical Registry
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
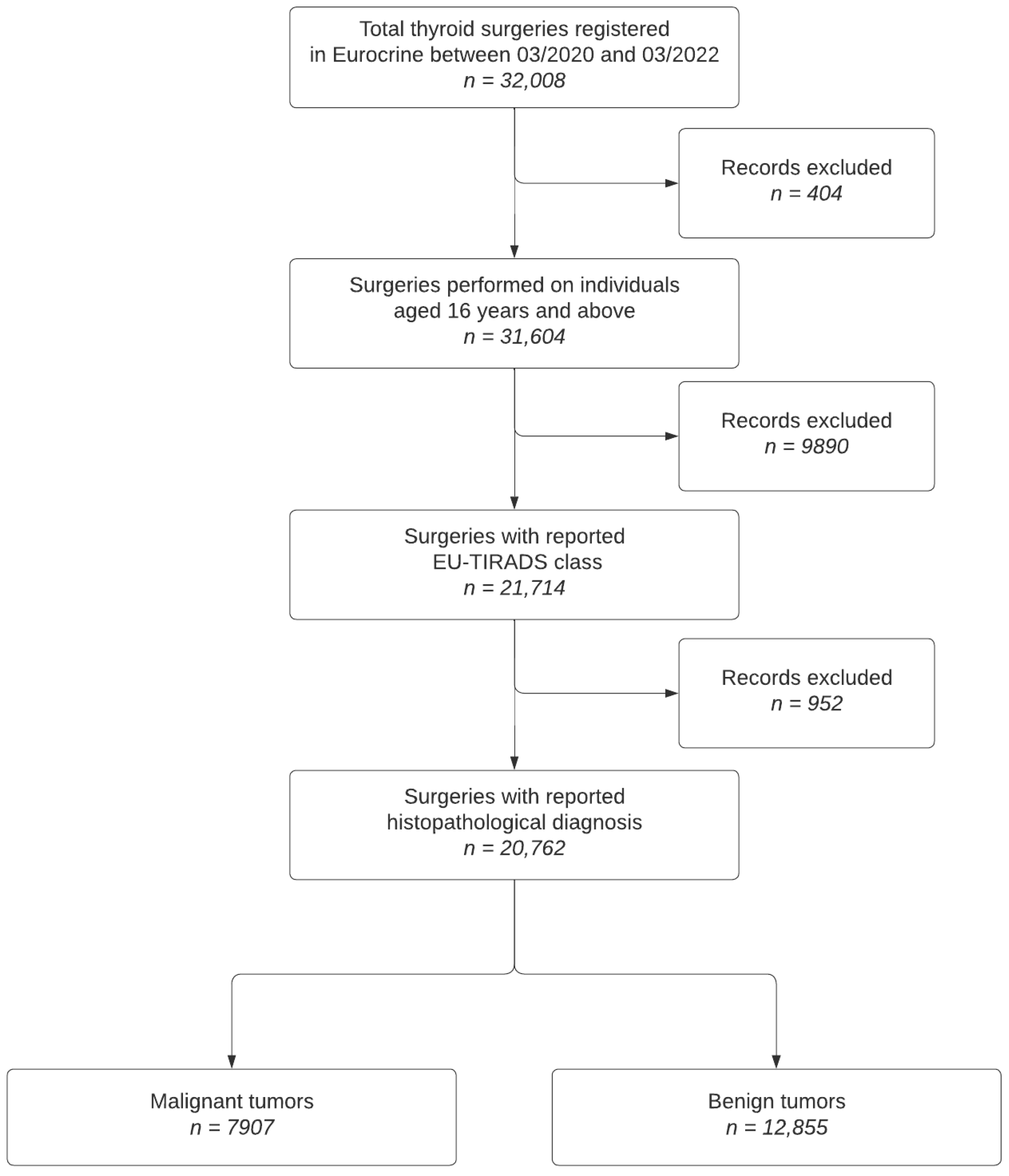
2.1. Study Population Inclusion and Exclusion Criteria
- Patients with thyroid nodules who underwent surgery between March 2020 and March 2022.
- Availability of preoperative EU-TIRADS scores and dominant nodule size.
- Patients without recorded EU-TIRADS classification.
- Incomplete data on primary and secondary histological diagnoses.
2.2. Study Outcomes
2.3. Statistical Methods
3. Results
3.1. EU-TIRADS Reporting Rate
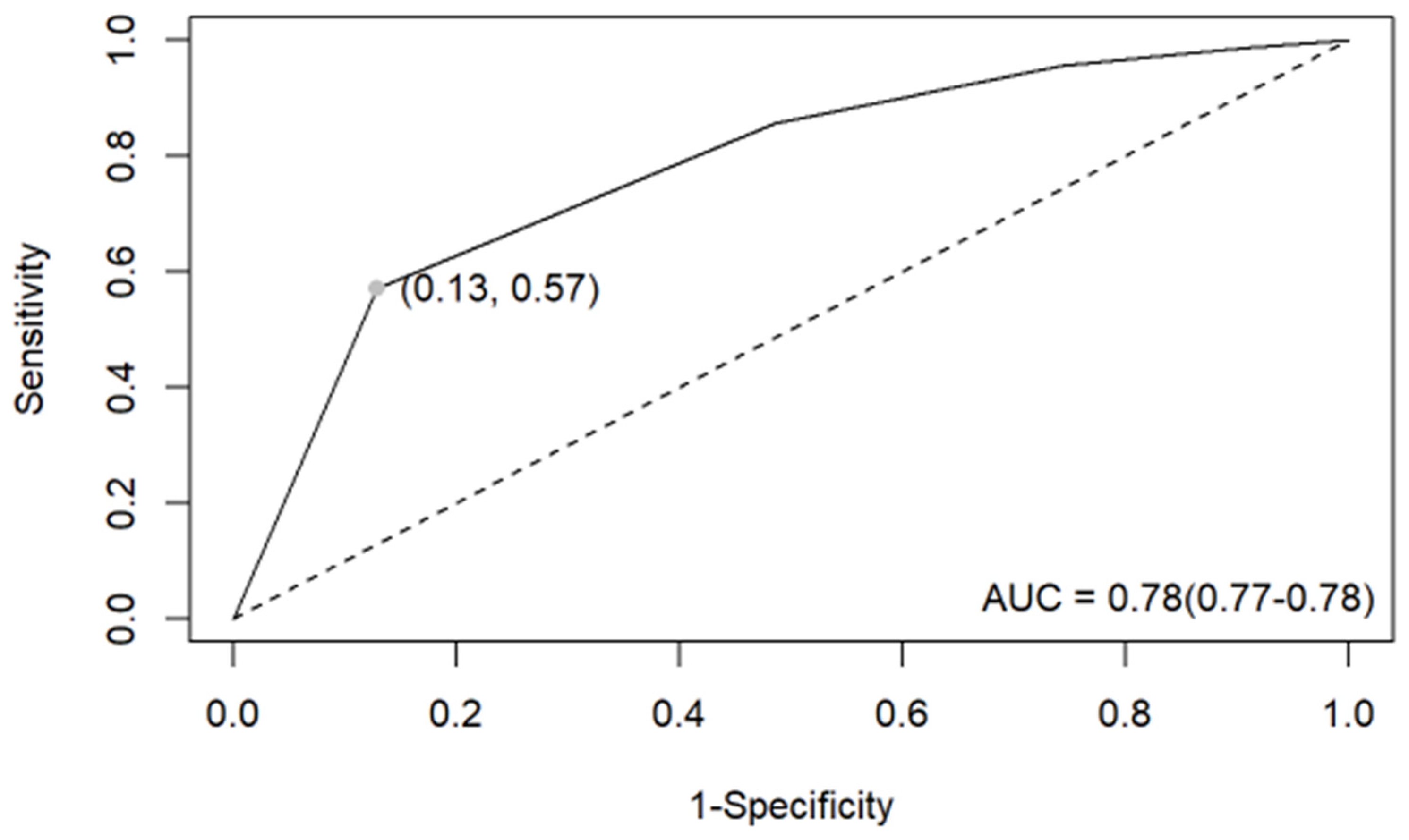
3.2. EU-TIRADS Diagnostic Performance
3.3. Recommended and Not Recommended FNABs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Xin, X.; Wang, L. A Bibliometric Analysis of 8271 Publications on Thyroid Nodules From 2000 to 2021. Front. Endocrinol. 2022, 13, 845776. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.S.; Gharib, H. Epidemiology of Thyroid Nodules. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Gharib, H.; Papini, E.; Paschke, R.; Duick, D.S.; Valcavi, R.; Hegedüs, L.; Vitti, P. AACE/AME/ETA Task Force on Thyroid Nodules American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr. Pract. 2010, 16 (Suppl. 1), 1–43. [Google Scholar] [CrossRef] [PubMed]
- Vanderpump, M.P.; Tunbridge, W.M.; French, J.M.; Appleton, D.; Bates, D.; Clark, F.; Grimley Evans, J.; Hasan, D.M.; Rodgers, H.; Tunbridge, F. The Incidence of Thyroid Disorders in the Community: A Twenty-Year Follow-up of the Whickham Survey. Clin. Endocrinol. (Oxf.) 1995, 43, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.; Xu, H.; Shang, W.; Dong, A. Systematic Review and Meta-Analysis of American College of Radiology TI-RADS Inter-Reader Reliability for Risk Stratification of Thyroid Nodules. Front. Oncol. 2022, 12, 840516. [Google Scholar] [CrossRef]
- Grant, E.G.; Tessler, F.N.; Hoang, J.K.; Langer, J.E.; Beland, M.D.; Berland, L.L.; Cronan, J.J.; Desser, T.S.; Frates, M.C.; Hamper, U.M.; et al. Thyroid Ultrasound Reporting Lexicon: White Paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. J. Am. Coll. Radiol. 2015, 12, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Han, K.H.; Yoon, J.H.; Moon, H.J.; Son, E.J.; Park, S.H.; Jung, H.K.; Choi, J.S.; Kim, B.M.; Kim, E.-K. Thyroid Imaging Reporting and Data System for US Features of Nodules: A Step in Establishing Better Stratification of Cancer Risk. Radiology 2011, 260, 892–899. [Google Scholar] [CrossRef]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.-H.; Lee, Y.H.; Lim, H.K.; Moon, W.-J.; Na, D.G.; Park, J.S.; et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid. J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Durante, C.; Grani, G.; Lamartina, L.; Filetti, S.; Mandel, S.J.; Cooper, D.S. The Diagnosis and Management of Thyroid Nodules: A Review. JAMA 2018, 319, 914–924. [Google Scholar] [CrossRef]
- Alshoabi, S.A.; Binnuhaid, A.A. Diagnostic Accuracy of Ultrasonography versus Fine-Needle-Aspiration Cytology for Predicting Benign Thyroid Lesions. Pak. J. Med. Sci. 2019, 35, 630–635. [Google Scholar] [CrossRef]
- Jarząb, B.; Dedecjus, M.; Lewiński, A.; Adamczewski, Z.; Bakuła-Zalewska, E.; Bałdys-Waligórska, A.; Barczyński, M.; Biskup-Frużyńska, M.; Bobek-Billewicz, B.; Bossowski, A.; et al. Diagnosis and Treatment of Thyroid Cancer in Adult Patients—Recommendations of Polish Scientific Societies and the National Oncological Strategy. 2022 Update [Diagnostyka i Leczenie Raka Tarczycy u Chorych Dorosłych—Rekomendacje Polskich Towarzystw Naukowych Oraz Narodowej Strategii Onkologicznej. Aktualizacja Na Rok 2022]. Endokrynol. Pol. 2022, 73, 173–300. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Gitto, S.; Cantisani, V.; Vallone, G.; Schiavone, C.; Papini, E.; Sconfienza, L.M. Use of the Thyroid Imaging Reporting and Data System (TIRADS) in Clinical Practice: An Italian Survey. Endocrine 2020, 68, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Ngu, R.; Royer, B.; Giovanella, L.; Bigorgne, C.; Simo, R.; Carroll, P.; Russ, G. A Multicentre Validation Study for the EU-TIRADS Using Histological Diagnosis as a Gold Standard. Clin. Endocrinol. (Oxf.) 2019, 91, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Dobruch-Sobczak, K.; Adamczewski, Z.; Szczepanek-Parulska, E.; Migda, B.; Woliński, K.; Krauze, A.; Prostko, P.; Ruchała, M.; Lewiński, A.; Jakubowski, W.; et al. Histopathological Verification of the Diagnostic Performance of the EU-TIRADS Classification of Thyroid Nodules-Results of a Multicenter Study Performed in a Previously Iodine-Deficient Region. J. Clin. Med. 2019, 8, E1781. [Google Scholar] [CrossRef]
- About Eurocrine|European Registry for Endocrine Surgery. Available online: https://eurocrine.eu/about-eurocrine (accessed on 4 September 2023).
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 7 August 2022).
- RStudio Team RStudio: Integrated Development Environment for R. Available online: http://www.rstudio.com/ (accessed on 7 August 2022).
- Vaccarella, S.; Franceschi, S.; Bray, F.; Wild, C.P.; Plummer, M.; Dal Maso, L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N. Engl. J. Med. 2016, 375, 614–617. [Google Scholar] [CrossRef]
- Grani, G.; Lamartina, L.; Ascoli, V.; Bosco, D.; Biffoni, M.; Giacomelli, L.; Maranghi, M.; Falcone, R.; Ramundo, V.; Cantisani, V.; et al. Reducing the Number of Unnecessary Thyroid Biopsies While Improving Diagnostic Accuracy: Toward the “Right” TIRADS. J. Clin. Endocrinol. Metab. 2019, 104, 95–102. [Google Scholar] [CrossRef]
- Ha, E.J.; Shin, J.H.; Na, D.G.; Jung, S.L.; Lee, Y.H.; Paik, W.; Hong, M.J.; Kim, Y.K.; Lee, C.Y. Comparison of the Diagnostic Performance of the Modified Korean Thyroid Imaging Reporting and Data System for Thyroid Malignancy with Three International Guidelines. Ultrasonography 2021, 40, 594–601. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Yan, C.; Liu, L.; Liao, Y.; Zeng, H.; Huang, W.; Li, Q.; Tao, N.; Zhou, J. Comparison of Diagnostic Accuracy and Utility of Artificial Intelligence-Optimized ACR TI-RADS and Original ACR TI-RADS: A Multi-Center Validation Study Based on 2061 Thyroid Nodules. Eur. Radiol. 2022, 32, 7733–7742. [Google Scholar] [CrossRef]
- Skowrońska, A.; Milczarek-Banach, J.; Wiechno, W.; Chudziński, W.; Żach, M.; Mazurkiewicz, M.; Miśkiewicz, P.; Bednarczuk, T. Accuracy of the European Thyroid Imaging Reporting and Data System (EU-TIRADS) in the Valuation of Thyroid Nodule Malignancy in Reference to the Post-Surgery Histological Results. Pol. J. Radiol. 2018, 83, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wu, Y.; Wu, R.-X.; Zhang, Y.-Z.; Gu, J.-Y.; Ye, X.-H.; Tang, W.; Xu, S.-H.; Liu, C.; Wu, X.-H. Validation and Comparison of Three Newly-Released Thyroid Imaging Reporting and Data Systems for Cancer Risk Determination. Endocrine 2019, 64, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Grani, G.; Radzina, M.; Guerra, V.; Giovanella, L.; Deandrea, M.; Ngu, R.; Durante, C.; Trimboli, P. Performance of EU-TIRADS in Malignancy Risk Stratification of Thyroid Nodules: A Meta-Analysis. Eur. J. Endocrinol. 2020, 183, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, J.; Kukulska, A.; Oczko-Wojciechowska, M.; Kotecka-Blicharz, A.; Drosik-Rutowicz, K.; Haras-Gil, M.; Jarzab, B.; Handkiewicz-Junak, D. Early Diagnosis of Low-Risk Papillary Thyroid Cancer Results Rather in Overtreatment Than a Better Survival. Front. Endocrinol. 2020, 11, 571421. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; Hutchinson, M.E.; Gonzalez-Losada, T.; McIver, B.; Reinalda, M.E.; Grant, C.S.; Thompson, G.B.; Sebo, T.J.; Goellner, J.R. Papillary Thyroid Microcarcinoma: A Study of 900 Cases Observed in a 60-Year Period. Surgery 2008, 144, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Inabnet, W.B.; Palazzo, F.; Sosa, J.A.; Kriger, J.; Aspinall, S.; Barczynski, M.; Doherty, G.; Iacobone, M.; Nordenstrom, E.; Scott-Coombes, D.; et al. Correlating the Bethesda System for Reporting Thyroid Cytopathology with Histology and Extent of Surgery: A Review of 21,746 Patients from Four Endocrine Surgery Registries Across Two Continents. World J. Surg. 2020, 44, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Bojunga, J.; Deandrea, M.; Frasca, F.; Imperiale, A.; Leoncini, A.; Paone, G.; Pitoia, F.; Rotondi, M.; Sadeghi, R.; et al. Reappraising the Role of Thyroid Scintigraphy in the Era of TIRADS: A Clinically-Oriented Viewpoint. Endocrine 2024. online ahead of print. [Google Scholar] [CrossRef]
- Scappaticcio, L.; Trimboli, P.; Iorio, S.; Maiorino, M.I.; Longo, M.; Croce, L.; Pignatelli, M.F.; Ferrandes, S.; Cozzolino, I.; Montella, M.; et al. Repeat Thyroid FNAC: Inter-Observer Agreement among High- and Low-Volume Centers in Naples Metropolitan Area and Correlation with the EU-TIRADS. Front. Endocrinol. 2022, 13, 1001728. [Google Scholar] [CrossRef]
- The National Health Fund of Poland–Statistics. Available online: https://statystyki.nfz.gov.pl/ (accessed on 18 October 2023).
- Medas, F.; Dobrinja, C.; Al-Suhaimi, E.A.; Altmeier, J.; Anajar, S.; Arikan, A.E.; Azaryan, I.; Bains, L.; Basili, G.; Bolukbasi, H.; et al. Effect of the COVID-19 Pandemic on Surgery for Indeterminate Thyroid Nodules (THYCOVID): A Retrospective, International, Multicentre, Cross-Sectional Study. Lancet. Diabetes Endocrinol. 2023, 11, 402–413. [Google Scholar] [CrossRef]
| Characteristics | Complete Data Set | Analytic Data Set |
|---|---|---|
| Total operations | 32,008 | 20,762 |
| Total patients | 31,703 | 20,691 |
| Females/males | 24,950/7058 | 16,508/4254 |
| Median age, years (min.–max.) | 51 (0–106) | 51 (16–106) |
| Indication for surgery: | ||
| Excluding malignancy | 12,914 (40%) | 8842 (43%) |
| Compression symptoms | 6643 (21%) | 4318 (21%) |
| Malignancy | 5657 (18%) | 4235 (20%) |
| Thyrotoxicosis | 5397 (17%) | 2817 (14%) |
| Other | 1397 (4.4%) | 550 (2.6%) |
| Type of surgery: | ||
| Thyroidectomy | 15,419 (48%) | 9465 (46%) |
| Unilateral lobectomy | 13,742 (43%) | 9647 (46%) |
| Other | 2847 (9%) | 1650 (8%) |
| Lymph node operation: | ||
| None | 25,709 (80%) | 16,218 (78%) |
| CLND a | 3746 (12%) | 3009 (14%) |
| CLND a + LLND b | 1344 (4.2%) | 984 (4.7%) |
| Other | 209 (3.8%) | 551 (2.7%) |
| Histopathological main diagnosis: | ||
| Nodular goiter | 9563 (30%) | 5346 (26%) |
| Papillary cancer | 8581 (27%) | 6766 (33%) |
| Follicular adenoma | 5527 (17%) | 4513 (22%) |
| Graves’ disease | 2827 (8.8%) | 1569 (7.6%) |
| Oncocytic adenoma | 749 (2.3%) | 589 (2.8%) |
| Follicular cancer | 593 (1.9%) | 395 (1.9%) |
| Medullary cancer | 423 (1.3%) | 319 (1.5%) |
| Lymphocytic thyroiditis | 522 (1.6%) | 280 (1.3%) |
| Oncocytic carcinoma | 241 (0.8%) | 183 (0.9%) |
| NIFTP | 217 (0.7%) | 133 (0.6%) |
| Other malignancies | 199 (0.6%) | 111 (0.5%) |
| Total malignant neoplasms | 10,254 (32.0%) | 7907 (38.1%) |
| Histopathological Type | EU-TIRADS 1 (n = 93) | EU-TIRADS 2 (n = 243) | EU-TIRADS 3 (n = 796) | EU-TIRADS 4 (n = 2259) | EU-TIRADS 5 (n = 4516) |
|---|---|---|---|---|---|
| Papillary | 79 (1%) | 205 (3%) | 592 (9%) | 1816 (27%) | 4074 (60%) |
| Follicular | 3 (1%) | 18 (5%) | 86 (22%) | 207 (52%) | 81 (20%) |
| Medullary | 1 (0%) | 6 (2%) | 35 (11%) | 54 (17%) | 223 (70%) |
| Oxyphylic | 2 (1%) | 4 (2%) | 30 (16%) | 101 (55%) | 46 (25%) |
| NIFTP | 4 (3%) | 9 (7%) | 48 (36%) | 52 (39%) | 20 (15%) |
| Other | 4 (4%) | 1 (1%) | 5 (4%) | 29 (26%) | 72 (65%) |
| Characteristics | Overall (N = 7907) | FNA Not Recommended (n = 4145 1) | FNA Recommended (n = 3517 1) |
|---|---|---|---|
| Histological type | |||
| Papillary | 6766 (86%) | 3850 (93%) | 2836 (81%) |
| Follicular | 395 (5.0%) | 91 (2.2%) | 294 (8.4%) |
| Medullary | 319 (4.0%) | 154 (3.7%) | 158 (4.5%) |
| Oxyphylic | 183 (2.3%) | 37 (0.9%) | 144 (4.1%) |
| Other | 111 (1.4%) | 13 (0.3%) | 84 (2.4%) |
| Primary tumor | |||
| pT1a | 3502 (44%) | 3407 (82%) | 42 (1.2%) |
| pT1b | 2260 (29%) | 607 (15%) | 1629 (46%) |
| pT2 | 1267 (16%) | 57 (1.4%) | 1198 (34%) |
| pT3a | 425 (5.4%) | 34 (0.8%) | 383 (11%) |
| pT3b or higher | 301 (3.8%) | 38 (0.9%) | 258 (7.3%) |
| pTx | 152 (1.9%) | 2 (<0.1%) | 7 (0.2%) |
| Regional lymph node | |||
| pN0 | 3497 (44%) | 2076 (50%) | 1385 (39%) |
| pN1a | 1414 (18%) | 636 (15%) | 768 (22%) |
| pN1b | 787 (10.0%) | 197 (4.8%) | 574 (16%) |
| pNx | 2209 (28%) | 1236 (30%) | 790 (22%) |
| Distant metastasis | |||
| pM0 | 6535 (83%) | 3521 (85%) | 2967 (84%) |
| pM1 | 96 (1.2%) | 12 (0.3%) | 80 (2.3%) |
| pMx | 1276 (16%) | 612 (15%) | 470 (13%) |
| Clinical risk 2 | |||
| Minimal | 2607 (33.0%) | 2541 (61.3%) | 24 (0.7%) |
| Low–moderate | 4297 (54.3%) | 1369 (33.0%) | 2743 (78.0%) |
| High | 1003 (12.7%) | 235 (5.7%) | 750 (21.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellmann, A.R.; Wiśniewski, P.; Śledziński, M.; Raffaelli, M.; Kobiela, J.; Barczyński, M. The European Thyroid Imaging and Reporting Data System as a Remedy for the Overdiagnosis and Overtreatment of Thyroid Cancer: Results from the EUROCRINE Surgical Registry. Cancers 2024, 16, 2237. https://doi.org/10.3390/cancers16122237
Hellmann AR, Wiśniewski P, Śledziński M, Raffaelli M, Kobiela J, Barczyński M. The European Thyroid Imaging and Reporting Data System as a Remedy for the Overdiagnosis and Overtreatment of Thyroid Cancer: Results from the EUROCRINE Surgical Registry. Cancers. 2024; 16(12):2237. https://doi.org/10.3390/cancers16122237
Chicago/Turabian StyleHellmann, Andrzej Rafał, Piotr Wiśniewski, Maciej Śledziński, Marco Raffaelli, Jarosław Kobiela, and Marcin Barczyński. 2024. "The European Thyroid Imaging and Reporting Data System as a Remedy for the Overdiagnosis and Overtreatment of Thyroid Cancer: Results from the EUROCRINE Surgical Registry" Cancers 16, no. 12: 2237. https://doi.org/10.3390/cancers16122237
APA StyleHellmann, A. R., Wiśniewski, P., Śledziński, M., Raffaelli, M., Kobiela, J., & Barczyński, M. (2024). The European Thyroid Imaging and Reporting Data System as a Remedy for the Overdiagnosis and Overtreatment of Thyroid Cancer: Results from the EUROCRINE Surgical Registry. Cancers, 16(12), 2237. https://doi.org/10.3390/cancers16122237








