The Safety of Novel Therapies in Chronic Lymphocytic Leukemia in the Era of Intermittent Fasting: A Pharmacology-Based Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methodology
3. Types of Intermittent Fasting
4. First-Line Novel Therapies
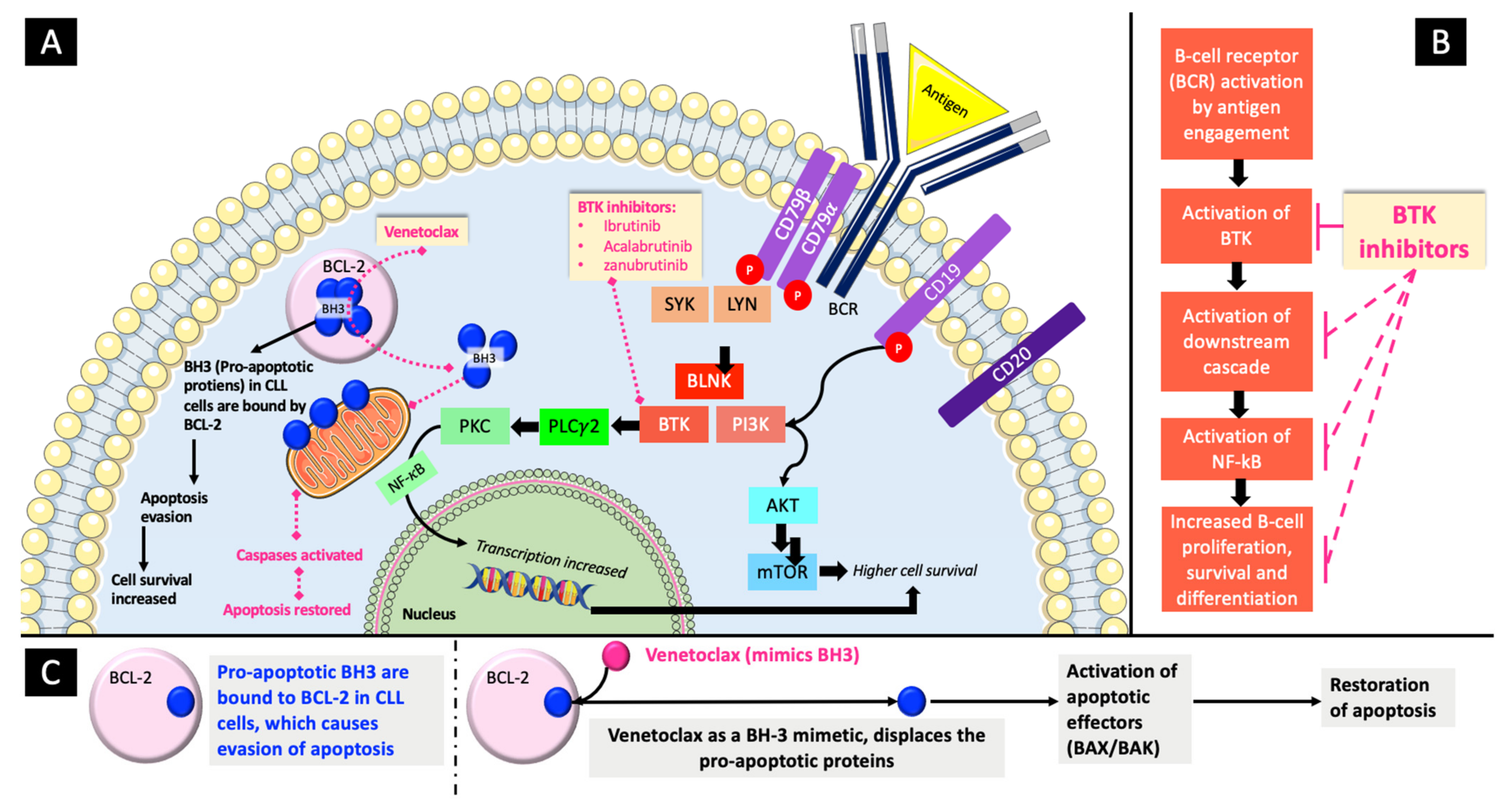
4.1. BTK Inhibitors
4.2. BCL-2 Inhibitors
5. TLS in CLL
5.1. TLS in CLL Patients Treated with Novel Agents
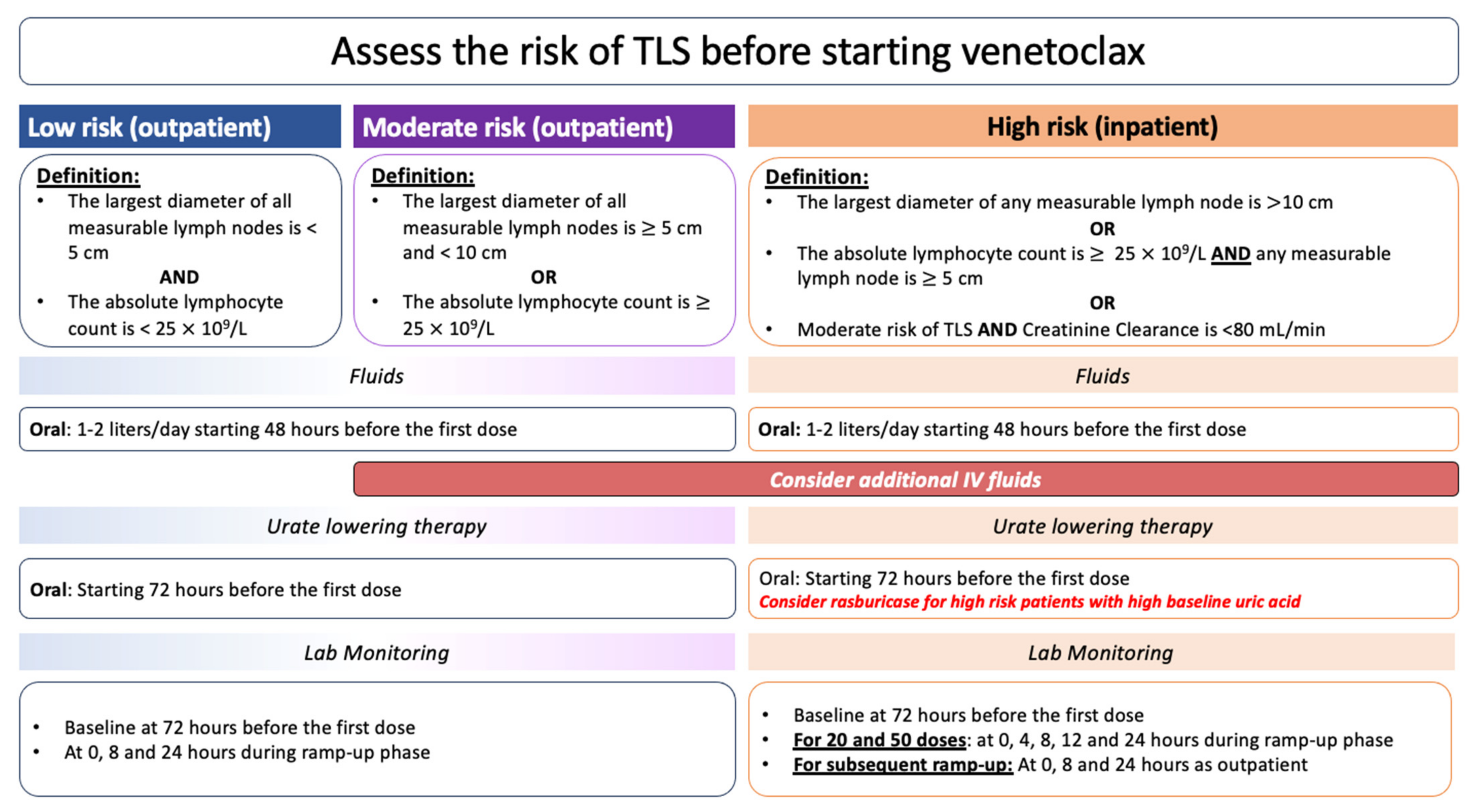
5.2. TLS Prevention in CLL Patients Treated with Novel Agents
6. GIB in CLL
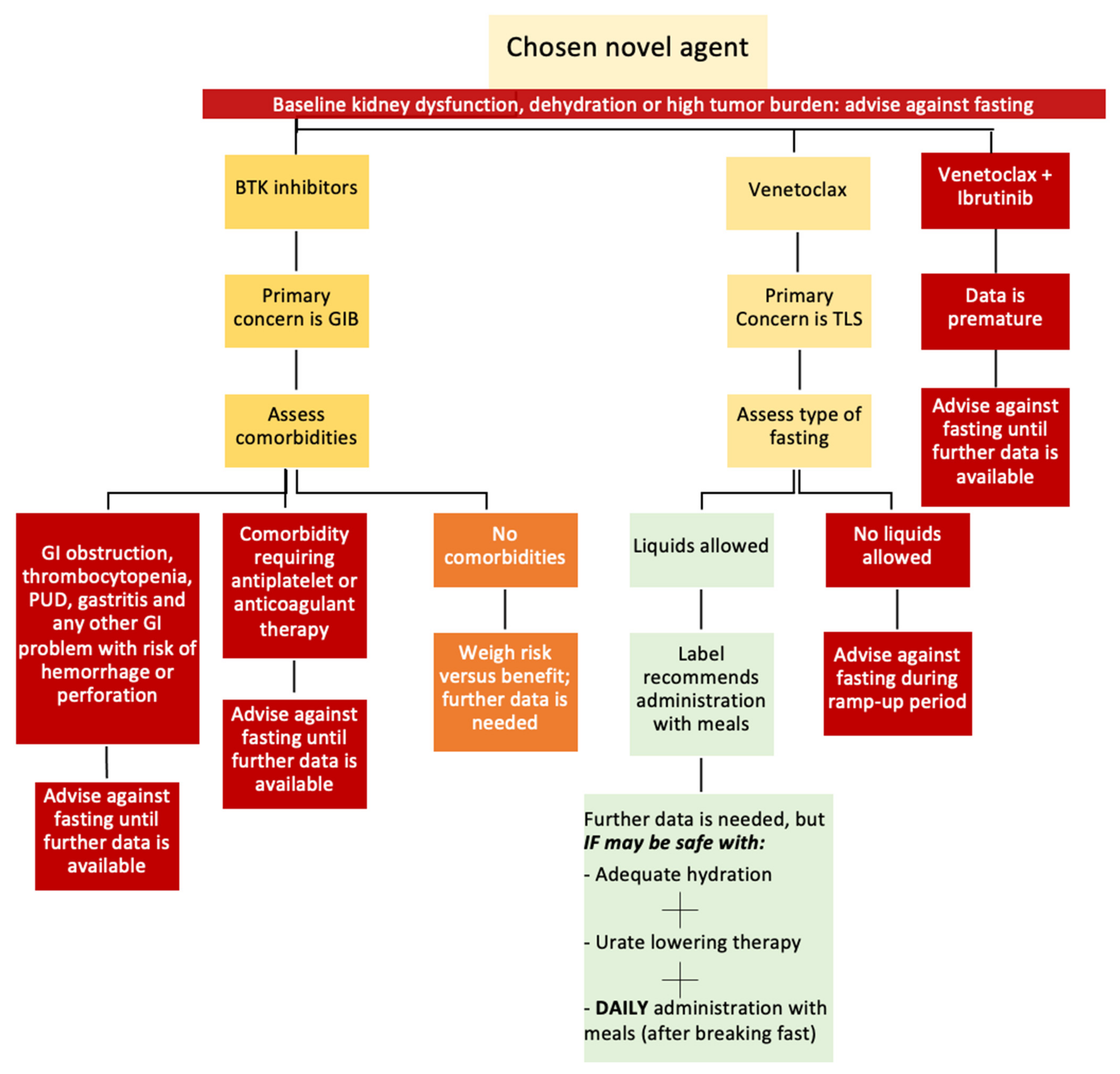
7. Challenges for Fasting in CLL Patients Treated with Novel Agents
7.1. The Need for Aggressive Hydration in Fluid-Restricting Fasting Practices
7.2. The Risks of GIB with BTK Inhibitors and Fasting
7.3. Possible Interactions with Food
7.4. Fasting Practices for a Day or More
8. Pathway for Decision of Fasting
- Venetoclax and TLS: Attaining adequate hydration is a necessity that may challenge fluid-restricted fasting during the venetoclax ramp-up phase.
- BTK inhibitors and GIB: Identifying the risk factors for GIB for each patient is vital. In addition, the increased gastric acidity during fasting periods (fluid-restricted and fluid-liberal IF) can increase the risk of GIB.
- The fasting patterns practiced by CLL patients should accommodate the daily administration of iburinib, zanubrutinib, and venetoclax (after food) and the twice-daily administration of acalabrutinib.
- Comorbidities that can increase the risks for GIB and TLS should be considered before permitting CLL patients to fast.
9. Discussion and Future Directions
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2017; Volume 2. [Google Scholar]
- ASCO Cancer. Net [Internet]. Leukemia—Chronic Lymphocytic—CLL: Statistics. 2023. Available online: https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics#:~:text=In%202023,%20an%20estimated%2018,740,is%20very%20rare%20in%20children (accessed on 15 May 2023).
- Patel, K.; Pagel, J.M. Current and future treatment strategies in chronic lymphocytic leukemia. J. Hematol. Oncol. 2021, 14, 69. [Google Scholar] [CrossRef]
- Weide, R.; Feiten, S.; Chakupurakal, G.; Friesenhahn, V.; Kleboth, K.; Köppler, H.; Lutschkin, J.; van Roye, C.; Thomalla, J.; Heymanns, J. Survival improvement of patients with chronic lymphocytic leukemia (CLL) in routine care 1995–2017. Leuk. Lymphoma 2019, 61, 557–566. [Google Scholar] [CrossRef]
- Lin, S.; Liu, H.; Tan, R.; Zhang, W.; He, C.; Rong, Y.; Huang, Z.; Yu, J.; Wang, Y.; Qi, Y.; et al. Abstract 2531: FCN-683, a novel second-generation BCL-2 inhibitor, is highly potent, selective and efficacious against clinically relevant venetoclax-resistant mutations. Am. Assoc. Cancer Res. 2023, 83, 2531. [Google Scholar] [CrossRef]
- Howard, S.C.; Trifilio, S.; Gregory, T.K.; Baxter, N.; McBride, A. Tumor lysis syndrome in the era of novel and targeted agents in patients with hematologic malignancies: A systematic review. Ann. Hematol. 2016, 95, 563–573. [Google Scholar] [CrossRef]
- Burger, J.A.; Chiorazzi, N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013, 34, 592–601. [Google Scholar] [CrossRef]
- Von Hundelshausen, P.; Siess, W. Bleeding by Bruton Tyrosine Kinase-Inhibitors: Dependency on Drug Type and Disease. Cancers 2021, 13, 1103. [Google Scholar] [CrossRef]
- Shatzel, J.J.; Olson, S.R.; Tao, D.L.; McCarty, O.J.T.; Danilov, A.V.; DeLoughery, T.G. Ibrutinib-associated bleeding: Pathogenesis, management and risk reduction strategies. J. Thromb. Haemost. 2018, 15, 835–847. [Google Scholar] [CrossRef]
- St-Pierre, F.; Ma, S. Use of BTK Inhibitors in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL): A Practical Guidance. Blood Lymphat. Cancer Targets Ther. 2022, 12, 81–98. [Google Scholar] [CrossRef]
- Salter, B.; Burns, I.; Fuller, K.; Eshaghpour, A.; Lionel, A.C.; Crowther, M. Tyrosine kinase inhibitors and tumor lysis syndrome in hematologic malignancies: A systemic review. Eur. J. Haematol. 2022, 109, 166–181. [Google Scholar] [CrossRef]
- Antic, D.; Milic, N.; Chatzikonstantinou, T.; Scarfò, L.; Otasevic, V.; Rajovic, N.; Allsup, D.; Cabrero, A.A.; Andres, M.; Gonzales, M.B.; et al. Thrombotic and bleeding complications in patients with chronic lymphocytic leukemia and severe COVID-19: A study of ERIC, the European Research Initiative on CLL. J. Hematol. Oncol. 2022, 15, 116. [Google Scholar] [CrossRef]
- Sharman, J.P.; Biondo, J.M.L.; Boyer, M.; Fischer, K.; Hallek, M.; Jiang, D.; Kater, A.P.; Lurà, M.P.; Wierda, W.G. A review of the incidence of tumor lysis syndrome in patients with chronic lymphocytic leukemia treated with venetoclax and debulking strategies. eJHaem 2022, 3, 492–506. [Google Scholar] [CrossRef]
- Yassin, M.A.; Ghasoub, R.S.; Aldapt, M.B.; Abdulla, M.A.; Chandra, P.; Shwaylia, H.M.; Nashwan, A.J.; Kassem, N.A.; Akiki, S.J. Effects of Intermittent Fasting on Response to Tyrosine Kinase Inhibitors (TKIs) in Patients with Chronic Myeloid Leukemia: An Outcome of European LeukemiaNet Project. Cancer Control 2021, 28, 10732748211009256. [Google Scholar] [CrossRef]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Cardiometabolic Benefits of Intermittent Fasting. Annu. Rev. Nutr. 2021, 41, 333–361. [Google Scholar] [CrossRef]
- Gabel, K.; Cares, K.; Varady, K.; Gadi, V.; Tussing-Humphreys, L. Current Evidence and Directions for Intermittent Fasting During Cancer Chemotherapy. Adv. Nutr. Int. Rev. J. 2022, 13, 667–680. [Google Scholar] [CrossRef]
- Clifton, K.K.; Ma, C.X.; Fontana, L.; Peterson, L.L.; Fontana, L.; Peterson, M.L.L. Intermittent fasting in the prevention and treatment of cancer. CA Cancer J. Clin. 2021, 71, 527–546. [Google Scholar] [CrossRef]
- Tiwari, S.; Sapkota, N.; Han, Z. Effect of fasting on cancer: A narrative review of scientific evidence. Cancer Sci. 2022, 113, 3291–3302. [Google Scholar] [CrossRef]
- Nista, E.C.; Del Gaudio, A.; Del Vecchio, L.E.; Mezza, T.; Pignataro, G.; Piccioni, A.; Gasbarrini, A.; Franceschi, F.; Candelli, M. Pancreatic Cancer Resistance to Treatment: The Role of Microbiota. Biomedicines 2023, 11, 157. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.; Gao, Z. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm. Sin. B 2021, 11, 1789–1812. [Google Scholar] [CrossRef]
- Faitová, T.; Svanberg, R.; Da Cunha-Bang, C.; Ilett, E.E.; Jørgensen, M.; Noguera-Julian, M.; Paredes, R.; MacPherson, C.R.; Niemann, C.U. The gut microbiome in patients with chronic lymphocytic leukemia. Haematologica 2022, 107, 2238–2243. [Google Scholar] [CrossRef]
- Leiper, J.B.; Molla, A.M.; Molla, A.M. Effects on health of fluid restriction during fasting in Ramadan. Eur. J. Clin. Nutr. 2003, 57, S30–S38. [Google Scholar] [CrossRef]
- Gupta, A.; Moore, J.A. Tumor Lysis Syndrome. JAMA Oncol. 2018, 4, 895. [Google Scholar] [CrossRef]
- Iraki, L.; Abkari, A.; Vallot, T.; Amrani, N.; Khlifa, R.H.; Jellouli, K.; Hakkou, F. Effect of Ramadan fasting on intragastric pH recorded during 24 hours in healthy subjects. Gastroenterol. Clin. Biol. 1997, 21, 813–819. [Google Scholar]
- Özkan, S.; Durukan, P.; Akdur, O.; Vardar, A.; Torun, E.; Ikizceli, I. Does Ramadan Fasting Increase Acute Upper Gastrointestinal Haemorrhage? J. Int. Med. Res. 2009, 37, 1988–1993. [Google Scholar] [CrossRef]
- Templeman, I.; Smith, H.A.; Chowdhury, E.; Chen, Y.-C.; Carroll, H.; Johnson-Bonson, D.; Hengist, A.; Smith, R.; Creighton, J.; Clayton, D.; et al. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci. Transl. Med. 2021, 13, eabd8034. [Google Scholar] [CrossRef]
- Mandal, S.; Simmons, N.; Awan, S.; Chamari, K.; Ahmed, I. Intermittent fasting: Eating by the clock for health and exercise performance. BMJ Open Sport Exerc. Med. 2022, 8, e001206. [Google Scholar] [CrossRef]
- Burger, J.A. Bruton Tyrosine Kinase Inhibitors. Cancer J. 2019, 25, 386–393. [Google Scholar] [CrossRef]
- Singh, S.P.; Dammeijer, F.; Hendriks, R.W. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer 2018, 17, 57. [Google Scholar] [CrossRef]
- Ahn, I.E.; Brown, J.R. Targeting Bruton’s Tyrosine Kinase in CLL. Front. Immunol. 2021, 12, 687458. [Google Scholar] [CrossRef]
- Mihalyova, J.; Jelinek, T.; Growkova, K.; Hrdinka, M.; Simicek, M.; Hajek, R. Venetoclax: A new wave in hematooncology. Exp. Hematol. 2018, 61, 10–25. [Google Scholar] [CrossRef]
- Annemans, L.; Moeremans, K.; Lamotte, M.; Conde, J.G.; Berg, H.v.D.; Myint, H.; Pieters, R.; Uyttebroeck, A. Incidence, medical resource utilisation and costs of hyperuricemia and Tumour lysis syndrome in patients with acute Leukaemia and non-hodgkin’s lymphoma in four European countries. Leuk. Lymphoma 2003, 44, 77–83. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 15 May 2023).
- Pession, A.; Masetti, R.; Gaidano, G.; Tosi, P.; Rosti, G.; Aglietta, M.; Specchia, G.; Porta, F.; Pane, F. Risk evaluation, prophylaxis, and treatment of tumor lysis syndrome: Consensus of an Italian expert panel. Adv. Ther. 2011, 28, 684–697. [Google Scholar] [CrossRef]
- Cairo, M.S.; Coiffier, B.; Reiter, A.; Younes, A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: An expert TLS panel consensus. Br. J. Haematol. 2010, 149, 578–586. [Google Scholar] [CrossRef]
- Cheson, B.D.; Enschede, S.H.; Cerri, E.; Desai, M.; Potluri, J.; Lamanna, N.; Tam, C. Tumor Lysis Syndrome in Chronic Lymphocytic Leukemia with Novel Targeted Agents. Oncologist 2017, 22, 1283–1291. [Google Scholar] [CrossRef]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 374, 311–322. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Hallek, M. Preventing and monitoring for tumor lysis syndrome and other toxicities of venetoclax during treatment of chronic lymphocytic leukemia. Hematol. Educ. Program Am. Soc. Hematol. 2020, 2020, 357–362. [Google Scholar] [CrossRef]
- Sarno, J. Prevention and Management of Tumor Lysis Syndrome in Adults with Malignancy. J. Adv. Pract. Oncol. 2013, 4, 101–106. [Google Scholar]
- Jones, G.L.; Will, A.; Jackson, G.H.; Webb, N.J.A.; Rule, S.; British Committee for Standards in Haematology. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br. J. Haematol. 2013, 169, 661–671. [Google Scholar] [CrossRef]
- Roeker, L.E.; Fox, C.P.; Eyre, T.A.; Brander, D.M.; Allan, J.N.; Schuster, S.J.; Nabhan, C.; Hill, B.T.; Shah, N.N.; Lansigan, F.; et al. Tumor Lysis, Adverse Events, and Dose Adjustments in 297 Venetoclax-Treated CLL Patients in Routine Clinical Practice. Clin. Cancer Res. 2019, 25, 4264–4270. [Google Scholar] [CrossRef]
- Ozturk, E.; Ozunal, I.E. A Rare Side Effect of Ibrutinib: Tumor Lysis Syndrome. Medeni. Med. J. 2021, 36, 176. [Google Scholar]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kaźmierczak, M.; Lamanna, N.; O’brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2022, 388, 319–332. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illés, A.; Kay, N.; et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef]
- Tam, C.S.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Jacobs, R.W.; Opat, S.; Barr, P.M.; Tedeschi, A.; Trentin, L.; Bannerji, R.; et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: Primary analysis of the CAPTIVATE FD cohort. Blood 2022, 139, 3278–3289. [Google Scholar] [CrossRef]
- Food and Drug Administration. The FDA Adverse Events Reporting System (FAERS) Public Dashboard. Available online: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070093.htm (accessed on 1 July 2023).
- Coiffier, B.; Altman, A.; Pui, C.-H.; Younes, A.; Cairo, M.S. Guidelines for the management of pediatric and adult tumor lysis syndrome: An evidence-based review. J. Clin. Oncol. 2008, 26, 2767–2778. [Google Scholar] [CrossRef]
- Abu-Alfa, A.K.; Younes, A. Tumor lysis syndrome and acute kidney injury: Evaluation, prevention, and management. Am. J. Kidney Dis. 2010, 55, S1–S13. [Google Scholar] [CrossRef] [PubMed]
- Georgantopoulos, P.; Yang, H.; Norris, L.B.; Bennett, C.L. Major hemorrhage in chronic lymphocytic leukemia patients in the US Veterans Health Administration system in the pre-ibrutinib era: Incidence and risk factors. Cancer Med. 2019, 8, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Alattar, M.L.; Ciccone, M.; Gaballa, M.R.; Vitale, C.; Badoux, X.C.; Manoukian, G.; Keating, M.J.; Kroll, M.H.; Ferrajoli, A. Bleeding diathesis associated with acquired von Willebrand Syndrome in three patients with chronic lymphocytic leukemia. Leuk. Lymphoma 2015, 56, 3452–3454. [Google Scholar] [CrossRef]
- Fattizzo, B.; Barcellini, W. Autoimmune Cytopenias in Chronic Lymphocytic Leukemia: Focus on Molecular Aspects. Front. Oncol. 2020, 9, 1435. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.J.; Ferrajoli, A. Unusual complications in the management of chronic lymphocytic leukemia. Am. J. Hematol. 2022, 97, S26–S34. [Google Scholar] [CrossRef]
- Tagliaferri, A.R.; Melki, G.; Baddoura, W. Chronic Lymphocytic Leukemia Causing Gastric Ulcer Perforation: A Case Presentation and Literature Review. Cureus 2023, 15, e36026. [Google Scholar] [CrossRef]
- Tsang, M.; Parikh, S.A. A Concise Review of Autoimmune Cytopenias in Chronic Lymphocytic Leukemia. Curr. Hematol. Malign. Rep. 2018, 12, 29–38. [Google Scholar] [CrossRef]
- Kaptein, A.; de Bruin, G.; Hoek, M.E.-V.; van de Kar, B.; de Jong, A.; Gulrajani, M.; Demont, D.; Covey, T.; Mittag, D.; Barf, T. Potency and Selectivity of BTK Inhibitors in Clinical Development for B-Cell Malignancies. Blood 2018, 132, 1871. [Google Scholar] [CrossRef]
- O’brien, S.M.; Brown, J.R.; Byrd, J.C.; Furman, R.R.; Ghia, P.; Sharman, J.P.; Wierda, W.G. Monitoring and Managing BTK Inhibitor Treatment-Related Adverse Events in Clinical Practice. Front. Oncol. 2021, 11, 720704. [Google Scholar] [CrossRef]
- Jayasekara, K.; Kulasooriya, P.; Wijayasiri, K.; Rajapakse, E.; Dulshika, D.; Bandara, P.; Fried, L.; De Silva, A.; Albert, S. Relevance of heat stress and dehydration to chronic kidney disease (CKDu) in Sri Lanka. Prev. Med. Rep. 2019, 15, 100928. [Google Scholar] [CrossRef] [PubMed]
- Persynaki, A.; Karras, S.; Pichard, C. Unraveling the metabolic health benefits of fasting related to religious beliefs: A narrative review. Nutrition 2017, 35, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Morales-Suarez-Varela, M.; Sánchez, E.C.; Peraita-Costa, I.; Llopis-Morales, A.; Soriano, J.M. Intermittent Fasting and the Possible Benefits in Obesity, Diabetes, and Multiple Sclerosis: A Systematic Review of Randomized Clinical Trials. Nutrients 2021, 13, 3179. [Google Scholar] [CrossRef]
- Food and Drug Administration. VENCLEXTA (venetoclax) Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208573s000lbl.pdf (accessed on 1 June 2023).
- Tariq, S.; Tariq, S.; Khan, M.; Azhar, A.; Baig, M. Venetoclax in the Treatment of Chronic Lymphocytic Leukemia: Evidence, Expectations, and Future Prospects. Cureus 2020, 12, e8908. [Google Scholar] [CrossRef]
- Food and Drug Administration. BRUKINSA (zanubrutinib) Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213217s005lbl.pdf (accessed on 1 June 2023).
- Tam, C.S.; Ou, Y.C.; Trotman, J.; Opat, S. Clinical pharmacology and PK/PD translation of the second-generation Bruton’s tyrosine kinase inhibitor, zanubrutinib. Expert Rev. Clin. Pharmacol. 2021, 14, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. IMBRUVICA (ibrutinib) Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/205552s030,210563s006lbl.pdf (accessed on 1 June 2023).
- De Jong, J.; Sukbuntherng, J.; Skee, D.; Murphy, J.; O’brien, S.; Byrd, J.C.; James, D.; Hellemans, P.; Loury, D.J.; Jiao, J.; et al. The effect of food on the pharmacokinetics of oral ibrutinib in healthy participants and patients with chronic lymphocytic leukemia. Cancer Chemother. Pharmacol. 2015, 75, 907–916. [Google Scholar] [CrossRef]
- Food and Drug Administration. CALQUENCE (acalabrutinib) Capsules Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/210259s009lbl.pdf (accessed on 1 June 2023).
- Food and Drug Administration. CALQUENCE (acalabrutinib) Tablets Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/216387Orig2s000Correctedlbl.pdf (accessed on 1 June 2023).
- De Groot, S.; Vreeswijk, M.P.G.; Welters, M.J.P.; Gravesteijn, G.; Boei, J.J.W.A.; Jochems, A.; Houtsma, D.; Putter, H.; Van Der Hoeven, J.J.M.; Nortier, J.W.R.; et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: A randomized pilot study. BMC Cancer 2015, 15, 652. [Google Scholar] [CrossRef]
- Safdie, F.M.; Dorff, T.; Quinn, D.; Fontana, L.; Wei, M.; Lee, C.; Cohen, P.; Longo, V.D. Fasting and cancer treatment in humans: A case series report. Aging 2009, 1, 988–1007. [Google Scholar] [CrossRef]
- Dorff, T.B.; Groshen, S.; Garcia, A.; Shah, M.; Tsao-Wei, D.; Pham, H.; Cheng, C.-W.; Brandhorst, S.; Cohen, P.; Wei, M.; et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer 2016, 16, 360. [Google Scholar] [CrossRef] [PubMed]
- Badar, T.; Ismail, A.; AlShanqeeti, A. Safety and Feasibility of Muslim Fasting While Receiving Chemotherapy. IOSR J. Pharm. 2014, 4, 15–20. [Google Scholar]
- Ferro, Y.; Maurotti, S.; Tarsitano, M.G.; Lodari, O.; Pujia, R.; Mazza, E.; Lascala, L.; Russo, R.; Pujia, A.; Montalcini, T. Therapeutic Fasting in Reducing Chemotherapy Side Effects in Cancer Patients: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2666. [Google Scholar] [CrossRef] [PubMed]
- Belay, Y.; Yirdaw, K.; Enawgaw, B. Tumor Lysis Syndrome in Patients with Hematological Malignancies. J. Oncol. 2017, 2017, 9684909. [Google Scholar] [CrossRef]
- Hu, X.; Xia, K.; Dai, M.; Han, X.; Yuan, P.; Liu, J.; Liu, S.; Jia, F.; Chen, J.; Jiang, F.; et al. Intermittent fasting modulates the intestinal microbiota and improves obesity and host energy metabolism. npj Biofilms Microbiomes 2023, 9, 19. [Google Scholar] [CrossRef]
| Fluid-Liberal IF *: Fasting Practices That Allow Liquid Intake | Fluid-Restricted IF *: Fasting Practices That Prohibit Liquid Intake |
|---|---|
|
|
|
|
| |
|
|
|
| Week/Dose | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 → Onward |
|---|---|---|---|---|---|
| 400 mg/day | |||||
| 200 mg/day | |||||
| 100 mg/day | |||||
| 50 mg/day | |||||
| 20 mg/day |
| Trial | ELEVATE-RR | ALPINE | CAPTIVATE | ||
|---|---|---|---|---|---|
| Comparison | Ibrutinib (n = 263) | Acalabrutinib (n = 266) | Ibrutinib (n = 324) | Zanubrutinib (n = 324) | Ibrutinib + Venetoclax (n = 159) |
| Any grade, n (%) | 1 (0.4) | 1 (0.4) | 0 | 1 (0.3) | 0 * |
| Grade ≥ 3–n (%) | 1 (0.4) | 1 (0.4) | 0 | 1 (0.3) | 0 * |
| Novel Agent | Ibrutinib | Acalabrutinib | Zanubrutinib | Venetoclax |
|---|---|---|---|---|
| Total number of all adverse events reported by June 2023 | 63,316 | 3694 | 760 | 35,886 |
| Cases of TLS reported as monotherapies in CLL | ||||
| Total number of cases | 73 | 36 | 3 | 195 |
| Total number of serious cases | 71 | 36 | 3 | 192 |
| Deaths | 20 | 2 | 0 | 68 |
| Trial | ELEVATE-RR | ALPINE | CAPTIVATE | ||
|---|---|---|---|---|---|
| Comparison | Ibrutinib (n = 263) | Acalabrutinib (n = 266) | Ibrutinib (n = 324) | Zanubrutinib (n = 324) | Ibrutinib + Venetoclax (n = 159) |
| Hemorrhage | |||||
| Any grade, n (%) | 135 (51.3) | 101 (38) | 134 (41.4) | 137 (42.3) | Not reported |
| Grade ≥ 3–n (%) | 12 (4.6) | 10 (3.8) | 12 (3.7) | 11 (3.4) | |
| Major hemorrhage | |||||
| Any grade, n (%) | 14 (5.3) | 12 (4.5) | 14 (4.3) | 12 (3.7) | 3 (2) |
| Grade ≥ 3–n (%) | 12 (4.6) | 10 (3.8) | 12 (3.7) | 11 (3.4) | 2 (1) |
| Novel Agent | Ibrutinib | Acalabrutinib | Zanubrutinib | Venetoclax |
|---|---|---|---|---|
| Total number of all adverse events reported by June 2023 | 63,316 | 3694 | 760 | 35,886 |
| Cases of GIB reported as monotherapies in CLL | ||||
| Total number of cases, n (concomitant antiplatelet or anticoagulant) | 191 (207) | 5 (NA) | 1 (NA) | 19 (NA) |
| Total number of serious cases, n (concomitant antiplatelet or anticoagulant) | 189 (205) | 5 (NA) | 1 (NA) | 19 (NA) |
| Deaths, n (concomitant antiplatelet or anticoagulant) | 26 (29) | 0 | 0 | 10 (NA) |
| BTK Inhibitor | Ibrutinib | Acalabrutinib | Zanubrutinib | |
|---|---|---|---|---|
| PK Data |
| Capsules: For high-fat and high-caloric meals:
| Tablets: For high-fat and high-caloric meals:
| No significant clinical effect |
| Drug Label | Can be given without regard to food * | Capsules and tablets: Can be given without regard to food | Can be given without regard to food | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benkhadra, M.; Fituri, N.; Aboukhalaf, S.; Ghasoub, R.; Mattar, M.; Alfarsi, K.; Alshemmari, S.; Yassin, M.A. The Safety of Novel Therapies in Chronic Lymphocytic Leukemia in the Era of Intermittent Fasting: A Pharmacology-Based Review. Cancers 2024, 16, 2079. https://doi.org/10.3390/cancers16112079
Benkhadra M, Fituri N, Aboukhalaf S, Ghasoub R, Mattar M, Alfarsi K, Alshemmari S, Yassin MA. The Safety of Novel Therapies in Chronic Lymphocytic Leukemia in the Era of Intermittent Fasting: A Pharmacology-Based Review. Cancers. 2024; 16(11):2079. https://doi.org/10.3390/cancers16112079
Chicago/Turabian StyleBenkhadra, Maria, Nuha Fituri, Soha Aboukhalaf, Rola Ghasoub, Mervat Mattar, Khalil Alfarsi, Salem Alshemmari, and Mohamed A. Yassin. 2024. "The Safety of Novel Therapies in Chronic Lymphocytic Leukemia in the Era of Intermittent Fasting: A Pharmacology-Based Review" Cancers 16, no. 11: 2079. https://doi.org/10.3390/cancers16112079
APA StyleBenkhadra, M., Fituri, N., Aboukhalaf, S., Ghasoub, R., Mattar, M., Alfarsi, K., Alshemmari, S., & Yassin, M. A. (2024). The Safety of Novel Therapies in Chronic Lymphocytic Leukemia in the Era of Intermittent Fasting: A Pharmacology-Based Review. Cancers, 16(11), 2079. https://doi.org/10.3390/cancers16112079








