Helium Ion Therapy for Advanced Juvenile Nasopharyngeal Angiofibroma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Tumor and Organs at Risk Delineation
2.3. Clinical Proton Treatment Planning
2.4. Retrospective Helium Ion Treatment Planning
2.5. Dosimetric Evaluation of Treatment Plans
2.6. Assessment of Normal Tissue Complication Probabilities
2.7. Statistical Analysis
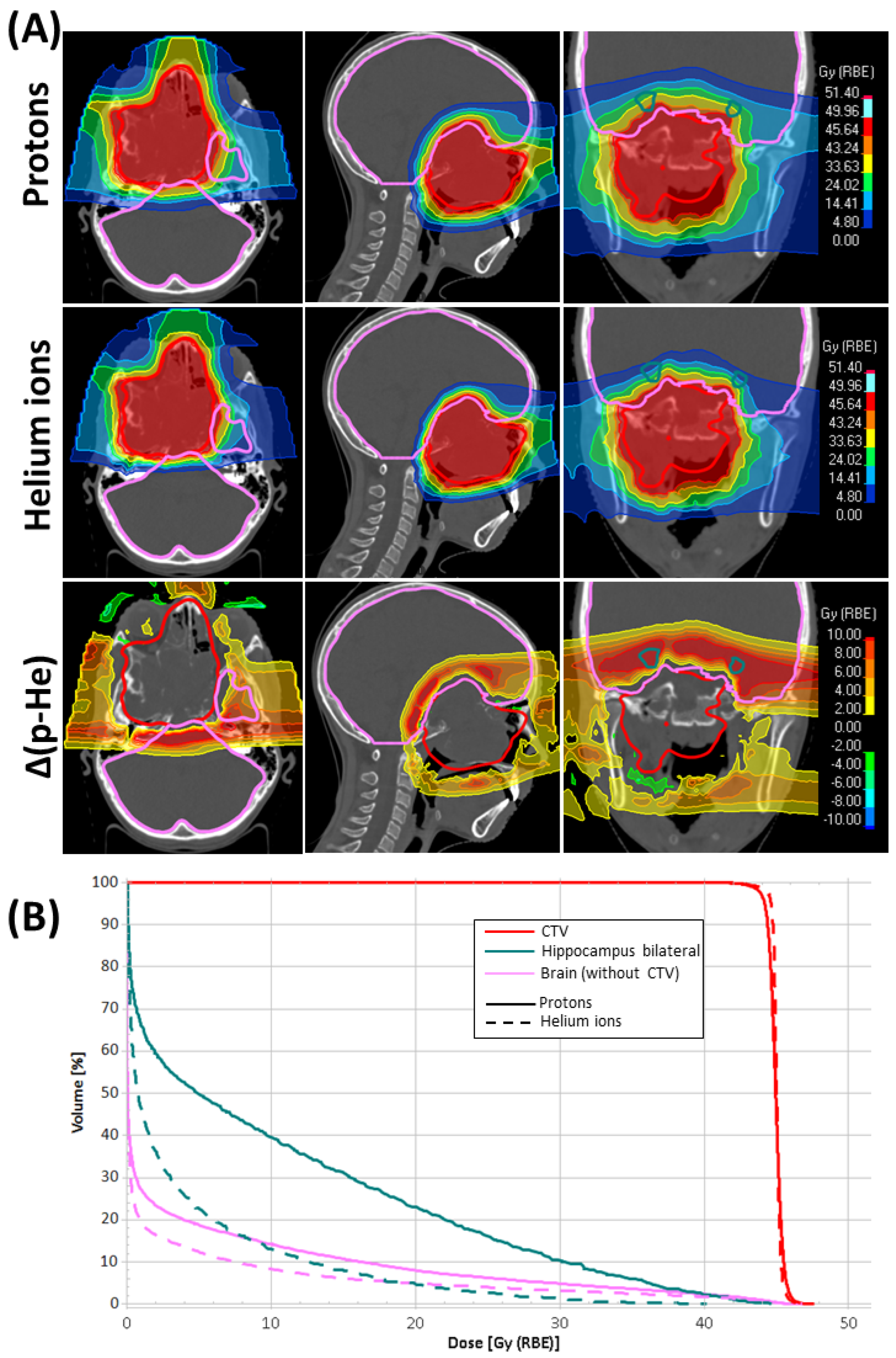
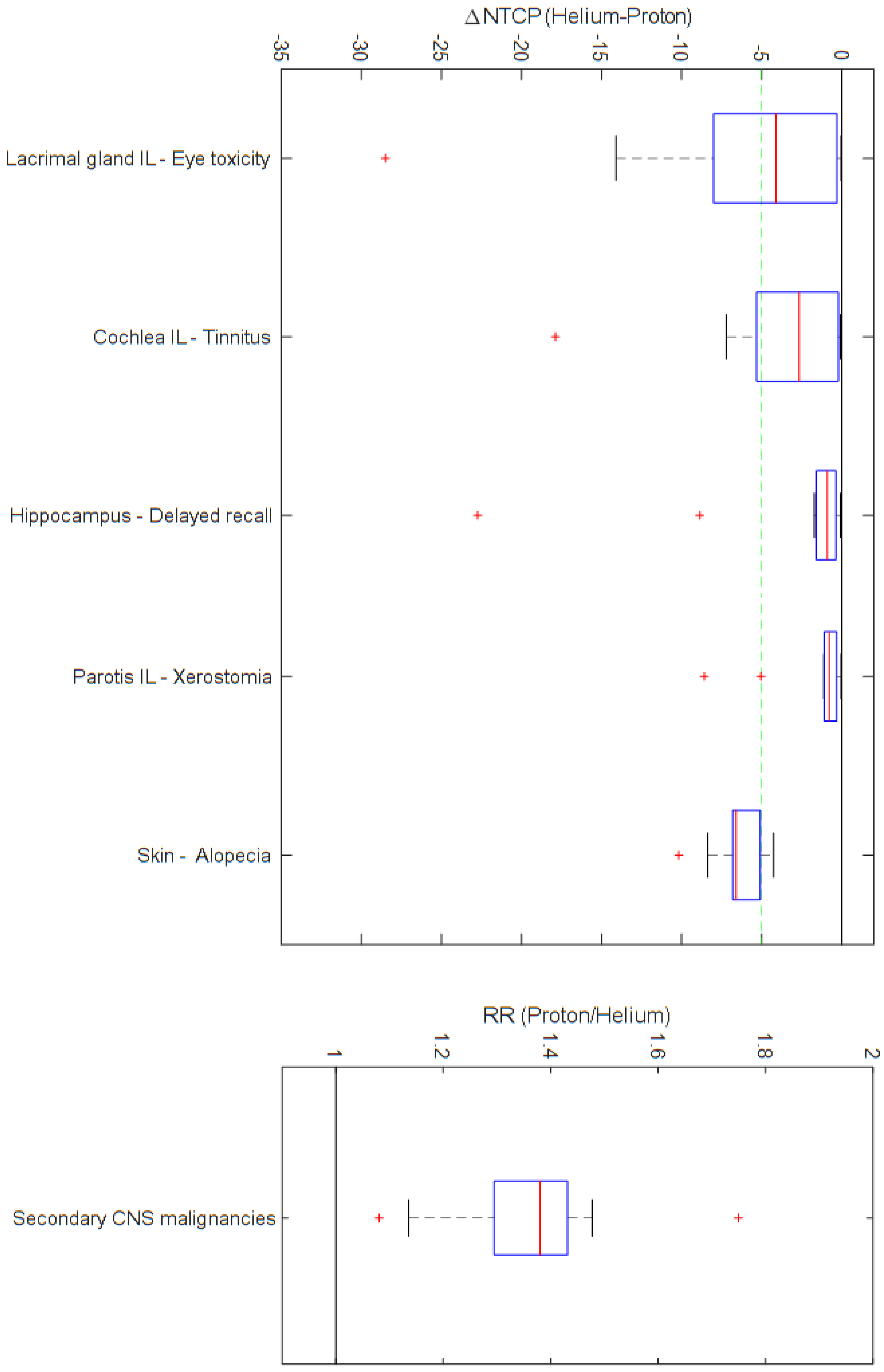
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez, F.; Triantafyllou, A.; Snyderman, C.H.; Hunt, J.L.; Suarez, C.; Lund, V.J.; Strojan, P.; Saba, N.F.; Nixon, I.J.; Devaney, K.O.; et al. Nasal juvenile angiofibroma: Current perspectives with emphasis on management. Head Neck 2017, 39, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Safadi, A.; Schreiber, A.; Fliss, D.M.; Nicolai, P. Juvenile Angiofibroma: Current Management Strategies. J. Neurol. Surg. B Skull Base 2018, 79, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Benson, R.; Bhasker, S.; Mohanti, B.K. Long-term treatment outcomes of juvenile nasopharyngeal angiofibroma treated with radiotherapy. Acta Otorhinolaryngol. Ital. 2015, 35, 75–79. [Google Scholar]
- Chakraborty, S.; Ghoshal, S.; Patil, V.M.; Oinam, A.S.; Sharma, S.C. Conformal radiotherapy in the treatment of advanced juvenile nasopharyngeal angiofibroma with intracranial extension: An institutional experience. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Mitin, T.; Zietman, A.L. Promise and pitfalls of heavy-particle therapy. J. Clin. Oncol. 2014, 32, 2855–2863. [Google Scholar] [CrossRef]
- Laprie, A.; Hu, Y.; Alapetite, C.; Carrie, C.; Habrand, J.L.; Bolle, S.; Bondiau, P.Y.; Ducassou, A.; Huchet, A.; Bertozzi, A.I.; et al. Paediatric brain tumours: A review of radiotherapy, state of the art and challenges for the future regarding protontherapy and carbontherapy. Cancer Radiother. 2015, 19, 775–789. [Google Scholar] [CrossRef]
- Combs, S.E.; Kessel, K.A.; Herfarth, K.; Jensen, A.; Oertel, S.; Blattmann, C.; Ecker, S.; Hoess, A.; Martin, E.; Witt, O.; et al. Treatment of pediatric patients and young adults with particle therapy at the Heidelberg Ion Therapy Center (HIT): Establishment of workflow and initial clinical data. Radiat. Oncol. 2012, 7, 170. [Google Scholar] [CrossRef]
- Hoeltgen, L.; Tessonnier, T.; Meixner, E.; Hoegen, P.; Kim, J.Y.; Deng, M.; Seidensaal, K.; Held, T.; Herfarth, K.; Debus, J.; et al. Proton Therapy for Advanced Juvenile Nasopharyngeal Angiofibroma. Cancers 2023, 15, 5022. [Google Scholar] [CrossRef]
- Tessonnier, T.; Mairani, A.; Brons, S.; Haberer, T.; Debus, J.; Parodi, K. Experimental dosimetric comparison of (1)H, (4)He, (12)C and (16)O scanned ion beams. Phys. Med. Biol. 2017, 62, 3958–3982. [Google Scholar] [CrossRef]
- Knausl, B.; Fuchs, H.; Dieckmann, K.; Georg, D. Can particle beam therapy be improved using helium ions?—A planning study focusing on pediatric patients. Acta Oncol. 2016, 55, 751–759. [Google Scholar] [CrossRef]
- Mairani, A.; Mein, S.; Blakely, E.; Debus, J.; Durante, M.; Ferrari, A.; Fuchs, H.; Georg, D.; Grosshans, D.R.; Guan, F.; et al. Roadmap: Helium ion therapy. Phys. Med. Biol. 2022, 67, 15TR02. [Google Scholar] [CrossRef] [PubMed]
- Wickert, R.; Tessonnier, T.; Deng, M.; Adeberg, S.; Seidensaal, K.; Hoeltgen, L.; Debus, J.; Herfarth, K.; Harrabi, S.B. Radiotherapy with Helium Ions Has the Potential to Improve Both Endocrine and Neurocognitive Outcome in Pediatric Patients with Ependymoma. Cancers 2022, 14, 5865. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, S.G.; Tessonnier, T.; Hoeltgen, L.; Meixner, E.; Harrabi, S.; Horner-Rieber, J.; Haberer, T.; Abdollahi, A.; Debus, J.; Mairani, A. Exploring Helium Ions’ Potential for Post-Mastectomy Left-Sided Breast Cancer Radiotherapy. Cancers 2024, 16, 410. [Google Scholar] [CrossRef]
- Tessonnier, T.; Ecker, S.; Besuglow, J.; Naumann, J.; Mein, S.; Longarino, F.K.; Ellerbrock, M.; Ackermann, B.; Winter, M.; Brons, S.; et al. Commissioning of Helium Ion Therapy and the First Patient Treatment with Active Beam Delivery. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Eekers, D.B.; In’t Ven, L.; Roelofs, E.; Postma, A.; Alapetite, C.; Burnet, N.G.; Calugaru, V.; Compter, I.; Coremans, I.E.M.; Hoyer, M.; et al. The EPTN consensus-based atlas for CT- and MR-based contouring in neuro-oncology. Radiother. Oncol. 2018, 128, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Chera, B.S.; Amdur, R.J.; Patel, P.; Mendenhall, W.M. A radiation oncologist’s guide to contouring the hippocampus. Am. J. Clin. Oncol. 2009, 32, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H.; Blakely, E.; Carabe-Fernandez, A.; Carlson, D.J.; Das, I.J.; Dong, L.; Grosshans, D.; Held, K.D.; Mohan, R.; Moiseenko, V.; et al. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med. Phys. 2019, 46, e53–e78. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Constine, L.S.; Deasy, J.O.; Eisbruch, A.; Jackson, A.; Marks, L.B.; Ten Haken, R.K.; Yorke, E.D. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): An introduction to the scientific issues. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Mairani, A.; Magro, G.; Tessonnier, T.; Bohlen, T.T.; Molinelli, S.; Ferrari, A.; Parodi, K.; Debus, J.; Haberer, T. Optimizing the modified microdosimetric kinetic model input parameters for proton and (4)He ion beam therapy application. Phys. Med. Biol. 2017, 62, N244–N256. [Google Scholar] [CrossRef]
- D’Souza, W.D.; Rosen, I.I. Nontumor integral dose variation in conventional radiotherapy treatment planning. Med. Phys. 2003, 30, 2065–2071. [Google Scholar] [CrossRef]
- Merchant, T.E.; Kiehna, E.N.; Li, C.; Shukla, H.; Sengupta, S.; Xiong, X.; Gajjar, A.; Mulhern, R.K. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Stavinoha, P.L.; Rongthong, W.; Brodin, N.P.; McGovern, S.L.; El Naqa, I.; Palmer, J.D.; Vennarini, S.; Indelicato, D.J.; Aridgides, P.; et al. Neurocognitive Effects and Necrosis in Childhood Cancer Survivors Treated with Radiation Therapy: A PENTEC Comprehensive Review. Int. J. Radiat. Oncol. Biol. Phys. 2021, 119, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Hermann, B.P.; Mehta, M.P.; Tome, W.A. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, G.; Grassberger, C.; Samers, J.; Dwyer, M.; Wiltshire, K.; Daly, P.; Alvarez, B.; Campbell, B.A.; Kerr, A.J.; Kron, T.; et al. Central Endocrine Complications among Childhood Cancer Survivors Treated with Radiation Therapy: A PENTEC Comprehensive Review. Int. J. Radiat. Oncol. Biol. Phys. 2023, 119, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Dell’Oro, M.; Wilson, P.; Short, M.; Hua, C.H.; Merchant, T.E.; Bezak, E. Normal tissue complication probability modeling to guide individual treatment planning in pediatric cranial proton and photon radiotherapy. Med. Phys. 2022, 49, 742–755. [Google Scholar] [CrossRef]
- Batth, S.S.; Sreeraman, R.; Dienes, E.; Beckett, L.A.; Daly, M.E.; Cui, J.; Mathai, M.; Purdy, J.A.; Chen, A.M. Clinical-dosimetric relationship between lacrimal gland dose and ocular toxicity after intensity-modulated radiotherapy for sinonasal tumours. Br. J. Radiol. 2013, 86, 20130459. [Google Scholar] [CrossRef]
- Dutz, A.; Luhr, A.; Agolli, L.; Troost, E.G.C.; Krause, M.; Baumann, M.; Vermeren, X.; Geismar, D.; Schapira, E.F.; Bussiere, M.; et al. Development and validation of NTCP models for acute side-effects resulting from proton beam therapy of brain tumours. Radiother. Oncol. 2019, 130, 164–171. [Google Scholar] [CrossRef]
- Houweling, A.C.; Philippens, M.E.; Dijkema, T.; Roesink, J.M.; Terhaard, C.H.; Schilstra, C.; Ten Haken, R.K.; Eisbruch, A.; Raaijmakers, C.P. A comparison of dose-response models for the parotid gland in a large group of head-and-neck cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.F.; Yeh, S.A.; Chao, P.J.; Chang, L.; Chiu, C.L.; Ting, H.M.; Wang, H.Y.; Huang, Y.J. Normal tissue complication probability modeling for cochlea constraints to avoid causing tinnitus after head-and-neck intensity-modulated radiation therapy. Radiat. Oncol. 2015, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- De Marzi, L.; Feuvret, L.; Boule, T.; Habrand, J.L.; Martin, F.; Calugaru, V.; Fournier-Bidoz, N.; Ferrand, R.; Mazal, A. Use of gEUD for predicting ear and pituitary gland damage following proton and photon radiation therapy. Br. J. Radiol. 2015, 88, 20140413. [Google Scholar] [CrossRef]
- Burman, C.; Kutcher, G.J.; Emami, B.; Goitein, M. Fitting of normal tissue tolerance data to an analytic function. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Moteabbed, M.; Yock, T.I.; Paganetti, H. The risk of radiation-induced second cancers in the high to medium dose region: A comparison between passive and scanned proton therapy, IMRT and VMAT for pediatric patients with brain tumors. Phys. Med. Biol. 2014, 59, 2883–2899. [Google Scholar] [CrossRef]
- Schneider, U.; Kaser-Hotz, B. Radiation risk estimates after radiotherapy: Application of the organ equivalent dose concept to plateau dose-response relationships. Radiat. Environ. Biophys. 2005, 44, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, S.; Briganti, G.; Mancuso, M.; Saran, A. Neurocognitive Decline Following Radiotherapy: Mechanisms and Therapeutic Implications. Cancers 2020, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Turnquist, C.; Harris, B.T.; Harris, C.C. Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neurooncol. Adv. 2020, 2, vdaa057. [Google Scholar] [CrossRef] [PubMed]
- Rube, C.E.; Raid, S.; Palm, J.; Rube, C. Radiation-Induced Brain Injury: Age Dependency of Neurocognitive Dysfunction Following Radiotherapy. Cancers 2023, 15, 2999. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, S.; Ekuni, D.; Tomofuji, T.; Azuma, T.; Kataoka, K.; Yamane, M.; Iwasaki, Y.; Morita, M. Relationship between xerostomia and gingival condition in young adults. J. Periodontal Res. 2015, 50, 74–79. [Google Scholar] [CrossRef]
- Stolze, J.; Teepen, J.C.; Raber-Durlacher, J.E.; Loonen, J.J.; Kok, J.L.; Tissing, W.J.E.; de Vries, A.C.H.; Neggers, S.; van Dulmen-den Broeder, E.; van den Heuvel-Eibrink, M.M.; et al. Prevalence and Risk Factors for Hyposalivation and Xerostomia in Childhood Cancer Survivors Following Different Treatment Modalities—A Dutch Childhood Cancer Survivor Study Late Effects 2 Clinical Study (DCCSS LATER 2). Cancers 2022, 14, 3379. [Google Scholar] [CrossRef]
- Trendowski, M.R.; Baedke, J.L.; Sapkota, Y.; Travis, L.B.; Zhang, X.; El Charif, O.; Wheeler, H.E.; Leisenring, W.M.; Robison, L.L.; Hudson, M.M.; et al. Clinical and genetic risk factors for radiation-associated ototoxicity: A report from the Childhood Cancer Survivor Study and the St. Jude Lifetime Cohort. Cancer 2021, 127, 4091–4102. [Google Scholar] [CrossRef]
- Stokkevag, C.H.; Journy, N.; Vogelius, I.R.; Howell, R.M.; Hodgson, D.; Bentzen, S.M. Radiation Therapy Technology Advances and Mitigation of Subsequent Neoplasms in Childhood Cancer Survivors. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 681–696. [Google Scholar] [CrossRef]
- Inskip, P.D.; Sigurdson, A.J.; Veiga, L.; Bhatti, P.; Ronckers, C.; Rajaraman, P.; Boukheris, H.; Stovall, M.; Smith, S.; Hammond, S.; et al. Radiation-Related New Primary Solid Cancers in the Childhood Cancer Survivor Study: Comparative Radiation Dose Response and Modification of Treatment Effects. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 800–807. [Google Scholar] [CrossRef]
- Withrow, D.R.; Anderson, H.; Armstrong, G.T.; Hawkins, M.; Journy, N.; Neglia, J.P.; de Vathaire, F.; Tucker, M.A.; Inskip, P.D.; Brenner, A.V.; et al. Pooled Analysis of Meningioma Risk Following Treatment for Childhood Cancer. JAMA Oncol. 2022, 8, 1756–1764. [Google Scholar] [CrossRef]
- Yonai, S.; Matsufuji, N.; Kanai, T.; Matsui, Y.; Matsushita, K.; Yamashita, H.; Numano, M.; Sakae, T.; Terunuma, T.; Nishio, T.; et al. Measurement of neutron ambient dose equivalent in passive carbon-ion and proton radiotherapies. Med. Phys. 2008, 35, 4782–4792. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Karger, C.P.; Feuerhake, A.; Nikoghosyan, A.; Combs, S.E.; Jakel, O.; Edler, L.; Scholz, M.; Debus, J. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Mandrillon, J.; Abs, M.; Cailliau, P.; Deprez, S.; Donzel, X.; Goose, G.; Jongen, Y.; Kleeven, W.; Koffel, L.; Nuttens, V. Status on NHa C400 cyclotron for hadrontherapy. JACoW 2022, 2022, 264–268. [Google Scholar]
- Iwata, Y.; Shirai, T.; Mizushima, K.; Matsuba, S.; Yang, Y.; Noda, E.; Urata, M.; Muramatsu, M.; Katagiri, K.; Yonai, S. Design of a compact superconducting accelerator for advanced heavy-ion therapy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2023, 1053, 168312. [Google Scholar] [CrossRef]
| Patient, Tumor and Treatment Characteristics | |
|---|---|
| Median patient age | 14 (range 12–21) years |
| Radkowski tumor stage | III |
| Median GTV volume | 35 (range 7–166) cm3 |
| Treatment setting | |
| Definitive setting | n = 6 (55%) |
| Postoperative setting | n = 5 (45%) |
| Number of treatment beams | |
| Two beams | n = 3 (27%) |
| Three beams | n = 5 (46%) |
| Four beams | n = 3 (27%) |
| Treatment room | |
| Isocentric gantry | n = 3 (27%) |
| Fixed 90° beam line | n = 8 (73%) |
| Organ at Risk | Complication | Formula | Parameters | Publication |
|---|---|---|---|---|
| Brain | IQ estimation (Wechsler Intelligence Scale) | time = time since radiotherapy (set at 5 years) age = age of the patient at the time of radiotherapy Dmean = average dose | Merchant 2006 [21] | |
| IQ estimation at a long follow-up time | age = age of the patient at the time of radiotherapy Dmean = average dose in 2 Gy (RBE) equivalent dose using an α/β = 3 Gy | Mahajan 2021 [22] | ||
| Neurocognitive impairment estimation at a long follow-up time (IQ < 85) | a = 3.39 n = 1/a TD50 = 33.5 Gy (RBE) m = 0.28 α/β = 3 Gy vi, corresponding to a volume fraction of a structure receiving a dose Di in 2 Gy (RBE) equivalent dose using an α/β = 3 Gy | Mahajan 2021 [22] | ||
| Infratentorial brain | IQ estimation (Wechsler Intelligence Scale) | time = time since radiotherapy (set at 5 years) age = age of the patient at the time of radiotherapy Dmean = average dose | Merchant 2006 [21] | |
| Supratentorial brain | IQ estimation (Wechsler Intelligence Scale) | time = time since radiotherapy (set at 5 years) age = age of the patient at the time of radiotherapy Dmean = average dose | Merchant 2006 [21] | |
| Cochlea (IL/CL) | Tinnitus (grade ≥ 2, 1–2 years post radiotherapy) | n = 1.0 TD50 = 46.52 Gy (RBE) m = 0.35 α/β = 3.25 Gy vi, corresponding to a volume fraction of a structure receiving a dose Di in 2 Gy equivalent dose using an α/β = 3.25 Gy | Dell’Oro 2021 [25] adapted from Lee 2015 [29] and De Marzi 2015 [30] | |
| Hearing loss (grade ≥ 1–2, 2 years post radiotherapy) | n = 1.0 TD50 = 55.57 Gy (RBE) m = 0.14 α/β = 3.25 Gy vi, corresponding to a volume fraction of a structure receiving a dose Di in 2 Gy (RBE) equivalent dose using an α/β = 3.25 Gy | |||
| Hippocampus bilateral | Delayed recall (Wechsler Memory Scale III Word Lists, 1.5 years post radiotherapy) | D40% = minimum dose received in 40% of the structure volume, in 2 Gy (RBE) equivalent dose using an α/β = 2.0 Gy TD50 = 14.88 Gy (RBE) m = 0.54 | Gondi 2012 [23] | |
| Pituitary | Endocrine dysfunction (grade ≥ 1–2, 2 years post radiotherapy) | n = 0.25 TD50 = 60.6 Gy (RBE) m = 0.15 α/β = 2.5 Gy vi, corresponding to a volume fraction of a structure receiving a dose Di in 2 Gy (RBE) equivalent dose using an α/β = 2.5 Gy | Dell’Orro 2021 [25] adapted from De Marzi 2015 [30] | |
| GH deficiency (5 years post radiotherapy) | TD50 = 27.2 Gy (RBE) γ = 0.5 D2% = minimum dose received in 2% of the structure, in 2 Gy (RBE) equivalent dose using an α/β = 3 Gy | Wheeler 2023 [24] | ||
| Hypothyroidism (5 years post radiotherapy) | TD50 = 39.2 Gy (RBE) γ = 0.75 D2% = minimum dose received in 2% of the structure, in 2 Gy (RBE) equivalent dose using an α/β = 3 Gy | |||
| ACTH-deficiency (5 years post radiotherapy) | TD50 = 58.0 Gy (RBE) γ = 0.74 D2% = minimum dose received in 2% of the structure, in 2 Gy (RBE) equivalent dose using an α/β = 3 Gy | |||
| Lacrimal gland IL | Ocular toxicity (grade ≥ 2, acute toxicity) | Dmax = minimum dose received in 0.03 cm3 of the structure β0 = −5.174 β1 = 0.205 Gy−1 | Batth 2013 [26] | |
| Lens (IL/CL) | Cataract (5 years post radiotherapy) | a = 3.33 n = 1/a TD50 = 18.0 Gy (RBE) m = 0.27 vi, corresponding to a volume fraction of a structure receiving a dose Di | Burman 1991 [31] | |
| Parotis (IL/CL) | Xerostomia (1 year post radiotherapy | n = 1.0 TD50 = 39.9 Gy (RBE) m = 0.4 vi, corresponding to a volume fraction of a structure receiving a dose Di | Houweling 2010 [28] | |
| Skin | Alopecia (grade ≥ 2, acute toxicity) | D5% = minimum dose received in 5% of the volume structure β0 = −1.33 β1 = 0.08 Gy−1 | Dutz 2019 [27] | |
| Erythema (grade ≥ 2, acute toxicity) | V35 = volume of the structure in cm3 receiving a minimum dose of 35 Gy (RBE) β0 = −1.54 β1 = 0.06 cm−3 |
| Helium | Proton | Δabs (Helium–Proton) | Δrel (Helium–Proton) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | p-Value | ||
| CTV | D0.03cm3 | 103.6 | ± | 0.6 | 105.3 | ± | 2.2 | −1.7 | ± | 1.8 | −1.6 | ± | 1.7 | 0.001 |
| D2% | 101.8 | ± | 0.5 | 102.8 | ± | 0.6 | −1.1 | ± | 0.5 | −1.0 | ± | 0.5 | 0.001 | |
| D95% | 98.4 | ± | 1.2 | 97.2 | ± | 1.8 | 1.2 | ± | 0.8 | 1.2 | ± | 0.9 | 0.003 | |
| V95% | 99.4 | ± | 1.3 | 98.0 | ± | 4.2 | 1.4 | ± | 3.0 | 1.6 | ± | 3.5 | 0.020 | |
| V107% | 0.0 | ± | 0.0 | 0.1 | ± | 0.2 | −0.1 | ± | 0.2 | - | ± | - | 0.016 | |
| HI | 2.9 | ± | 1.3 | 5.0 | ± | 1.8 | −2.1 | ± | 0.8 | −42.9 | ± | 11.2 | 0.001 | |
| CI | 0.57 | ± | 0.19 | 0.57 | ± | 0.18 | 0.01 | ± | 0.04 | 0.3 | ± | 6.0 | 1.000 | |
| Helium | Proton | Δabs (Helium–Proton) | Δrel (Helium–Proton) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | p-Value | ||
| Brain (without CTV) | D0.03cm3 | 45.1 | ± | 0.8 | 45.4 | ± | 0.9 | −0.3 | ± | 0.4 | −0.6 | ± | 0.9 | 0.039 |
| Dmean | 2.2 | ± | 1.1 | 2.9 | ± | 1.4 | −0.8 | ± | 0.5 | −26.5 | ± | 9.5 | 0.001 | |
| V10 | 6.8 | ± | 3.6 | 11.1 | ± | 6.5 | −4.3 | ± | 3.9 | −35.1 | ± | 16.2 | 0.001 | |
| V15 | 4.4 | ± | 2.3 | 7.0 | ± | 3.8 | −2.6 | ± | 2.0 | −34.6 | ± | 13.8 | 0.001 | |
| V20 | 3.0 | ± | 1.6 | 4.6 | ± | 2.5 | −1.5 | ± | 1.0 | −32.5 | ± | 9.4 | 0.001 | |
| ID | 3538.1 | ± | 1815.5 | 4812.2 | ± | 2337.4 | −1274.1 | ± | 828.4 | |||||
| Brain supratentorial | D0.03cm3 | 45.5 | ± | 0.5 | 46.0 | ± | 0.6 | −0.6 | ± | 0.4 | −1.1 | ± | 0.8 | 0.003 |
| Dmean | 2.4 | ± | 1.2 | 3.3 | ± | 1.6 | −0.9 | ± | 0.8 | −25.7 | ± | 1.8 | 0.001 | |
| V10 | 7.8 | ± | 4.4 | 12.2 | ± | 7.3 | −4.4 | ± | 4.2 | −32.8 | ± | 14.0 | 0.001 | |
| V15 | 5.1 | ± | 2.8 | 7.7 | ± | 4.3 | −2.6 | ± | 2.1 | −31.5 | ± | 8.0 | 0.001 | |
| V20 | 3.7 | ± | 2.1 | 5.1 | ± | 2.9 | −1.5 | ± | 1.0 | −27.9 | ± | 16.8 | 0.001 | |
| ID | 3409.0 | ± | 1839.4 | 4619.1 | ± | 2391.3 | −1210.1 | ± | 924.3 | |||||
| Brain infratentorial | D0.03cm3 | 43.0 | ± | 6.5 | 43.3 | ± | 6.5 | −0.4 | ± | 0.6 | −0.8 | ± | 4.9 | 0.067 |
| Dmean | 1.9 | ± | 1.1 | 2.6 | ± | 1.4 | −0.8 | ± | 0.5 | −29.5 | ± | 8.9 | 0.001 | |
| V10 | 5.3 | ± | 3.2 | 8.0 | ± | 4.4 | −2.7 | ± | 1.6 | −36.0 | ± | 6.3 | 0.001 | |
| V15 | 4.2 | ± | 2.8 | 6.2 | ± | 3.8 | −2.0 | ± | 1.3 | −35.0 | ± | 12.2 | 0.001 | |
| V20 | 3.3 | ± | 2.5 | 4.8 | ± | 3.3 | −1.5 | ± | 1.1 | −33.4 | ± | 21.7 | 0.001 | |
| ID | 472.0 | ± | 289.0 | 657.0 | ± | 343.2 | −185.0 | ± | 102.5 | |||||
| Brainstem | D0.03cm3 | 32.9 | ± | 10.3 | 35.8 | ± | 8.7 | −2.9 | ± | 2.7 | −9.6 | ± | 9.9 | 0.002 |
| Dmean | 4.8 | ± | 3.9 | 7.7 | ± | 4.8 | −2.9 | ± | 1.7 | −41.7 | ± | 15.2 | 0.001 | |
| ID | 134.4 | ± | 108.7 | 213.5 | ± | 137.1 | −79.1 | ± | 50.4 | |||||
| Hippocampus bilateral | D0.03cm3 | 20.9 | ± | 13.0 | 28.8 | ± | 11.6 | −7.9 | ± | 4.5 | −32.7 | ± | 17.8 | 0.001 |
| D40% | 1.6 | ± | 3.1 | 4.0 | ± | 5.3 | −2.4 | ± | 2.7 | −65.7 | ± | 14.7 | 0.001 | |
| Dmean | 2.8 | ± | 2.7 | 5.6 | ± | 3.9 | −2.8 | ± | 1.8 | −54.1 | ± | 14.4 | 0.001 | |
| ID | 11.4 | ± | 10.0 | 23.1 | ± | 14.5 | −11.6 | ± | 7.6 | |||||
| Hippocampus CL | D0.03cm3 | 12.5 | ± | 13.9 | 18.7 | ± | 15.4 | −6.2 | ± | 4.2 | −50.3 | ± | 25.7 | 0.001 |
| Dmean | 1.8 | ± | 2.2 | 3.7 | ± | 3.9 | −1.9 | ± | 2.1 | −57.9 | ± | 15.8 | 0.001 | |
| ID | 3.7 | ± | 4.4 | 7.3 | ± | 7.2 | −3.7 | ± | 3.9 | |||||
| Hippocampus IL | D0.03cm3 | 19.9 | ± | 13.6 | 27.9 | ± | 12.1 | −8.0 | ± | 5.0 | −35.2 | ± | 20.4 | 0.001 |
| Dmean | 3.9 | ± | 3.5 | 7.7 | ± | 4.9 | −3.8 | ± | 2.4 | −53.1 | ± | 14.8 | 0.001 | |
| ID | 7.8 | ± | 6.2 | 15.7 | ± | 9.3 | −8.0 | ± | 5.2 | |||||
| Pituitary | D0.03cm3 | 39.2 | ± | 8.1 | 41.1 | ± | 6.5 | −1.8 | ± | 2.2 | −5.2 | ± | 7.6 | 0.001 |
| Dmean | 36.3 | ± | 11.4 | 37.9 | ± | 10.1 | −1.7 | ± | 1.7 | −6.1 | ± | 8.8 | 0.003 | |
| ID | 14.6 | ± | 7.3 | 15.3 | ± | 7.2 | −0.7 | ± | 0.6 | |||||
| Chiasma | D0.03cm3 | 36.5 | ± | 12.3 | 37.6 | ± | 11.0 | −1.1 | ± | 1.4 | −5.3 | ± | 9.0 | 0.007 |
| Dmean | 25.2 | ± | 15.0 | 27.0 | ± | 14.2 | −1.7 | ± | 2.1 | −11.5 | ± | 12.7 | 0.024 | |
| ID | 38.7 | ± | 26.6 | 41.8 | ± | 28.2 | −3.1 | ± | 5.1 | |||||
| Optic nerve CL | D0.03cm3 | 36.3 | ± | 10.8 | 36.9 | ± | 10.4 | −0.6 | ± | 1.2 | −2.2 | ± | 4.5 | 0.102 |
| Dmean | 26.2 | ± | 13.3 | 27.8 | ± | 12.3 | −1.6 | ± | 2.0 | −9.2 | ± | 12.2 | 0.010 | |
| ID | 30.6 | ± | 33.0 | 31.8 | ± | 32.1 | −1.2 | ± | 1.9 | |||||
| Optic nerve IL | D0.03cm3 | 41.8 | ± | 2.7 | 41.9 | ± | 2.9 | −0.2 | ± | 0.6 | −0.4 | ± | 1.5 | 0.377 |
| Dmean | 34.4 | ± | 9.0 | 35.4 | ± | 8.0 | −1.0 | ± | 1.2 | −3.6 | ± | 4.6 | 0.054 | |
| ID | 35.1 | ± | 28.0 | 35.8 | ± | 27.2 | −0.7 | ± | 1.1 | |||||
| Eye CL | D0.03cm3 | 14.2 | ± | 10.5 | 19.1 | ± | 9.0 | −4.9 | ± | 4.1 | −32.0 | ± | 29.8 | 0.001 |
| Dmean | 4.0 | ± | 3.6 | 6.1 | ± | 4.3 | −2.1 | ± | 1.6 | −43.0 | ± | 25.4 | 0.001 | |
| V10 | 11.1 | ± | 15.6 | 19.1 | ± | 23.4 | −8.0 | ± | 9.8 | −66.7 | ± | 31.4 | 0.001 | |
| ID | 36.9 | ± | 33.5 | 56.4 | ± | 41.3 | −19.5 | ± | 15.6 | |||||
| Eye IL | D0.03cm3 | 26.9 | ± | 12.4 | 28.6 | ± | 11.3 | −1.6 | ± | 3.5 | −8.3 | ± | 17.3 | 0.123 |
| Dmean | 9.4 | ± | 6.8 | 11.1 | ± | 7.2 | −1.8 | ± | 1.3 | −22.3 | ± | 17.9 | 0.001 | |
| V10 | 36.6 | ± | 28.5 | 43.1 | ± | 30.2 | −7.5 | ± | 5.9 | −35.5 | ± | 34.8 | 0.001 | |
| ID | 87.2 | ± | 65.0 | 103.4 | ± | 69.4 | −16.2 | ± | 12.0 | |||||
| Lens CL | D0.03cm3 | 2.3 | ± | 2.1 | 4.2 | ± | 3.6 | −1.9 | ± | 2.2 | −47.6 | ± | 25.1 | 0.001 |
| Dmean | 1.7 | ± | 1.8 | 3.2 | ± | 2.8 | −1.5 | ± | 1.8 | −47.9 | ± | 27.7 | 0.001 | |
| ID | 0.3 | ± | 0.3 | 0.7 | ± | 0.6 | −0.3 | ± | 0.4 | |||||
| Lens IL | D0.03cm3 | 4.9 | ± | 5.8 | 6.8 | ± | 7.3 | −0.1 | ± | 1.9 | −37.5 | ± | 20.2 | 0.001 |
| Dmean | 3.6 | ± | 4.2 | 5.3 | ± | 5.3 | −1.6 | ± | 1.5 | −39.8 | ± | 18.8 | 0.001 | |
| ID | 0.8 | ± | 1.0 | 1.1 | ± | 1.4 | −0.4 | ± | 0.4 | |||||
| Lacrimal gland CL | D0.03cm3 | 3.6 | ± | 4.0 | 5.5 | ± | 4.8 | −1.9 | ± | 1.8 | −41.6 | ± | 28.5 | 0.001 |
| Dmean | 1.8 | ± | 2.3 | 3.0 | ± | 3.6 | −1.2 | ± | 1.7 | −40.1 | ± | 37.5 | 0.007 | |
| ID | 1.9 | ± | 3.0 | 3.3 | ± | 5.5 | −1.4 | ± | 2.6 | |||||
| Lacrimal gland IL | D0.03cm3 | 10.1 | ± | 8.9 | 13.2 | ± | 10.4 | −3.1 | ± | 2.7 | −28.2 | ± | 18.3 | 0.001 |
| Dmean | 5.2 | ± | 4.9 | 7.1 | ± | 6.3 | −1.9 | ± | 1.8 | −31.3 | ± | 16.8 | 0.001 | |
| ID | 4.5 | ± | 5.3 | 6.3 | ± | 7.3 | −1.8 | ± | 2.1 | |||||
| Cochlea CL | D0.03cm3 | 4.9 | ± | 6.1 | 8.1 | ± | 6.7 | −3.3 | ± | 2.6 | −52.6 | ± | 24.1 | 0.002 |
| Dmean | 4.1 | ± | 5.4 | 7.3 | ± | 6.1 | −3.1 | ± | 2.5 | −55.3 | ± | 23.5 | 0.002 | |
| ID | 0.5 | ± | 0.7 | 0.9 | ± | 0.8 | −0.4 | ± | 0.3 | |||||
| Cochlea IL | D0.03cm3 | 17.1 | ± | 12.0 | 22.7 | ± | 13.7 | −5.6 | ± | 4.5 | −29.5 | ± | 17.2 | 0.001 |
| Dmean | 14.7 | ± | 10.3 | 20.7 | ± | 12.4 | −5.9 | ± | 4.3 | −32.9 | ± | 16.5 | 0.001 | |
| ID | 2.1 | ± | 2.2 | 2.8 | ± | 2.6 | −0.8 | ± | 0.7 | |||||
| Parotis CL | D0.03cm3 | 11.3 | ± | 5.5 | 16.0 | ± | 6.4 | −4.7 | ± | 3.8 | −29.5 | ± | 18.0 | 0.002 |
| Dmean | 3.9 | ± | 3.6 | 6.8 | ± | 4.1 | −2.8 | ± | 2.2 | −45.4 | ± | 23.6 | 0.002 | |
| ID | 74.7 | ± | 74.9 | 125.1 | ± | 93.7 | −50.4 | ± | 45.3 | |||||
| Parotis IL | D0.03cm3 | 23.1 | ± | 13.1 | 26.9 | ± | 11.9 | −3.8 | ± | 2.8 | −17.9 | ± | 13.3 | 0.001 |
| Dmean | 8.1 | ± | 7.9 | 10.6 | ± | 8.9 | −2.5 | ± | 1.8 | −31.4 | ± | 15.7 | 0.001 | |
| ID | 120.5 | ± | 109.8 | 159.4 | ± | 122.8 | −38.9 | ± | 25.9 | |||||
| Skin | D0.03cm3 | 32.2 | ± | 10.7 | 34.0 | ± | 9.3 | −1.8 | ± | 2.4 | −6.7 | ± | 9.0 | 0.019 |
| V10 | 32.4 | ± | 27.9 | 49.4 | ± | 30.9 | −17.0 | ± | 5.8 | −31.2 | ± | 6.3 | 0.001 | |
| V15 | 15.3 | ± | 15.7 | 27.5 | ± | 26.5 | −12.2 | ± | 11.9 | −41.0 | ± | 15.4 | 0.001 | |
| V20 | 7.6 | ± | 8.5 | 13.2 | ± | 12.7 | −5.6 | ± | 5.0 | −51.7 | ± | 21.6 | 0.002 | |
| ID | 876.4 | ± | 51.5.5 | 1235.5 | ± | 624.1 | −359.1 | ± | 118.2 | |||||
| Helium | Proton | Δabs (Helium–Proton) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ at Risk | Complication | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | p-Value |
| Cochlea IL | Tinnitus | 3.0 | ± | 3.6 | 7.0 | ± | 7.6 | −4.0 | ± | 5.3 | 0.001 |
| Hippocampus (bilateral) | Delayed recall | 4.6 | ± | 3.5 | 8.0 | ± | 10.2 | −3.5 | ± | 6.9 | 0.001 |
| Lacrimal gland IL | Ocular toxicity | 11.8 | ± | 18.5 | 18.1 | ± | 27.0 | −6.3 | ± | 8.6 | 0.001 |
| Lens IL | Cataract | 3.4 | ± | 10.5 | 7.4 | ± | 22.9 | −3.9 | ± | 12.9 | 0.001 |
| Parotis IL | Xerostomia | 3.9 | ± | 5.5 | 5.6 | ± | 7.6 | −1.7 | ± | 2.7 | 0.001 |
| Skin | Alopecia | 38.0 | ± | 9.4 | 44.5 | ± | 10.2 | −6.4 | ± | 1.7 | 0.001 |
| RR (Proton/Helium) | |||||||||||
| Mean | ± | SD | p-value | ||||||||
| Brain | Secondary malignancies | 1.4 | ± | 0.2 | 0.001 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoeltgen, L.; Meixner, E.; Hoegen-Saßmannshausen, P.; Kim, J.-Y.; Deng, M.; Seidensaal, K.; Held, T.; Herfarth, K.; Haberer, T.; Debus, J.; et al. Helium Ion Therapy for Advanced Juvenile Nasopharyngeal Angiofibroma. Cancers 2024, 16, 1993. https://doi.org/10.3390/cancers16111993
Hoeltgen L, Meixner E, Hoegen-Saßmannshausen P, Kim J-Y, Deng M, Seidensaal K, Held T, Herfarth K, Haberer T, Debus J, et al. Helium Ion Therapy for Advanced Juvenile Nasopharyngeal Angiofibroma. Cancers. 2024; 16(11):1993. https://doi.org/10.3390/cancers16111993
Chicago/Turabian StyleHoeltgen, Line, Eva Meixner, Philipp Hoegen-Saßmannshausen, Ji-Young Kim, Maximilian Deng, Katharina Seidensaal, Thomas Held, Klaus Herfarth, Thomas Haberer, Jürgen Debus, and et al. 2024. "Helium Ion Therapy for Advanced Juvenile Nasopharyngeal Angiofibroma" Cancers 16, no. 11: 1993. https://doi.org/10.3390/cancers16111993
APA StyleHoeltgen, L., Meixner, E., Hoegen-Saßmannshausen, P., Kim, J.-Y., Deng, M., Seidensaal, K., Held, T., Herfarth, K., Haberer, T., Debus, J., Mairani, A., Harrabi, S., & Tessonnier, T. (2024). Helium Ion Therapy for Advanced Juvenile Nasopharyngeal Angiofibroma. Cancers, 16(11), 1993. https://doi.org/10.3390/cancers16111993






