Recommendations for the Management of Patients with Hairy-Cell Leukemia and Hairy-Cell Leukemia-like Disorders: A Work by French-Speaking Experts and French Innovative Leukemia Organization (FILO) Group
Abstract
Simple Summary
Abstract
1. Introduction
2. Hairy-Cell Leukemia
- Epidemiology of HCL
- -
- -
- The incidence rate was 0.3 per 100,000 for HCL and 0.2 per 100,000 for HCL-V in the United States [28]. It was lower in non-Hispanic/Blacks, Hispanics, and Asians/Pacific Islanders. In 2018, the number of new cases was estimated at 300 in France. Incidence between 1990 and 2018 remained relatively stable despite the aging of the population and better access to diagnostic tools [29].
- -
- The male-to-female sex ratio is 5:1.
- -
- At diagnosis, the median age is 63 years for men and 59 years for women.
- -
- HCL Diagnosis
3. Complete Blood Count (CBC) with Careful Search for Hairy Cells (HCs)
- -
- A careful examination of the PB smear is necessary for the identification of HCs. HCL patients usually have a low white blood count and the tumor burden is usually low.
- -
- CBC may show one or more cytopenias of varying severity: anemia, neutropenia or thrombocytopenia.
- -
- Lymphocytosis is uncommon [73].
- -
- Unlike HCL-like disorders, monocytopenia is often present in HCL: it can be masked by automated hematology analyzers, which frequently identify HCs as monocytes [74].
- -
- The morphology of HCs is characteristic. HCs have long, fine and circumferential villi, a mature and homogeneous chromatin, and occasional nucleoli. HCs are medium-sized lymphocytes with an abundant, poorly defined, weakly and heterogeneously basophilic cytoplasm. The cytoplasmic projections that give hairy appearance are narrow and circumferential. The nucleus is often round, oval, or kidney-shaped and the chromatin is dispersed with rare or inconspicuous nucleoli. Even if HCs are in low number in the PB, their identification has a strong value for the diagnosis.
4. Trephine Bone Marrow Biopsy with Dry Marrow Aspirates
- -
- BM can be difficult to aspirate due to reticulin fibrosis.
- -
- As in the international consensus guidelines [75], we strongly recommend BM trephine biopsy (with immunostaining for CD20, DBA44, TRAP, VE-1, Cyclin D1 and Annexin-A1). It evaluates the degree of BM infiltration and is useful when assessing the response after treatment.
- -
- Medullary tumor infiltrate is commonly interstitial with honeycomb appearance [76].
5. Flow Cytometry (FCM)
- -
- An immunophenotype can be carried out on PB or BM after mononuclear cell concentration by gradient density to improve sensitivity. HC can be localized very close to the monocyte gate. At the very least, a marker panel should combine markers for analysis of the B-cell lineage (CD19, CD20), for immunoglobulin light chain isotype restriction, and markers indicative of HCs (CD11c, CD25, CD103, CD123).
- -
- The four markers CD11c, CD25, CD103 and CD123 define the HCL immunophenotypic score [2] (one point is given to each expressed marker), which distinguishes HCL that are ≥3 in 98% of cases from other HCL-like disorders with a low score <3. CD103 expression could be negative, weak or with bimodal expression [77,78].
- -
- -
- The expression of CD38, present in one-third of HCL, confers a poor prognosis [82] as in CLL.
- -
- On the contrary, HCs are usually negative for CD5, CD10, CD23 and CD27.
- -
6. Cytogenetics and Molecular Analysis
- -
- We do not recommend performing a karyotype at diagnosis of HCL, even if it may show several abnormalities. In fact, the presence or absence of these abnormalities will not modify the type of treatment.
- -
- We recommend BRAF testing for diagnosis. BRAF testing should be carried out whenever possible in the BM aspirate. The BRAFV600E mutation is present in more than 90% of HCL [3] and is usually absent in HCL-like disorders. The integration of molecular data is essential [80]. The method used to look for BRAFV600E mutation is left to the centers. In case of BRAFV600E negativity, an extended sequencing of BRAF (exon 11 and 15) is recommended because of the possible use of targeted treatments. Some patients have alternative BRAF mutations close to the valine in position 600, which impairs the specifically targeted BRAFV600E molecular testing [86].
- -
- Preservation of cells and serum in a cell bank is also recommended before any treatment for further analysis and a better understanding of the pathophysiology of the disease.
7. Other tests: Biochemistry, Viral Studies, Imagery
- -
- LDH and β2 microglobulin have a prognostic value.
- -
- Screening for hemolysis (direct antiglobulin test, haptoglobin, unconjugated bilirubin and lactic dehydrogenase) and immunodeficiency risk by HIV, hepatitis B and C serology is also necessary.
- -
- Chest radiograph or computed tomography (CT) scan (chest abdomen and pelvis) is likewise necessary before any treatment.
- -
- Magnetic resonance imaging (MRI) can be necessary in case of symptomatic and extramedullary manifestations; it must be completed by histologic analysis of the involved tissues.
- -
- Prognostic factors
- -
- Leukocytosis > 10 × 109/L,
- -
- Bulky spleen extending >10 cm below costal margin,
- -
- Unmutated (UM) IGHV profile (>98%),
- -
- Use of the VH4-34 repertory,
- -
- Mutation of the TP53 gene
- -
- In case of relapse, it is necessary to perform:
- -
- Next-generation sequencing (NGS) with a review of different molecular abnormalities (BRAF, MAP2K1, TP53),
- -
- IGHV mutational status and IGHV repertory,
- -
- TP53 status to evaluate prognosis and adapt targeted therapies.
- Assessment of response and measurable residual disease (MRD)
- -
- Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) are defined in the international guidelines [75] and must be used in clinical practice. CR is defined by resolution of palpable splenomegaly, near normalization of PB count and eradication of HC from the BM. The definition of responses is described in Table 2.
- -
- The BM trephine biopsy should be performed between 4 and 6 months after administering CDA and after obtaining a clinical and hematological response with pentostatin.
- -
- Immunohistochemical (IHC) measurable residual disease (MRD) analysis of BM biopsy using B-lineage or specific HCL antibodies (VE-1, DBA.44, TRAP) is correlated to relapse [91,92,93]. The sensitivity of IHC (nearly 1% or less if dual-staining) is overcome by high-throughput technologies such as FCM and molecular analysis for detecting MRD [94,95]. Patient-specific RQ-PCR based on clone-specific IGH is very sensitive to quantify MRD but laborious [96]. Because BRAFV600E seems to be the primary event in HCL [97,98], an interesting alternative is allele-specific qPCR of DropletDigitalPCR (ddPCR) against BRAFV600E [19,95,99,100]. A recent consensus guideline insisted that MRD must be performed on the first pull BM aspiration because of poor medullary infiltration and limited PB involvement [101].
8. HCL-like Disorders
- -
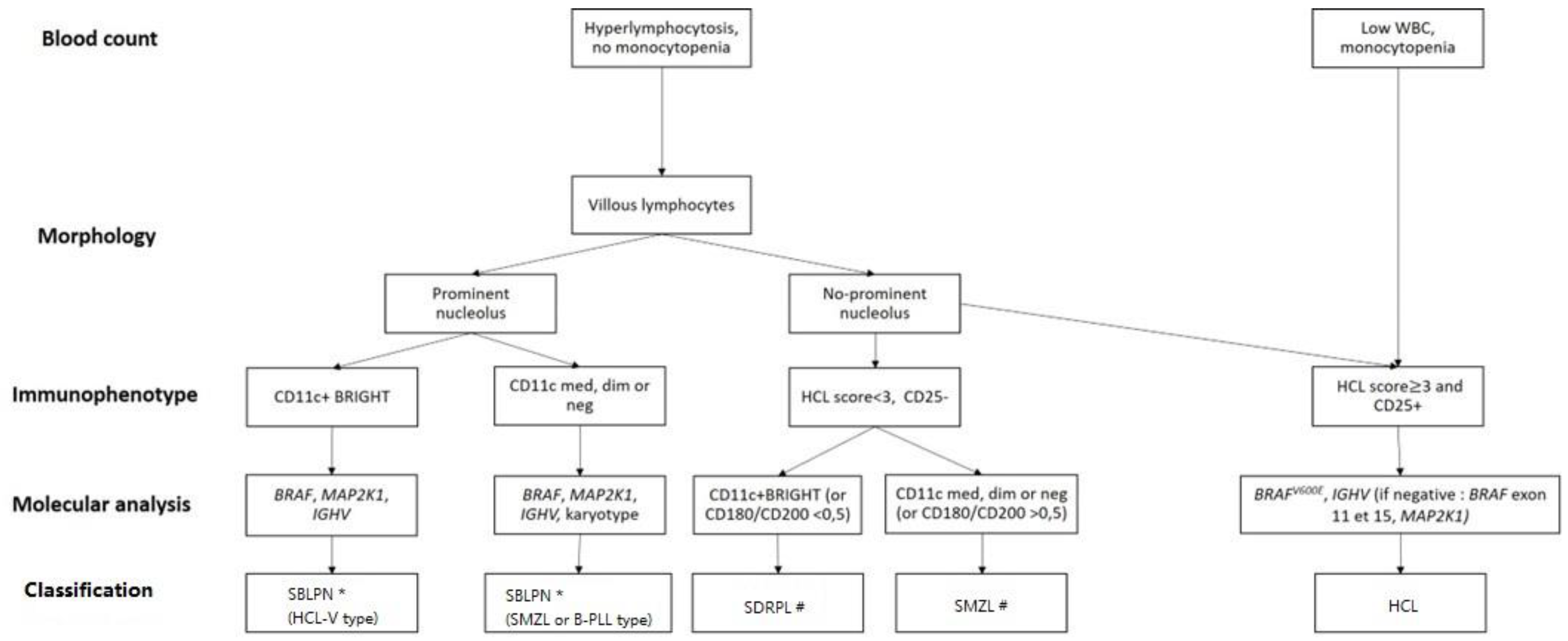
- As differential diagnosis between all these villous proliferations may be difficult, we propose here a simplified algorithm based on the integration (Figure 1 and Table 3) of clinical, morphologic, histologic, immunophenotypic and molecular data that are required to ensure the best classification, the correct diagnosis and therefore the best therapeutic management.
- -
- The peripheral blood picture in HCL-V is monomorphic, with morphology of the abnormal lymphoid cells between hairy cells and prolymphocytes. The proportion of atypical lymphoid cells ranges from 20% to 95% and in most cases accounts for more than 50% of mononuclear cells. The cells are medium to large in size and have an abundant basophilic cytoplasm with circumferential hair-like cytoplasmic projections. The nucleus has a prominent vesicular nucleolus and a condensed chromatin. In HCL-V, the expression of CD25 is negative in all cases at diagnosis and the expression of CD103 and CD123 is usually dim or negative. The expression of CD11c is positive and bright.
- -
- Distinction between SDRPL and HCL-V may be challenging. However, cytomorphologic blood examination of SDRPL should find numerous villous lymphocytes (always > 20%, often > 60%). The nucleolus is present but not prominent [105]. Immunophenotypic features find high expression of CD11c and CD180, CD27 negativity and diminished expression of CD200 [79,106]. The CD200/CD180 ratio is equal or below 0.5 [79]. Unlike HCL, HCL-V and SDRPL, the splenic involvement of SMZL affects the white pulp and not the red pulp of the spleen.
- -
- To avoid diagnostic splenectomy, SMZL may be distinguished from SDRPL or HCL-V thanks to cytomorphologic examination: SMZL leukemic cells have more clumped chromatin, less prolonged villi with a broad base, and villous lymphocytes represent less than 20% of lymphomatous circulated cells [107]. The immunophenotypic profile differs due to diminished expression of CD11c, positivity of CD27, and medium expression of CD180 and CD200 [79,106]. Mutational landscape is dominated by KLF2 and NOTCH2 mutations (in about 40% and 20% of cases, respectively) [108,109,110,111]. The IGHV repertory is biased with overrepresentation of VH1-2 in one-third of cases [112]. The nucleolated form of SMZL that could correspond to the evolution of the indolent form is now classified with HCL-V and B-PLL in the SBLPN group [113,114].
- -
- The Japanese variant form of hairy-cell leukemia (HCL-JV) is more frequent in Japan than classical HCL. However, this entity is very rare; only 17 cases have been published so far, and they are closer to SDRPL [115]. The median age at diagnosis is 75 years. Men are more affected (sex ratio 3.2:1). Splenomegaly is recurrent, lymph nodes are rare and the HCs do not have prominent nucleoli. As in HCL-V and SDRPL, lymphocytosis is frequent, and there is no expression of CD25 [115]. Further investigations should be carried out to confirm the specific pattern of HCL-JV or if they are related to SDRPL.
9. Treatment
- Hairy-Cell Leukemia
- -
- In first-line and fit patients, we recommend CDAR regimen with CDA plus rituximab (R). The data with P plus rituximab are limited [115]. CDA plus R was demonstrated to be more effective than PNA in monotherapy, allowing to reach undetectable MRD (uMRD) in most patients and to achieve more durable responses.
- -
- The simultaneous combination of R + CDA (i.e., R started at day 1 of CDA) was shown to be superior to the sequential schedule of CDA followed by R (i.e., R started 6 months later). R is given at day 1 of CDA for 8 weekly infusions [116]. The overall response rate (ORR) and the CR rate were both 100%, with 97% of patients reaching uMRD.
- -
- -
- PNAs in monotherapy remain an option as the patients may achieve a durable response even if they do not reach uMRD. CDA is given subcutaneously at 0.1–0.14 mg/kg/d once per day for 5 days. Pentostatin is given intravenously at 4 mg/m2 once every 2 weeks for one year. If there is no response at 6 months, P should be stopped and another treatment should be discussed [11,119].
- -
- In the recently updated real-life and retrospective French HCL cohort, the ORR and the CR rate were 100% and 83%, respectively, in patients receiving PNA in the front-line setting. Median RFS was 163 months with CDA and 159 months with P [12]. The five-year overall survival (OS) was 97% for patients treated with CDA and 86% for those treated with P. Similarly, in an Italian study including 513 patients treated with CDA in first line, the ORR and CR rate were 91.8% and 65.3%, respectively [120]. Median RFS was 12.2 years and five-year OS 95.3%.
- -
- In case of renal insufficiency, CDA is contra-indicated if clearance is ≤ 50 mL/min and P if clearance is < 60 mL/min because of a lack of data and a potential risk of increased toxicity as PNAs and their metabolites are mainly excreted renally. There are no specific data in the literature for patients with HCL and chronic kidney disease. In this case, in order to achieve CR with uMRD, particular caution is advised and risks/benefits should be carefully evaluated if administration of PNA is considered; alternatively, if BRAF is mutated, vemurafenib could be used.
- -
- In case of relapse, a new complete work-up is needed, including a complete physical examination, CBC, trephine BM biopsy, and FCM. We also recommend BRAF testing again, the study of IGHV mutational profile, use or not of the VH4-34 repertory and the TP53 status. UM IGHV, VH4-34 usage and TP53 alterations confer an unfavorable prognosis in HCL [89,90,132]. IGHVs are unmutated in 10% of HCL cases, which are associated with resistance to CDA, shorter event-free survival (EFS), high white blood cell count, splenomegaly, and p53 dysfunction [89]. VH4-34 usage occurs in 10% of HCL and 40% of HCL-V and is associated with high white blood cell count, UM IGHV, a lower response rate to CDA, and shorter PFS and OS [90]. TP53 mutations are present in 0–2% of HCL and 30% of HCL-V and confer a poor prognosis in HCL [132,133,134].
- -
- Type of first-line treatment, duration of response, quality of response, toxicities of previous treatment, age and comorbidities must be considered for the choice of second-line treatment in HCL.
10. Third Line Treatment and beyond
- -
- In patients in third line and beyond, we do not recommend the use of PNA again, especially if recurrence of HCL has occurred within 2–5 years. The use of several courses of PNA is associated with cumulative toxicities linked to immunosuppression, especially infectious diseases and second primary malignancies [12,16,139,140,141,142,143,144,145,146]. HCL becomes less responsive to PNA at each line, with lower response rates and shorter PFS [12,13,14].
11. Supportive Care in All Cases
- HCL-like disorders
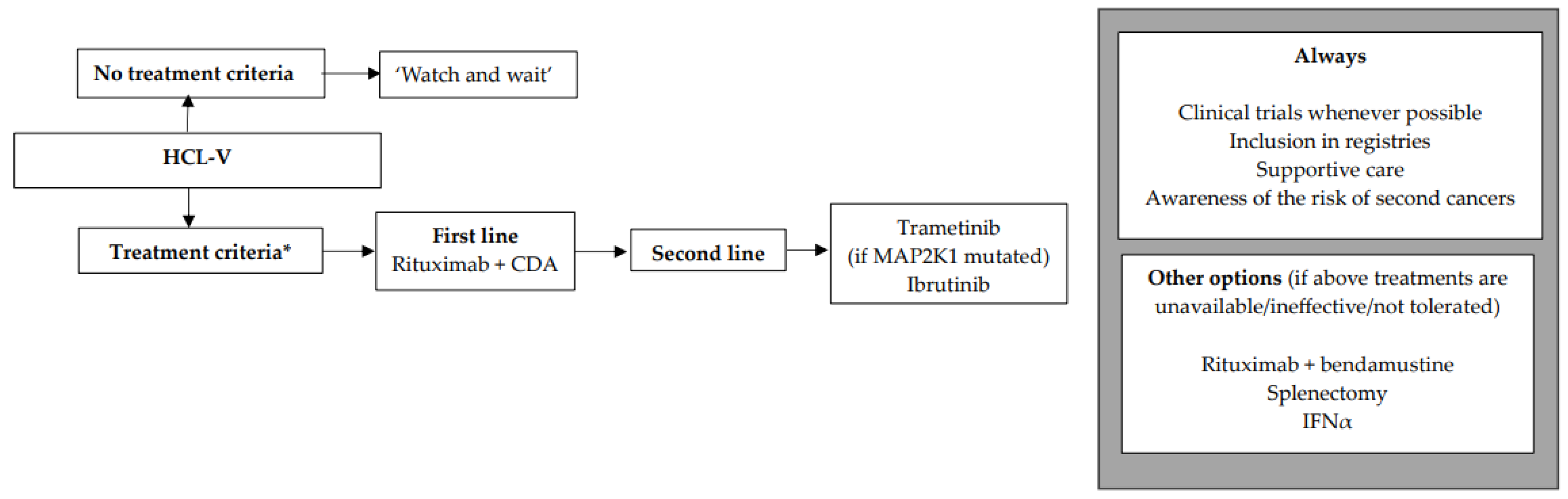
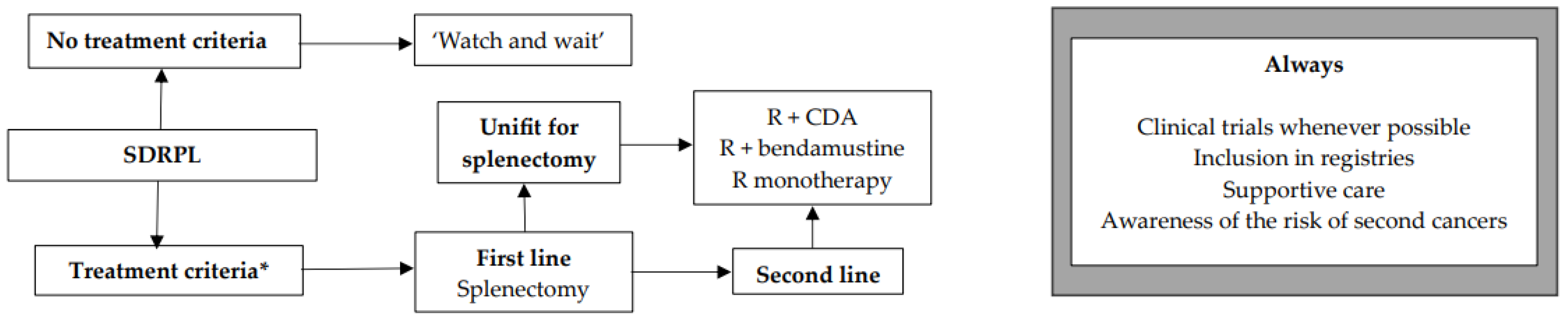
12. SDRPL
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouroncle, B.A.; Wiseman, B.K.; Doan, C.A. Leukemic reticuloendotheliosis. Blood 1958, 13, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Matutes, E.; Morilla, R.; Owusu-Ankomah, K.; Houliham, A.; Meeus, P.; Catovsky, D. The immunophenotype of hairy cell leukemia (HCL). Proposal for a scoring system to distinguish HCL from B-cell disorders with hairy or villous lymphocytes. Leuk. Lymphoma 1994, 14 (Suppl. S1), 57–61. [Google Scholar] [PubMed]
- Tiacci, E.; Trifonov, V.; Schiavoni, G.; Holmes, A.; Kern, W.; Martelli, M.P.; Pucciarini, A.; Bigerna, B.; Pacini, R.; Wells, V.A.; et al. BRAF Mutations in Hairy-Cell Leukemia. N. Engl. J. Med. 2011, 364, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Maitre, E.; Bertrand, P.; Maingonnat, C.; Viailly, P.-J.; Wiber, M.; Naguib, D.; Salaün, V.; Cornet, E.; Damaj, G.; Sola, B.; et al. New generation sequencing of targeted genes in the classical and the variant form of hairy cell leukemia highlights mutations in epigenetic regulation genes. Oncotarget 2018, 9, 28866–28876. [Google Scholar] [CrossRef] [PubMed]
- Sivina, M.; Kreitman, R.J.; Arons, E.; Ravandi, F.; Burger, J.A. The bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) blocks hairy cell leukaemia survival, proliferation and B cell receptor signalling: A new therapeutic approach. Br. J. Haematol. 2014, 166, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Sivina, M.; Burger, J.A. The importance of the tissue microenvironment in hairy cell leukemia. Best. Pract. Res. Clin. Haematol. 2015, 28, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Tiacci, E.; Schiavoni, G.; Martelli, M.P.; Boveri, E.; Pacini, R.; Tabarrini, A.; Zibellini, S.; Santi, A.; Pettirossi, V.; Fortini, E.; et al. Constant activation of the RAF-MEK-ERK pathway as a diagnostic and therapeutic target in hairy cell leukemia. Haematologica 2013, 98, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Mintz, U.; Golomb, H.M. Splenectomy as initial therapy in twenty-six patients with leukemic reticuloendotheliosis (hairy cell leukemia). Cancer Res. 1979, 39, 2366–2370. [Google Scholar] [PubMed]
- Quesada, J.R.; Reuben, J.; Manning, J.T.; Hersh, E.M.; Gutterman, J.U. Alpha interferon for induction of remission in hairy-cell leukemia. N. Engl. J. Med. 1984, 310, 15–18. [Google Scholar] [CrossRef]
- Piro, L.D.; Carrera, C.J.; Carson, D.A.; Beutler, E. Lasting remissions in hairy-cell leukemia Induced by a single Infusion of 2-Chlorodeoxyadenosine. N. Engl. J. Med. 1990, 322, 1117–1121. [Google Scholar] [CrossRef]
- Grever, M.; Kopecky, K.; Foucar, M.K.; Head, D.; Bennett, J.M.; Hutchison, R.E.; Corbett, W.E.; Cassileth, P.A.; Habermann, T.; Golomb, H. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: An intergroup study. J. Clin. Oncol. 1995, 13, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Paillassa, J.; Cornet, E.; Noel, S.; Tomowiak, C.; Lepretre, S.; Vaudaux, S.; Dupuis, J.; Devidas, A.; Joly, B.; Petitdidier-Lionnet, C.; et al. Analysis of a cohort of 279 patients with hairy-cell leukemia (HCL): 10 years of follow-up. Blood Cancer J. 2020, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Bohn, J.P.; Neururer, S.; Pirklbauer, M.; Pircher, A.; Wolf, D. Hairy cell leukemia patients have a normal life expectancy—A 35-year single-center experience and comparison with the general population. Cancers 2022, 14, 1242. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Pellegrini, C.; Stefoni, V.; Derenzini, E.; Gandolfi, L.; Broccoli, A.; Argnani, L.; Quirini, F.; Pileri, S.; Baccarani, M. Hairy cell leukemia: Evaluation of the long-term outcome in 121 patients. Cancer 2010, 116, 4788–4792. [Google Scholar] [CrossRef] [PubMed]
- Hisada, M.; Chen, B.E.; Jaffe, E.S.; Travis, L.B. Second cancer incidence and cause-specific mortality among 3104 patients with hairy cell leukemia: A population-based study. J. Natl. Cancer Inst. 2007, 99, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Benz, R.; Arn, K.; Andres, M.; Pabst, T.; Baumann, M.; Novak, U.; Hitz, F.; Hess, U.; Zenhaeusern, R.; Chalandon, Y.; et al. Prospective long-term follow-up after first-line subcutaneous cladribine in hairy cell leukemia: A SAKK trial. Blood Adv. 2020, 4, 3699. [Google Scholar] [CrossRef] [PubMed]
- Tiacci, E.; Park, J.H.; De Carolis, L.; Chung, S.S.; Broccoli, A.; Scott, S.; Zaja, F.; Devlin, S.; Pulsoni, A.; Chung, Y.R.; et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N. Engl. J. Med. 2015, 373, 1733–1747. [Google Scholar] [CrossRef]
- TiaTiacci, E.; De Carolis, L.; Simonetti, E.; Merluzzi, M.; Bennati, A.; Perriello, V.M.; Pucciarini, A.; Santi, A.; Venanzi, A.; Pettirossi, V.; et al. Safety and efficacy of the BRAF inhibitor dabrafenib in relapsed or refractory hairy cell leukemia: A pilot phase-2 clinical trial. Leukemia 2021, 35, 3314–3318. [Google Scholar] [CrossRef] [PubMed]
- Tiacci, E.; De Carolis, L.; Simonetti, E.; Capponi, M.; Ambrosetti, A.; Lucia, E.; Antolino, A.; Pulsoni, A.; Ferrari, S.; Zinzani, P.L.; et al. Vemurafenib plus rituximab in refractory or relapsed hairy-cell leukemia. N. Engl. J. Med. 2021, 384, 1810–1823. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Moreau, P.; Ravandi, F.; Hutchings, M.; Gazzah, A.; Michallet, A.-S.; Wainberg, Z.A.; Stein, A.; Dietrich, S.; de Jonge, M.J.A.; et al. Dabrafenib plus trametinib in patients with relapsed/refractory BRAF V600E mutation-positive hairy cell leukemia. Blood 2023, 141, 996–1006. [Google Scholar] [CrossRef]
- Rogers, K.A.; Andritsos, L.A.; Wei, L.; McLaughlin, E.M.; Ruppert, A.S.; Anghelina, M.; Blachly, J.S.; Call, T.G.; Chihara, D.; Dauki, A.M.; et al. Phase 2 study of ibrutinib in classic and variant hairy cell leukemia. Blood. 2021, 137, 3473–3483. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Trotman, J.; Opat, S.S.; Stern, J.C.; Allewelt, H.; By, K.; Novotny, W.; Huang, J.; Tedeschi, A. Zanubrutinib for the treatment of relapsed/refractory hairy cell leukemia. Blood Adv. 2023, 7, 2884–2887. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Dearden, C.; Zinzani, P.L.; Delgado, J.; Robak, T.; le Coutre, P.D.; Gjertsen, B.T.; Troussard, X.; Roboz, G.J.; Karlin, L.; et al. Moxetumomab pasudotox in heavily pre-treated patients with relapsed/refractory hairy cell leukemia (HCL): Long-term follow-up from the pivotal trial. J. Hematol. Oncol. 2020, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Cornet, E.; Delmer, A.; Feugier, P.; Garnache-Ottou, F.; Ghez, D.; Leblond, V.; Levy, V.; Maloisel, F.; Re, D.; Zini, J.-M.; et al. Recommendations of the SFH (French Society of Haematology) for the diagnosis, treatment and follow-up of hairy cell leukaemia. Ann. Hematol. 2014, 93, 1977–1983. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tadmor, T.; Polliack, A. Epidemiology and environmental risk in hairy cell leukemia. Best. Pract. Res. Clin. Haematol. 2015, 28, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, L.; Newton, P.; Ross, R.K. Epidemiology of hairy cell leukemia in Los Angeles County. Cancer Res. 1990, 50, 3605–3609. [Google Scholar] [PubMed]
- Wiber, M.; Maitre, E.; Poncet, J.-M.; Duchenet, V.; Damaj, G.; Cornet, E.; Troussard, X. A population-based study of hairy cell leukemia over a period of 20 years. Cancer Treat. Res. Commun. 2020, 25, 100236. [Google Scholar] [CrossRef] [PubMed]
- Teras, L.R.; DeSantis, C.E.; Cerhan, J.R.; Morton, L.M.; Jemal, A.; Flowers, C.R. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J. Clin. 2016, 66, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Defossex, G.; Le Guader- Peyrou, S.; Uhry, Z.; Grosclaude, P.; Colonna, M.; Dantony, E.; Delafosse, P.; Molinie, F.; Woronoff, A.-S.; Bouvier, A.-M.; et al. Estimations Nationales de L’incidence et de la Mortalité par Cancer en France Métropolitaine Entre 1990 et 2018. Etude à Partir des Registres des Cancers du Réseau Francim. Volume 2: Hémopathies Malignes; INCA: Billancourt, France, 2019. [Google Scholar]
- Hardell, L.; Eriksson, M.; Nordstrom, M. Exposure to pesticides as risk factor for non-Hodgkin’s lymphoma and hairy cell leukemia: Pooled analysis of two Swedish case-control studies. Leuk. Lymphoma 2002, 43, 1043–1049. [Google Scholar] [CrossRef]
- Aristeguieta, C.; de Perio, M.A. Three cases of hairy cell leukemia in coal miners. Leuk. Lymphoma 2011, 52, 2391–2392. [Google Scholar] [CrossRef]
- Monnereau, A.; Slager, S.L.; Hughes, A.M.; Smith, A.; Glimelius, B.; Habermann, T.M.; Berndt, S.I.; Staines, A.; Norman, A.D.; Cerhan, J.R.; et al. Medical history, lifestyle, and occupational risk factors for hairy cell leukemia: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Clavel, J.; Hémon, D.; Mandereau, L.; Delemotte, B.; Séverin, F.; Flandrin, G. Farming, pesticide use and hairy-cell leukemia. Scand. J. Work. Environ. Health 1996, 22, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Clavel, J.; Mandereau, L.; Cordier, S.; Le Goaster, C.; Hémon, D.; Conso, F.; Flandrin, G. Hairy cell leukaemia, occupation, and smoking. Br. J. Haematol. 1995, 91, 154–161. [Google Scholar] [CrossRef]
- Golomb, H.M.; Hadad, L.J. Infectious complications in 127 patients with hairy cell leukemia. Am. J. Hematol. 1984, 16, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Kraut, E. Infectious complications in hairy cell leukemia. Leuk. Lymphoma 2011, 52, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Kraut, E.H. Clinical manifestations and infectious complications of hairy-cell leukaemia. Best. Pract. Res. Clin. Haematol. 2003, 16, 33–40. [Google Scholar] [CrossRef]
- Green, L.; Coumbe, A.; Sawicka, E.; De Lord, C. Mycobacterium kansasii in a patient with hairy cell leukaemia. Br. J. Haematol. 2009, 144, 2. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Kartsios, C.; Spyrou, A.; Loukidis, K.; Miyakis, S.; Pervana, S.; Makridis, C.; Kioumi, A.; Korantzis, I. Isolated splenic mycobacterial disease: A cause of persistent fever in a hairy cell leukemia patient. Case Rep. Gastroenterol. 2010, 4, 330–334. [Google Scholar] [CrossRef]
- Ramasamy, C.; Dubashi, B.; Sree Rekha, J.; Basu, D.; Jain, A.; Dutta, T.K. Atypical mycobacterial infection in hairy cell leukemia treated with cladribine. Indian. J. Hematol. Blood Transfus. 2014, 30, 59–61. [Google Scholar] [CrossRef][Green Version]
- Thaker, H.; Neilly, I.J.; Saunders, P.G.; Magee, J.G.; Snow, M.H.; Ong, E.L.C. Remember mycobacterial disease in hairy cell leukaemia (HCL). J. Infect. 2001, 42, 213–214. [Google Scholar] [CrossRef]
- Filho, R.J.d.L.C.; de Carvalho Portela, N.; Neto, A.M.P.; Teixeira Henderson, M.N.R.; Pinheiro, R.F. Nontuberculous mycobacterium genital infection mimicking donovanosis in a patient with hairy cell leukemia. Leuk. Res. 2011, 35, e44-5. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.C.; Edmunds, H.S. Listeria monocytogenes infection in hairy cell leukemia: A case report and literature review. Case Rep. Hematol. 2018, 2018, 5616898. [Google Scholar] [CrossRef] [PubMed]
- Ikebe, T.; Sasaki, H.; Takata, H.; Miyazaki, Y.; Ohtsuka, E.; Saburi, Y.; Ogata, M.; Shirao, K. Toxoplasmic encephalitis with untreated hairy cell leukemia variant. Intern. Med. 2016, 55, 3175–3180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bunce, P.E.; Yang, L.; Chun, S.; Zhang, S.X.; Trinkaus, M.A.; Matukas, L.M. Disseminated sporotrichosis in a patient with hairy cell leukemia treated with amphotericin B and posaconazole. Med. Mycol. 2012, 50, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Damaj, G.; Kuhnowski, F.; Marolleau, J.-P.; Bauters, F.; Leleu, X.; Yakoub-Agha, I. Risk factors for severe infection in patients with hairy cell leukemia: A long-term study of 73 patients. Eur. J. Haematol. 2009, 83, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Dinçol, G.; Kahraman, R. Cryptococcus neoformans meningitis in a patient with hairy cell leukemia. Am. J. Hematol. 2006, 81, 387. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, T.; Polliack, A. Hairy cell leukemia: Uncommon clinical features, unusual sites of involvement and some rare associations. Best. Pract. Res. Clin. Haematol. 2015, 28, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.A.; Engels, E.A. Autoimmune conditions and hairy cell leukemia: An exploratory case-control study. J. Hematol. Oncol. 2010, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Dasanu, C.A.; Van den Bergh, M.; Pepito, D.; Alvarez Argote, J. Autoimmune disorders in patients with hairy cell leukemia: Are they more common than previously thought? Curr. Med. Res. Opin. 2015, 31, 17–23. [Google Scholar] [CrossRef]
- Viens, D.; St-Hilaire, È.; Beauregard, P.; Dufresne, J.; Knecht, H. Successful treatment of warm antibody (IgG/C3 positive) autoimmune hemolytic anemia in hairy-cell leukemia with 2-CdA in the elderly. Leuk. Lymphoma 2008, 49, 1424–1426. [Google Scholar] [CrossRef]
- Moullet, I.; Salles, G.; Dumontet, C.; Bastion, Y.; Morel, D.; Felman, P.; Coiffier, B. Sever immune thombocytopenic purpura and haemolytic anaemia in a hairy-cell leukaemia patient. Eur. J. Haematol. 1995, 54, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Güler, N.; Kansu, E.; Türker, A.; Barişta, I.; Kanra, T. Severe autoimmune hemolytic anemia in hairy cell leukemia. Eur. J. Haematol. 1997, 58, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, C.A.; Golde, D.W. Autoimmune disease in hairy-cell leukaemia: Clinical syndromes and treatment. Br. J. Haematol. 1985, 61, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Broccoli, A.; Gandolfi, L.; Pellegrini, C.; Agostinelli, C.; Argnani, L.; Zinzani, P.L. Leukocytoclastic vasculitis associated with hairy cell leukemia at diagnosis: A case report and review of the literature. Tumori J. 2016, 102, S124–S127. [Google Scholar] [CrossRef] [PubMed]
- Hasler, P.; Kistler, H.; Gerber, H. Vasculitides in hairy cell leukemia. Semin. Arthritis Rheum. 1995, 25, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Spann, C.R.; Callen, J.P.; Yam, L.T.; Apgar, J.T. Cutaneous leukocytoclastic vasculitis complicating hairy cell leukemia (leukemic reticuloendotheliosis). Arch. Dermatol. 1986, 122, 1057–1059. [Google Scholar] [CrossRef] [PubMed]
- Ozkok, A.; Elcioglu, O.C.; Akpinar, T.S.; Nalcaci, M. Vasculitis in a patient with hairy cell leukemia. Intern. Med. 2011, 50, 2713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carpenter, M.T.; West, S.G. Polyarteritis nodosa in hairy cell leukemia: Treatment with interferon-alpha. J. Rheumatol. 1994, 21, 1150–1152. [Google Scholar] [PubMed]
- Vankalakunti, M.; Joshi, K.; Jain, S.; Nada, R.; Radotra, B.D.; Varma, S. Polyarteritis nodosa in hairy cell leukaemia: An autopsy report. J. Clin. Pathol. 2007, 60, 1181–1182. [Google Scholar] [CrossRef]
- Oksuz, M.F.; Coskun, B.N.; Tufan, A.N.; Orucoglu, N.; Dalkilic, E.; Nazlıoğlu, H.; Pehlivan, Y. Hairy cell leukemia presenting initially with symptoms of Behçet’s disease. Int. J. Rheum. Dis. 2014, 17, 689–692. [Google Scholar] [CrossRef]
- Vernhes, J.P.; Schaeverbeke, T.; Fach, J.; Lequen, L.; Bannwarth, B.; Dehais, J. Chronic immunity-driven polyarthritis in hairy cell leukemia. Report of a case and review of the literature. Rev. Rhum. Engl. Ed. 1997, 64, 578–581. [Google Scholar] [PubMed]
- Rossi, D.; Franceschetti, S.; Cerri, M.; Conconi, A.; Lunghi, M.; Capello, D.; Cantello, R.; Gaidano, G. Hairy cell leukaemia complicated by anti-MAG paraproteinemic demyelinating neuropathy: Resolution of neurological syndrome after cladribrine treatment. Leuk. Res. 2007, 31, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Yonal-Hindilerden, I.; Hindilerden, F.; Bulut-Dereli, S.; Yıldız, E.; Dogan, I.O.; Nalcaci, M. Hairy cell leukemia presenting with isolated skeletal involvement successfully treated by radiation therapy and cladribine: A case report and review of the literature. Case Rep. Hematol. 2015, 2015, 803921. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.M.; Matsuda, K.; Wadhwa, V.; Hewitt, D.; Almiski, M.; Johnston, J.B.; Banerji, V. Multifocal brain involvement in a patient with hairy cell leukemia successfully treated with rituximab and cladribine. Blood Adv. 2017, 1, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Robak, E.; Jesionek-Kupnicka, D.; Robak, T. Skin changes in hairy cell leukemia. Ann. Hematol. 2021, 100, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Notarfranchi, L.; Russo, F.; Re, F.; Mancini, C.; Martella, E.; Falini, B.; Aversa, F.; Tiacci, E. Hairy cell leukaemia mimicking multiple myeloma. Lancet Oncol. 2019, 20, e187. [Google Scholar] [CrossRef] [PubMed]
- Cailly, L.; Gruchet, C.; Maitre, E.; Guidez, S.; Delwail, V.; Systchenko, T.; Moya, N.; Sabirou, F.; Levy, A.; Bobin, A.; et al. Hairy cell leukemia with isolated bone lesions. Clin. Case Rep. 2023, 11, e7343. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.E.; Raju, A.R.; Jacob, A.; Hildebrandt, G.C. Case report: A case of classic hairy cell leukemia with CNS involvement treated with vemurafenib. Front. Oncol. 2022, 12, 1100577. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Jesionek-Kupnicka, D.; Kupnicki, P.; Polliack, A.; Robak, T. Bone lesions in hairy cell leukemia: Diagnosis and treatment. Eur. J. Haematol. 2020, 105, 682–691. [Google Scholar] [CrossRef]
- Lembersky, B.C.; Ratain, M.J.; Golomb, H.M. Skeletal complications in hairy cell leukemia: Diagnosis and therapy. J. Clin. Oncol. 1988, 6, 1280–1284. [Google Scholar] [CrossRef]
- Rosen, D.S.; Smith, S.; Gurbuxani, S.; Yamini, B. Extranodal hairy cell leukemia presenting in the lumbar spine: Case report. J. Neurosurg. Spine 2008, 9, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Frassoldati, A.; Lamparelli, T.; Federico, M.; Annino, L.; Capnist, G.; Pagnucco, G.; Dini, E.; Resegotti, L.; Damasiot, E.E.; Silingardi, V. Hairy cell leukemia: A clinical review based on 725 cases of the Italian Cooperative Group (ICGHCL). Leuk. Lymphoma 1994, 13, 307–316. [Google Scholar] [CrossRef]
- Seghezzi, M.; Manenti, B.; Previtali, G.; Gianatti, A.; Dominoni, P.; Buoro, S. A specific abnormal scattergram of peripheral blood leukocytes that may suggest hairy cell leukemia. Clin. Chem. Lab. Med. 2018, 56, e108–e111. [Google Scholar] [CrossRef] [PubMed]
- Grever, M.R.; Abdel-Wahab, O.; Andritsos, L.A.; Banerji, V.; Barrientos, J.; Blachly, J.S.; Call, T.G.; Catovsky, D.; Dearden, C.; Demeter, J.; et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood 2017, 129, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Wotherspoon, A.; Attygalle, A.; Sena Teixeira Mendes, L. Bone marrow and splenic histology in hairy cell leukaemia. Best. Pract. Res. Clin. Haematol. 2015, 28, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cui, H.; Jin, L.; Zhao, M.; Shen, W. CD103-CD23+ classical hair cell leukemia: A case report and review of the literature. Medicine 2021, 100, e28262. [Google Scholar] [CrossRef] [PubMed]
- De Propris, M.S.; Musiu, P.; Intoppa, S.; Nardacci, M.G.; Pucciarini, A.; Santi, A.; Peragine, N.; Canichella, M.; De Luca, M.L.; D’Elia, G.M.; et al. Hairy cell leukaemia with low CD103 expression: A rare but important diagnostic pitfall. Br. J. Haematol. 2022, 198, e28–e31. [Google Scholar] [CrossRef]
- Favre, R.; Manzoni, D.; Traverse-Glehen, A.; Verney, A.; Jallades, L.; Callet-Bauchu, E.; Sujobert, P.; Salles, G.; Baseggio, L. Usefulness of CD200 in the differential diagnosis of SDRPL, SMZL, and HCL. Int. J. Lab. Hematol. 2018, 40, e59–e62. [Google Scholar] [CrossRef]
- Maitre, E.; Cornet, E.; Salaün, V.; Kerneves, P.; Chèze, S.; Repesse, Y.; Damaj, G.; Troussard, X. Immunophenotypic analysis of hairy cell leukemia (HCL) and hairy cell leukemia-like (HCL-like) disorders. Cancers 2022, 14, 1050–1064. [Google Scholar] [CrossRef]
- Pillai, V.; Pozdnyakova, O.; Charest, K.; Li, B.; Shahsafaei, A.; Dorfman, D.M. CD200 flow cytometric assessment and semiquantitative immunohistochemical staining distinguishes hairy cell leukemia from hairy cell leukemia-variant and other B-cell lymphoproliferative disorders. Am. J. Clin. Pathol. 2013, 140, 536–543. [Google Scholar] [CrossRef]
- Poret, N.; Fu, Q.; Guihard, S.; Cheok, M.; Miller, K.; Zeng, G.; Quesnel, B.; Troussard, X.; Galiègue-Zouitina, S.; Shelley, C.S. CD38 in hairy cell leukemia is a marker of poor prognosis and a new target for therapy. Cancer Res. 2015, 75, 3902–3911. [Google Scholar] [CrossRef]
- Jain, D.; Dorwal, P.; Gajendra, S.; Pande, A.; Mehra, S.; Sachdev, R. CD5 positive hairy cell leukemia: A rare case report with brief review of literature. Cytometry B Clin. Cytom. 2016, 90, 467–472. [Google Scholar] [CrossRef]
- Wang, L.; Tadros, A.S.; Hoh, C.K.; Wang, H.Y. CD10-positive hairy cell leukemia involving multiple deep lymph nodes. Clin. Lymphoma Myeloma Leuk. 2016, 16, e51–e53. [Google Scholar] [CrossRef]
- Anghelina, M.; Epperla, N.; Rogers, K.A.; Guo, L.; Zhao, Q.; Blachly, J.S.; Lucas, D.M.; Oakes, C.C.; Lozanski, G.; Grever, M.R.; et al. Down-regulation of CD25 antigen in hairy cell leukemia patients after treatment. Blood 2018, 132, 4143. [Google Scholar] [CrossRef]
- Maitre, E.; Macro, M.; Troussard, X. Hairy cell leukaemia with unusual BRAF mutations. J. Cell Mol. Med. 2023, 27, 2626–2630. [Google Scholar] [CrossRef] [PubMed]
- Itchaki, G.; Gurevich, K.; Gorenberg, M.; Tadmor, T. The role of PET CT in hairy cell leukemia. HemaSphere 2022, 6, 1764–1765. [Google Scholar] [CrossRef]
- Doma, A.; Škerget, M.; Žagar, I. 18F-FDG PET/CT for staging and evaluation of therapy in a patient with unusual hairy cell leukemia presentation. Clin. Nucl. Med. 2019, 44, e458–e460. [Google Scholar] [CrossRef]
- Forconi, F.; Sozzi, E.; Cencini, E.; Zaja, F.; Intermesoli, T.; Stelitano, C.; Rigacci, L.; Gherlinzoni, F.; Cantaffa, R.; Baraldi, A.; et al. Hairy cell leukemias with unmutated IGHV genes define the minor subset refractory to single-agent cladribine and with more aggressive behavior. Blood 2009, 114, 4696–4702. [Google Scholar] [CrossRef] [PubMed]
- Arons, E.; Suntum, T.; Stetler-Stevenson, M.; Kreitman, R.J. VH4-34+ hairy cell leukemia, a new variant with poor prognosis despite standard therapy. Blood 2009, 114, 4687–4695. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.K.; Sun, X.; Yuan, C.M.; Stetler-Stevenson, M.; Kreitman, R.J.; Maric, I. Usefulness of dual immunohistochemistry staining in detection of hairy cell leukemia in bone marrow. Am. J. Clin. Pathol. 2019, 153, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, S.; Tallman, M.S.; Hakimian, D.; Peterson, L. Minimal residual disease may predict bone marrow relapse in patients with hairy cell leukemia treated with 2-chlorodeoxyadenosine. Blood 1996, 87, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.; Sharpe, R.; Robbins, B.; Spinosa, J.; Leopard, J.; Saven, A.; Piro, L. Immunomorphologic analysis of bone marrow biopsies after treatment with 2-chlorodeoxyadenosine for hairy cell leukemia. Blood 1994, 84, 4310–4315. [Google Scholar] [CrossRef] [PubMed]
- Ottou, F.G.; Chandesris, M.; Lhermitte, L.; Callens, C.; Beldjord, K.; Garrido, M.; Bedin, A.; Brouzes, C.; Villemant, S.; Rubio, M.; et al. Peripheral blood 8 colour flow cytometry monitoring of hairy cell leukaemia allows detection of high-risk patients. Br. J. Haematol. 2014, 166, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Broccoli, A.; Terragna, C.; Nanni, L.; Martello, M.; Armuzzi, S.; Agostinelli, C.; Morigi, A.; Casadei, B.; Pellegrini, C.; Stefoni, V.; et al. Droplet digital polymerase chain reaction for the assessment of disease burden in hairy cell leukemia. Hematol. Oncol. 2022, 40, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Arons, E.; Margulies, I.; Sorbara, L.; Raffeld, M.; Stetler-Stevenson, M.; Pastan, I.; Kreitman, R.J. Minimal residual disease in hairy cell leukemia patients assessed by clone-specific polymerase chain reaction. Clin. Cancer Res. 2006, 12, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Rueda, L.Y.M.; Bryant, D.; Tapper, W.J.; Weston-Bell, N.J.; Wedge, D.C.; Ansari-Pour, N.; Sahota, S.S. The genomic landscape and clonal evolutionary trajectory of classical hairy cell leukemia. Leukemia 2023, 37, 929–933. [Google Scholar] [CrossRef]
- Chung, S.S.; Kim, E.; Park, J.H.; Chung, Y.R.; Lito, P.; Teruya-Feldstein, J.; Hu, W.; Beguelin, W.; Monette, S.; Duy, C.; et al. Hematopoietic stem cell origin of BRAFV600E mutations in hairy cell leukemia. Sci. Transl. Med. 2014, 6, 238ra71. [Google Scholar] [CrossRef]
- Guerrini, F.; Paolicchi, M.; Ghio, F.; Ciabatti, E.; Grassi, S.; Salehzadeh, S.; Ercolano, G.; Metelli, M.R.; Del Re, M.; Iovino, L.; et al. The droplet digital PCR: A new valid molecular approach for the assessment of B-RAF V600E mutation in hairy cell leukemia. Front. Pharmacol. 2016, 7, 363. [Google Scholar] [CrossRef]
- Tiacci, E.; Schiavoni, G.; Forconi, F.; Santi, A.; Trentin, L.; Ambrosetti, A.; Cecchini, D.; Sozzi, E.; di Celle, P.F.; Di Bello, C.; et al. Simple genetic diagnosis of hairy cell leukemia by sensitive detection of the BRAF-V600E mutation. Blood 2012, 119, 192–195. [Google Scholar] [CrossRef]
- Ravandi, F.; Kreitman, R.J.; Tiacci, E.; Andritsos, L.; Banerji, V.; Barrientos, J.C.; Bhat, S.A.; Blachly, J.S.; Broccoli, A.; Call, T.; et al. Consensus opinion from an international group of experts on measurable residual disease in hairy cell leukemia. Blood Cancer J. 2022, 12, 165. [Google Scholar] [CrossRef]
- Ravandi, F. MRD in HCL: Does it matter? Blood 2018, 131, 2277–2278. [Google Scholar] [CrossRef] [PubMed]
- Bohn, J.P.; Dietrich, S. Treatment of classic hairy cell leukemia: Targeting minimal residual disease beyond cladribine. Cancers 2022, 14, 956. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Hernandez, A.; Moturi, K.; Hanson, V.; Andritsos, L.A. Hairy cell leukemia: Where are we in 2023? Curr. Oncol. Rep. 2023, 25, 833. [Google Scholar] [CrossRef] [PubMed]
- Traverse-Glehen, A.; Baseggio, L.; Bauchu, E.C.; Morel, D.; Gazzo, S.; Ffrench, M.; Verney, A.; Rolland, D.; Thieblemont, C.; Magaud, J.P.; et al. Splenic red pulp lymphoma with numerous basophilic villous lymphocytes: A distinct clinicopathologic and molecular entity? Blood 2008, 111, 2253–2260. [Google Scholar] [CrossRef]
- Traverse-Glehen, A.; Verney, A.; Gazzo, S.; Jallades, L.; Chabane, K.; Hayette, S.; Coiffier, B.; Callet-Bauchu, E.; Ffrench, M.; Felman, P.; et al. Splenic diffuse red pulp lymphoma has a distinct pattern of somatic mutations amongst B-cell malignancies. Leuk. Lymphoma 2016, 58, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Traverse-Glehen, A.; Baseggio, L.; Salles, G.; Coiffier, B.; Felman, P.; Berger, F. Splenic diffuse red pulp small-B cell lymphoma: Toward the emergence of a new lymphoma entity. Discov. Med. 2012, 13, 253–265. [Google Scholar] [PubMed]
- Clipson, A.; Wang, M.; de Leval, L.; Ashton-Key, M.; Wotherspoon, A.; Vassiliou, G.; Bolli, N.; Grove, C.; Moody, S.; Escudero-Ibarz, L.; et al. KLF2 mutation is the most frequent somatic change in splenic marginal zone lymphoma and identifies a subset with distinct genotype. Leukemia 2015, 29, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Kiel, M.J.; Velusamy, T.; Betz, B.L.; Zhao, L.; Weigelin, H.G.; Chiang, M.Y.; Huebner-Chan, D.R.; Bailey, N.G.; Yang, D.T.; Bhagat, G.; et al. Whole-genome sequencing identifies recurrent somatic NOTCH2 mutations in splenic marginal zone lymphoma. J. Exp. Med. 2012, 209, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Trifonov, V.; Fangazio, M.; Bruscaggin, A.; Rasi, S.; Spina, V.; Monti, S.; Vaisitti, T.; Arruga, F.; Famà, R.; et al. The coding genome of splenic marginal zone lymphoma: Activation of NOTCH2 and other pathways regulating marginal zone development. J. Exp. Med. 2012, 209, 1537–1551. [Google Scholar] [CrossRef]
- Jallades, L.; Baseggio, L.; Sujobert, P.; Huet, S.; Chabane, K.; Callet-Bauchu, E.; Verney, A.; Hayette, S.; Desvignes, J.-P.; Salgado, D.; et al. Exome sequencing identifies recurrent BCOR gene alterations and the absence of KLF2, TNFAIP3 and MYD88 mutations in splenic diffuse red pulp small B-cell lymphoma. Haematologica 2017, 102, 1758–1766. [Google Scholar] [CrossRef]
- Baliakas, P.; Strefford, J.C.; Bikos, V.; Parry, M.; Stamatopoulos, K.; Oscier, D. Splenic marginal-zone lymphoma: Ontogeny and genetics. Leuk. Lymphoma 2015, 56, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Harada, T.; Lang, L.; Saga, T.; Kanagawa, M.; Matsuda, R.; Yashiro, S.; Kano, S.; Sasaki, Y.; Nakamine, H. Hairy cell Leukemia-Japanese variant: Report of a patient and literature Review. Int. J. Surg. Pathol. 2022, 30, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Chihara, D.; Arons, E.; Stetler-Stevenson, M.; Yuan, C.M.; Wang, H.-W.; Zhou, H.; Raffeld, M.; Xi, L.; Steinberg, S.M.; Feurtado, J.; et al. Randomized phase II study of first-line cladribine with concurrent or delayed rituximab in patients with hairy cell leukemia. J. Clin. Oncol. 2020, 38, 1527. [Google Scholar] [CrossRef] [PubMed]
- Lauria, F.; Cencini, E.; Forconi, F. Alternative methods of cladribine administration. Leuk. Lymphoma 2011, 52, 34–37. [Google Scholar] [CrossRef]
- von Rohr, A.; Schmitz, S.-F.H.; Tichelli, A.; Hess, U.; Piguet, D.; Wernli, M.; Frickhofen, N.; Konwalinka, G.; Zulian, G.; Ghielmini, M.; et al. Treatment of hairy cell leukemia with cladribine (2-chlorodeoxyadenosine) by subcutaneous bolus injection: A phase II study. Ann. Oncol. 2002, 13, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Grever, M.R. How I treat hairy cell leukemia. Blood 2010, 115, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Criscuolo, M.; Broccoli, A.; Piciocchi, A.; Varettoni, M.; Galli, E.; Anastasia, A.; Cantonetti, M.; Trentin, L.; Kovalchuk, S.; et al. Long-term follow-up of cladribine treatment in hairy cell leukemia: 30-year experience in a multicentric Italian study. Blood Cancer J. 2022, 12, 109. [Google Scholar] [CrossRef]
- Lauria, F.; Lenoci, M.; Annino, L.; Raspadori, D.; Marotta, G.; Bocchia, M.; Forconi, F.; Gentili, S.; La Manda, M.; Marconcini, S.; et al. Efficacy of anti-CD20 monoclonal antibodies (Mabthera) in patients with progressed hairy cell leukemia. Haematologica 2001, 86, 1046–1050. [Google Scholar]
- Hagberg, H. Chimeric monoclonal anti-CD20 antibody (rituximab)--an effective treatment for a patient with relapsing hairy cell leukaemia. Med. Oncol. 1999, 16, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, H.; Lundholm, L. Rituximab, a chimaeric anti-CD20 monoclonal antibody, in the treatment of hairy cell leukaemia. Br. J. Haematol. 2001, 115, 609–611. [Google Scholar] [CrossRef]
- Nieva, J.; Bethel, K.; Saven, A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood 2003, 102, 810–813. [Google Scholar] [CrossRef]
- Thomas, D.A.; O’Brien, S.; Bueso-Ramos, C.; Faderl, S.; Keating, M.J.; Giles, F.J.; Cortes, J.; Kantarjian, H.M. Rituximab in relapsed or refractory hairy cell leukemia. Blood 2003, 102, 3906–3911. [Google Scholar] [CrossRef]
- Zenhäusern, R.; Simcock, M.; Gratwohl, A.; Hess, U.; Bargetzi, M.; Tobler, A.; Swiss Group for Clinical Cancer Research (SAKK). Rituximab in patients with hairy cell leukemia relapsing after treatment with 2-chlorodeoxyadenosine (SAKK 31/98). Haematologica 2008, 93, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Malfuson, J.V.; Fagot, T.; Konopacki, J.; Souleau, B.; Cremades, S.; de Revel, T. Which role for rituximab in hairy cell leukemia? Reflections on six cases. Acta Haematol. 2010, 123, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Bohn, J.; Pircher, A.; Wanner, D.; Vill, D.; Foeger, B.; Wolf, D.; Steurer, M. Low-dose vemurafenib in hairy cell leukemia patients with active infection. Am. J. Hematol. 2019, 94, E180. [Google Scholar] [CrossRef] [PubMed]
- Grever, M.; Andritsos, L.; Banerji, V.; Barrientos, J.C.; Bhat, S.; Blachly, J.S.; Call, T.; Cross, M.; Dearden, C.; Demeter, J.; et al. Hairy cell leukemia and COVID-19 adaptation of treatment guidelines. Leukemia 2021, 35, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Yu, T.; James, L.; Feurtado, J.; Eager, H.; Ortiz, O.S.; Gould, M.; Mauter, J.; Zhou, H.; Burbelo, P.D.; et al. COVID-19 in patients with classic and variant hairy cell leukemia. Blood Adv. 2023, 7, 7161–7168. [Google Scholar] [CrossRef]
- Maleka, A.; Enblad, G.; Sjörs, G.; Lindqvist, A.; Ullenhag, G.J. Treatment of metastatic malignant melanoma with vemurafenib during pregnancy. J. Clin. Oncol. 2013, 31, e192–e193. [Google Scholar] [CrossRef]
- König, E.; Kusser, W.; Day, C.; Porzsolt, F.; Glickman, B.; Messer, G.; Schmid, M.; de Châtel, R.; Marcsek, Z.; Demeter, J. p53 mutations in hairy cell leukemia. Leukemia 2000, 14, 706–711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hockley, S.L.; Else, M.; Morilla, A.; Wotherspoon, A.; Dearden, C.; Catovsky, D.; Gonzalez, D.; Matutes, E. The prognostic impact of clinical and molecular features in hairy cell leukaemia variant and splenic marginal zone lymphoma. Br. J. Haematol. 2012, 158, 347–354. [Google Scholar] [CrossRef]
- Durham, B.H.; Getta, B.; Dietrich, S.; Taylor, J.; Won, H.; Bogenberger, J.M.; Scott, S.; Kim, E.; Chung, Y.R.; Chung, S.S.; et al. Genomic analysis of hairy cell leukemia identifies novel recurrent genetic alterations. Blood 2017, 130, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, S.; Pircher, A.; Endris, V.; Peyrade, F.; Wendtner, C.-M.; Follows, G.A.; Hüllein, J.; Jethwa, A.; Ellert, E.; Walther, T.; et al. BRAF inhibition in hairy cell leukemia with low-dose vemurafenib. Blood 2016, 127, 2847–2855. [Google Scholar] [CrossRef]
- Lin, A.Y.; Dinner, S.N. Moxetumomab pasudotox for hairy cell leukemia: Preclinical development to FDA approval. Blood Adv. 2019, 3, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Dearden, C.; Zinzani, P.L.; Delgado, J.; Karlin, L.; Robak, T.; Gladstone, D.E.; le Coutre, P.; Dietrich, S.; Gotic, M.; et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia 2018, 32, 1768–1777. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Tallman, M.S.; Robak, T.; Coutre, S.; Wilson, W.H.; Stetler-Stevenson, M.; FitzGerald, D.J.; Santiago, L.; Gao, G.; Lanasa, M.C.; et al. Minimal residual hairy cell leukemia eradication with moxetumomab pasudotox: Phase 1 results and long-term follow-up. Blood 2018, 131, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Saven, A.; Burian, C.; Koziol, J.A.; Piro, L.D. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood 1998, 92, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Goodman, G.R.; Burian, C.; Koziol, J.A.; Saven, A. Extended follow-up of patients with hairy cell leukemia after treatment with cladribine. J. Clin. Oncol. 2003, 21, 891–896. [Google Scholar] [CrossRef]
- Dasanu, C.A.; Ichim, T.; Alexandrescu, D.T. Inherent and iatrogenic immune defects in hairy cell leukemia: Revisited. Expert. Opin. Drug Saf. 2010, 9, 55–64. [Google Scholar] [CrossRef]
- Paillassa, J.; Troussard, X. Patients with relapsed/refractory hairy-cell leukemia. Cancer Rep. 2021, 5, e1495. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.F.; Kurzrock, R.; Freireich, E.J.; Estey, E.H. 2-chlorodeoxyadenosine induces durable remissions and prolonged suppression of CD4+ lymphocyte counts in patients with hairy cell leukemia. Blood 1994, 83, 2906–2911. [Google Scholar] [CrossRef] [PubMed]
- Bastie, J.N.; Cazals-Hatem, D.; Daniel, M.T.; D’Agay, M.F.; Rabian, C.; Glaisner, S.; Noel-Walter, M.P.; Dabout, D.; Flandrin, G.; Dombret, H.; et al. Five years follow-up after 2-chloro deoxyadenosine treatment in thirty patients with hairy cell leukemia: Evaluation of minimal residual disease and CD4+ Lymphocytopenia after treatment. Leuk. Lymphoma 1999, 35, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Hilbe, W.; Geisen, F.; Thaler, J.; Konwalinka, G. T Cells and natural killer cells after treatment of hairy cell leukaemia with 2-Chlorodeoxyadenosine. Acta Haematol. 1997, 97, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Paltiel, O.; Adler, B.; Barchana, M.; Dann, E.J. A population-based study of hairy cell leukemia in Israel. Eur. J. Haematol. 2006, 77, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Burotto, M.; Stetler-Stevenson, M.; Arons, E.; Zhou, H.; Wilson, W.; Kreitman, R.J. Bendamustine and rituximab in relapsed and refractory hairy cell leukemia. Clin. Cancer Res. 2013, 19, 6313–6321. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Arons, E.; Stetler-Stevenson, M.; Miller, K.B. Response of hairy cell leukemia to bendamustine. Leuk. Lymphoma 2011, 52, 1153. [Google Scholar] [CrossRef] [PubMed]
- Zaja, F.; Diloreto, C.; Amoroso, V.; Salmaso, F.; Russo, D.; Silvestri, F.; Fanin, R.; Damiani, D.; Infanti, L.; Mariuzzi, L.; et al. BCL-2 immunohistochemical evaluation in B-cell chronic lymphocytic leukemia and hairy cell leukemia before treatment with fludarabine and 2-chloro-deoxy-adenosine. Leuk. Lymphoma 1998, 28, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.D.; Wendtner, C.-M.; Roberts, A.W.; Jurczak, W.; et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016, 17, 768–778. [Google Scholar] [CrossRef]
- Dinardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Vereertbrugghen, A.; Colado, A.; Gargiulo, E.; Bezares, R.F.; Grecco, H.F.; Cordini, G.; Custidiano, M.d.R.; Francois, J.-H.; Berchem, G.; Borge, M.; et al. In vitro sensitivity to venetoclax and microenvironment protection in hairy cell leukemia. Front. Oncol. 2021, 11, 2595. [Google Scholar] [CrossRef]
- Tiacci, E.; De Carolis, L.; Santi, A.; Falini, B. Venetoclax in relapsed or refractory hairy-cell leukemia. N. Engl. J. Med. 2023, 388, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Kampmeier, P.; Spielberger, R.; Dickstein, J.; Mick, R.; Golomb, H.; Vardiman, J.W. Increased incidence of second neoplasms in patients treated with interferon alpha 2b for hairy cell leukemia: A clinicopathologic assessment. Blood 1994, 83, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
- Federico, M.; Zinzani, P.L.; Frassoldati, A.; Vinceti, M.; Modè, A.; Annino, L.; Chisesi, T.; Pagnucco, G.; Invernizzi, R.; Spriano, M.; et al. Risk of second cancer in patients with hairy cell leukemia: Long-term follow-up. J. Clin. Oncol. 2002, 20, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Kurzrock, R.; Strom, S.S.; Estey, E.; O’Brien, S.; Keating, M.J.; Jiang, H.; Adams, T.; Talpaz, M. Second cancer risk in hairy cell leukemia: Analysis of 350 patients. J. Clin. Oncol. 1997, 15, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Au, W.Y.; Klasa, R.J.; Gallagher, R.; Le, N.; Gascoyne, R.D.; Connors, J.M. Second malignancies in patients with hairy cell leukemia in british columbia: A 20-year experience. Blood 1998, 92, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Chihara, D.; Arons, E.; Stetler-Stevenson, M.; Yuan, C.M.; Wang, H.-W.; Zhou, H.; Raffeld, M.; Xi, L.; Steinberg, S.M.; Feurtado, J.C.; et al. Long term follow-up of a phase II study of cladribine with concurrent rituximab with hairy cell leukemia variant. Blood Adv. 2021, 5, 4807–4816. [Google Scholar] [CrossRef] [PubMed]
- Andritsos, L.A.; Grieselhuber, N.R.; Anghelina, M.; Rogers, K.A.; Roychowdhury, S.; Reeser, J.W.; Timmers, C.D.; Freud, A.G.; Blachly, J.S.; Lucas, D.M.; et al. Trametinib for the treatment of IGHV4-34, MAP2K1-mutant variant hairy cell leukemia. Leuk. Lymphoma 2018, 59, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Visentin, A.; Imbergamo, S.; Frezzato, F.; Pizzi, M.; Bertorelle, R.; Scomazzon, E.; Berno, T.; Riva, M.; Piva, E.; Facco, M.; et al. Bendamustine plus rituximab is an effective first-line treatment in hairy cell leukemia variant: A report of three cases. Oncotarget 2017, 8, 110727. [Google Scholar] [CrossRef]
- Imoto, N.; Koyama, D.; Sugiura, I.; Kurahashi, S. Long-term follow-up after rituximab plus bendamustine in a patient with relapsed or refractory hairy cell leukemia variant: A case report. Medicine 2021, 100, e24457. [Google Scholar] [CrossRef]
- Kanellis, G.; Mollejo, M.; Montes-Moreno, S.; Rodriguez-Pinilla, S.-M.; Cigudosa, J.C.; Algara, P.; Montalban, C.; Matutes, E.; Wotherspoon, A.; Piris, M.A. Splenic diffuse red pulp small B-cell lymphoma: Revision of a series of cases reveals characteristic clinico-pathological features. Haematologica 2010, 95, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.; Navarro, A.; Martinez-Trillos, A.; Molina-Urra, R.; Gonzalez-Farre, B.; Salaverria, I.; Nadeu, F.; Enjuanes, A.; Clot, G.; Costa, D.; et al. NOTCH1, TP53, and MAP2K1 mutations in splenic diffuse red pulp small B-cell lymphoma are associated with progressive disease. Am. J. Surg. Pathol. 2016, 40, 192–201. [Google Scholar] [CrossRef] [PubMed]

| Diagnosis | General Practice |
|---|---|
| For diagnosis/classification | |
| CBC and blood smear revision (nucleolus) | All cases |
| Immunophenotyping (HCL score assessment) | All cases |
| BRAFV600E mutational status | All cases (IHC or molecular analysis #) |
| Bone marrow trephine biopsy | All cases |
| IGHV repertory and mutational status | Recommended if BRAFWT or if R/R cases |
| Extended mutational landscape (BRAF exon 11 and 15, MAP2K1, KRAS, NRAS, HRAS) | Recommended if BRAFWT |
| TP53 mutational status | Recommended if R/R cases |
| Cytogenetic analysis | Not recommended |
| Pretreatment assessment | |
| History, physical examination (performans status) | All cases |
| Creatinine clearance | All cases |
| Direct antiglobulin test, haptoglobin, unconjugated bilirubin and lactic dehydrogenase | All cases |
| Transaminases, hepatitis B and C serology | All cases |
| Preservation of cells and serum in a tumor bank | Recommended |
| Chest radiograph or CT scan (chest abdomen and pelvis) | Recommended |
| MRI/PET CT scan | In symptomatic patients with unusual extramedullary localizations |
| Assessment for MRD | Recommended |
| Response | Definition |
|---|---|
| Complete response (CR) | Regression of splenomegaly on physical examination. Near normalization of blood count without transfusion: Hb > 11 g/dL, platelets > 100 G/L, neutrophils > 1.5 G/L. Absence of hairy-cell on blood smear and bone marrow examination. |
| Complete response with MRD negativity (CR MRD-) | CR and MRD negativity by IHC or FCM on bone marrow examination. |
| Partial response (PR) | Regression of at least 50% of splenomegaly on physical examination. Near normalization of blood count without transfusion: Hb > 11 g/dL, platelets > 100 G/L, neutrophils > 1.5 G/L. Regression of at least 50% of bone marrow infiltration by hairy cells. |
| Stable disease (SD) | Patients who do not met criteria for CR, PR ou PD. |
| Progressive disease (PD) | Increase of at least 25% of splenomegaly on physical examination and/or decline of at least 25% of hematological parameters and/or increase of symptoms. |
| Hematologic complete response (HCR) | Regression of splenomegaly on physical examination. Near normalization of blood count without transfusion: Hb > 11 g/dL, platelets > 100 G/L, neutrophils > 1.5 G/L. |
| Hematologic partial response (HPR) | Regression of at least 50% of splenomegaly on physical examination. Near normalization of blood count without transfusion: Hb > 11 g/dL, platelets > 100 G/L, neutrophils > 1.5 G/L. |
| HCL | HCLv | SDRPL | SZML | ||
|---|---|---|---|---|---|
| Epidemiology | Incidence | 0.3 | 0.2 | ND | 0.2 |
| Sex ratio M/F | 4 | 1.6 | 1.6 | 0.5 | |
| Median age at diagnosis | 55 | 70 | 77 | 62 | |
| Clinical data | Infections | Yes | Yes | Yes | Yes |
| Splenomegaly | Yes | Yes | Yes | Yes | |
| B symptoms | rare | rare | 1/3 | 1/4 | |
| Complete blood count | Cytopenia | Yes | Yes | Yes | Yes |
| Monocytopenia | Yes | No | No | No | |
| Lymphocytosis | ≤10% | ≥90% | ≥50% | ≥50% | |
| Cell morphology | Villi | Long, fine and circumferential | Long, fine and circumferential, sometimes shaggy | Long, large, broad base and polar | Small and polar |
| Nucleoli | Occasional, inconspicuous | Constant, prominent | Occasional | Small | |
| Chromatin | Mature, homogene | Mature, homogene | Condensed | Condensed | |
| Immunophenotype | CD5 | - | - | - | +/− |
| CD11c | +Bright | +(63%) | +(>90%) | +dim (33–67%) | |
| CD23 | - | - | - | - | |
| CD25 | +Bright | −(>90%) | −(>90%) | −(78–88%) | |
| CD27 | −(>90%) | ND | −(81%) | +(89%) | |
| CD103 | +Bright | +(65–100%) | −(62–84%) | −(>90%) | |
| CD123 | +Bright | −(60%)/dim | −(50–84%) | −(75%)/dim | |
| CD180 | + | ND | + Bright | + | |
| CD200 | +Bright | - | - | - | |
| Histology | Bone marrow infiltration | fibrosis, intrasinusoidal | interstitial, intrasinusoidal | interstitial, intrasinusoidal | intrasinusoidal, nodular |
| Spleen infiltration | Red pulp | Red pulp | Red pulp | White pulp | |
| Immunohistochemistry | Annexin A1 | + | - | - | - |
| DBA 44 | + | + | + | + | |
| VE 1 | + | - | - | - | |
| Cyclin D1 | + | +/− | - | - | |
| Cytogenetics | Abnormal karyotype | 40% | rare | 30% | 80% |
| Abnormalities | del(17p), del(7q), +12 | del(17p), del(7q), +12, complex | del(7q), +3, +18, complex | del(7q), +3, +18 | |
| IGHV | Mutated | 83–90% | 46–73% | 79% | 59–68% |
| Repertory | VH3-30, VH3-23, VH4-34 | VH4-34 | VH4-34, VH3-23 | VH1-2, VH4-34 | |
| Genetics | ARID1A | 4–5% | 4% | 8% | ND |
| BCOR | 0–5% | 0% | 24% | 2% | |
| BRAF V600E | 70–100% | 0% | 0–2% | 0–2% | |
| CCND3 | 0% | 13% | 21–24% | 13% | |
| CDKN1B | 10–16% | 0% | 4% | ND | |
| CREBBP | 5–6% | 12–25% | ND | ND | |
| KDM6A | 0–2% | 12–50% | 2% | 0% | |
| KLF2 | 13–16% | 0% | 2–4% | 20–30% | |
| KMT2C | 15% | 25% | ND | ND | |
| MAP2K1 | 0–22% | 38–42% | 7–12% | 0% | |
| MYD88 | 0% | ND | 0% | 9% | |
| NOTCH1 | 4–13% | 0% | 2% | 9% | |
| NOTCH2 | 0–4% | 0% | 10% | 17–25% | |
| TNFAIP3 | 0% | ND | 0% | 20% | |
| TP53 | 2–28% | 8–38% | 5–15% | 13–25% | |
| U2AF1 | 0% | 13% | ND | ND |
| Regimen | Details |
|---|---|
| CDA | sc 0.1–0.14 mg/kg/d once per day for 5 days |
| CDA + R | CDA sc 0.1–0.14 mg/kg/d once per day for 5 days + R iv 375 mg/m2, 8 weekly infusions, the first one started at day 1 of CDA |
| Pentostatin (P) | iv 4 mg/m2 once every 2 weeks for one year. If there is no response at 6 months, P should be stopped and another treatment should be discussed |
| Vemurafenib | 960 mg twice daily for 16–18 weeks |
| Vemurafenib + R | vemurafenib 960 mg twice daily for 8 weeks + R 375 mg/m2 8 infusions over 18 weeks (started at day 1 of vemurafenib) |
| Dabrafenib + Trametinib | dabrafenib 150 mg twice daily + trametinib 2 mg once daily until disease progression or unacceptable toxicity |
| Trametinib | 2 mg once daily until disease progression or unacceptable toxicity |
| Moxetumomab pasudotox | iv 40 µg/kg on days 1, 3 and 5 for a maximum of six cycles of 28 days |
| Ibrutinib | 420 mg/d or 840 mg/d until disease progression or unacceptable toxicity |
| Venetoclax | 400 mg/d (after a ramp-up phase), maximum 12 28-day cycles |
| Bendamustine + R | bendamustine iv 90 mg/m2 at day 1 and day 2 + R iv 375 mg/m2 at day 1; 6 28-day cycles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paillassa, J.; Maitre, E.; Belarbi Boudjerra, N.; Madani, A.; Benlakhal, R.; Matthes, T.; Van Den Neste, E.; Cailly, L.; Inchiappa, L.; Bekadja, M.A.; et al. Recommendations for the Management of Patients with Hairy-Cell Leukemia and Hairy-Cell Leukemia-like Disorders: A Work by French-Speaking Experts and French Innovative Leukemia Organization (FILO) Group. Cancers 2024, 16, 2185. https://doi.org/10.3390/cancers16122185
Paillassa J, Maitre E, Belarbi Boudjerra N, Madani A, Benlakhal R, Matthes T, Van Den Neste E, Cailly L, Inchiappa L, Bekadja MA, et al. Recommendations for the Management of Patients with Hairy-Cell Leukemia and Hairy-Cell Leukemia-like Disorders: A Work by French-Speaking Experts and French Innovative Leukemia Organization (FILO) Group. Cancers. 2024; 16(12):2185. https://doi.org/10.3390/cancers16122185
Chicago/Turabian StylePaillassa, Jérôme, Elsa Maitre, Nadia Belarbi Boudjerra, Abdallah Madani, Raihane Benlakhal, Thomas Matthes, Eric Van Den Neste, Laura Cailly, Luca Inchiappa, Mohammed Amine Bekadja, and et al. 2024. "Recommendations for the Management of Patients with Hairy-Cell Leukemia and Hairy-Cell Leukemia-like Disorders: A Work by French-Speaking Experts and French Innovative Leukemia Organization (FILO) Group" Cancers 16, no. 12: 2185. https://doi.org/10.3390/cancers16122185
APA StylePaillassa, J., Maitre, E., Belarbi Boudjerra, N., Madani, A., Benlakhal, R., Matthes, T., Van Den Neste, E., Cailly, L., Inchiappa, L., Bekadja, M. A., Tomowiak, C., & Troussard, X. (2024). Recommendations for the Management of Patients with Hairy-Cell Leukemia and Hairy-Cell Leukemia-like Disorders: A Work by French-Speaking Experts and French Innovative Leukemia Organization (FILO) Group. Cancers, 16(12), 2185. https://doi.org/10.3390/cancers16122185






