Nanomaterial-Driven Precision Immunomodulation: A New Paradigm in Therapeutic Interventions
Abstract
Simple Summary
Abstract
1. Introduction
2. Immunomodulatory Strategies in Drug Delivery
2.1. Targeting Immune Cells
| Nanocarrier Type | Targeted Immune Cells | Surface Ligands/Antibodies/Peptides | Functional Outcome | Examples of Nanomaterial-Based Systems | References |
|---|---|---|---|---|---|
| Liposomes | Dendritic Cells | Mannose Receptors | Enhanced Antigen Presentation | Liposomes loaded with tumor antigens and CD40 ligands | [67,68] |
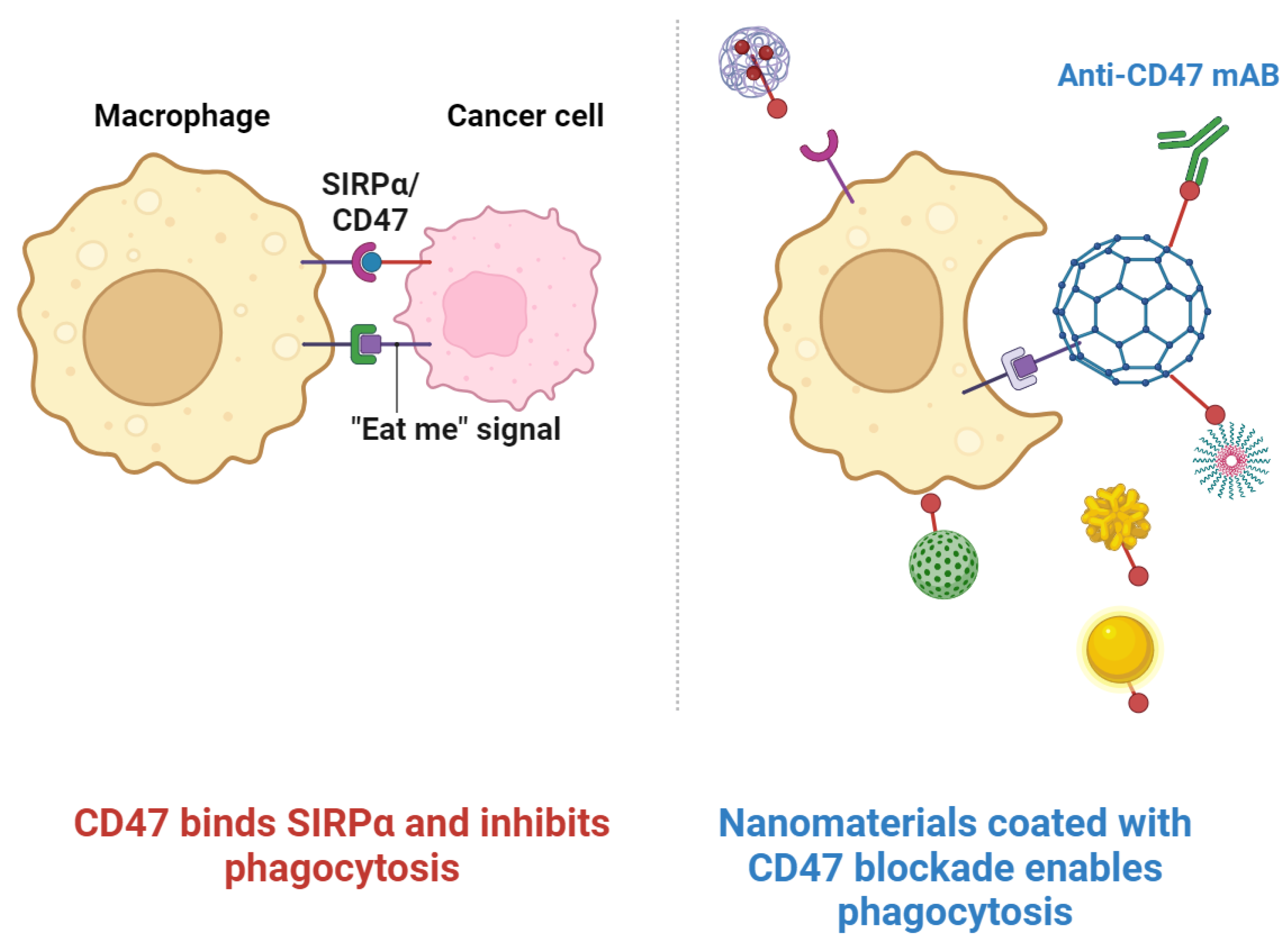
| Polymeric NPs | Macrophages | CD47-SIRPα Interactions | Inhibition of Phagocytosis | Polymer-based NPs with CD47 for macrophage evasion | [69,70] |
| Macrophages | LPS Mimics | Enhanced Immune Activation | Polymer-based NPs with LPS mimics for macrophages | [71,72,73] | |
| Monocytes | Anti-inflammatory Cytokines | Repolarization of Monocytes | Polymer-based NPs delivering IL-10 | [74,75] | |
| Lipid NPs | T-Cells | T Cell Receptor Ligands | T Cell Activation | Lipid NPs coated with TCR ligands | [76,77] |
| Regulatory T-Cells | TGF-β Receptor Blockade | Suppression of Treg Functionality | Lipid-based NPs with TGF-β receptor inhibitors | [78,79] | |
| Neutrophils | CXCR2 Ligands | Neutrophil Chemotaxis Inhibition | Lipid NPs with CXCR2 ligands | [80,81] | |
| Gold NPs | Natural Killer Cells | Natural Cytotoxicity Receptors | Increased Cytotoxic Activity | Gold NPs conjugated with NK cell ligands | [82,83] |
| Inorganic NPs | B Cells | CD20 Antibodies | Targeted B Cell Depletion | Silica NPs functionalized with CD20 antibodies | [84,85] |
| Tumor- Infiltrating Lymphocytes | PD-1 Antibodies | Reinvigoration of TILs | Mesoporous silica NPs with PD-1 antibodies | [86,87] | |
| Metal/Polymeric Hybrid NPs | Dendritic Cells | Toll-like Receptor (TLR) Ligands | Activation of Dendritic Cells | Hybrid NPs with Toll-like receptor ligands | [88,89] |
| Carbon-Based Nanomaterials | Various | Various | Various Applications in Medicine | Single- and multi-walled carbon nanotubes, graphene oxide, fullerenes, and nanodiamonds for drug delivery and imaging | [90] |
| Graphene-Based Nanomaterials | Cancer Cells | Hyaluronic Acid | pH-Responsive Drug Delivery | Hyaluronic-acid-decorated graphene oxide nanohybrids for drug delivery | [91] |
| Superparamagnetic Iron Oxide NPs | Scavenger Receptor | Surface Polymer Coating | Immune Recognition Modulation | Surface-modified iron oxide NPs interacting with scavenger receptors | [92,93] |
2.2. Modulating Immune Signaling Pathways
2.3. Nanomaterials and the Intricate Network of Immune Signaling Pathways
2.4. Mechanisms of Action and Advantages of Lipid-Based Nanomaterials
2.5. Biodegradable and Biocompatible Polymers
2.6. Inorganic NPs for Immunomodulatory Drug Delivery
3. Immunomodulatory Drug Delivery Systems in Cancer Treatment
4. Nanomaterial-Based Approaches for Treating Autoimmune Diseases and Chronic Inflammation
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; El-Tanani, M.; Hromić-Jahjefendić, A.; Kapoor, D.N.; Goyal, R.; et al. Nanomaterials and Their Impact on the Immune System. Int. J. Mol. Sci. 2023, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, J.; Huang, L. Modulation of tumor microenvironment for immunotherapy: Focus on nanomaterial-based strategies. Theranostics 2020, 10, 3099–3117. [Google Scholar] [CrossRef] [PubMed]
- Dupoiron, D.; Duarte, R.; Carvajal, G.; Aubrun, F.; Eldabe, S. Rationale and Recent Advances in Targeted Drug Delivery for Cancer Pain: Is It Time to Change the Paradigm? Pain Physician 2022, 25, E414–E425. [Google Scholar] [PubMed]
- Shen, Q.; Du, Y. A comprehensive review of advanced drug delivery systems for the treatment of rheumatoid arthritis. Int. J. Pharm. 2023, 635, 122698. [Google Scholar] [CrossRef] [PubMed]
- Quarterman, J.C.; Geary, S.M.; Salem, A.K. Evolution of drug-eluting biomedical implants for sustained drug delivery. Eur. J. Pharm. Biopharm. 2021, 159, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Fayzullin, A.; Bakulina, A.; Mikaelyan, K.; Shekhter, A.; Guller, A. Implantable Drug Delivery Systems and Foreign Body Reaction: Traversing the Current Clinical Landscape. Bioengineering 2021, 8, 205. [Google Scholar] [CrossRef]
- Dash, A.K.; Cudworth, G.C., 2nd. Therapeutic applications of implantable drug delivery systems. J. Pharm. Toxicol. Methods 1998, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, H.; Jangde, R.K. Current updated review on preparation of polymeric nanoparticles for drug delivery and biomedical applications. Next Nanotechnol. 2023, 2, 100013. [Google Scholar] [CrossRef]
- Mulhern, O.; Harrington, B.; Bowie, A.G. Modulation of innate immune signalling pathways by viral proteins. Adv. Exp. Med. Biol. 2009, 666, 49–63. [Google Scholar] [CrossRef]
- Han, S.; Quach, T.; Hu, L.; Wahab, A.; Charman, W.N.; Stella, V.J.; Trevaskis, N.L.; Simpson, J.S.; Porter, C.J.H. Targeted delivery of a model immunomodulator to the lymphatic system: Comparison of alkyl ester versus triglyceride mimetic lipid prodrug strategies. J. Control. Release 2014, 177, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Huang, S.; Gartung, A.; Cortés-Puch, I.; Sime, P.J.; Phipps, R.P.; Serhan, C.N.; Hammock, B.D. Inflammation resolution: A dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020, 39, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Cazzamalli, S.; Corso, A.D.; Neri, D. Targeted Delivery of Cytotoxic Drugs: Challenges, Opportunities and New Developments. Chimia 2017, 71, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Sathish, J.G.; Sethu, S.; Bielsky, M.-C.; de Haan, L.; French, N.S.; Govindappa, K.; Green, J.; Griffiths, C.E.M.; Holgate, S.; Jones, D.; et al. Challenges and approaches for the development of safer immunomodulatory biologics. Nat. Rev. Drug Discov. 2013, 12, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.K.; Li, C.; Rosenberg, A.S.; Kishnani, P.S. Immunological challenges and approaches to immunomodulation in Pompe disease: A literature review. Ann. Transl. Med. 2019, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.G.; Greenberg, N. Harmonization and Standardization: Where Are We Now? J. Appl. Lab. Med. 2021, 6, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Sushnitha, M.; Evangelopoulos, M.; Tasciotti, E.; Taraballi, F. Cell membrane-based biomimetic nanoparticles and the immune system: Immunomodulatory interactions to therapeutic applications. Front. Bioeng. Biotechnol. 2020, 8, 627. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Blum, N.T.; Lin, J.; Qu, J.; Huang, P. Biomaterial scaffold-based local drug delivery systems for cancer immunotherapy. Sci. Bull. 2020, 65, 1489–1504. [Google Scholar] [CrossRef]
- Jindal, A.; Sarkar, S.; Alam, A. Nanomaterials-Mediated Immunomodulation for Cancer Therapeutics. Front. Chem. 2021, 9, 629635. [Google Scholar] [CrossRef]
- Alagarsamy, K.N.; Mathan, S.; Yan, W.; Rafieerad, A.; Sekaran, S.; Manego, H.; Dhingra, S. Carbon Nanomaterials for Cardiovascular Theranostics: Promises and Challenges. Bioact. Mater. 2021, 6, 2261–2280. [Google Scholar] [CrossRef]
- Seth, A.; Gholami Derami, H.; Gupta, P.; Wang, Z.; Rathi, P.; Gupta, R.; Cao, T.; Morrissey, J.J.; Singamaneni, S. Polydopamine–mesoporous silica core–shell nanoparticles for combined photothermal immunotherapy. ACS Appl. Mater. Interfaces 2020, 12, 42499–42510. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Figueiredo, P.; Bauleth-Ramos, T.; Correia, A.; Santos, H.A. Immunostimulation and Immunosuppression: Nanotechnology on the Brink. Small Methods 2018, 2, 1700347. [Google Scholar] [CrossRef]
- Ding, F.; Zhang, S.; Chen, Q.; Feng, H.; Ge, Z.; Zuo, X.; Fan, C.; Li, Q.; Xia, Q. Immunomodulation With Nucleic Acid Nanodevices. Small 2023, 19, 2206228. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S. Nanomaterials in tumor immunotherapy: New strategies and challenges. Mol. Cancer 2023, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Duo, Y.; Fu, J.; Qiu, M.; Sun, Z.; Adah, D.; Kang, J.; Xie, Z.; Fan, T.; Bao, S.; et al. Nano-immunotherapy: Unique mechanisms of nanomaterials in synergizing cancer immunotherapy. Nano Today 2021, 36, 101023. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Novel Tumor-Targeting Nanoparticles for Cancer Treatment-A Review. Int. J. Mol. Sci. 2022, 23, 5253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhang, F.; Peng, Y.; Xie, T.; Wang, Y.; Lan, Y. Current Progress in Cancer Treatment Using Nanomaterials. Front. Oncol. 2022, 12, 930125. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Abedi-Gaballu, F.; Abbaspour, S.; Ghasabi, M.; Yekta, R.; Shirjang, S.; Dehghan, G.; Hamblin, M.R.; Baradaran, B. Hyaluronic acid-decorated liposomal nanoparticles for targeted delivery of 5-fluorouracil into HT-29 colorectal cancer cells. J. Cell Physiol. 2020, 235, 6817–6830. [Google Scholar] [CrossRef]
- Rad, L.M.; Arellano, G.; Podojil, J.R.; O’Konek, J.J.; Shea, L.D.; Miller, S.D. Engineering nanoparticle therapeutics for food allergy. J. Allergy Clin. Immunol. 2024, 153, 549–559. [Google Scholar] [CrossRef]
- Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2007, 119, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-S.; Tang, K.; Lv, J. Peptide–drug conjugate-based novel molecular drug delivery system in cancer. Trends Pharmacol. Sci. 2021, 42, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Gocheva, G.; Ivanova, A. A look at receptor–ligand pairs for active-targeting drug delivery from crystallographic and molecular dynamics perspectives. Mol. Pharm. 2019, 16, 3293–3321. [Google Scholar] [CrossRef]
- Sujka, M.; Pankiewicz, U.; Kowalski, R.; Nowosad, K.; Noszczyk-Nowak, A. Porous starch and its application in drug delivery systems. Polim. W Med. 2018, 48, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Pacardo, D.B.; Ligler, F.S.; Gu, Z. Programmable nanomedicine: Synergistic and sequential drug delivery systems. Nanoscale 2015, 7, 3381–3391. [Google Scholar] [CrossRef] [PubMed]
- Demetzos, C.; Pippa, N. Advanced drug delivery nanosystems (aDDnSs): A mini-review. Drug Deliv. 2014, 21, 250–257. [Google Scholar] [CrossRef]
- Mobeen, H.; Safdar, M.; Fatima, A.; Afzal, S.; Zaman, H.; Mehdi, Z. Emerging applications of nanotechnology in context to immunology: A comprehensive review. Front. Bioeng. Biotechnol. 2022, 10, 1024871. [Google Scholar] [CrossRef]
- Chuang, S.T.; Conklin, B.; Stein, J.B.; Pan, G.; Lee, K.-B. Nanotechnology-enabled immunoengineering approaches to advance therapeutic applications. Nano Converg. 2022, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Prim. 2022, 2, 24. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, X.; Cao, T.; Deng, H.; Tang, X.; Lin, Q.; Zhou, Q. Immune cell membrane-based biomimetic nanomedicine for treating cancer metastasis. Acta Pharm. Sin. B 2023, 13, 2464–2482. [Google Scholar] [CrossRef]
- Cruz, L.J.; Tacken, P.J.; Rueda, F.; Domingo, J.C.; Albericio, F.; Figdor, C.G. Targeting nanoparticles to dendritic cells for immunotherapy. Methods Enzym. 2012, 509, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, Y.; Xin, Y.; Jiang, C.; Yan, B.; Zhai, S. Interactions Between Nanoparticles and Dendritic Cells: From the Perspective of Cancer Immunotherapy. Front. Oncol. 2018, 8, 404. [Google Scholar] [CrossRef]
- Simon, J.; Fichter, M.; Kuhn, G.; Brückner, M.; Kappel, C.; Schunke, J.; Klaus, T.; Grabbe, S.; Landfester, K.; Mailänder, V. Achieving dendritic cell subset-specific targeting in vivo by site-directed conjugation of targeting antibodies to nanocarriers. Nano Today 2022, 43, 101375. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Guo, M.; Xu, J.; Wu, F.; Fan, J.; Huang, Q.; Yang, G.; Lv, Z.; Wang, X.; Jin, Y. Nanoparticles Targeting Macrophages as Potential Clinical Therapeutic Agents Against Cancer and Inflammation. Front. Immunol. 2019, 10, 1998. [Google Scholar] [CrossRef]
- Lôbo, G.; Paiva, K.L.R.; Silva, A.L.G.; Simões, M.M.; Radicchi, M.A.; Báo, S.N. Nanocarriers Used in Drug Delivery to Enhance Immune System in Cancer Therapy. Pharmaceutics 2021, 13, 1167. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Minko, T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 2021, 18, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Abdelmoneem, M.A.; Hassanin, I.A.; Abd Elwakil, M.M.; Elnaggar, M.A.; Mokhtar, S.; Fang, J.-Y.; Elkhodairy, K.A. Lactoferrin, a multi-functional glycoprotein: Active therapeutic, drug nanocarrier & targeting ligand. Biomaterials 2020, 263, 120355. [Google Scholar]
- Heimall, J. The Adaptive Cellular Immune Response: T Cells and Cytokines. UpToDate. 2022. Available online: https://www.uptodate.com/contents/the-adaptive-cellular-immune-response-t-cells-and-cytokines (accessed on 15 May 2024).
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Gupta, J.; Quadros, M.; Momin, M. Mesoporous silica nanoparticles: Synthesis and multifaceted functionalization for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2023, 81, 104305. [Google Scholar] [CrossRef]
- Noureddine, A.; Maestas-Olguin, A.; Tang, L.; Corman-Hijar, J.I.; Olewine, M.; Krawchuck, J.A.; Tsala Ebode, J.; Edeh, C.; Dang, C.; Negrete, O.A.; et al. Future of Mesoporous Silica Nanoparticles in Nanomedicine: Protocol for Reproducible Synthesis, Characterization, Lipid Coating, and Loading of Therapeutics (Chemotherapeutic, Proteins, siRNA and mRNA). ACS Nano 2023, 17, 16308–16325. [Google Scholar] [CrossRef] [PubMed]
- Eloy, J.O.; Petrilli, R.; Trevizan, L.N.F.; Chorilli, M. Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Faham, A.; Altin, J.G. Ag-bearing liposomes engrafted with peptides that interact with CD11c/CD18 induce potent Ag-specific and antitumor immunity. Int. J. Cancer 2011, 129, 1391–1403. [Google Scholar] [CrossRef]
- Hama, S.; Sakai, M.; Itakura, S.; Majima, E.; Kogure, K. Rapid modification of antibodies on the surface of liposomes composed of high-affinity protein A-conjugated phospholipid for selective drug delivery. Biochem. Biophys. Rep. 2021, 27, 101067. [Google Scholar] [CrossRef] [PubMed]
- Ohradanova-Repic, A.; Nogueira, E.; Hartl, I.; Gomes, A.C.; Preto, A.; Steinhuber, E.; Mühlgrabner, V.; Repic, M.; Kuttke, M.; Zwirzitz, A. Fab antibody fragment-functionalized liposomes for specific targeting of antigen-positive cells. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 123–130. [Google Scholar] [CrossRef]
- Millozzi, F.; Papait, A.; Bouché, M.; Parolini, O.; Palacios, D. Nano-Immunomodulation: A New Strategy for Skeletal Muscle Diseases and Aging? Int. J. Mol. Sci. 2023, 24, 1175. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Rius, A.; Desai, A.; Yuen, D.; Johnston, A.P.R.; Voelcker, N.H. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat. Nanotechnol. 2021, 16, 37–46. [Google Scholar] [CrossRef]
- Yousefpour, P.; Ni, K.; Irvine, D.J. Targeted modulation of immune cells and tissues using engineered biomaterials. Nat. Rev. Bioeng. 2023, 1, 107–124. [Google Scholar] [CrossRef]
- Wang, D.-R.; Wu, X.-L.; Sun, Y.-L. Therapeutic targets and biomarkers of tumor immunotherapy: Response versus non-response. Signal Transduct. Target. Ther. 2022, 7, 331. [Google Scholar] [CrossRef]
- Immune discovery aplenty at twenty. Nat. Rev. Immunol. 2021, 21, 613. [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.C.; Sarkizova, S.; Hacohen, N. Systems Immunology: Learning the Rules of the Immune System. Annu. Rev. Immunol. 2018, 36, 813–842. [Google Scholar] [CrossRef]
- Brameshuber, M.; Klotzsch, E.; Ponjavic, A.; Sezgin, E. Understanding immune signaling using advanced imaging techniques. Biochem. Soc. Trans. 2022, 50, 853–866. [Google Scholar] [CrossRef]
- Bonaguro, L.; Schulte-Schrepping, J.; Ulas, T.; Aschenbrenner, A.C.; Beyer, M.; Schultze, J.L. A guide to systems-level immunomics. Nat. Immunol. 2022, 23, 1412–1423. [Google Scholar] [CrossRef]
- Yuba, E.; Fukaya, Y.; Yanagihara, S.; Kasho, N.; Harada, A. Development of Mannose-Modified Carboxylated Curdlan-Coated Liposomes for Antigen Presenting Cell Targeted Antigen Delivery. Pharmaceutics 2020, 12, 754. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiao, W.; Elbahnasawy, M.A.; Bao, X.; Zheng, Q.; Gong, L.; Zhou, Y.; Yang, S.; Fang, A.; Farag, M.M.S.; et al. Optimization of the Linker Length of Mannose-Cholesterol Conjugates for Enhanced mRNA Delivery to Dendritic Cells by Liposomes. Front. Pharm. 2018, 9, 980. [Google Scholar] [CrossRef]
- Liu, J.; Xavy, S.; Mihardja, S.; Chen, S.; Sompalli, K.; Feng, D.; Choi, T.; Agoram, B.; Majeti, R.; Weissman, I.L.; et al. Targeting macrophage checkpoint inhibitor SIRPα for anticancer therapy. JCI Insight 2020, 5, e134728. [Google Scholar] [CrossRef]
- Yu, W.-B.; Ye, Z.-H.; Chen, X.; Shi, J.-J.; Lu, J.-J. The development of small-molecule inhibitors targeting CD47. Drug Discov. Today 2021, 26, 561–568. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Kraynak, C.A.; Huang, W.; Bender, E.C.; Wang, J.-L.; Hanafy, M.S.; Cui, Z.; Suggs, L.J. Apoptotic body-inspired nanoparticles target macrophages at sites of inflammation to support an anti-inflammatory phenotype shift. Int. J. Pharm. 2022, 618, 121634. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wan, S.; Yang, S.; Hu, H.; Zhang, C.; Lai, J.; Zhou, J.; Chen, W.; Tang, X.; Luo, J.; et al. Macrophage cell membrane-based nanoparticles: A new promising biomimetic platform for targeted delivery and treatment. J. Nanobiotechnology 2022, 20, 542. [Google Scholar] [CrossRef]
- Cai, D.; Gao, W.; Li, Z.; Zhang, Y.; Xiao, L.; Xiao, Y. Current Development of Nano-Drug Delivery to Target Macrophages. Biomedicines 2022, 10, 1203. [Google Scholar] [CrossRef]
- Chaintreuil, P.; Kerreneur, E.; Bourgoin, M.; Savy, C.; Favreau, C.; Robert, G.; Jacquel, A.; Auberger, P. The generation, activation, and polarization of monocyte-derived macrophages in human malignancies. Front. Immunol. 2023, 14, 1178337. [Google Scholar] [CrossRef] [PubMed]
- Pinto, I.S.; Cordeiro, R.A.; Faneca, H. Polymer- and lipid-based gene delivery technology for CAR T cell therapy. J. Control. Release 2023, 353, 196–215. [Google Scholar] [CrossRef] [PubMed]
- Est-Witte, S.E.; Livingston, N.K.; Omotoso, M.O.; Green, J.J.; Schneck, J.P. Nanoparticles for generating antigen-specific T cells for immunotherapy. Semin. Immunol. 2021, 56, 101541. [Google Scholar] [CrossRef]
- Konkel, J.E.; Zhang, D.; Zanvit, P.; Chia, C.; Zangarle-Murray, T.; Jin, W.; Wang, S.; Chen, W. Transforming Growth Factor-β Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity 2017, 46, 660–674. [Google Scholar] [CrossRef]
- Polanczyk, M.J.; Walker, E.; Haley, D.; Guerrouahen, B.S.; Akporiaye, E.T. Blockade of TGF-β signaling to enhance the antitumor response is accompanied by dysregulation of the functional activity of CD4(+)CD25(+)Foxp3(+) and CD4(+)CD25(-)Foxp3(+) T cells. J. Transl. Med. 2019, 17, 219. [Google Scholar] [CrossRef]
- Delobel, P.; Ginter, B.; Rubio, E.; Balabanian, K.; Lazennec, G. CXCR2 intrinsically drives the maturation and function of neutrophils in mice. Front. Immunol. 2022, 13, 1005551. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Gouwy, M.; Proost, P. Neutrophil chemoattractant receptors in health and disease: Double-edged swords. Cell. Mol. Immunol. 2020, 17, 433–450. [Google Scholar] [CrossRef]
- Shamalov, K.; Meir, R.; Motiei, M.; Popovtzer, R.; Cohen, C.J. Noninvasive Tracking of Natural Killer Cells Using Gold Nanoparticles. ACS Omega 2021, 6, 28507–28514. [Google Scholar] [CrossRef] [PubMed]
- Murugan, D.; Murugesan, V.; Panchapakesan, B.; Rangasamy, L. Nanoparticle Enhancement of Natural Killer (NK) Cell-Based Immunotherapy. Cancers 2022, 14, 5438. [Google Scholar] [CrossRef] [PubMed]
- Kläsener, K.; Jellusova, J.; Andrieux, G.; Salzer, U.; Böhler, C.; Steiner, S.N.; Albinus, J.B.; Cavallari, M.; Süß, B.; Voll, R.E.; et al. CD20 as a gatekeeper of the resting state of human B cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2021342118. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wu, G.; Huang, X.; Ma, Y.; Zhang, Y.; Song, Q.; Xie, M.; Sun, Y.; Huang, Y.; Huang, Z.; et al. Efficacy and safety of new anti-CD20 monoclonal antibodies versus rituximab for induction therapy of CD20+ B-cell non-Hodgkin lymphomas: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 3255. [Google Scholar] [CrossRef] [PubMed]
- Bevins, N.J.; Okamura, R.; Montesion, M.; Adashek, J.J.; Goodman, A.M.; Kurzrock, R. Tumor Infiltrating Lymphocyte Expression of PD-1 Predicts Response to Anti-PD-1/PD-L1 Immunotherapy. J. Immunother. Precis. Oncol. 2022, 5, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, J.; Inozume, T.; Sax, N.; Ariyasu, R.; Ishikawa, M.; Yamashita, K.; Kawazu, M.; Ueno, T.; Irie, T.; Tanji, E.; et al. PD-1 blockade therapy promotes infiltration of tumor-attacking exhausted T cell clonotypes. Cell Rep. 2022, 38, 110331. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I.; Ravindran, R.; Stewart, S.; Alam, M.; Kwissa, M. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Napolitani, G.; Rinaldi, A.; Bertoni, F.; Sallusto, F.; Lanzavecchia, A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1–polarizing program in dendritic cells. Nat. Immunol. 2005, 6, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef]
- Song, E.; Han, W.; Li, C.; Cheng, D.; Li, L.; Liu, L.; Zhu, G.; Song, Y.; Tan, W. Hyaluronic acid-decorated graphene oxide nanohybrids as nanocarriers for targeted and pH-responsive anticancer drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 11882–11890. [Google Scholar] [CrossRef]
- Kim, Y.E.; Kim, J. ROS-scavenging therapeutic hydrogels for modulation of the inflammatory response. ACS Appl. Mater. Interfaces 2021, 14, 23002–23021. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Karmali, P.P.; Mukthavaram, R.; Kesari, S.; Kouznetsova, V.L.; Tsigelny, I.F.; Simberg, D. Direct recognition of superparamagnetic nanocrystals by macrophage scavenger receptor SR-AI. ACS Nano 2013, 7, 4289–4298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.R.; Devan, A.R.; Nair, B.; Vinod, B.S.; Nath, L.R. Harnessing the immune system against cancer: Current immunotherapy approaches and therapeutic targets. Mol. Biol. Rep. 2021, 48, 8075–8095. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Wang, J.; Yang, Y.-G.; Zhang, Y.; Sun, T. Advances in dendritic cell targeting nano-delivery systems for induction of immune tolerance. Front. Bioeng. Biotechnol. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, H.; Iwashita, S.; Kuraishi, T.; Goto, A.; Fuse, N.; Ueno, H.; Nimura, M.; Oyama, T.; Tang, C.; Watanabe, R.; et al. cGMP signaling pathway that modulates NF-κB activation in innate immune responses. iScience 2021, 24, 103473. [Google Scholar] [CrossRef] [PubMed]
- Strzelec, M.; Detka, J.; Mieszczak, P.; Sobocińska, M.K.; Majka, M. Immunomodulation-a general review of the current state-of-the-art and new therapeutic strategies for targeting the immune system. Front. Immunol. 2023, 14, 1127704. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Srinivas, S.P.; Kumawat, M.; Daima, H.K. Ligand-based surface engineering of nanomaterials: Trends, challenges, and biomedical perspectives. OpenNano 2024, 15, 100194. [Google Scholar] [CrossRef]
- Khan, A.; Dias, F.; Neekhra, S.; Singh, B.; Srivastava, R. Designing and Immunomodulating Multiresponsive Nanomaterial for Cancer Theranostics. Front. Chem. 2020, 8, 631351. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Q.; Wang, Y.; Zhao, X.; Gao, K.; Liu, Q.; Zhao, Y.; Zhang, Z.; Zheng, Y.; Cao, J.; et al. In Situ Modification of the Tumor Cell Surface with Immunomodulating Nanoparticles for Effective Suppression of Tumor Growth in Mice. Adv. Mater. 2019, 31, e1902542. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Sun, Q. The Expression Profile of Human Peripheral Blood Mononuclear Cell miRNA Is Altered by Antibody-Dependent Enhancement of Infection with Dengue Virus Serotype 3. Virol. J. 2018, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Meng, J.; Li, Y.; Yang, C.; Hou, Y.; Tang, W.; McHugh, K.J.; Jing, L. Nanotechnology-Enhanced Immunotherapy for Metastatic Cancer. Innovation 2021, 2, 100174. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumawat, M.; Gogoi, H.; Madhyastha, H.; Lichtfouse, E.; Daima, H.K. Engineered Nanomaterials for Immunomodulation: A Review. Acs Appl. Bio Mater. 2024, 7, 727–751. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, D.Y.; Huang, L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Nano-enabled immunomodulation. Nat. Nanotechnol. 2021, 16, 1. [CrossRef] [PubMed]
- Dararatana, N.; Seidi, F.; Hamel, J.; Crespy, D. Controlling release kinetics of pH-responsive polymer nanoparticles. Polym. Chem. 2020, 11, 1752–1762. [Google Scholar] [CrossRef]
- Takemoto, H.; Nishiyama, N. Construction of nanomaterials based on pH-responsive polymers for effective tumor delivery. Polym. J. 2021, 53, 1353–1360. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Shi, R.; Liu, H. Multifunctional mesoporous silica nanoparticles for biomedical applications. Signal Transduct. Target. Ther. 2023, 8, 435. [Google Scholar] [CrossRef]
- Khatun, S.; Putta, C.L.; Hak, A.; Rengan, A.K. Immunomodulatory nanosystems: An emerging strategy to combat viral infections. Biomater. Biosyst. 2023, 9, 100073. [Google Scholar] [CrossRef]
- Bochicchio, S.; Lamberti, G.; Barba, A.A. Polymer-Lipid Pharmaceutical Nanocarriers: Innovations by New Formulations and Production Technologies. Pharmaceutics 2021, 13, 198. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Kelly, H.G.; Dagley, L.F.; Reynaldi, A.; Schlub, T.E.; Spall, S.K.; Bell, C.A.; Cui, J.; Mitchell, A.J.; Lin, Z. Person-specific biomolecular coronas modulate nanoparticle interactions with immune cells in human blood. ACS Nano 2020, 14, 15723–15737. [Google Scholar] [CrossRef] [PubMed]
- Sofias, A.M.; Andreassen, T.; Hak, S. Nanoparticle ligand-decoration procedures affect in vivo interactions with immune cells. Mol. Pharm. 2018, 15, 5754–5761. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Roy, K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng. Transl. Med. 2016, 1, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J.; Zhang, H.; Ye, J.; Moore, C.; Lu, C.; Fang, Y.; Fu, Y.-X.; Li, B. Concurrent delivery of immune checkpoint blockade modulates T cell dynamics to enhance neoantigen vaccine-generated antitumor immunity. Nat. Cancer 2022, 3, 437–452. [Google Scholar] [CrossRef]
- Kubli, S.P.; Berger, T.; Araujo, D.V.; Siu, L.L.; Mak, T.W. Beyond immune checkpoint blockade: Emerging immunological strategies. Nat. Rev. Drug Discov. 2021, 20, 899–919. [Google Scholar] [CrossRef]
- Pognan, F.; Beilmann, M.; Boonen, H.C.M.; Czich, A.; Dear, G.; Hewitt, P.; Mow, T.; Oinonen, T.; Roth, A.; Steger-Hartmann, T.; et al. The evolving role of investigative toxicology in the pharmaceutical industry. Nat. Rev. Drug Discov. 2023, 22, 317–335. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, N.; Ai, F.; Wang, Z.; Zhu, G. Nanomaterial-mediated platinum drug-based combinatorial cancer therapy. VIEW 2021, 2, 20200030. [Google Scholar] [CrossRef]
- Babbitt, C.W.; Moore, E.A. Sustainable nanomaterials by design. Nat. Nanotechnol. 2018, 13, 621–623. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, W.; Zhang, J.; Liu, T.; Xing, J.; Zhang, H.; Tang, D. Nanomaterial-Based Drug Delivery Systems: A New Weapon for Cancer Immunotherapy. Int. J. Nanomed. 2022, 17, 4677–4696. [Google Scholar] [CrossRef]
- Longo, A.; Longo, V.; Colombo, P. Nanoparticles in allergen immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 576–582. [Google Scholar] [CrossRef]
- Ma, X.; Liang, X.; Li, Y.; Feng, Q.; Cheng, K.; Ma, N.; Zhu, F.; Guo, X.; Yue, Y.; Liu, G.; et al. Modular-designed engineered bacteria for precision tumor immunotherapy via spatiotemporal manipulation by magnetic field. Nat. Commun. 2023, 14, 1606. [Google Scholar] [CrossRef] [PubMed]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Bril, M.; Fredrich, S.; Kurniawan, N.A. Stimuli-responsive materials: A smart way to study dynamic cell responses. Smart Mater. Med. 2022, 3, 257–273. [Google Scholar] [CrossRef]
- Mohapatra, A.; Uthaman, S.; Park, I.K. External and Internal Stimuli-Responsive Metallic Nanotherapeutics for Enhanced Anticancer Therapy. Front. Mol. Biosci. 2020, 7, 597634. [Google Scholar] [CrossRef] [PubMed]
- Vinchhi, P.; Rawal, S.U.; Patel, M.M. Chapter 13-External stimuli-responsive drug delivery systems. In Drug Delivery Devices and Therapeutic Systems; Chappel, E., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 267–288. [Google Scholar]
- Pan, T.; Lu, D.; Xin, H.; Li, B. Biophotonic probes for bio-detection and imaging. Light Sci. Appl. 2021, 10, 124. [Google Scholar] [CrossRef]
- Yu, H.; Peng, Y.; Yang, Y.; Li, Z.-Y. Plasmon-enhanced light–matter interactions and applications. npj Comput. Mater. 2019, 5, 45. [Google Scholar] [CrossRef]
- Kinney, M.A.; McDevitt, T.C. Emerging strategies for spatiotemporal control of stem cell fate and morphogenesis. Trends Biotechnol. 2013, 31, 78–84. [Google Scholar] [CrossRef]
- Chagri, S.; Ng, D.Y.W.; Weil, T. Designing bioresponsive nanomaterials for intracellular self-assembly. Nat. Rev. Chem. 2022, 6, 320–338. [Google Scholar] [CrossRef]
- Egan, P.; Sinko, R.; LeDuc, P.R.; Keten, S. The role of mechanics in biological and bio-inspired systems. Nat. Commun. 2015, 6, 7418. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Chen, F. pH-responsive drug-delivery systems. Chem. Asian J. 2015, 10, 284–305. [Google Scholar] [CrossRef] [PubMed]
- Hiruta, Y. Poly(N-isopropylacrylamide)-based temperature- and pH-responsive polymer materials for application in biomedical fields. Polym. J. 2022, 54, 1419–1430. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Wagner, J.; Gößl, D.; Ustyanovska, N.; Xiong, M.; Hauser, D.; Zhuzhgova, O.; Hočevar, S.; Taskoparan, B.; Poller, L.; Datz, S.; et al. Mesoporous Silica Nanoparticles as pH-Responsive Carrier for the Immune-Activating Drug Resiquimod Enhance the Local Immune Response in Mice. ACS Nano 2021, 15, 4450–4466. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.N.; Zhang, C.Q.; Wang, W.; Wang, P.C.; Zhou, J.P.; Liang, X.J. pH-responsive mesoporous silica nanoparticles employed in controlled drug delivery systems for cancer treatment. Cancer Biol. Med. 2014, 11, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Zimlichman, E.; Nicklin, W.; Aggarwal, R.; Bates, D.W. Health care 2030: The coming transformation. NEJM Catal. Innov. Care Deliv. 2021, 2. [Google Scholar]
- Boone, C.G.; Pickett, S.T.A.; Bammer, G.; Bawa, K.; Dunne, J.A.; Gordon, I.J.; Hart, D.; Hellmann, J.; Miller, A.; New, M.; et al. Preparing interdisciplinary leadership for a sustainable future. Sustain. Sci. 2020, 15, 1723–1733. [Google Scholar] [CrossRef]
- Brain, D.; Plant-Hately, A.; Heaton, B.; Arshad, U.; David, C.; Hedrich, C.; Owen, A.; Liptrott, N.J. Drug delivery systems as immunomodulators for therapy of infectious disease: Relevance to COVID-19. Adv. Drug Deliv. Rev. 2021, 178, 113848. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef] [PubMed]
- Syama, K.; Jakubek, Z.J.; Chen, S.; Zaifman, J.; Tam, Y.Y.C.; Zou, S. Development of lipid nanoparticles and liposomes reference materials (II): Cytotoxic profiles. Sci. Rep. 2022, 12, 18071. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Guo, J.; Huang, Q. Liposomes for Tumor Targeted Therapy: A Review. Int. J. Mol. Sci. 2023, 24, 2643. [Google Scholar] [CrossRef]
- Pandey, S.; Shaikh, F.; Gupta, A.; Tripathi, P.; Yadav, J.S. A Recent Update: Solid Lipid Nanoparticles for Effective Drug Delivery. Adv. Pharm. Bull. 2022, 12, 17–33. [Google Scholar] [CrossRef]
- Gull, N.; Arshad, F.; Naikoo, G.A.; Hassan, I.U.; Pedram, M.Z.; Ahmad, A.; Aljabali, A.A.A.; Mishra, V.; Satija, S.; Charbe, N. Recent advances in anticancer activity of novel plant extracts and compounds from Curcuma longa in hepatocellular carcinoma. J. Gastrointest. Cancer 2023, 54, 368–390. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.A.; Alsaadi, M.; Aljabali, A.A. Recent updates in curcumin delivery. J. Liposome Res. 2023, 33, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, H.H.; Felimban, R.; Almaghrabi, S.; Hasaballah, N. Nanoemulsions: Formulation, characterization, biological fate, and potential role against COVID-19 and other viral outbreaks. Colloid Interface Sci. Commun. 2021, 45, 100533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, S.; Hu, Y.; Zhao, X.; Lee, R.J. Application of lipid-based nanoparticles in cancer immunotherapy. Front. Immunol. 2022, 13, 967505. [Google Scholar] [CrossRef] [PubMed]
- Bunea, A.-I.; Harloff-Helleberg, S.; Taboryski, R.; Nielsen, H.M. Membrane interactions in drug delivery: Model cell membranes and orthogonal techniques. Adv. Colloid Interface Sci. 2020, 281, 102177. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, J.; Shen, R.; Lin, J.; Li, S.; Lu, X.; Stelzel, J.L.; Kong, J.; Cheng, L.; Vuong, I.; et al. Screening for lipid nanoparticles that modulate the immune activity of helper T cells towards enhanced antitumour activity. Nat. Biomed. Eng. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lu, A.; Wang, X.; Belhadj, Z.; Wang, J.; Zhang, Q. A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives. Acta Pharm. Sin. B 2021, 11, 2396–2415. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ye, Q.; Gu, H.; Chen, Z. The role of lipid metabolism in tumor immune microenvironment and potential therapeutic strategies. Front. Oncol. 2022, 12, 984560. [Google Scholar] [CrossRef] [PubMed]
- Alhariri, M.; Azghani, A.; Omri, A. Liposomal antibiotics for the treatment of infectious diseases. Expert Opin. Drug Deliv. 2013, 10, 1515–1532. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gao, L.; Wang, Y.; Xu, B.; Maswikiti, E.P.; Li, H.; Zheng, P.; Tao, P.; Xiang, L.; Gu, B.; et al. A Forgotten Corner in Cancer Immunotherapy: The Role of Lipids. Front. Oncol. 2021, 11, 751086. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.M.L.; Farber, D.L. The Whole Body as the System in Systems Immunology. iScience 2020, 23, 101509. [Google Scholar] [CrossRef] [PubMed]
- Tsakiri, M.; Zivko, C.; Demetzos, C.; Mahairaki, V. Lipid-based nanoparticles and RNA as innovative neuro-therapeutics. Front. Pharm. 2022, 13, 900610. [Google Scholar] [CrossRef]
- Dacoba, T.G.; Olivera, A.; Torres, D.; Crecente-Campo, J.; Alonso, M.J. Modulating the immune system through nanotechnology. Semin. Immunol. 2017, 34, 78–102. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Koirala, P.; Chen, S.-P.R.; Boer, J.C.; Khalil, Z.G.; Deceneux, C.; Goodchild, G.; Lu, L.; Faruck, M.O.; Shalash, A.O.; Bashiri, S.; et al. Polymeric Nanoparticles as a Self-Adjuvanting Peptide Vaccine Delivery System: The Role of Shape. Adv. Funct. Mater. 2023, 33, 2209304. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Lee, C.H.; Park, S.H.; Lim, Y.T. Nanoparticle-based delivery strategies of multifaceted immunomodulatory RNA for cancer immunotherapy. J. Control. Release 2022, 343, 564–583. [Google Scholar] [CrossRef]
- Tng, D.J.H.; Low, J.G.H. Current status of silica-based nanoparticles as therapeutics and its potential as therapies against viruses. Antivir. Res 2023, 210, 105488. [Google Scholar] [CrossRef]
- Barroso, M.M. Quantum dots in cell biology. J. Histochem. Cytochem. 2011, 59, 237–251. [Google Scholar] [CrossRef]
- Alshammari, B.H.; Lashin, M.M.A.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and inorganic nanomaterials: Fabrication, properties and applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Mohammapdour, R.; Ghandehari, H. Mechanisms of immune response to inorganic nanoparticles and their degradation products. Adv. Drug Deliv. Rev. 2022, 180, 114022. [Google Scholar] [CrossRef]
- Reddy, N.; Hernandez-Ilizaliturri, F.J.; Deeb, G.; Roth, M.; Vaughn, M.; Knight, J.; Wallace, P.; Czuczman, M.S. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br. J. Haematol. 2008, 140, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guan, J.; Song, R.; Zhang, X.; Mao, S. Physicochemical properties of nanoparticles affecting their fate and the physiological function of pulmonary surfactants. Acta Biomater. 2022, 140, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges, and prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Leong, W.; Leong, K.W.; Chen, C.; Zhao, Y. Walking the line: The fate of nanomaterials at biological barriers. Biomaterials 2018, 174, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Yoon, M.S.; Lee, Y.J.; Shin, H.J.; Park, C.W.; Han, S.B.; Jung, J.K.; Kim, J.S.; Shin, D.H. Recent Advances and Challenges in Controlling the Spatiotemporal Release of Combinatorial Anticancer Drugs from Nanoparticles. Pharmaceutics 2020, 12, 1156. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Turkmen Koc, S.N.; Rezaei Benam, S.; Aral, I.P.; Shahbazi, R.; Ulubayram, K. Gold nanoparticles-mediated photothermal and photodynamic therapies for cancer. Int. J. Pharm. 2024, 655, 124057. [Google Scholar] [CrossRef]
- Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M.; Rahdar, A. Functional Nanomaterials in Biomedicine: Current Uses and Potential Applications. ChemMedChem 2022, 17, e202200142. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.C.; Wang, X. Discovery in clinical and translational medicine. Clin. Transl. Med. 2021, 11, e568. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Demetzos, C. Promising Nanotechnology Approaches in Treatment of Autoimmune Diseases of Central Nervous System. Brain Sci. 2020, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Peate, I. Pathophysiology applied to nursing: The basis for disease and illness. Br. J. Nurs. 2022, 31, 72–74. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- van Leent, M.M.T.; Priem, B.; Schrijver, D.P.; de Dreu, A.; Hofstraat, S.R.J.; Zwolsman, R.; Beldman, T.J.; Netea, M.G.; Mulder, W.J.M. Regulating trained immunity with nanomedicine. Nat. Rev. Mater. 2022, 7, 465–481. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, Y.; Wang, T.; Sun, M.; Chen, X. Nanomaterials as Smart Immunomodulator Delivery System for Enhanced Cancer Therapy. ACS Biomater. Sci. Eng. 2020, 6, 4774–4798. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R.; Raj, A.; Tseng, T.H.; Xiao, H.; Nguyen, R.; Mohammed, F.S.; Halder, S.; Xu, M.; Wu, M.J.; Bao, S.; et al. Biocompatibility of nanomaterials and their immunological properties. Biomed. Mater. 2021, 16, 042005. [Google Scholar] [CrossRef] [PubMed]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Farcal, L.; Hardy, B.; Vázquez-Campos, S.; Hristozov, D.; Marcomini, A.; Lynch, I.; Valsami-Jones, E.; Alenius, H.; Savolainen, K. Advanced tools for the safety assessment of nanomaterials. Nat. Nanotechnol. 2018, 13, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef]
- Lukin, I.; Erezuma, I.; Desimone, M.F.; Zhang, Y.S.; Dolatshahi-Pirouz, A.; Orive, G. Nanomaterial-based drug delivery of immunomodulatory factors for bone and cartilage tissue engineering. Biomater. Adv. 2023, 154, 213637. [Google Scholar] [CrossRef] [PubMed]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth Coating of Nanoparticles in Drug-Delivery Systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef]
- Lee, D.; Huntoon, K.; Lux, J.; Kim, B.Y.S.; Jiang, W. Engineering nanomaterial physical characteristics for cancer immunotherapy. Nat. Rev. Bioeng. 2023, 1, 499–517. [Google Scholar] [CrossRef]
- Hassan, S.; Prakash, G.; Ozturk, A.; Saghazadeh, S.; Sohail, M.F.; Seo, J.; Dockmeci, M.; Zhang, Y.S.; Khademhosseini, A. Evolution and Clinical Translation of Drug Delivery Nanomaterials. Nano Today 2017, 15, 91–106. [Google Scholar] [CrossRef] [PubMed]
| NPs Type | Advantages | Disadvantage |
|---|---|---|
| Organic Dye NPs | High biocompatibility and low toxicity | Lower payload than for other nanomaterials |
| Versatile surface chemistry for functionalization and targeting | Prone to photodegradation and photobleaching | |
| Strong optical properties for imaging and phototherapy applications | Challenges in achieving long-term stability in physiological environments | |
| Silica NPs | Rigid, tunable, and porous structure for high drug loading and controlled delivery | Concerns regarding long-term biodegradation and persistence |
| Good biocompatibility and low immunogenicity | Poor encapsulation of hydrophobic drugs | |
| Amenable to surface modifications for targeted delivery and imaging | Promotion of nonspecific interaction with biological components | |
| Metal NPs | Unique physicochemical properties, like plasmonic and magnetic responsiveness | Biocompatibility concerns and potential toxicity issues, especially with heavy metal-based NPs |
| Multimodal imaging and the combination of photothermal/photodynamic therapy is possible | Challenges: precise control over size, shape, and surface properties | |
| Easy synthesis and surface functionalization | Low stability and predisposition to aggregation in biological environments |
| Immunotherapy Paradigm | Mechanism of Action | Clinical Efficacy | Side Effects |
|---|---|---|---|
| Immune Checkpoint Inhibitors | Binding of inhibitory checkpoints (e.g., PD-1/PD-L1, CTLA-4) to liberate antitumor immune responses | Proved effective in numerous malignancies, including melanoma, non-small cell lung cancer, and renal cell carcinoma | Immune-related adverse events, such as dermatitis, colitis, and pneumonitis |
| Chimeric Antigen Receptor (CAR) T cell Therapy | Genetic manipulation of patient’s own T cells with the gene for chimeric antigen receptors recognizing tumor-specific antigens | Excellent responses were observed in hematologic cancers, particularly in relapsed/refractory B-cell acute lymphoblastic leukemia and non-Hodgkin’s lymphoma | Cytokine release syndrome, neurotoxicity, and on-target/off-tumor effects |
| Cancer Vaccines | Stimulation of specific immune responses against tumor-associated antigens by vaccination | Limited success in solid tumors, with some exceptions in prostate cancer (Sipuleucel-T) and melanoma (T-VEC) | Local reactions at the site of injection, flu-like symptoms, and autoimmune reactions |
| Adoptive Cell Transfer | Infusion of ex vivo expanded autologous tumor-infiltrating lymphocytes (TILs) or genetically engineered T cells | Impressive responses were seen in melanoma and selected solid tumors | Cytokine release syndrome and graft-versus-host disease (with allogeneic T cells) |
| Oncolytic Viral Therapy | Selective replication of viruses in tumor cells and their lysis, leading to immune activation | Preliminary results in clinical trials for malignancies such as melanoma, glioblastoma, and advanced solid tumors | Local inflammation at the tumor site, flu-like symptoms, potential for viral shedding |
| Checkpoint Inhibitor Therapy in Combination with Chemotherapy | Concurrent delivery of immune checkpoint inhibitors with conventional chemotherapy agents to augment antitumor immune responses | Improved overall survival and progression-free survival in multiple cancers, including lung cancer, triple-negative breast cancer, and bladder cancer | Enhanced potential for adverse events related to chemotherapy, including cytopenia, nausea, and alopecia; potential for immune-related adverse events in an additive manner |
| Bispecific Antibodies | Act as the bridge between the tumor cells and immune effector cells via dual binding of CD3-positive T cells and tumor-specific antigens | Proven clinical benefit in hematological malignancies, especially acute lymphoblastic leukemia and multiple myeloma; evolving evidence in solid tumors | Cytokine release syndrome; potential for off-tumor toxicities |
| Modulating the Tumor Micro- environment | Alteration of the tumor microenvironment to potentiate immune responses to the tumor | Preliminary evidence of potential efficacy in overcoming immunosuppressive barriers in the tumor microenvironment and enhancing responses to immunotherapy | Risk of exacerbating autoimmune reactions; potential for off-target effects on normal tissues |
| Dendritic Cell Vaccines | Usage of dendritic cells loaded with tumor antigens to induce specific immune responses | Some clinical success to date but ongoing research to optimize the strategy of dendritic cell vaccines | Localized reactions at the injection site, flu-like symptoms, and possibility for autoimmune reactions |
| Strengths | Weaknesses | Opportunities | Threats |
|---|---|---|---|
| Precision Targeting: Nanomaterials offer precise delivery of immunomodulatory agents, potentially reducing treatment frequency. | Complex Design: Design esign intricacies extend to maintaining stability and consistency during storage and transportation. | Personalized Medicine: Advancements in immune profiling enable tailored treatments and identification of individual immune signatures. | Regulatory Hurdles: Strict regulations may lead to delays in clinical translation and commercialization, necessitating comprehensive safety assessments. |
| Enhanced Efficacy: Nanocarriers enable controlled release, optimizing immune modulation and treatment outcomes. | Potential Toxicity: Thorough safety assessments must account for potential biodegradability issues and tissue clearance of nanomaterials. | Multidisciplinary Collaboration: The collaboration of nanotechnology and immunology experts generates innovative solutions and strategies for immunotherapy. | Immunogenicity: the risk of immune reactions triggered by nanomaterials could impact both the efficacy and safety of treatment. |
| Diverse Applications: Nanomaterials find application in cancer therapy, autoimmune diseases, and inflammation, expanding the scope of precision medicine. | Manufacturing Challenges: Scaling up nanomaterial production while ensuring consistent quality remains a challenge, affecting widespread adoption. | Drug Combination Therapy: Nanocarriers enable synergistic effects and novel combinations, offering avenues for enhanced treatment strategies. | Resistance Development: Prolonged usage of nanomaterials might lead to immune cell resistance, potentially reducing therapeutic effectiveness. |
| Immune Cell Modulation: Nanomaterials allow targeted modulation of immune cells, promoting precise immune responses and homeostasis. | Biodistribution Variability: Variability in nanoparticle distribution among individuals could impact treatment outcomes and response rates. | Minimized Side Effects: Accurate targeting reduces off-target effects, limiting damage to healthy tissues and improving patient tolerance. | Long-term Effects: The potential accumulation of nanomaterials in tissues might lead to unforeseen long-term effects on health and the environment. |
| Nanomaterial | Immune Signaling Pathway | Mechanism of Action | Applications | Challenges and Considerations |
|---|---|---|---|---|
| Liposomes | Cytokine Modulation | Encapsulation and controlled release of cytokines | Cancer immunotherapy, Autoimmune disorders | Variability in release kinetics, the potential for immunogenicity |
| Gold NPs | Immune Checkpoint Blockade | Surface functionalization with checkpoint inhibitors | Cancer immunotherapy | Optimal dosage, potential off-target effects |
| Lipid NPs | Cytokine Modulation | siRNA delivery for cytokine modulation | Inflammatory diseases, Vaccination | Intracellular delivery efficiency, stability |
| Polymeric NPs | Immune Checkpoint Blockade | Controlled release of checkpoint inhibitors | Cancer immunotherapy | Long-term biocompatibility, controlled release optimization |
| Quantum Dots | Cytokine Modulation | Photo stimulation-induced cytokine production | Immunomodulation | Phototoxicity, long-term effects |
| Carbon Nanotubes | Immune Checkpoint Blockade | Functionalization for checkpoint inhibition | Cancer immunotherapy | Biodistribution, biodegradation |
| Micelles | Toll-like Receptor Modulation | Encapsulation of TLR agonists | Vaccine adjuvants, Immunotherapy | Stability in physiological conditions, potential for TLR activation |
| Magnetic NPs | Macrophage Activation | Magnetic targeting of macrophages | Drug delivery, Immunotherapy | Optimal magnetic field strength, potential for non-specific binding |
| DNA NPs | Antigen Presentation | Display of antigens on DNA scaffolds | Vaccines, Immunotherapy | Immunogenicity, stability in biological environments |
| Protein NPs | Immune Cell Activation | Presentation of immunostimulatory proteins | Cancer immunotherapy, Vaccines | Protein stability, the potential for immune recognition |
| Hybrid NPs | Dual Modulation | Combination of different immune modulation strategies | Autoimmune disorders, Cancer immunotherapy | Optimization of hybrid composition and properties, potential for off-target effects |
| Stimulus | Trigger Mechanism | Nanomaterials and Systems | Immunomodulation Applications | Advancements and Challenges |
|---|---|---|---|---|
| Light | Photothermal Effects | Gold NPs, Carbon Nanotubes | Cancer immunotherapy (e.g., PD-L1 targeting) | Enhanced tissue penetration, e.g., NIR-II |
| Photochemical Reactions | Liposomes (encapsulating photosensitizers), Quantum Dots | Photodynamic immunotherapy (e.g., ROS induction) | Spatiotemporal precision, photochemical stability | |
| Temperature | Thermosensitive Polymers | Lipid-based NPs (e.g., liposomes) | Fever-range activation for controlled inflammation | External control, systemic effects |
| pH | pH-Responsive Polymers | Polymeric NPs (e.g., micelles) | pH-triggered drug delivery in tumor microenvironment | pH-responsive release kinetics, stability |
| pH-Activated Nanomaterials | Mesoporous Silica NPs | pH-dependent cytokine modulation | pH range compatibility, controlled release | |
| Enzymatic Activity | Enzyme-Responsive Systems | Lipid-based Nanovesicles (e.g., exosomes) | Wound healing, enzyme-targeted immunomodulation | Enzyme specificity, stability |
| Redox Potential | Redox-Responsive Nanomaterials | Nanogels, Liposomes | Oxidative stress modulation in autoimmune disorders | Intracellular release, bioavailability |
| Radiological | Radiation | Radioactive NPs | Cancer immunotherapy, Tumor ablation | Targeted delivery to tumors, radiation dose optimization |
| Ultrasound | Acoustic Waves | Ultrasound Contrast Agents, Microbubbles | Drug delivery, Immunomodulation | Non-invasive, targeted delivery, safety concerns |
| Magnetic Fields | Magnetic Forces | Magnetic NPs | Immunomagnetic targeting, Drug delivery | Targeted delivery, magnetic field strength optimization |
| Electric Fields | Electrical Signals | Electroconductive Nanomaterials | Neuromodulation, Tissue regeneration | Precise control, Biocompatibility |
| Mechanical Strain | Physical Stress | Nanocomposite Hydrogels, NPs | Tissue engineering, Regenerative medicine | Mechanical properties, biodegradability |
| Nanomaterial | Mechanisms of Action and Advantages | Examples of Systems and Therapeutic Applications |
|---|---|---|
| Liposomes | Encapsulation: efficiently encapsulate hydrophilic and hydrophobic drugs. Biocompatibility: low toxicity and immunogenicity. Surface modification for site-specific drug delivery. | Encapsulation of cytokines (e.g., IL-2) for cancer immunotherapy. Co-delivery of tumor antigens and adjuvants for cancer vaccines [145]. |
| Lipid NPs | Increased Drug Loading Capacity Sustained Release: achieve controlled and prolonged release patterns. Cellular Uptake: facilitate efficient absorption by immune cells. | Lipid NPs loaded with siRNA targeting TNF-α for the treatment of inflammatory diseases. Lipid NPs loaded with curcumin for immune modulation in autoimmune disorders [146,147,148]. |
| Nanoemulsions | Nano-sized droplets, which exhibit stability and compatibility with the human body, possess various advantages in the field of biomedical research. One significant characteristic of these droplets is their versatility, as they can be employed for a wide range of administration techniques. Furthermore, the immunomodulatory properties of nano-sized droplets hold great potential in activating immune responses. | Enhancing vaccine responses through application of oil-in-water nanoemulsions as adjuvants. Skin cancer immunotherapy: topical delivery of resiquimod-loaded nanoemulsions [149]. |
| Polymeric NPs | Design Considerations | Case Studies and Efficacy in Immunomodulation |
|---|---|---|
| Biodegradable and biocompatible polymers | Biodegradability: select polymers with controlled degradation into non-toxic byproducts. Biocompatibility: minimize immunogenicity for enhanced safety. | PLGA NPs co-delivering tumor antigens and adjuvants, boosting cancer vaccine responses. Chitosan-based NPs as tolerogenic carriers in autoimmune disease management. |
| Design considerations | Size Optimization: tailor nanoparticle size for efficient cellular uptake and lymphatic drainage, influencing immune cell interaction. Surface Modification: functionalize surfaces for targeted delivery to specific immune cell populations. Controlled Release: implement sustained release strategies for prolonged immunomodulation. | PEGylated polymeric NPs with tailored size delivering miRNA for inflammation regulation in colitis. Zwitterionic polymer-coated NPs achieving controlled release of checkpoint inhibitors, enhancing anticancer immune response. |
| Case studies and efficacy | PLGA-based NPs loaded with immune-modulating agents exhibit improved tumor regression in murine models. | PCL NPs loaded with anti-inflammatory cytokines showcase reduced joint inflammation in arthritis models. pH-responsive polymeric NPs effectively release immunomodulatory drugs in response to the tumor microenvironment. |
| Cancer Immunotherapy | Nanomaterial-Based Systems | Case Studies in Efficacy |
|---|---|---|
| Nanomaterial-Based Systems | Liposomes, lipid NPs, and polymer-based NPs engineered for targeted drug delivery and sustained release. Surface functionalization with ligands for enhanced tumor cell targeting and immune cell interaction. | Co-delivery of PD-1 inhibitors and tumor antigens via liposomes, achieving synergistic checkpoint blockade and antigen presentation. PLGA-based cancer vaccines inducing robust immune responses against specific tumor antigens. Gold NPs conjugated with CAR-T-cell-targeting ligands for improved tumor penetration. |
| Case Studies on Efficacy | Lipid-based NPs co-encapsulating checkpoint inhibitors and tumor antigens for potent antitumor immune responses. | Nanoemulsions delivering immune adjuvants, enhancing DC activation and immune memory in cancer vaccines. Polymeric NPs prolong CAR T-cell persistence and enable sustained tumor surveillance. |
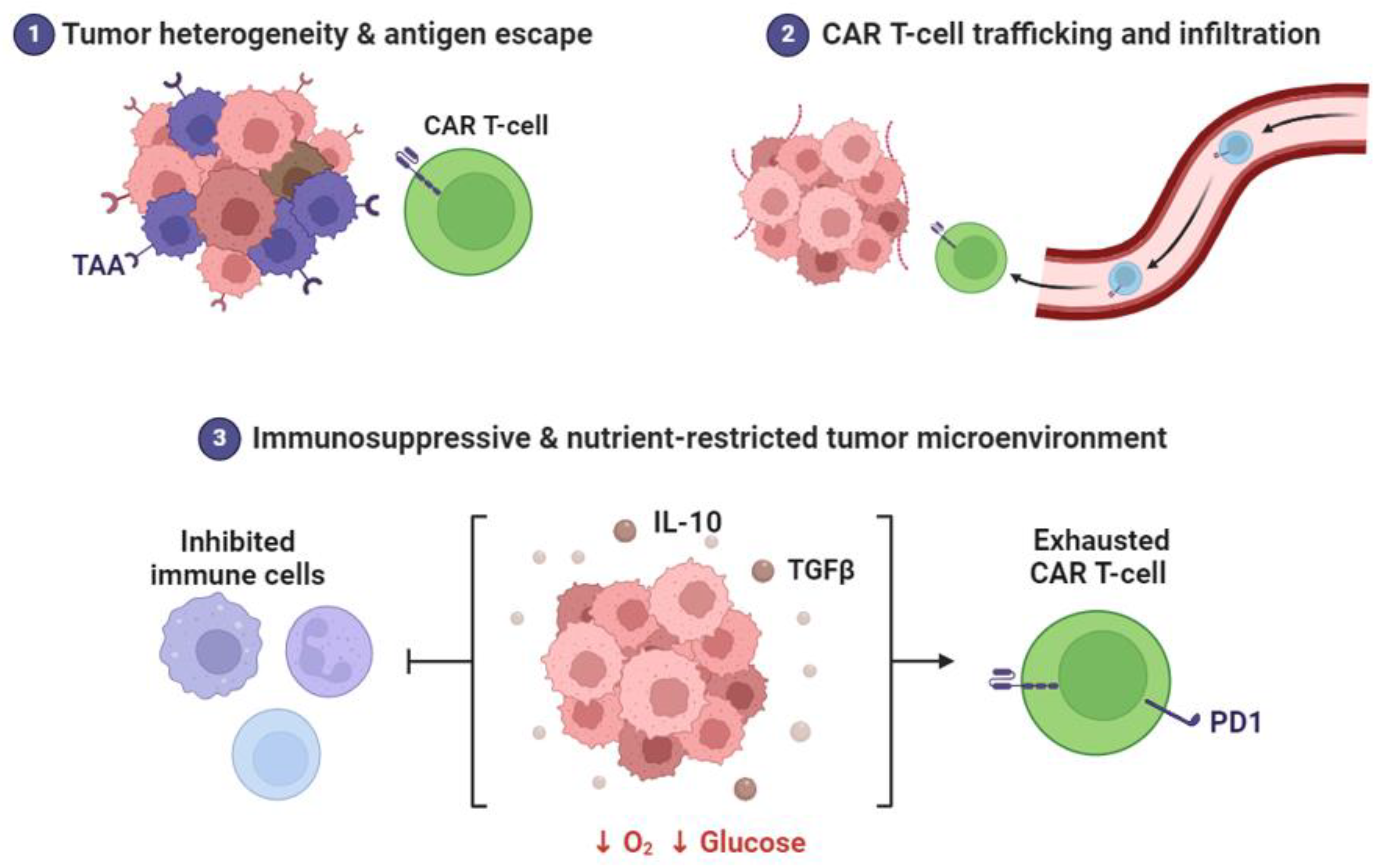
| Challenges and Future Directions | Optimizing nanoparticle properties for efficient tumor accumulation and controlled drug release. Overcoming immunosuppressive tumor microenvironment barriers for effective immune activation. | Nanoparticle-mediated combinatorial strategies, leveraging synergies between immunomodulators and conventional therapies. Personalized approaches tailoring nanoparticle formulations to individual patient profiles for optimized outcomes. |
| Biocompatibility and Toxicity | Nanomaterial Evaluation |
|---|---|
| Biocompatibility and toxicity considerations |
|
| Nanomaterial evaluation |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljabali, A.A.A.; Obeid, M.A.; Gammoh, O.; El-Tanani, M.; Mishra, V.; Mishra, Y.; Kapre, S.; Srivatsa Palakurthi, S.; Hassan, S.S.; Nawn, D.; et al. Nanomaterial-Driven Precision Immunomodulation: A New Paradigm in Therapeutic Interventions. Cancers 2024, 16, 2030. https://doi.org/10.3390/cancers16112030
Aljabali AAA, Obeid MA, Gammoh O, El-Tanani M, Mishra V, Mishra Y, Kapre S, Srivatsa Palakurthi S, Hassan SS, Nawn D, et al. Nanomaterial-Driven Precision Immunomodulation: A New Paradigm in Therapeutic Interventions. Cancers. 2024; 16(11):2030. https://doi.org/10.3390/cancers16112030
Chicago/Turabian StyleAljabali, Alaa A. A., Mohammad A. Obeid, Omar Gammoh, Mohamed El-Tanani, Vijay Mishra, Yachana Mishra, Sumedha Kapre, Sushesh Srivatsa Palakurthi, Sk. Sarif Hassan, Debaleena Nawn, and et al. 2024. "Nanomaterial-Driven Precision Immunomodulation: A New Paradigm in Therapeutic Interventions" Cancers 16, no. 11: 2030. https://doi.org/10.3390/cancers16112030
APA StyleAljabali, A. A. A., Obeid, M. A., Gammoh, O., El-Tanani, M., Mishra, V., Mishra, Y., Kapre, S., Srivatsa Palakurthi, S., Hassan, S. S., Nawn, D., Lundstrom, K., Hromić-Jahjefendić, A., Serrano-Aroca, Á., Redwan, E. M., Uversky, V. N., & Tambuwala, M. M. (2024). Nanomaterial-Driven Precision Immunomodulation: A New Paradigm in Therapeutic Interventions. Cancers, 16(11), 2030. https://doi.org/10.3390/cancers16112030











