Plasmatic Inactive IL-18 Predicts a Worse Overall Survival for Advanced Non-Small-Cell Lung Cancer with Early Metabolic Progression after Immunotherapy Initiation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. 18FDG PET-CT Exams
2.3. PERCIST Criteria
2.4. Blood Sample Collection
2.5. IL-18-Related Compound Measurement
2.6. Microarray
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
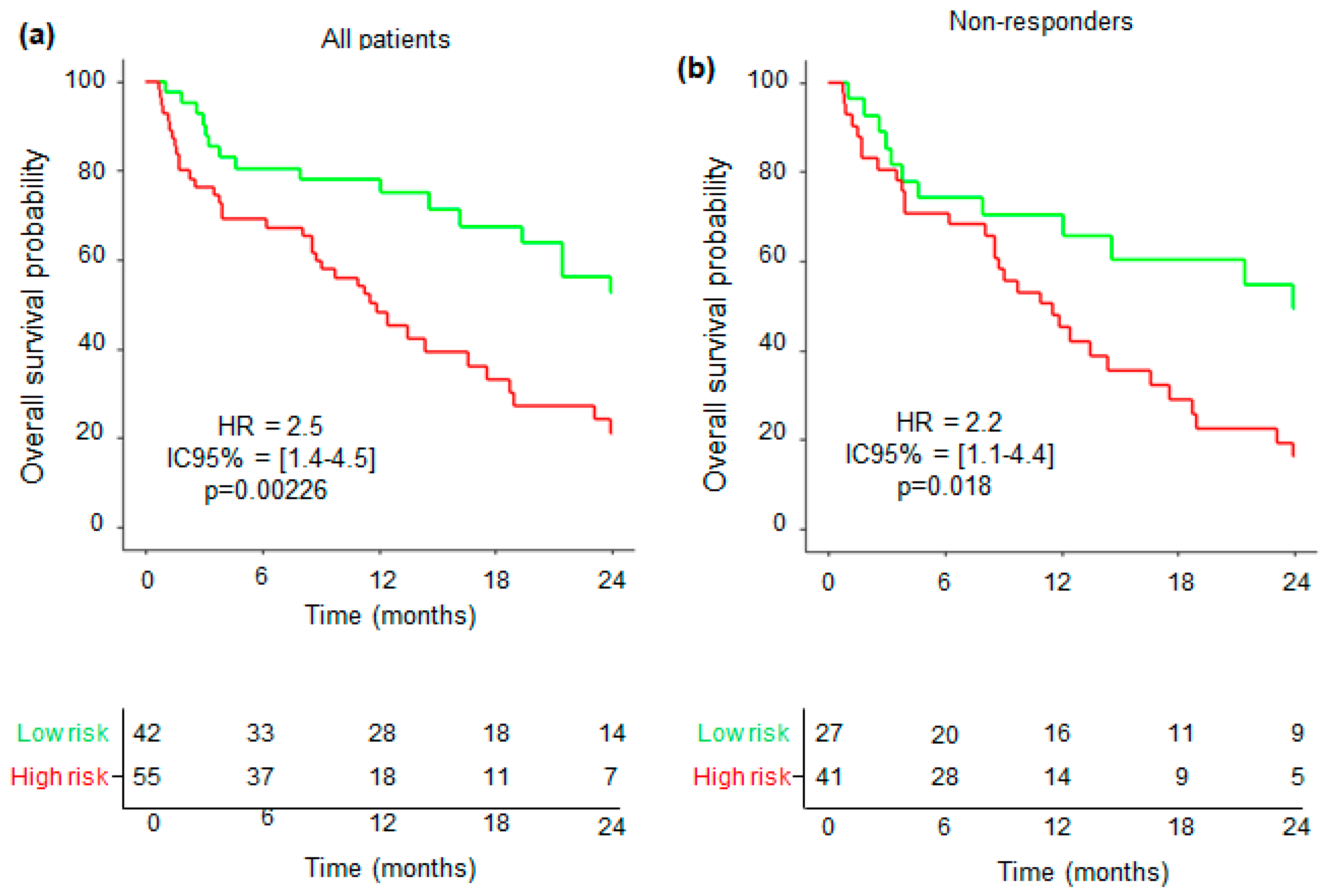
3.2. Tumor Response on PETinterim1 (PERSIST Criteria)
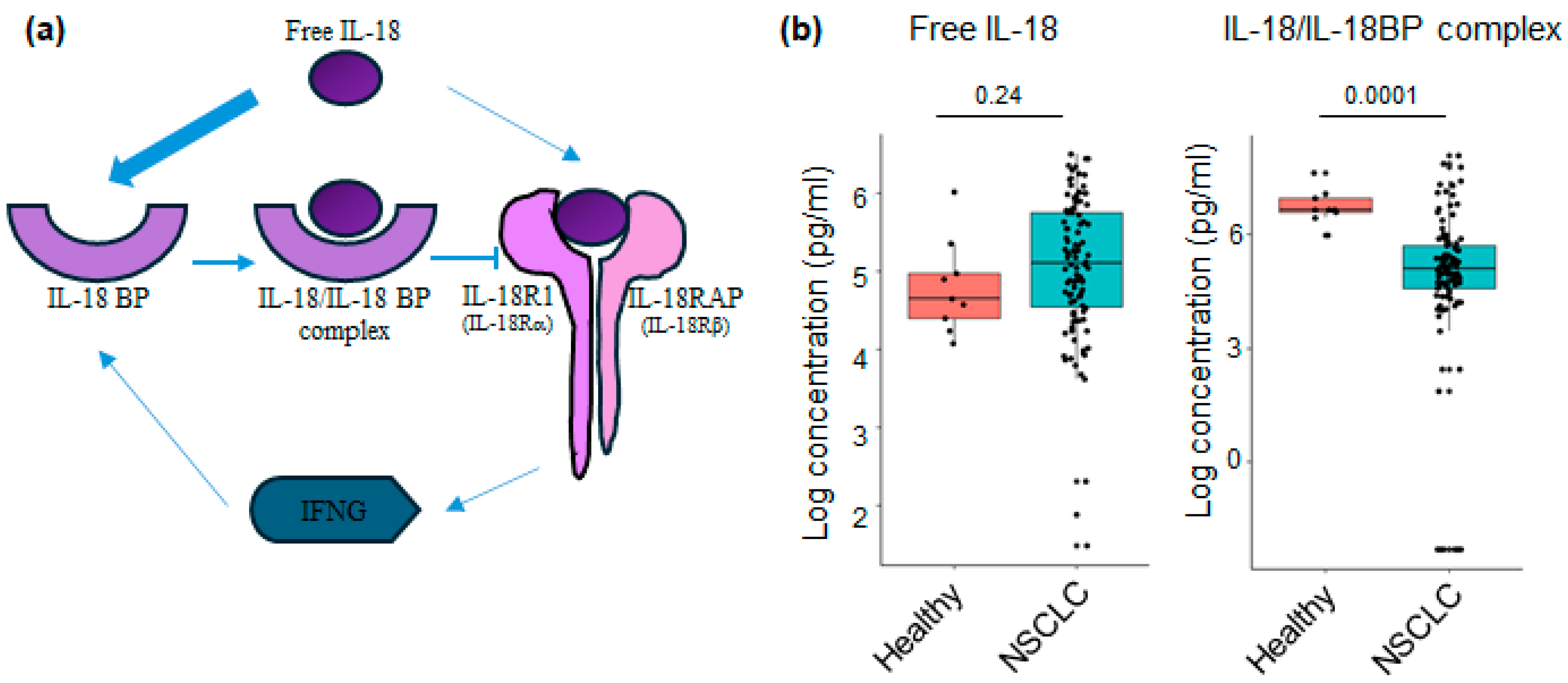
3.3. Levels of IL-18 Relative Analytes at Baseline in Plasma of NSCLC Patients
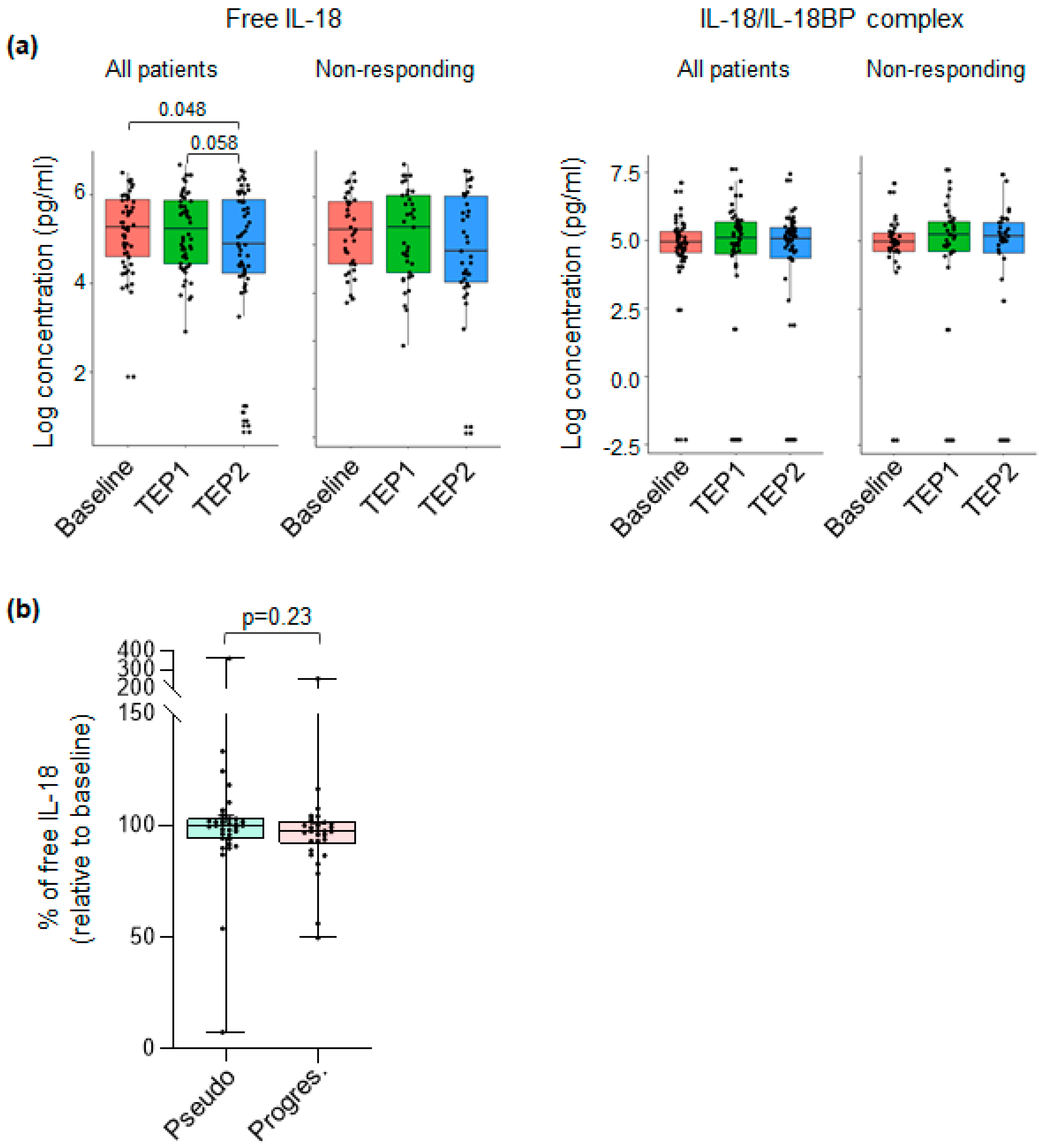
3.4. The Level of Plasmatic Free IL-18 Is Decreased in ICI-Treated NSCLC Patients
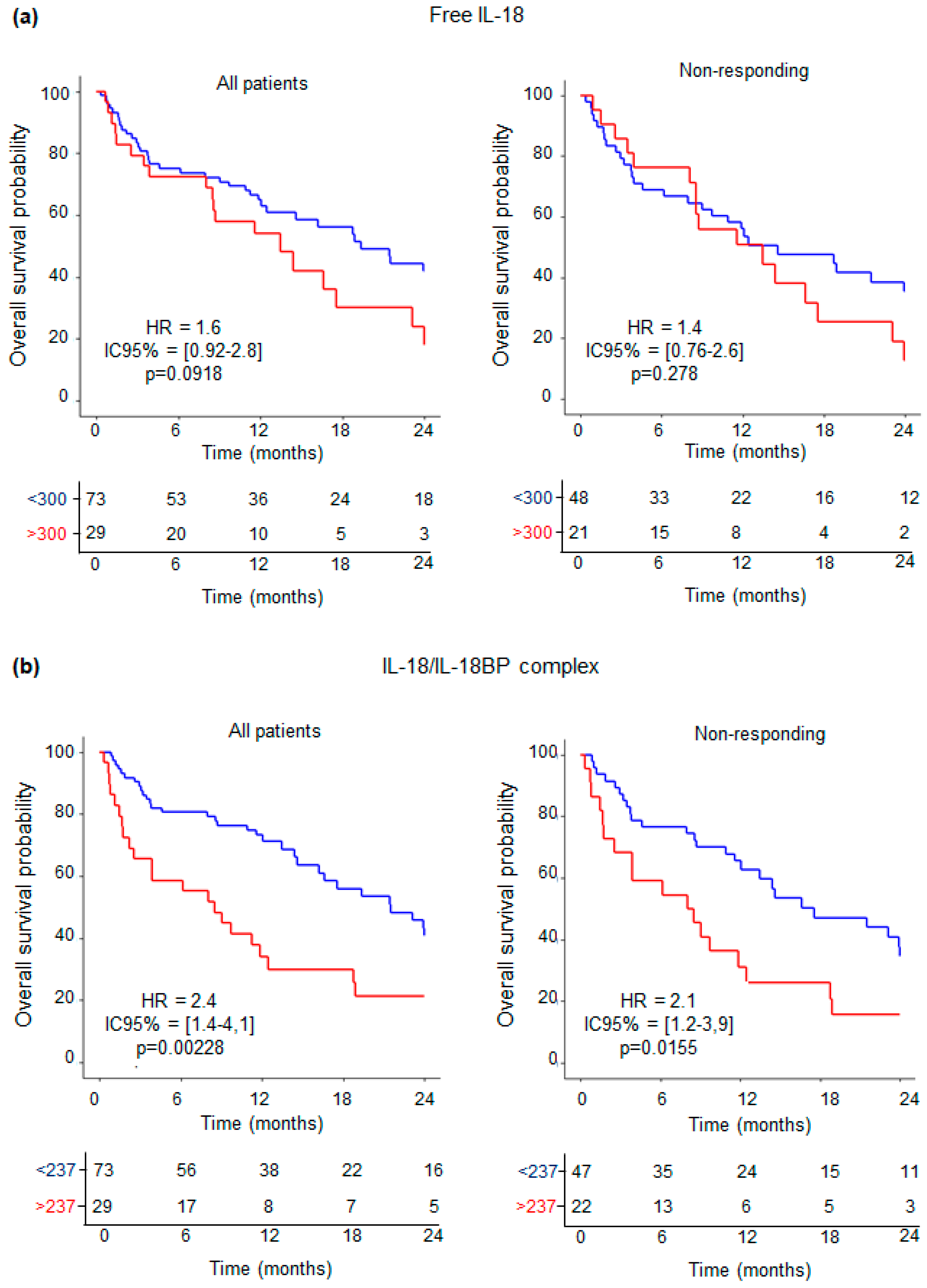
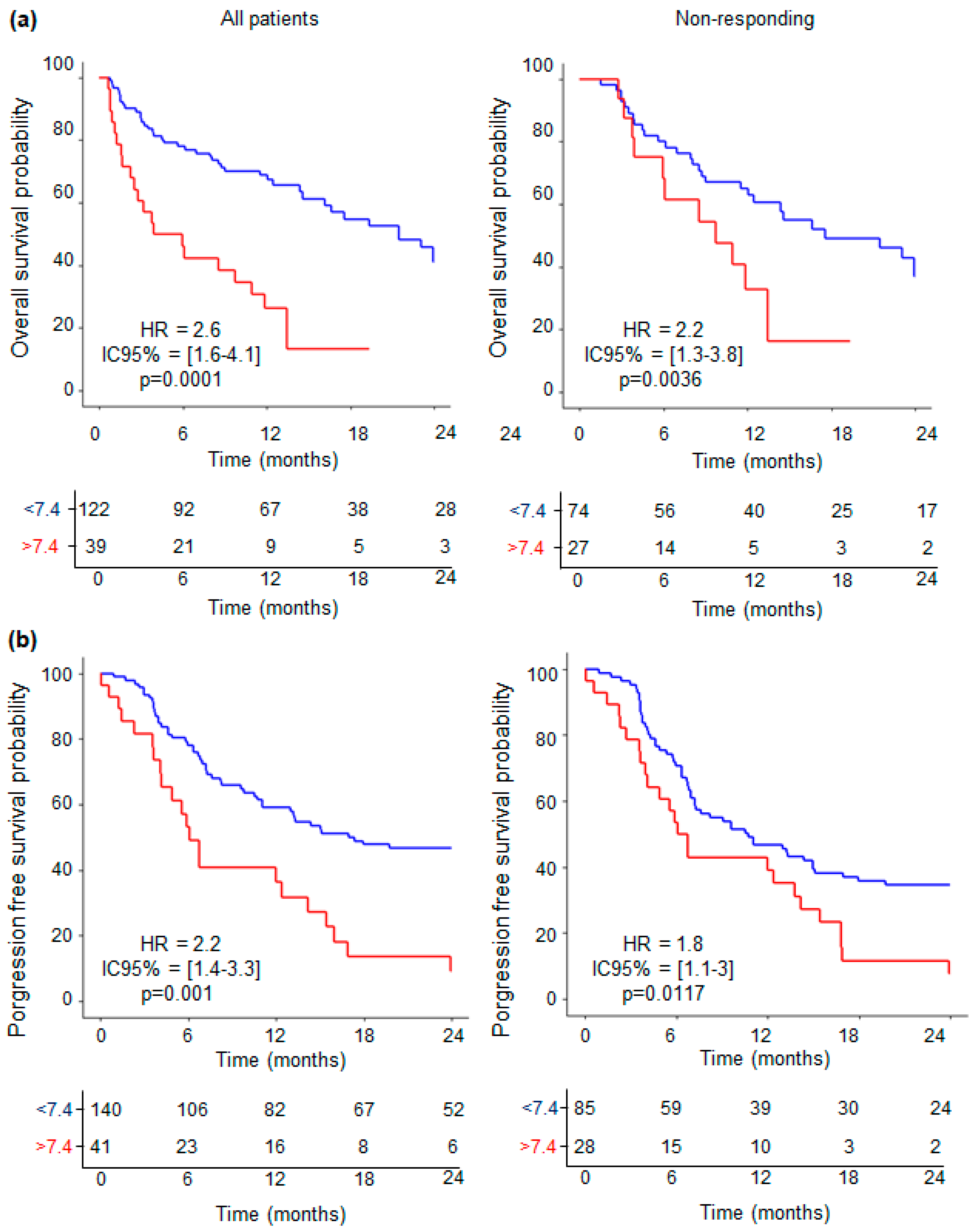
3.5. Levels of IL-18-Related Compounds and Their Association with Outcome of ICI-Treated NSCLC Patients
3.6. Predictive Value of Combined IL-18-Related Compounds—Neutrophil Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
References
- Zulfiqar, B.; Farooq, A.; Kanwal, S.; Asghar, K. Immunotherapy and Targeted Therapy for Lung Cancer: Current Status and Future Perspectives. Front. Pharmacol. 2022, 13, 1035171. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Mannani, R.; Heidarnejad Maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent Advances in Non-Small Cell Lung Cancer Targeted Therapy; an Update Review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef]
- Mino-Kenudson, M.; Schalper, K.; Cooper, W.; Dacic, S.; Hirsch, F.R.; Jain, D.; Lopez-Rios, F.; Tsao, M.S.; Yatabe, Y.; Beasley, M.B.; et al. Predictive Biomarkers for Immunotherapy in Lung Cancer: Perspective from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2022, 17, 1335–1354. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Tsutsi, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K. Cloning of a New Cytokine That Induces IFN-Gamma Production by T Cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, J.; Cai, Y.; Fu, S.; Zhang, N.; Fu, X.; Li, L. IFN-γ-Mediated Inhibition of Lung Cancer Correlates with PD-L1 Expression and Is Regulated by PI3K-AKT Signaling. Int. J. Cancer 2018, 143, 931–943. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 Binding Protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Janho Dit Hreich, S.; Hofman, P.; Vouret-Craviari, V. The Role of IL-18 in P2RX7-Mediated Antitumor Immunity. Int. J. Mol. Sci. 2023, 24, 9235. [Google Scholar] [CrossRef] [PubMed]
- Timperi, E.; Focaccetti, C.; Gallerano, D.; Panetta, M.; Spada, S.; Gallo, E.; Visca, P.; Venuta, F.; Diso, D.; Prelaj, A.; et al. IL-18 Receptor Marks Functional CD8+ T Cells in Non-Small Cell Lung Cancer. Oncoimmunology 2017, 6, e1328337. [Google Scholar] [CrossRef] [PubMed]
- Douguet, L.; Janho dit Hreich, S.; Benzaquen, J.; Seguin, L.; Juhel, T.; Dezitter, X.; Duranton, C.; Ryffel, B.; Kanellopoulos, J.; Delarasse, C.; et al. A Small-Molecule P2RX7 Activator Promotes Anti-Tumor Immune Responses and Sensitizes Lung Tumor to Immunotherapy. Nat. Commun. 2021, 12, 653. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Damsky, W.; El Weizman, O.; McGeary, M.K.; Hartmann, K.P.; Rosen, C.E.; Fischer, S.; Jackson, R.; Flavell, R.A.; Wang, J.; et al. IL-18BP Is a Secreted Immune Checkpoint and Barrier to IL-18 Immunotherapy. Nature 2020, 583, 609–614. [Google Scholar] [CrossRef]
- Netterberg, I.; Li, C.-C.; Molinero, L.; Budha, N.; Sukumaran, S.; Stroh, M.; Jonsson, E.N.; Friberg, L.E. A PK/PD Analysis of Circulating Biomarkers and Their Relationship to Tumor Response in Atezolizumab-Treated Non-Small Cell Lung Cancer Patients. Clin. Pharmacol. Ther. 2019, 105, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122S–150S. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, P.; Chardin, D.; Martin, N.; Contu, S.; Hugonnet, F.; Otto, J.; Humbert, O. Total Metabolic Tumor Volume on 18F-FDG PET/CT Is a Game-Changer for Patients with Metastatic Lung Cancer Treated with Immunotherapy. J. Immunother. Cancer 2024, 12, e007628. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Hicks, R.J.; Dimitrakopoulou-Strauss, A.; Dercle, L.; Iravani, A.; Seban, R.D.; Sachpekidis, C.; Humbert, O.; Gheysens, O.; Glaudemans, A.W.J.M.; et al. Joint EANM/SNMMI/ANZSNM Practice Guidelines/Procedure Standards on Recommended Use of [18F]FDG PET/CT Imaging during Immunomodulatory Treatments in Patients with Solid Tumors Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2323–2341. [Google Scholar] [CrossRef] [PubMed]
- ISO 9001:2015; Quality Management Systems. ISO: Geneva, Switzerland, 2015.
- Migliorini, P.; Anzilotti, C.; Pratesi, F.; Quattroni, P.; Bargagna, M.; Dinarello, C.A.; Boraschi, D. Serum and Urinary Levels of IL-18 and Its Inhibitor IL-18BP in Systemic Lupus Erythematosus. Eur. Cytokine Netw. 2010, 21, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Schemper, M.; Smith, T.L. A Note on Quantifying Follow-up in Studies of Failure Time. Control Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Proceedings of the Second International Symposium of Information Theory, Tsahkadsor, Armenia, 2–8 September 1971; Springer: New York, NY, USA, 1973; pp. 267–281. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Valero, C.; Lee, M.; Hoen, D.; Weiss, K.; Kelly, D.W.; Adusumilli, P.S.; Paik, P.K.; Plitas, G.; Ladanyi, M.; Postow, M.A.; et al. Pretreatment Neutrophil-to-Lymphocyte Ratio and Mutational Burden as Biomarkers of Tumor Response to Immune Checkpoint Inhibitors. Nat. Commun. 2021, 12, 729. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, M.; Li, K.; Li, J.; Xu, L.; Xu, F.; Xu, Y.; Ren, D.; Zhang, J.; Liu, L. Immune-related Genes and Gene Sets for Predicting the Response to Anti-programmed Death 1 Therapy in Patients with Primary or Metastatic Non-small Cell Lung Cancer. Oncol. Lett. 2021, 22, 540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Zhang, T.; Yang, X.; Zhong, J.; Wang, Y.; Chi, Y.; Wu, M.; An, T.; Li, J.; et al. Plasma Cytokines Interleukin-18 and C-X-C Motif Chemokine Ligand 10 Are Indicative of the Anti-Programmed Cell Death Protein-1 Treatment Response in Lung Cancer Patients. Ann. Transl. Med. 2021, 9, 33. [Google Scholar] [CrossRef]
- Khatir, W.; Humbert, O.; Benzaquen, J.; Bontoux, C.; Neels, J.; Berland, L.; Rivera, F.A.G.; Allegra, M.; Salah, M.; Tanga, V.; et al. Identification of a Circulating Immunological Signature Predictive of Response to Immune Checkpoint Inhibitors in Patients with Advanced Non-small Cell Lung Cancer. Clin. Transl. Med. 2022, 12, e1018. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Liu, C.; Xu, C.; Lou, Y.; Chen, J.; Yang, Y.; Yagita, H.; Overwijk, W.W.; Lizée, G.; Radvanyi, L.; et al. PD-1 Blockade Enhances T-Cell Migration to Tumors by Elevating IFN-γ Inducible Chemokines. Cancer Res. 2012, 72, 5209–5218. [Google Scholar] [CrossRef]
- Humbert, O.; Bauckneht, M.; Gal, J.; Paquet, M.; Chardin, D.; Rener, D.; Schiazza, A.; Genova, C.; Schiappa, R.; Zullo, L.; et al. Prognostic Value of Immunotherapy-Induced Organ Inflammation Assessed on 18FDG PET in Patients with Metastatic Non-Small Cell Lung Cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3878–3891. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune Checkpoint Inhibitors: Recent Progress and Potential Biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Akinci Ozyurek, B.; Sahin Ozdemirel, T.; Buyukyaylaci Ozden, S.; Erdogan, Y.; Kaplan, B.; Kaplan, T. Prognostic Value of the Neutrophil to Lymphocyte Ratio (NLR) in Lung Cancer Cases. Asian Pac. J. Cancer Prev. 2017, 18, 1417–1421. [Google Scholar] [CrossRef]
- Moutafi, M.; Martinez-Morilla, S.; Divakar, P.; Vathiotis, I.; Gavrielatou, N.; Aung, T.N.; Yaghoobi, V.; Fernandez, A.I.; Zugazagoitia, J.; Herbst, R.S.; et al. Discovery of Biomarkers of Resistance to Immune Checkpoint Blockade in NSCLC Using High-Plex Digital Spatial Profiling. J. Thorac. Oncol. 2022, 17, 991–1001. [Google Scholar] [CrossRef]
| Characteristics | n, (Range) or (%) | |
|---|---|---|
| Age (years) | Median (range) | 66 (39–91) |
| Sex, n, (%) | Female | 88 (36.8%) |
| Male | 151 (63.2%) | |
| Smoking history, n, (%) | Smokers or smoking history | 221 (84%) |
| Nonsmoker | 42 (16%) | |
| Body Mass Index WHO categories | Underweight (<18.5) | 26 (15.3%) |
| Normal weight (18.5 to 24.9) | 88 (51.8%) | |
| Overweight (25 to 30) | 45 (26.5%) | |
| Obese (≥30) | 11 (6.5%) | |
| Unknown | 102 (37.5%) | |
| Tumor histology, n, (%) | Undifferentiated carcinoma | 11 (4.3%) |
| Adenocarcinoma | 200 (77.5%) | |
| Squamous cell carcinoma | 47 (18.2%) | |
| Tumor stage | IIIB | 23 (15%) |
| IV | 130 (85%) | |
| Immunotherapy, n, (%) | Nivolumab | 29 (20.8%) |
| Pembrolizumab | 104 (74.8%) | |
| Atezolizumab | 6 (4.3%) | |
| PD-L1 tumor expression, n (%) | >50% | 88 (51.5%) |
| 0% | 28 (16.4%) | |
| 1–49% | 55 (32.2%) | |
| Previous lung surgery, n, (%) | No | 84 (61.3) |
| Yes | 53 (19.4) | |
| Previous radiotherapy, n (%) | No | 84 (61.3%) |
| Yes | 53 (38.7%) | |
| Previous chemotherapy lines | No | 11 (4.04%) |
| 1st line | 88 (33.7%) | |
| 2nd line | 83 (31.8%) | |
| 3rd line | 90 (34.5%) | |
| PERCIST response | Complete response | 12 (5%) |
| Partial response | 55 (23.1%) | |
| Progressive disease | 66 (60%) | |
| Stable disease | 18 (7.6%) | |
| Unknown | 34 (12.5%) | |
| Pseudo progression | No | 106 (48%) |
| Yes | 115 (52%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janho dit Hreich, S.; Humbert, O.; Pacé-Loscos, T.; Schiappa, R.; Juhel, T.; Ilié, M.; Ferrari, V.; Benzaquen, J.; Hofman, P.; Vouret-Craviari, V. Plasmatic Inactive IL-18 Predicts a Worse Overall Survival for Advanced Non-Small-Cell Lung Cancer with Early Metabolic Progression after Immunotherapy Initiation. Cancers 2024, 16, 2226. https://doi.org/10.3390/cancers16122226
Janho dit Hreich S, Humbert O, Pacé-Loscos T, Schiappa R, Juhel T, Ilié M, Ferrari V, Benzaquen J, Hofman P, Vouret-Craviari V. Plasmatic Inactive IL-18 Predicts a Worse Overall Survival for Advanced Non-Small-Cell Lung Cancer with Early Metabolic Progression after Immunotherapy Initiation. Cancers. 2024; 16(12):2226. https://doi.org/10.3390/cancers16122226
Chicago/Turabian StyleJanho dit Hreich, Serena, Olivier Humbert, Tanguy Pacé-Loscos, Renaud Schiappa, Thierry Juhel, Marius Ilié, Victoria Ferrari, Jonathan Benzaquen, Paul Hofman, and Valérie Vouret-Craviari. 2024. "Plasmatic Inactive IL-18 Predicts a Worse Overall Survival for Advanced Non-Small-Cell Lung Cancer with Early Metabolic Progression after Immunotherapy Initiation" Cancers 16, no. 12: 2226. https://doi.org/10.3390/cancers16122226
APA StyleJanho dit Hreich, S., Humbert, O., Pacé-Loscos, T., Schiappa, R., Juhel, T., Ilié, M., Ferrari, V., Benzaquen, J., Hofman, P., & Vouret-Craviari, V. (2024). Plasmatic Inactive IL-18 Predicts a Worse Overall Survival for Advanced Non-Small-Cell Lung Cancer with Early Metabolic Progression after Immunotherapy Initiation. Cancers, 16(12), 2226. https://doi.org/10.3390/cancers16122226








