Lazertinib versus Platinum-Based Chemotherapy with Epidermal Growth Factor Receptor (EGFR)-Positive Non-Small-Cell Lung Cancer after Failing EGFR-Tyrosine Kinase Inhibitor: A Real-World External Comparator Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Data Sources
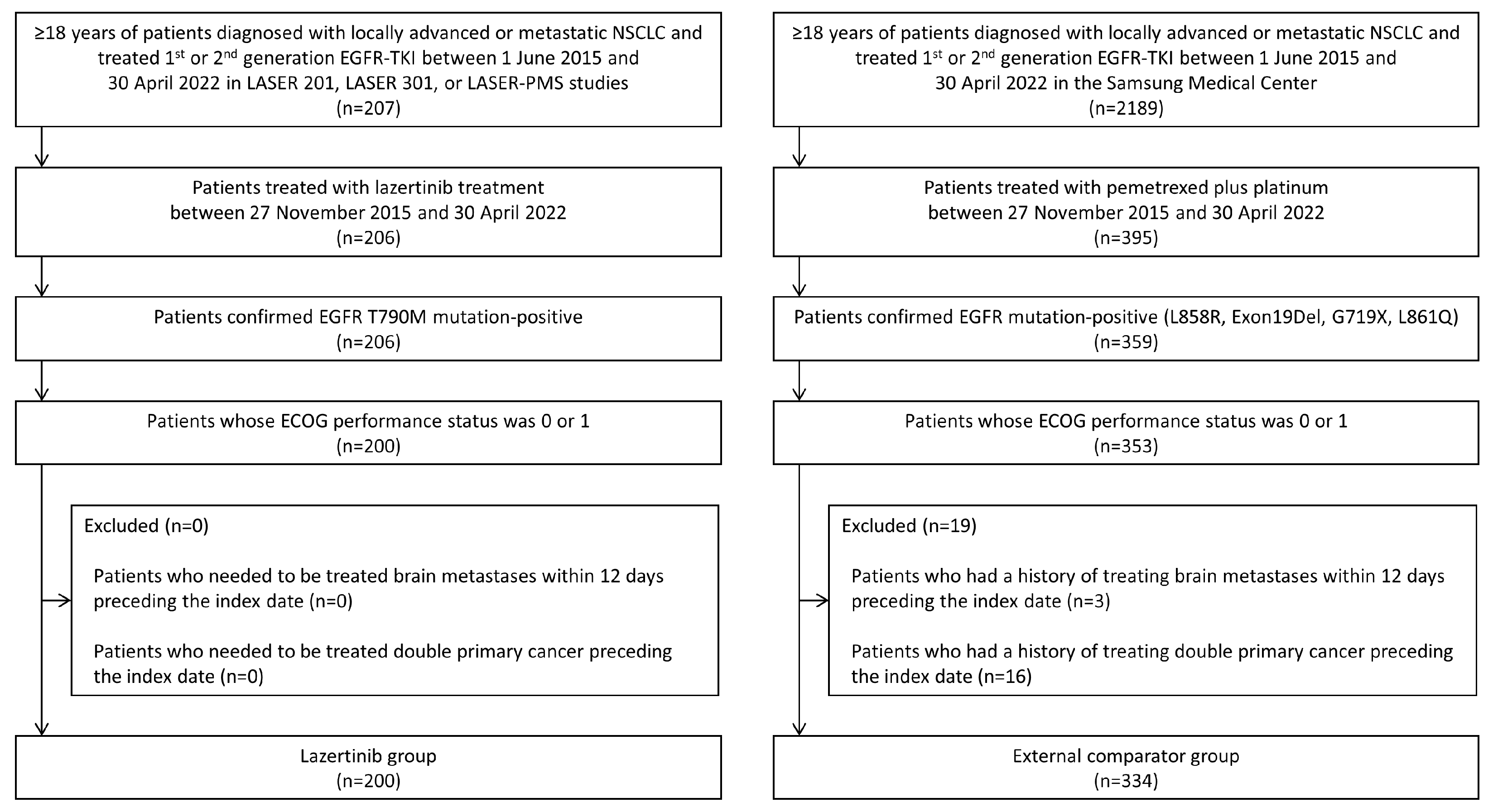
2.2. Study Population and Exposure
2.3. Outcomes
2.4. Potential Confounders
2.5. Statistical Analysis
3. Results
3.1. Study Population and Clinical Characteristics
3.2. Progression-Free Survival and Overall Survival
3.3. Objective Response Rate
3.4. Time to Treatment Discontinuation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, B.C.; Han, J.Y.; Kim, S.W.; Lee, K.H.; Cho, E.K.; Lee, Y.G.; Kim, D.W.; Kim, J.H.; Lee, G.W.; Lee, J.S.; et al. A Phase 1/2 Study of Lazertinib 240 mg in Patients with Advanced EGFR T790M-Positive NSCLC After Previous EGFR Tyrosine Kinase Inhibitors. J. Thorac. Oncol. 2022, 17, 558–567. [Google Scholar] [CrossRef]
- Cho, B.C.; Ahn, M.J.; Kang, J.H.; Soo, R.A.; Reungwetwattana, T.; Yang, J.C.; Cicin, I.; Kim, D.W.; Wu, Y.L.; Lu, S.; et al. Lazertinib Versus Gefitinib as First-Line Treatment in Patients With EGFR-Mutated Advanced Non-Small-Cell Lung Cancer: Results from LASER301. J. Clin. Oncol. 2023, 41, 4208–4217. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Seeger, J.D.; Davis, K.J.; Iannacone, M.R.; Zhou, W.; Dreyer, N.; Winterstein, A.G.; Santanello, N.; Gertz, B.; Berlin, J.A. Methods for external control groups for single arm trials or long-term uncontrolled extensions to randomized clinical trials. Pharmacoepidemiol. Drug Saf. 2020, 29, 1382–1392. [Google Scholar] [CrossRef]
- Burger, H.U.; Gerlinger, C.; Harbron, C.; Koch, A.; Posch, M.; Rochon, J.; Schiel, A. The use of external controls: To what extent can it currently be recommended? Pharm. Stat. 2021, 20, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jeong, O.; Chang, D.K.; Park, S.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Ahn, M.J.; Park, K. Real-time autOmatically updated data warehOuse in healThcare (ROOT): An innovative and automated data collection system. Transl. Lung Cancer Res. 2021, 10, 3865–3874. [Google Scholar] [CrossRef] [PubMed]
- Rassen, J.A.; Shelat, A.A.; Myers, J.; Glynn, R.J.; Rothman, K.J.; Schneeweiss, S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol. Drug Saf. 2012, 21 (Suppl. S2), 69–80. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohe, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.C.; et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Papadimitrakopoulou, V.A.; Mok, T.S.; Han, J.Y.; Ahn, M.J.; Delmonte, A.; Ramalingam, S.S.; Kim, S.W.; Shepherd, F.A.; Laskin, J.; He, Y.; et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann. Oncol. 2020, 31, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Najafzadeh, M.; Gagne, J.J.; Schneeweiss, S. Synergies from Integrating Randomized Controlled Trials and Real-World Data Analyses. Clin. Pharmacol. Ther. 2017, 102, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Gregg, K.; Davis, G.; Ndu, A.; Miller, V.; Vockley, J.; Ollivier, C.; Franolic, T.; Sakai, S. The Use of External Controls in FDA Regulatory Decision Making. Ther. Innov. Regul. Sci. 2021, 55, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.V.; Schneeweiss, S.; Initiative, R.-D.; Franklin, J.M.; Desai, R.J.; Feldman, W.; Garry, E.M.; Glynn, R.J.; Lin, K.J.; Paik, J.; et al. Emulation of Randomized Clinical Trials with Nonrandomized Database Analyses: Results of 32 Clinical Trials. JAMA 2023, 329, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.M.; Grimson, F.; Layton, D.; Pocock, S.; Kim, J. A Framework for Methodological Choice and Evidence Assessment for Studies Using External Comparators from Real-World Data. Drug Saf. 2020, 43, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Park, S.H.; Won, E.; Park, Y.R.; Kim, H.J. Propensity score matching: A conceptual review for radiology researchers. Korean J. Radiol. 2015, 16, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.B.; Xu, J.L.; Lou, Y.Q.; Chu, T.Q.; Zhong, H.; Han, B.H. Anlotinib or platinum-pemetrexed as second-line therapy in EGFR T790M-negative lung cancer. Ann. Palliat. Med. 2020, 9, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K.; Ahn, M.J. Pemetrexed plus platinum versus pemetrexed alone in non-small cell lung cancer patients who have progressed after first-line EGFR TKIs. Lung Cancer 2015, 90, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Wu, Y.L.; Nakagawa, K.; Kim, S.W.; Yang, J.J.; Ahn, M.J.; Wang, J.; Yang, J.C.; Lu, Y.; Atagi, S.; et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): A phase 3 randomised trial. Lancet Oncol. 2015, 16, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kuroda, H.; Oya, Y.; Shimizu, J.; Horio, Y.; Sakao, Y.; Hida, T.; Yatabe, Y. Clinical outcomes of platinum-based chemotherapy according to T790M mutation status in EGFR-positive non-small cell lung cancer patients after initial EGFR-TKI failure. Lung Cancer 2017, 109, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Hirsh, V.; Boyer, M.; Yang, J.C.; Mok, T.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: Overall survival data from the phase IIb LUX-Lung 7 trial. Ann. Oncol. 2017, 28, 270–277. [Google Scholar] [CrossRef] [PubMed]

| Before PS Matching | After PS Matching | |||||
|---|---|---|---|---|---|---|
| Lazertinib (n = 200) | External Comparator (n = 334) | aSD | Lazertinib (n = 156) | External Comparator (n = 156) | aSD | |
| Age, median (Q1–Q3) | 64 (56–71) | 63 (56–70) | 0.11 | 63.5 (55–70.5) | 62.5 (57–69) | 0.03 |
| Female sex, n (%) | 114 (57.0) | 191 (57.2) | 0.00 | 85 (54.5) | 87 (55.8) | 0.03 |
| No history of smoking, n (%) | 123 (61.5) | 213 (63.8) | 0.05 | 94 (60.3) | 94 (60.3) | 0.00 |
| ECOG performance status, n (%) | ||||||
| 0 | 41 (20.5) | 19 (5.7) | 0.45 | 16 (10.3) | 17 (10.9) | 0.02 |
| 1 | 159 (79.5) | 315 (94.3) | 140 (89.7) | 139 (89.1) | ||
| Adenocarcinoma tumor histology, n (%) | 194 (97.0) | 320 (95.8) | 0.06 | 151 (96.8) | 150 (96.1) | 0.03 |
| Brain metastasis, n (%) | 76 (38.0) | 90 (27.0) | 0.24 | 56 (35.9) | 56 (35.9) | 0.00 |
| EGFR mutation status, n (%) | ||||||
| L858R | 37 (18.5) | 145 (43.4) | 0.56 | 27 (17.3) | 67 (43.0) | 0.58 |
| Exon19Del | 80 (40.0) | 179 (53.6) | 0.27 | 68 (43.6) | 83 (53.2) | 0.19 |
| Others (G719X, L861Q, others, unknown) | 83 (41.5) | 19 (5.7) | 0.35 | 61 (39.1) | 8 (5.1) | 0.90 |
| Previous lines of systemic therapy, median (min–max) | 1 (1–10) | 1 (1–5) | 0.45 | 1 (1–5) | 1 (1–5) | 0.06 |
| Previous lines of EGFR-TKI treatment, median (min–max) | 1 (1–10) | 1 (1–5) | 0.36 | 1 (1–3) | 1 (1–5) | 0.03 |
| Type of previous EGFR-TKI treatment, median (min–max) | ||||||
| Gefitinib | 120 (60.0) | 117 (35.0) | 0.52 | 94 (60.3) | 54 (34.6) | 0.53 |
| Erlotinib | 25 (12.5) | 77 (23.1) | 0.28 | 15 (9.6) | 43 (27.6) | 0.47 |
| Afatinib | 61 (30.5) | 193 (57.8) | 0.57 | 46 (29.5) | 89 (57.1) | 0.58 |
| Time from immediate previous EGFR-TKI treatment, n (%) | 0.58 | 0.15 | ||||
| <30 days | 140 (70.0) | 167 (50.0) | 108 (69.2) | 117 (75.0) | ||
| ≥30 days | 47 (23.5) | 165 (49.4) | 45 (28.9) | 37 (23.7) | ||
| No | 13 (6.5) | 2 (0.6) | 3 (1.9) | 2 (1.3) | ||
| Duration of previous EGFR-TKI treatment, n (%) | 0.45 | 0.14 | ||||
| <6 months | 8 (4.0) | 59 (17.7) | 6 (3.9) | 11 (7.1) | ||
| ≥6 months | 192 (96.0) | 275 (82.3) | 150 (96.2) | 145 (93.0) | ||
| Adjusted HR (95% CI) a | Adjusted OR (95% CI) a | ||||
|---|---|---|---|---|---|
| PFS | OS | TTD | ORR | DCR | |
| Subgroup analyses | |||||
| Overall | 0.40 (0.29–0.55) | 0.45 (0.29–0.69) | 0.54 (0.39–0.75) | 1.92 (1.08–3.39) | 1.96 (0.93–4.13) |
| Age group | |||||
| <65 years | 0.40 (0.25–0.62) | 0.50 (0.27–0.91) | 0.55 (0.35–0.88) | 1.45 (0.66–3.17) | 0.98 (0.36–2.71) |
| ≥65 years | 0.40 (0.23–0.68) | 0.68 (0.34–1.36) | 0.74 (0.44–1.24) | 2.00 (0.81–4.98) | 2.16 (0.68–6.88) |
| Sex | |||||
| Male | 0.32 (0.18–0.58) | 0.36 (0.16–0.83) | 0.52 (0.29–0.96) | 3.05 (1.16–8.03) | 4.04 (0.91–18.00) |
| Female | 0.44 (0.28–0.69) | 0.65 (0.36–1.17) | 0.58 (0.37–0.92) | 1.81 (0.80–4.08) | 2.53 (0.92–6.94) |
| Smoking status | |||||
| Ever smoker | 0.38 (0.20–0.72) | 0.36 (0.15–0.84) | 0.54 (0.28–1.04) | 2.60 (0.87–7.75) | 1.94 (0.46–8.20) |
| Never smoker | 0.34 (0.22–0.51) | 0.55 (0.32–0.93) | 0.48 (0.32–0.73) | 2.06 (0.99–4.29) | 2.70 (1.02–7.11) |
| Sensitivity analyses | |||||
| Main analyses b | 0.40 (0.29–0.55) | 0.45 (0.29–0.69) | 0.54 (0.39–0.75) | 1.92 (1.08–3.39) | 1.96 (0.93–4.13) |
| Applying IPTW | 0.50 (0.43–0.58) | 0.62 (0.50–0.76) | 0.67 (0.58–0.79) | 1.62 (1.20–2.19) | 3.04 (2.01–4.60) |
| Restricted to the lazertinib group c | 0.37 (0.25–0.55) | 0.54 (0.33–0.87) | 0.46 (0.31–0.69) | 2.12 (1.04–4.32) | 2.72 (1.13–6.52) |
| Alternative PS model 1 d | 0.39 (0.27–0.55) | 0.51 (0.32–0.82) | 0.53 (0.38–0.75) | 1.79 (0.998–3.21) | 1.78 (0.84–3.76) |
| Alternative PS model 2 e | 0.46 (0.32–0.65) | 0.62 (0.39–0.99) | 0.63 (0.44–0.89) | 1.90 (1.05–3.42) | 2.09 (0.99–4.43) |
| Before PS Matching | After PS Matching | |||||
|---|---|---|---|---|---|---|
| Lazertinib (n = 206) | External Comparator (n = 334) | p-Value | Lazertinib (n = 156) | External Comparator (n = 156) | p-Value | |
| Type of response, n (%) | ||||||
| Complete response | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Partial response | 131 (65.5) | 157 (47.0) | 100 (64.1) | 74 (47.4) | ||
| Stable disease | 45 (22.5) | 96 (28.7) | 38 (24.4) | 47 (30.1) | ||
| Progressive diseases | 15 (7.5) | 75 (22.5) | 12 (7.7) | 32 (20.5) | ||
| Not evaluable | 3 (1.5) | 6 (1.8) | 2 (1.3) | 3 (1.9) | ||
| Unknown, n (%) | 6 (3.0) | 0 (0.0) | 4 (2.6) | 0 (0.0) | ||
| Objective response rate | ||||||
| No. of patients, n | 131 | 157 | 100 | 74 | ||
| Percentage of patients (95% CI) | 65.5 (58.9–72.1) | 47.0 (41.7–52.4) | 64.1 (56.6–71.6) | 47.4 (39.6–55.3) | ||
| Crude OR (95% CI) | 2.14 (1.49–3.07) | 1.00 (Reference) | <0.001 | 1.98 (1.26–3.12) | 1.00 (Reference) | 0.003 |
| Adjusted OR (95% CI) a | 1.89 (1.18–3.04) | 1.00 (Reference) | 0.009 | 1.92 (1.08–3.39) | 1.00 (Reference) | 0.026 |
| Disease control rate | ||||||
| No. of patients, n | 176 | 253 | 138 | 121 | ||
| Percentage of patients (95% CI) | 88.0 (83.5–92.5) | 75.8 (71.2–80.4) | 88.5 (83.5–93.5) | 77.6 (71.0–84.1) | ||
| Crude OR (95% CI) | 2.35 (1.43–3.85) | 1.00 (Reference) | <0.001 | 2.22 (1.20–4.12) | 1.00 (Reference) | 0.012 |
| Adjusted OR (95% CI) a | 1.98 (1.07–3.67) | 1.00 (Reference) | 0.029 | 1.96 (0.93–4.13) | 1.00 (Reference) | 0.077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lee, H.; Yoon, D.; Choi, E.-Y.; Woo, J.; Jo, B.; Kim, S.; Shin, J.-Y.; Jung, H.A. Lazertinib versus Platinum-Based Chemotherapy with Epidermal Growth Factor Receptor (EGFR)-Positive Non-Small-Cell Lung Cancer after Failing EGFR-Tyrosine Kinase Inhibitor: A Real-World External Comparator Study. Cancers 2024, 16, 2169. https://doi.org/10.3390/cancers16122169
Lee J, Lee H, Yoon D, Choi E-Y, Woo J, Jo B, Kim S, Shin J-Y, Jung HA. Lazertinib versus Platinum-Based Chemotherapy with Epidermal Growth Factor Receptor (EGFR)-Positive Non-Small-Cell Lung Cancer after Failing EGFR-Tyrosine Kinase Inhibitor: A Real-World External Comparator Study. Cancers. 2024; 16(12):2169. https://doi.org/10.3390/cancers16122169
Chicago/Turabian StyleLee, Junho, Hyesung Lee, Dongwon Yoon, Eun-Young Choi, Jieun Woo, Bobae Jo, Sohee Kim, Ju-Young Shin, and Hyun Ae Jung. 2024. "Lazertinib versus Platinum-Based Chemotherapy with Epidermal Growth Factor Receptor (EGFR)-Positive Non-Small-Cell Lung Cancer after Failing EGFR-Tyrosine Kinase Inhibitor: A Real-World External Comparator Study" Cancers 16, no. 12: 2169. https://doi.org/10.3390/cancers16122169
APA StyleLee, J., Lee, H., Yoon, D., Choi, E.-Y., Woo, J., Jo, B., Kim, S., Shin, J.-Y., & Jung, H. A. (2024). Lazertinib versus Platinum-Based Chemotherapy with Epidermal Growth Factor Receptor (EGFR)-Positive Non-Small-Cell Lung Cancer after Failing EGFR-Tyrosine Kinase Inhibitor: A Real-World External Comparator Study. Cancers, 16(12), 2169. https://doi.org/10.3390/cancers16122169





