Prostate Cancer Lung Metastasis: Clinical Insights and Therapeutic Strategies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
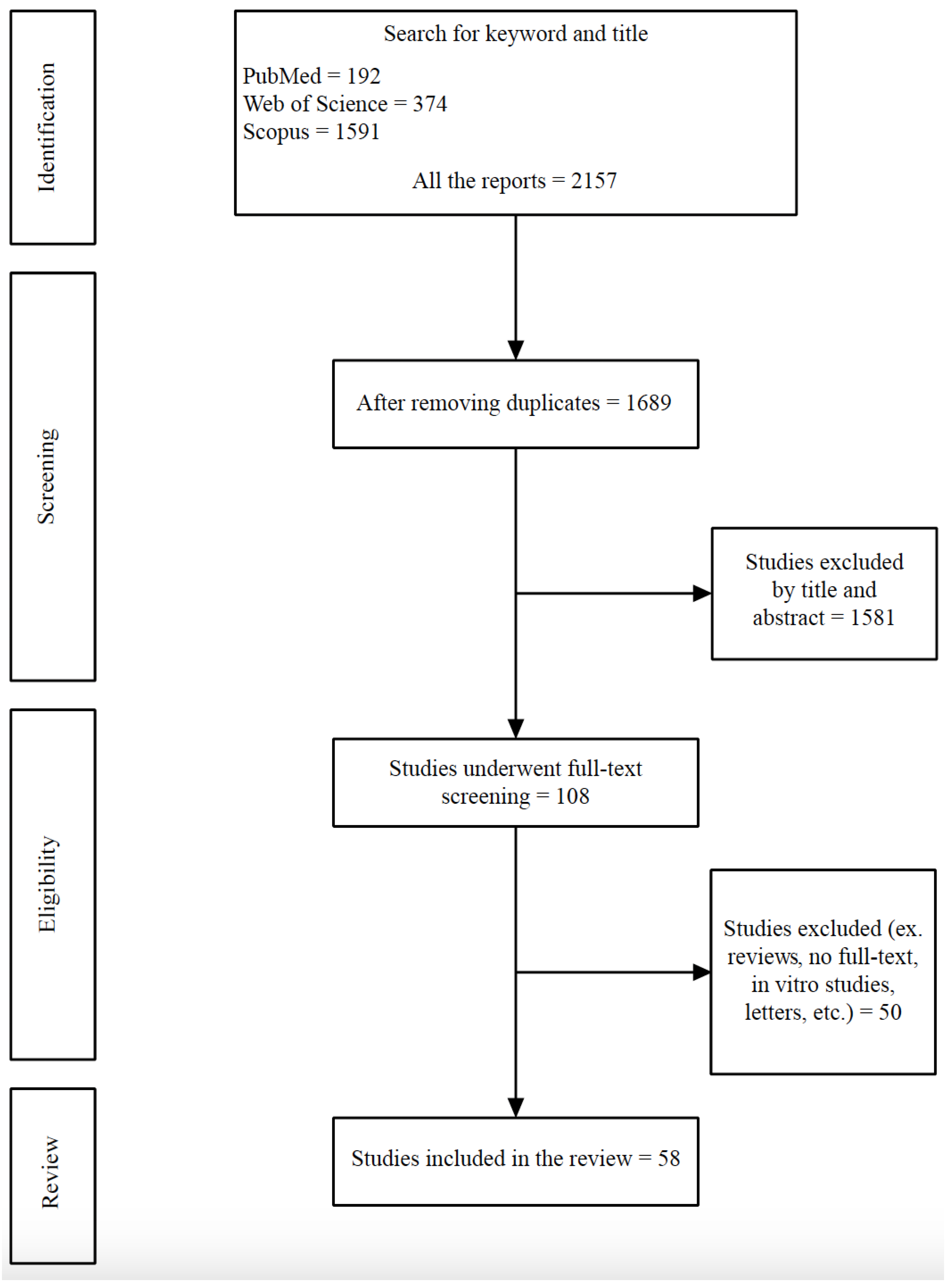
2.1. The Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
3. Prostate Cancer Lung Metastasis Presentations
3.1. Clinical and Laboratories
3.2. Diagnostic Methods
3.2.1. Imaging Modalities
3.2.2. Histopathology
3.2.3. Molecular and Genetics
4. Treatment Approaches
4.1. Systemic Therapies
4.2. Metastasis-Directed Therapies (MDT)
5. Prognosis and Oncological Outcomes
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Goodman, O.B.; Flaig, T.W.; Molina, A.; Mulders, P.F.A.; Fizazi, K.; Suttmann, H.; Li, J.; Kheoh, T.; de Bono, J.S.; Scher, H.I. Exploratory analysis of the visceral disease subgroup in a phase III study of abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2014, 17, 34–39. [Google Scholar] [CrossRef]
- Schymura, M.J.; Sun, L.; Percy-Laurry, A. Prostate cancer collaborative stage data items--their definitions, quality, usage, and clinical implications: A review of SEER data for 2004–2010. Cancer 2014, 120 (Suppl. 23), 3758–3770. [Google Scholar] [CrossRef] [PubMed]
- Scosyrev, E.; Messing, E.M.; Mohile, S.; Golijanin, D.; Wu, G. Prostate cancer in the elderly: Frequency of advanced disease at presentation and disease-specific mortality. Cancer 2012, 118, 3062–3070. [Google Scholar] [CrossRef]
- Norgaard, M.; Jensen, A.O.; Jacobsen, J.B.; Cetin, K.; Fryzek, J.P.; Sorensen, H.T. Skeletal related events, bone metastasis and survival of prostate cancer: A population-based cohort study in Denmark (1999 to 2007). J. Urol. 2010, 184, 162–167. [Google Scholar] [CrossRef]
- Rigaud, J.; Tiguert, R.; LE Normand, L.; Karam, G.; Glemain, P.; Buzelin, J.-M.; Bouchot, O. Prognostic value of bone scan in patients with metastatic prostate cancer treated initially with androgen deprivation therapy. J. Urol. 2002, 168 Pt 1, 1423–1426. [Google Scholar] [CrossRef]
- Nakamachi, H.; Suzuki, H.; Akakura, K.; Imamoto, T.; Ueda, T.; Ishihara, M.; Furuya, Y.; Ichikawa, T.; Igarashi, T.; Ito, H. Clinical significance of pulmonary metastasis in stage D2 prostate cancer patients. Prostate Cancer Prostatic Dis. 2002, 5, 159–163. [Google Scholar] [CrossRef]
- Pond, G.R.; Sonpavde, G.; de Wit, R.; Eisenberger, M.A.; Tannock, I.F.; Armstrong, A.J. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur. Urol. 2014, 65, 3–6. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef]
- Chen, H.; Stoltzfus, K.C.; Lehrer, E.J.; Horn, S.R.; Siva, S.; Trifiletti, D.M.; Meng, M.B.; Verma, V.; Louie, A.V.; Zaorsky, N.G. The Epidemiology of Lung Metastasis. Front. Med. 2021, 8, 723396. [Google Scholar] [CrossRef]
- Damjanovic, J.; Janssen, J.C.; Furth, C.; Diederichs, G.; Walter, T.; Amthauer, H.; Makowski, M.R. 68Ga-PSMA-PET/CT for the evaluation of pulmonary metastasis and opacities in patients with prostate cancer. Cancer Imaging 2018, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, K.C.; Batihan, G.; Kaya, S.O. Pulmonary metastasis in urogenital cancers: Surgical treatment and outcomes. Cir. Esp. (Engl. Ed.) 2023, 101, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Delbare, F.; Villeirs, G. Cavitary Lung Metastasis in Prostate Cancer. J. Belg. Soc. Radiol. 2022, 106, 137. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Iizuka, S.; Yoneda, T.; Otsuki, Y.; Nakamura, T. Solitary pulmonary nodule as the initial manifestation of isolated metastasis from prostate cancer without bone involvement: A case report. Int. J. Surg. Case Rep. 2022, 90, 106681. [Google Scholar] [CrossRef] [PubMed]
- Tohfe, M.; Baki, S.A.; Saliba, W.; Ghandour, F.; Ashou, R.; Ghazal, G.; Bahous, J.; Chamseddine, N. Metastatic prostate adenocarcinoma presenting with pulmonary symptoms: A case report and review of the literature. Cases J. 2008, 1, 316. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.H.; Higgins, J.P.; Brooks, J.D. Biochemical remission after resection of prostate cancer lung metastasis. Urology 2004, 63, 584–585. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, C.; Xu, A.; Wang, Z.; Liu, B. Acinar with ductal and mucinous adenocarcinoma of prostate cancer complicated with lung metastasis: A case report and literature review. Ann. Palliat. Med. 2021, 10, 2366–2370. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, E.B.; Buyukpinarbasili, N.; Ziyade, S.; Akman, T.; Turk, H.M.; Aydin, M. Incidental detection of prostate-specific antigen-negative metastatic prostate cancer initially presented with solitary pulmonary nodule on fluorodeoxyglucose positron emission tomography/computed tomography. Indian J. Nucl. Med. 2015, 30, 268–271. [Google Scholar] [CrossRef]
- Fukuoka, T. Prostate cancer in a patient with multiple pulmonary metastasis alone and respiratory symptoms. J. Rural. Med. 2014, 9, 27–31. [Google Scholar] [CrossRef]
- Gago, J.P.; Camara, G.; Dionisio, J.; Opiniao, A. Pulmonary metastasis as sole manifestation of relapse in previously treated localised prostate cancer: Three exceptional case reports. Ecancermedicalscience 2016, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Khandani, A.H.; Funkhouser, W.K.; Feins, R.; Socinski, M.A. Simultaneous FDG PET+/Glut1+ lung and FDG PET-/Glut1- subcarinal lymph node metastasis from prostate cancer. Ann. Nucl. Med. 2009, 23, 595–597. [Google Scholar] [CrossRef]
- Kirby, R. Case study: Management of advanced prostate cancer with soft tissue metastasis. Prostate Cancer Prostatic Dis. 2005, 8, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Maebayashi, T.; Abe, K.; Aizawa, T.; Sakaguchi, M.; Ishibash, N.; Fukushima, S.; Honma, T.; Kusumi, Y.; Matsui, T.; Kawata, N. Solitary pulmonary metastasis from prostate cancer with neuroendocrine differentiation: A case report and review of relevant cases from the literature. World J. Surg. Oncol. 2015, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Mantica, G.; Giavarra, M.; Perrone, V.; De Marchi, L.; Gennari, A.; Toncini, C.; Terrone, C. Curative Lung Metastasectomy Without Concomitant Androgen Deprivation Therapy in Oligometastatic Castration-resistant Prostate Cancer: A Case Report and Review of the Literature. Clin. Genitourin. Cancer 2020, 18, e295–e299. [Google Scholar] [CrossRef] [PubMed]
- Petras, A.F.; Wollett, F.C. Metastatic prostatic pulmonary nodules with normal bone image. J. Nucl. Med. 1983, 24, 1026–1027. [Google Scholar] [PubMed]
- Polistina, G.E.; Matarese, A.; Cariello, P.; Caroppo, D.; Zamparelli, A.S. Cavitary lung metastasis as relapse of prostate cancer. Respir. Med. Case Rep. 2020, 29, 100973. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Wang, L.A.; Wang, P.; Tong, D.; Liu, G.; Zhang, J.; Dai, N.; Zhang, Y.; Yuan, G.; Geary, K.; et al. Case Report: Co-Existence of BRCA2 and PALB2 Germline Mutations in Familial Prostate Cancer with Solitary Lung Metastasis. Front. Oncol. 2020, 10, 564694. [Google Scholar] [CrossRef] [PubMed]
- Tsakiridis, T.; Bonert, M.; Zukotynski, K.; Anagnostopoulos, A.E. Radiographic and metabolic evolution of prostate cancer lung metastasis detected by prostate-specific membrane antigen and fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography. World J. Nucl. Med. 2020, 19, 421–424. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Gao, R.W.; Ahmed, M.E.; Orme, J.J.; Rincón, M.M.; Harmsen, W.S.; Johnson, G.B.; Cassivi, S.D.; Kwon, E.D.; Phillips, R.M.; et al. Metastasis-Directed Therapy for Metachronous Lung Metastasis in Prostate Cancer. JU Open Plus 2023, 1, e00050. [Google Scholar] [CrossRef]
- Kase, A.M.; Copland JA 3rd Zhai, Q.; Tan, W. Complete response in Patients with Lung-Only Metastatic Prostate Cancer: Outcome Analysis. Clin. Genitourin. Cancer 2022, 20, e485–e489. [Google Scholar] [CrossRef]
- Kaneko, Y.; Kosaka, T.; Nakamura, K.; Mikami, S.; Nishihara, H.; Oya, M. Squamous cell carcinoma of the prostate with SMARCA4 alteration in a Japanese patient. IJU Case Rep. 2022, 5, 323–326. [Google Scholar] [CrossRef]
- Tarabaih, M.; Degheili, J.A.; Nasser, M. Isolated Solitary Lung Nodule in a Patient with Idiopathic Pulmonary Fibrosis and Concomitant Prostate Cancer: A Challenging Diagnosis. Cureus 2021, 13, e14218. [Google Scholar] [CrossRef] [PubMed]
- Yoshitake, H.; Oura, S.; Yamaguchi, T.; Makimoto, S. Solitary Lung Metastasis of Prostate Cancer with a Long Disease-Free Interval and Normal Prostate-Specific Antigen Level. Case Rep. Oncol. 2021, 14, 284–289. [Google Scholar] [CrossRef]
- Vaz, S.; Silva, A.; Oliveira, C.; Marques, R.; Galzerano, A.; Castillo-Martin, M. Impact of PSMA PET/CT in prostate cancer patient’s clinical management: A pictorial essay of interesting cases with histologic confirmation. Clin. Transl. Imaging 2020, 8, 207–226. [Google Scholar]
- Izawa, M.; Kosaka, T.; Nakamura, K.; Oba, J.; Hishida, T.; Hongo, H.; Mikami, S.; Nishihara, H.; Oya, M. Pulmonary metastasis secondary to abiraterone-resistant prostate cancer with homozygous deletions of BRCA2: First Japanese case. IJU Case Rep. 2021, 4, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.X.; Lei, L.; Zhu, Y.C.; Du, K.Q.; Li, X.F.; Chen, H.F.; Wang, W.X.; Xu, C.W. A prostate cancer patient with isolated lung metastasis: A case report. Transl. Cancer Res. 2020, 9, 2064–2068. [Google Scholar] [CrossRef]
- Ciriaco, P.; Briganti, A.; Bernabei, A.; Gandaglia, G.; Carretta, A.; Viola, C.; Montorsi, F.; Negri, G. Safety and Early Oncologic Outcomes of Lung Resection in Patients with Isolated Pulmonary Recurrent Prostate Cancer: A Single-center Experience. Eur. Urol. 2019, 75, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Katsui, M.; Ohigashi, T.; Kosaka, T.; Bessho, H.; Arakawa, T. Remarkable response to abiraterone acetate in castration-resistant prostate cancer patient with aggressive liver metastasis. IJU Case Rep. 2018, 2, 12–14. [Google Scholar] [CrossRef]
- Seniaray, N.; Verma, R.; Belho, E.; Malik, D.; Mahajan, H. Diffuse Pulmonary Metastasis from Prostate Cancer on 68Ga PSMA PET/CT. Clin. Nucl. Med. 2019, 44, 898–900. [Google Scholar] [CrossRef]
- Boschian, R.; Rizzo, M.; Zandona, L.; Trombetta, C.; Liguori, G. Pulmonary recurrence from prostate cancer and biochemical remission after metastasis directed therapy. A case report. Arch. Ital. Urol. Androl. 2018, 90, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Polverari, G.; Ceci, F.; Allen-Auerbach, M.; Gupta, P.; Fishbein, M.C.; Reiter, R.E.; Lee, J.M.; Hope, T.A.; Carroll, R.M.; Czernin, J.; et al. Solitary Mucinous Prostate Adenocarcinoma Lung Metastasis Detected by 68Ga-PSMA-11 PET/CT. Clin. Genitourin. Cancer 2019, 17, e53–e55. [Google Scholar] [CrossRef] [PubMed]
- Reinstatler, L.; Dupuis, J.; Dillon, J.L.; Black, C.C.; Phillips, J.D.; Hyams, E.S. Lung malignancy in prostate cancer: A report of both metastatic and primary lung lesions. Urol. Case Rep. 2018, 16, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Grosse Hokamp, N.; Kobe, C.; Linzenich, E.; Maintz, D.; Drzezga, A. Solitary PSMA-Positive Pulmonary Metastasis in Biochemical Relapse of Prostate Cancer. Clin. Nucl. Med. 2017, 42, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Mortier, D.; Baten, E.; Vandeurzen, K.; van Renterghem, K. The Benefit of a Surgical Resection of a Solitary Pulmonary Metastasis of Prostate Cancer after Radical Prostatectomy. Curr. Urol. 2017, 10, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.; Pai, R.; Parikh, R.A. Complete biochemical response after pulmonary metastasectomy in prostate adenocarcinoma. Exp. Hematol. Oncol. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, L.; Ceci, F.; Uprimny, C.; Kendler, D.; Virgolini, I. Detection of Sarcomatoid Lung Metastasis With 68GA-PSMA PET/CT in a Patient with Prostate Cancer. Clin. Nucl. Med. 2016, 41, 421–422. [Google Scholar] [CrossRef]
- Su, H.Y.; Chen, M.L.; Hsieh, P.J.; Hsieh, T.S.; Chao, I.M. Lung Metastasis from Prostate Cancer Revealed by 18F-FDG PET/CT Without Osseous Metastasis on Bone Scan. Clin. Nucl. Med. 2016, 41, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, Y.; Mitsuzuka, K.; Watanabe, M.; Kawamorita, N.; Yamada, S.; Kaiho, Y.; Ito, A.; Nakagawa, H.; Arai, Y. Chemotherapy with Gemcitabine and Cisplatin for Advanced Ductal Adenocarcinoma of the Prostate: Clinical Courses of Two Patients. Tohoku J. Exp. Med. 2015, 237, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Lococo, F.; Petrone, G.; Inzani, F.; Perotti, G.; Porziella, V.; Granone, P.; Rindi, G.; Giordano, A.; Rufini, V. Pulmonary neuroendocrine tumor incidentally detected by (18)F-CH PET/CT. Clin. Nucl. Med. 2013, 38, e196–e199. [Google Scholar] [CrossRef]
- Pepe, P.; Fraggetta, F.; Tornabene, F.; Nicolosi, M.; Aragona, F. Solitary lung metastasis after radical prostatectomy in presence of undetectable PSA. Arch. Ital. Urol. Androl. 2012, 84, 208–210. [Google Scholar]
- Wallis, C.J.; English, J.C.; Goldenberg, S.L. The role of resection of pulmonary metastasis from prostate cancer: A case report and literature review. Can. Urol. Assoc. J. 2011, 5, E104–E108. [Google Scholar] [CrossRef]
- Sakai, T.; Kimura, D.; Hatanaka, R.; Yamada, Y.; Tsushima, T.; Fukuda, I.; Kamata, Y. Surgical treated pulmonary metastasis from prostatic cancer; report of a case. Kyobu Geka Jpn. J. Thorac. Surg. 2010, 63, 340–343. [Google Scholar]
- Goto, T.; Maeshima, A.; Oyamada, Y.; Kato, R. Solitary pulmonary metastasis from prostate sarcomatoid cancer. World J. Surg. Oncol. 2010, 8, 101. [Google Scholar] [CrossRef]
- Boyer, B.P.; Boyer, M.J. An elusive tumor in a man who has evidence of prostate cancer metastasis. JAAPA 2009, 22, 22–24. [Google Scholar] [CrossRef]
- Pruthi, R.S.; Hubbard, J.S.; Kouba, E.; Wallen, E. Androgen-independent prostate cancer treated with resection of the solitary metastatic site. Urol. Int. 2007, 79, 371–373. [Google Scholar] [CrossRef]
- Maeda, T.; Tateishi, U.; Komiyama, M.; Fujimoto, H.; Watanabe, S.-I.; Terauchi, T.; Moriyama, N.; Arai, Y.; Sugimura, K.; Kakizoe, T. Distant metastasis of prostate cancer: Early detection of recurrent tumor with dual-phase carbon-11 choline positron emission tomography/computed tomography in two cases. Jpn. J. Clin. Oncol. 2006, 36, 598–601. [Google Scholar] [CrossRef]
- Hofland, C.A.; Bagg, M.D. An isolated pulmonary metastasis in prostate cancer. Mil. Med. 2000, 165, 973–974. [Google Scholar] [CrossRef]
- Kume, H.; Takai, K.; Kameyama, S.; Kawabe, K. Multiple pulmonary metastasis of prostatic carcinoma with little or no bone or lymph node metastasis. Report of two cases and review of the literature. Urol. Int. 1999, 62, 44–47. [Google Scholar] [CrossRef]
- Smith, C.P.; Sharma, A.; Ayala, G.; Cagle, P.; Kadmon, D. Solitary pulmonary metastasis from prostate cancer. J. Urol. 1999, 162, 2102. [Google Scholar] [CrossRef]
- Behrakis, P.; Koutsilieris, M. Pulmonary metastasis in metastatic prostate cancer: Host tissue-tumor cell interactions and response to hormone therapy. Anticancer. Res. 1997, 17, 1517–1518. [Google Scholar]
- Allen, F.J.; Van Velden, D.J. Haemospermia and nodular lung metastasis without bone involvement: An unusual presentation of prostate carcinoma. Br. J. Urol. 1996, 78, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.J.; Cowley, N. Pulmonary metastasis, an occult prostatic adenocarcinoma and delayed administration of antiandrogens. Clin. Oncol. (R. Coll. Radiol.) 1996, 8, 118–119. [Google Scholar] [CrossRef]
- Leibman, B.D.; Dillioglugil, O.; Wheeler, T.M.; Scardino, P.T. Distant metastasis after radical prostatectomy in patients without an elevated serum prostate specific antigen level. Cancer 1995, 76, 2530–2534. [Google Scholar] [CrossRef]
- Fabozzi, S.J.; Schellhammer, P.F.; el-Mahdi, A.M. Pulmonary metastasis from prostate cancer. Cancer 1995, 75, 2706–2709. [Google Scholar] [CrossRef] [PubMed]
- Cusan, L.; Gomez, J.; Dupont, A.; Diamond, P.; Lemay, M.; Labrie, F.; Moore, S. Metastatic prostate cancer pulmonary nodules: Beneficial effects of combination therapy and subsequent withdrawal of flutamide. Prostate 1994, 24, 257–261. [Google Scholar] [CrossRef]
- Eastham, J.A.; Esensten, M.L.; Wilson, T.G. Isolated pulmonary metastasis from prostatic adenocarcinoma. West. J. Med. 1993, 159, 489–490. [Google Scholar]
- Rockey, K.E.; Graham, T.E. Prostate adenocarcinoma metastatic to the lung. Postgrad. Med. 1990, 87, 199–205, 208. [Google Scholar] [CrossRef]
- Bromberg, W.D.; Gaylis, F.D.; Bauer, K.D.; Schaeffer, A.J. Isolated pulmonary metastasis from carcinoma of the prostate: A case report and deoxyribonucleic acid analysis using flow cytometry. J. Urol. 1989, 141, 137–139. [Google Scholar] [CrossRef]
- Panella, J.; Mintzer, R.A. Multiple calcified pulmonary nodules in an elderly man. JAMA 1980, 244, 2559–2560. [Google Scholar] [CrossRef]
- Varkarakis, M.J.; Winterberger, A.R.; Gaeta, J.; Moore, R.H.; Murphy, G.P. Lung metastasis in prostatic carcinoma. Clin. Significance Urol. 1974, 3, 447–452. [Google Scholar]
- Alumkal, J.J.; Chowdhury, S.; Loriot, Y.; Sternberg, C.N.; de Bono, J.S.; Tombal, B.; Carles, J.; Flaig, T.W.; Dorff, T.B.; Phung, D.; et al. Effect of Visceral Disease Site on Outcomes in Patients with Metastatic Castration-resistant Prostate Cancer Treated with Enzalutamide in the PREVAIL Trial. Clin. Genitourin. Cancer 2017, 15, 610–617.e3. [Google Scholar] [CrossRef]
- Lindell, M.M.; Doubleday, L.C.; von Eschenbach, A.C.; Libshitz, H.I. Mediastinal metastases from prostatic carcinoma. J. Urol. 1982, 128, 331–334. [Google Scholar] [CrossRef]
- Miseria, S.; Torresi, U.; Menichetti, E.T.; Tummarello, D.; Baldelli, S.; Murer, B.; Cenerino, R. Lymphangitic carcinomas of the lung as presentation of prostatic cancer. A case report. Tumori 1991, 77, 86–89. [Google Scholar] [CrossRef]
- Miller, K.S.; Miller, J.M. Imaging case of the month. Pulmonary lymphangitic carcinomatosis from adenocarcinoma of the prostate. Md. Med. J. 1994, 43, 989–990. [Google Scholar]
- Wu, J.W.; Chiles, C. Lymphangitic carcinomatosis from prostate carcinoma. J. Comput. Assist. Tomogr. 1999, 23, 761–763. [Google Scholar] [CrossRef]
- Cilento, M.A.; Hocking, C.M. Cystic and cavitating lung lesions as a presenting finding of metastatic prostate cancer. Med. J. Aust. 2021, 214, 459.e1. [Google Scholar] [CrossRef]
- Lubin, D.J.; Holden, S.B.; Rettig, M.B.; Reiter, R.E.; King, C.R.; Lee, J.M.; Wallace, D.W.; Calais, J. Prostate Cancer Pulmonary Metastasis Presenting as a Ground-Glass Pulmonary Nodule on 68Ga-PSMA-11 PET/CT. Clin. Nucl. Med. 2019, 44, e353–e356. [Google Scholar] [CrossRef]
- Christie-Large, M.; James, S.L.; Tiessen, L.; Davies, A.M.; Grimer, R.J. Imaging strategy for detecting lung metastasis at presentation in patients with soft tissue sarcomas. Eur. J. Cancer 2008, 44, 1841–1845. [Google Scholar] [CrossRef]
- Cardinale, L.; Ardissone, F.; Novello, S.; Busso, M.; Solitro, F.; Longo, M.; Sardo, D.; Giors, M.; Fava, C. The pulmonary nodule: Clinical and radiological characteristics affecting a diagnosis of malignancy. Radiol. Med. 2009, 114, 871–889. [Google Scholar] [CrossRef]
- Henschke, C.I. Early lung cancer action project: Overall design and findings from baseline screening. Cancer 2000, 89 (Suppl. 11), 2474–2482. [Google Scholar] [CrossRef]
- Kobayashi, N.; Idiyatullin, D.; Corum, C.; Weber, J.; Garwood, M.; Sachdev, D. SWIFT MRI enhances detection of breast cancer metastasis to the lung. Magn. Reson. Med. 2015, 73, 1812–1819. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, R.; Wang, W.; Zhao, Y.; Liu, X. 68Ga-PSMA PET/CT for the evaluation of metastasis in patients with prostate cancer: A systematic review and meta-analysis. Hell. J. Nucl. Med. 2022, 25, 297–311. [Google Scholar]
- Damjanovic, J.; Janssen, J.C.; Prasad, V.; Diederichs, G.; Walter, T.; Brenner, W.; Makowski, M.R. 68Ga-PSMA-PET/CT for the evaluation of liver metastasis in patients with prostate cancer. Cancer Imaging 2019, 19, 37. [Google Scholar] [CrossRef]
- Pyka, T.; Weirich, G.; Einspieler, I.; Maurer, T.; Theisen, J.; Hatzichristodoulou, G.; Schwamborn, K.; Schwaiger, M.; Eiber, M. 68Ga-PSMA-HBED-CC PET for Differential Diagnosis of Suggestive Lung Lesions in Patients with Prostate Cancer. J. Nucl. Med. 2016, 57, 367–371. [Google Scholar] [CrossRef]
- Swanton, C.; Soria, J.-C.; Bardelli, A.; Biankin, A.; Caldas, C.; Chandarlapaty, S.; de Koning, L.; Dive, C.; Feunteun, J.; Leung, S.-Y.; et al. Consensus on precision medicine for metastatic cancers: A report from the MAP conference. Ann. Oncol. 2016, 27, 1443–1448. [Google Scholar] [CrossRef]
- Shenderov, E.; Velho, P.I.; Awan, A.H.; Wang, H.; Mirkheshti, N.; Lotan, T.L.; Carducci, M.A.; Pardoll, D.M.; Eisenberger, M.A.; Antonarakis, E.S. Genomic and clinical characterization of pulmonary-only metastatic prostate cancer: A unique molecular subtype. Prostate 2019, 79, 1572–1579. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, L.; Wang, Z. PCA3 and TMPRSS2-ERG gene fusions as diagnostic biomarkers for prostate cancer. Chin. J. Cancer Res. 2016, 28, 65–71. [Google Scholar]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef]
- Sumiyoshi, T.; Chi, K.N.; Wyatt, A.W. Clinical implications of genomic alterations in metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 310–322. [Google Scholar] [CrossRef]
- Fonseca, N.M.; Van der Eecken, K.; Herberts, C.; Verbeke, S.; Ng, S.W.; Lumen, N.; Ritch, E.; Murtha, A.J.; Bernales, C.Q.; Schönlau, E.; et al. Genomic Features of Lung-Recurrent Hormone-Sensitive Prostate Cancer. JCO Precis. Oncol. 2022, 6, e2100543. [Google Scholar] [CrossRef]
- Kosaka, T.; Hongo, H.; Aimono, E.; Matsumoto, K.; Hayashida, T.; Mikami, S.; Nishihara, H.; Oya, M. A first Japanese case of neuroendocrine prostate cancer accompanied by lung and brain metastasis with somatic and germline BRCA2 mutation. Pathol. Int. 2019, 69, 715–720. [Google Scholar] [CrossRef]
- Loriot, Y.; Fizazi, K.; de Bono, J.S.; Forer, D.; Hirmand, M.; Scher, H.I. Enzalutamide in castration-resistant prostate cancer patients with visceral disease in the liver and/or lung: Outcomes from the randomized controlled phase 3 AFFIRM trial. Cancer 2017, 123, 253–262. [Google Scholar] [CrossRef]
- Scher, H.I.; Beer, T.M.; Higano, C.S.; Anand, A.; Taplin, M.-E.; Efstathiou, E.; Rathkopf, D.; Shelkey, J.; Yu, E.Y.; Alumkal, J.; et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet 2010, 375, 1437–1446. [Google Scholar] [CrossRef]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Gupta, M.; Karthikeyan, G.; Choudhury, P.S.; Sharma, A.; Singh, A.; Rawal, S. Is (177)Lu-PSMA an effective treatment modality for mCRPC patients with bone and visceral metastasis? Hell. J. Nucl. Med. 2020, 23, 312–320. [Google Scholar]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D’Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy with (177)Lu-PSMA-I&T in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 75, 920–926. [Google Scholar]
- Kessel, K.; Seifert, R.; Schäfers, M.; Weckesser, M.; Schlack, K.; Boegemann, M.; Rahbar, K. Second line chemotherapy and visceral metastasis are associated with poor survival in patients with mCRPC receiving (177)Lu-PSMA-617. Theranostics 2019, 9, 4841–4848. [Google Scholar] [CrossRef]
- Zhang, J.; Kulkarni, H.R.; Singh, A.; Baum, R.P. Complete Regression of Lung Metastasis in a Patient with Metastatic Castration-Resistant Prostate Cancer Using 177Lu-PSMA Radioligand Therapy. Clin. Nucl. Med. 2020, 45, e48–e50. [Google Scholar] [CrossRef] [PubMed]
- Franzese, C.; Zucali, P.A.; Di Brina, L.; D’Agostino, G.; Navarria, P.; Franceschini, D.; Santoro, A.; Scorsetti, M. The efficacy of Stereotactic body radiation therapy and the impact of systemic treatments in oligometastatic patients from prostate cancer. Cancer Med. 2018, 7, 4379–4386. [Google Scholar] [CrossRef] [PubMed]
- Berkovic, P.; De Meerleer, G.; Delrue, L.; Lambert, B.; Fonteyne, V.; Lumen, N.; Decaestecker, K.; Villeirs, G.; Vuye, P.; Ost, P. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastasis: Deferring androgen deprivation therapy. Clin. Genitourin. Cancer 2013, 11, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ost, P.; Jereczek-Fossa, B.A.; Van As, N.; Zilli, T.; Muacevic, A.; Olivier, K.; Henderson, D.; Casamassima, F.; Orecchia, R.; Surgo, A.; et al. Progression-free Survival Following Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Treatment-naive Recurrence: A Multi-institutional Analysis. Eur. Urol. 2016, 69, 9–12. [Google Scholar] [CrossRef]
- Conteduca, V.; Caffo, O.; Fratino, L.; Lo Re, G.; Basso, U.; D’Angelo, A.; Donini, M.; Verderame, F.; Ratta, R.; Procopio, G.; et al. Impact of visceral metastasis on outcome to abiraterone after docetaxel in castration-resistant prostate cancer patients. Future Oncol. 2015, 11, 2881–2891. [Google Scholar] [CrossRef]
| Articles | Year of Publication | Type of Study | Number of Cases | Age/Median | Pathology | Gleason Score/Median | PSA/Median | Symptoms | Lines of Treatment | Prognosis | Survival (Months) | Methods of Diagnosing | Number of Met | Met’s Location | Concomitant Met |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mahmoud, et al. [30] | 2023 | Retrospective | 75 | 69 | Adeno | 7 | 4 | N/A | ADT, Surgical resection, Chemo | 72% Survived | N/A | Chest CT, PET/CT | Multiple | Bilateral | N/A |
| Ceylan, et al. [13] | 2023 | Case report | 2 | 57.11 | Adeno | N/A | N/A | Asymptomatic | Surgical resection | Died | 6 | Chest CT, PET/CT | N/A | N/A | N/A |
| Kase, et al. [31] | 2022 | Case series | 8 | N/A | Adeno | 7 | 3.1 | N/A | ADT, Surgical resection | Survived | 64 | Chest CT | Multiple | Bilateral | N/A |
| Delbare, et al. [14] | 2022 | Case report | 1 | 75 | Adeno | 7 | 6 | Asymptomatic | N/A | N/A | N/A | Chest CT, F18-PSMA-PET/CT | Multiple | Bilateral | L.N |
| Kaneko, et al. [32] | 2022 | Case report | 1 | 79 | SCC | N/A | 0.12 | Asymptomatic | Chemo | Died | N/A | Chest CT, SCC antigen | Multiple | Bilateral | Liver, L.N |
| Kosaka, et al. [15] | 2022 | Case report | 1 | 61 | Adeno | 7 | 0.76 | Asymptomatic | ADT, RT, Surgical resection | Survived | 84 | Chest CT, PET/CT | Multiple | Bilateral | None |
| Cui, et al. [18] | 2021 | Case report | 1 | 72 | Adeno | 8 | 12.64 | Frequent urination | ADT | Survived | 12 | Chest CT | Solitary | Right | None |
| Tarabaih, et al. [33] | 2021 | Case report | 1 | 70 | Adeno | 7 | 1.97 | Asymptomatic | Surgical resection | Survived | N/A | Chest CT | Solitary | Right base | None |
| Yoshitake, et al. [34] | 2021 | Case report | 1 | 83 | Adeno | 5 | N/A | Asymptomatic | Surgical resection | Survived | N/A | Chest CT | 2 | Bilateral | None |
| Carrilho Vaz, et al. [35] | 2020 | Case reports | 4 | 77, 76, 76, 75 | Adeno | N/A, N/A, 7, N/A | 1.69, 7.55, 6.36, 2.6 | N/A | ADT, ARPI, Chemo, Surgical resection | Survived | N/A, 4, 9, N/A | Ga-PSMA PET/CT | 1- Multiple, 2-Solitary, 3- Multiple, 4-Solitary | 1- Bilateral, 2- Left upper, 3- Bilateral, 4- Right | N/A |
| Izawa, et al. [36] | 2020 | Case report | 1 | 62 | Adeno | 9 | 4.25 | Asymptomatic | VATS, Chemo | Survived | 10 | Chest CT | Multiple | Bilateral | None |
| Tang, et al. [28] | 2020 | Case report | 1 | 48 | Adeno | 8 | 3.03 | Hematuria | ADT, Chemo | Survived | 19 | Chest CT, PET/CT | 2 | Right | None |
| Tsakiridis, et al. [29] | 2020 | Case report | 1 | 75 | Adeno | 7 | 5 | Shortness of breath | ADT, ARPI, SBRT | Survived | 48 | Chest CT, 18F-NaF-PET/CT, 18F-DCFPyL PET/CT | 2 | Left lower, Left hilar | None |
| Polistina, et al. [27] | 2020 | Case report | 1 | 74 | Adeno | 9 | N/A | Weight loss | N/A | N/A | N/A | Chest CT, 18F PET/CT, FDG PET/CT | Multiple | Bilateral | N/A |
| Wu, et al. [37] | 2020 | Case report | 1 | 74 | Adeno | 8 | 2 | Asymptomatic | ADT | Survived | 36 | Chest CT | Solitary | Right | N/A |
| Ciriaco, et al. [38] | 2019 | Case series | 9 | 61 | Adeno | 8 | 1.66 | Asymptomatic | RT, Surgical resection | Survived | 24.5 | Ga-PSMA PET/CT | 4 Solitary, 5 multiple | Left, Right | None |
| Mosca, et al. [25] | 2019 | Case report | 1 | 63 | Adeno | 8 | 1.32 | Cough | ADT, Surgical resection | Survived | 32 | Chest CT, PET/CT | 2 | Left | None |
| Katsui, et al. [39] | 2019 | Case report | 1 | 62 | Adeno | 8 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Seniaray, et al. [40] | 2019 | Case report | 1 | 63 | Adeno | 8 | 189.2 | Asymptomatic | N/A | N/A | N/A | Ga PSMA PET/CT | Multiple | Bilateral | L.N |
| Boschian, et al. [41] | 2018 | Case report | 1 | 69 | Adeno | 7 | 0.4 | Asymptomatic | Surgical resection | Survived | 36 | FDG-PET/CT | Solitary | Left | N/A |
| Damjanovic, et al. [12] | 2018 | Retrospective | 34 | 70.6 | Adeno | 9 | 123.6 | N/A | ADT, Chemo, RT | N/A | N/A | Ga-PSMA-PET/CT | Multiple | Bilateral | N/A |
| Polverari, et al. [42] | 2018 | Case report | 1 | 78 | Adeno | 7 | 0.3 | Asymptomatic | Surgical resection | N/A | N/A | 68Ga-PSMA-11 PET/CT, FDG PET/CT | Solitary | Upper right | None |
| Reinstatler, et al. [43] | 2018 | Case report | 1 | 60 | Adeno | 8 | 44 | Asymptomatic | ADT, Surgical resection, Chemo | N/A | N/A | Chest CT | Multiple | Left | L.N |
| Hokamp, et al. [44] | 2017 | Case report | 1 | 63 | Adeno | N/A | 1.60 | Asymptomatic | ADT, Surgical resection | Survived | N/A | 68Ga PSMA PET/CT | Solitary | Right | L.N |
| Mortier, et al. [45] | 2017 | Case report | 1 | 82 | Adeno | 6 | 3.32 | Asymptomatic | ADT, Surgical resection | Survived | 12 | Chest CT, PET/CT | Solitary | Right | N/A |
| Rush, et al. [46] | 2017 | Case report | 1 | 70 | Adeno | 8 | 2.9 | Asymptomatic | Surgical resection | Survived | 24 | Chest CT, PET-CT | Solitary | Left | N/A |
| Gago, et al. [21] | 2016 | Case reports | 3 | 63, 62, 79 | Adeno | 9, 7, 7 | 12.3, N/A, 2 | Cough | ADT, Surgical resection, Chemo | Died, Survived, Survived | 24, 60, 5 | Chest CT, PET/CT, Endobronchial US | Multiple | Right, Left, Bilateral | None |
| Geraldo, et al. [47] | 2016 | Case report | 1 | 60 | Sarcomatoid | 8 | 4.63 | Asymptomatic | Surgical resection | Survived | 12 | Ga-PSMA PET/CT, F-FDG PET/CT | Solitary | Left | L.N |
| Hung-Yi Su, et al. [48] | 2016 | Case report | 1 | 54 | Adeno | 7 | 11.08 | Asymptomatic | Surgical resection | Survived | N/A | F-FDG PET/CT | 3 | Right, mediastinum | L.N |
| Erdogan, et al. [19] | 2015 | Case report | 1 | 71 | Adeno | 7 | 3.83 | Chest pain | Surgical resection | N/A | N/A | FDG PET/CT | Solitary | Right | Bone |
| Kamiyama, et al. [49] | 2015 | Case report | 2 | 59, 69 | Adeno | 8, 9 | 0, 5.6 | Asymptomatic | ADT, Chemo | Died | 6 | Chest CT | Multiple | N/A | L.N, Bone, Brain |
| Maebayashi, et al. [24] | 2015 | Case report | 1 | 50 | Adeno | 9 | N/A | Bloody sputum | Chemo, RT, Surgical resection | Died | 30 | Chest CT, PET/CT | Solitary | Left | Systemic |
| Fukuoka, et al. [20] | 2014 | Case report | 1 | 87 | Adeno | 7 | 66.6 | Cough | ADT | Died | 18 | CXR, Chest-CT | Multiple | Bilateral | Liver |
| Treglia, et al. [50] | 2013 | Case report | 1 | 68 | Neuroendocrine | N/A | N/A | N/A | Surgical resection | N/A | N/A | PET/CT | N/A | N/A | N/A |
| Pepe, et al. [51] | 2012 | Case report | 1 | 75 | Adeno | 7 | 0 | Asymptomatic | Surgical resection | Survived | N/A | FDG PET/CT | N/A | N/A | N/A |
| Wallis, et al. [52] | 2011 | Case report | 1 | 53 | Adeno | 9 | N/A | Asymptomatic | Surgical resection | Survived | 12 | Chest CT, PET/CT | Multiple | Right | None |
| Sakai, et al. [53] | 2010 | Case report | 1 | 74 | Adeno | N/A | N/A | Surgical resection | N/A | N/A | N/A | N/A | N/A | N/A | |
| Goto, et al. [54] | 2010 | Case report | 1 | 73 | Sarcomatoid | 9 | N/A | Asymptomatic | ADT, Surgical resection | Survived | 10 | Chest CT, Bronchoscopy, PET scan | Solitary | Right | None |
| Khandani, et al. [22] | 2009 | Case report | 1 | 78 | Adeno | 8 | 8.5 | Chest pain and hemoptysis | Surgical resection | Survived | N/A | Chest CT, FDG PET/CT | Solitary | Left | Subcarinal L.N |
| Boyer, et al. [55] | 2009 | Case report | 1 | 65 | Adeno | 9 | 10.56 | Asymptomatic | ADT, Surgical resection | Survived | 11 | Chest CT | Solitary | Upper left | N/A |
| Pruthi, et al. [56] | 2007 | Case report | 1 | 72 | Adeno | 6 | 1.9 | Asymptomatic | ADT, Surgical resection | Survived | 36 | Chest CT, PET CT, Bone scan | Solitary | Right | None |
| Maeda, et al. [57] | 2006 | Case report | 1 | 71 | Adeno | N/A | 4 | Asymptomatic | Surgical resection | Survived | N/A | Carbon-11 Choline PET-/CT | Solitary | Left upper | None |
| Kirby, et al. [23] | 2005 | Case report | 1 | 59 | Adeno | 9 | 23 | Dyspnea and hemoptysis | ADT | Survived | 7 | Chest CT | Multiple | Right | None |
| Chao, et al. [17] | 2004 | Case report | 1 | 68 | Adeno | 9 | 0.4 | Cough and dyspnea | ADT, Surgical resection | Survived | 144 | CXR | Solitary | Left lower | None |
| Hofland, et al. [58] | 2000 | Case report | 1 | 49 | Adeno | 9 | 1 | Asymptomatic | Surgical resection | Lost to follow-up | 10 | CXR, Chest CT | Solitary | Left lower | L.N |
| Kume, et al. [59] | 1999 | Case report | 2 | 56 | N/A | N/A | N/A | N/A | Bilateral orchiectomy, ADT | Survived | 88, 32 | CXR, Chest CT | Multiple | Bilateral | None, Bone |
| Smith, et al. [60] | 1999 | Case report | 1 | 70 | Adeno | 9 | 2.1 | Asymptomatic | Surgical resection | Survived | 12 | CXR, Chest CT | Solitary | Right lower | None |
| Behrakis, et al. [61] | 1997 | Case report | 1 | 71 | N/A | N/A | N/A | N/A | ADT | Survived | 8 | CXR | Multiple | N/A | N/A |
| Allen, et al. [62] | 1996 | Case report | 1 | 59 | Adeno | N/A | N/A | N/A | Bilateral orchiectomy | Survived | 3 | CXR | Multiple | Bilateral | None |
| Harris, et al. [63] | 1996 | Case report | 1 | 76 | Adeno | N/A | 42.6 | Asymptomatic | ADT | Survived | 60 | CXR | Multiple | Bilateral | None |
| Leibman, et al. [64] | 1995 | Case report | 1 | 78 | Adeno | 7 | 0.4 | Asymptomatic | ADT, Chemo | Died | 20 | CXR, Chest CT | 3 | 2 Right, 1 Left | Brain |
| Fabozzi, et al. [65] | 1995 | Retrospective | 47 | N/A | Adeno | 7 | N/A | N/A | ADT, Orchiectomy, Chemotherapy | N/A | HSPC: 25, CRPC: 13 | CXR | N/A | N/A | N/A |
| Cusan, et al. [66] | 1994 | Case report | 1 | 60 | Adeno | N/A | N/A | Asymptomatic | Flutamide, thoracotomy | Survived | 25 | CXR, Chest CT | Multiple | Bilateral | None |
| Eastham, et al. [67] | 1993 | Case report | 1 | 69 | Adeno | 7 | N/A | Asymptomatic | Bilateral orchiectomy | Survived | N/A | CXR | Multiple | Bilateral | None |
| Rockey, et al. [68] | 1990 | Case report | 1 | 83 | Adeno | N/A | N/A | N/A | ADT | N/A | N/A | Chest CT | Solitary | Left | None |
| Bromberg, et al. [69] | 1989 | Case report | 1 | N/A | N/A | 6 | N/A | N/A | Bilateral orchiectomy, Wedge resection | Survived | 13 | N/A | N/A | N/A | N/A |
| Petras, et al. [26] | 1983 | Case report | 1 | 59 | N/A | N/A | N/A | Cough | Bilateral orchiectomy | Survived | N/A | CXR, Bone scan | Multiple | Bilateral | Bone |
| Panella, et al. [70] | 1980 | Case report | 1 | 76 | Adeno | N/A | N/A | N/A | RT, bilateral orchiectomy, chemo | N/A | N/A | CXR, transthoracic needle biopsy | Multiple | Bilateral | N/A |
| Varkarakis, et al. [71] | 1974 | Retrospective | 26 | 64 | N/A | N/A | N/A | N/A | ADT, RT | Died | 14.9 | CXR | N/A | N/A | Bone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.M.; Moustafa, A.; Day, C.; Ahmed, M.E.; Zeina, W.; Marzouk, U.M.; Basourakos, S.; Haloi, R.; Mahon, M.; Muniz, M.; et al. Prostate Cancer Lung Metastasis: Clinical Insights and Therapeutic Strategies. Cancers 2024, 16, 2080. https://doi.org/10.3390/cancers16112080
Mahmoud AM, Moustafa A, Day C, Ahmed ME, Zeina W, Marzouk UM, Basourakos S, Haloi R, Mahon M, Muniz M, et al. Prostate Cancer Lung Metastasis: Clinical Insights and Therapeutic Strategies. Cancers. 2024; 16(11):2080. https://doi.org/10.3390/cancers16112080
Chicago/Turabian StyleMahmoud, Ahmed M., Amr Moustafa, Carter Day, Mohamed E. Ahmed, Wael Zeina, Usama M. Marzouk, Spyridon Basourakos, Rimki Haloi, Mindie Mahon, Miguel Muniz, and et al. 2024. "Prostate Cancer Lung Metastasis: Clinical Insights and Therapeutic Strategies" Cancers 16, no. 11: 2080. https://doi.org/10.3390/cancers16112080
APA StyleMahmoud, A. M., Moustafa, A., Day, C., Ahmed, M. E., Zeina, W., Marzouk, U. M., Basourakos, S., Haloi, R., Mahon, M., Muniz, M., Childs, D. S., Orme, J. J., Riaz, I. B., Kendi, A. T., Stish, B. J., Davis, B. J., Kwon, E. D., & Andrews, J. R. (2024). Prostate Cancer Lung Metastasis: Clinical Insights and Therapeutic Strategies. Cancers, 16(11), 2080. https://doi.org/10.3390/cancers16112080






