Effect of Radiotherapy on the Right Ventricular Function in Lung Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
- -
- Age over 18;
- -
- Histologically proven and unresectable lung cancer;
- -
- Planned treatment with radiochemotherapy or chemotherapy;
- -
- A written patient consent for the study participation.
- -
- Planned or previous surgical treatment of lung cancer;
- -
- History of potentially cardiotoxic oncological treatment;
- -
- No written consent for the study participation.
- -
- New LV ejection fraction (LVEF) reduction to <40% indicates severe LV cardiotoxicity;
- -
- New LVEF reduction by ≥10% to a LVEF of 40–49% or new LVEF reduction by <10% to a LVEF of 40–49% and either new relative decline in LV GLS by >15% from baseline or new rise in cardiac biomarkers indicate moderate LV cardiotoxicity;
- -
- LVEF ≥ 50% and a new relative decline in GLS by >15% from baseline and/or a new rise in cardiac biomarkers indicate mild LV cardiotoxicity.
3. Statistical Analysis
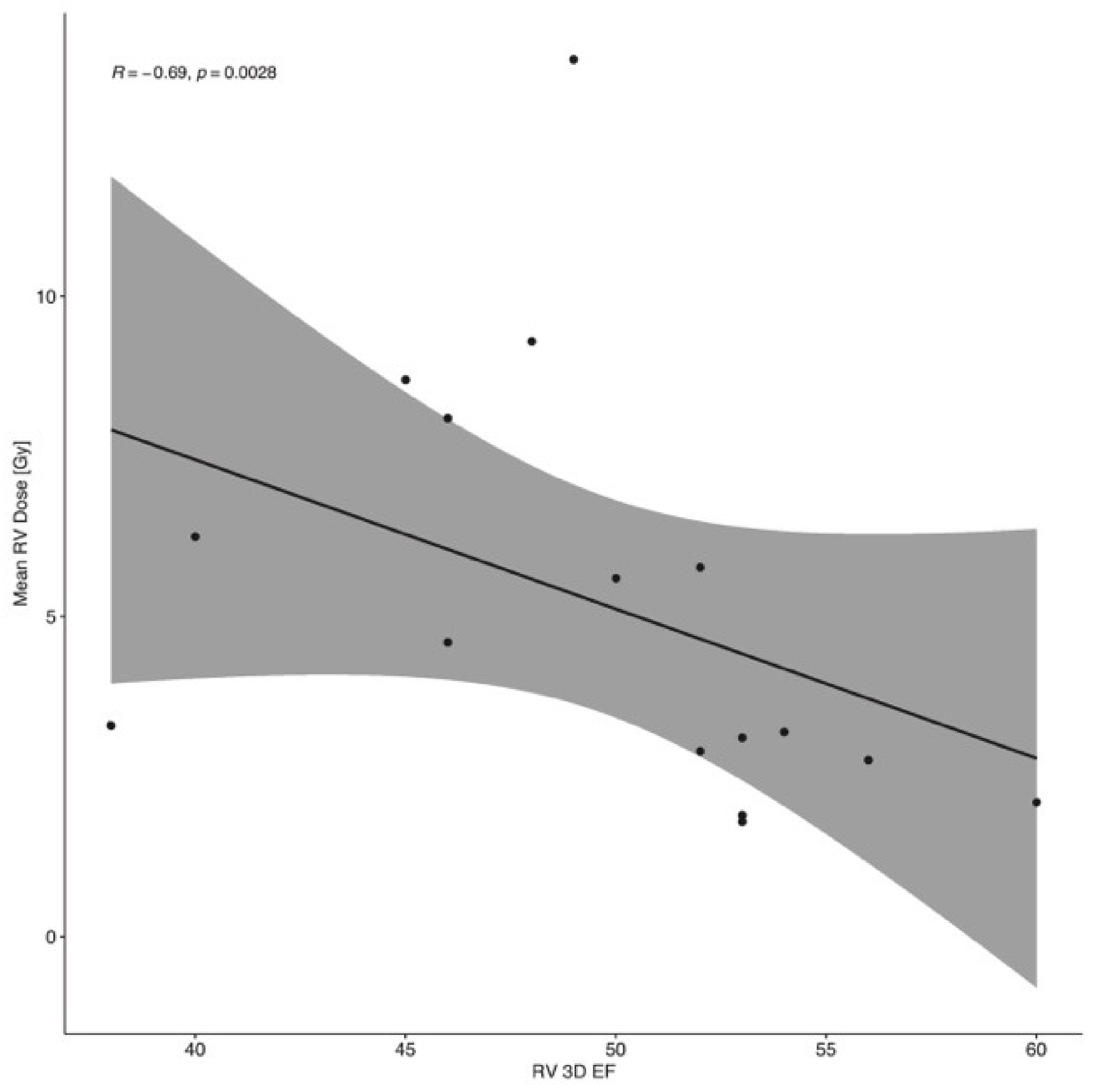
4. Results
5. Discussion
6. Limitations of the Study
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Eblan, M.J.; Deal, A.M.; Lipner, M.; Zagar, T.M.; Wang, Y.; Mavroidis, P.; Lee, C.B.; Jensen, B.C.; Rosenman, J.G.; et al. Cardiac Toxicity After Radiotherapy for Stage III Non–Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J. Clin. Oncol. 2017, 35, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Atkins, K.M.; Rawal, B.; Chaunzwa, T.L.; Lamba, N.; Bitterman, D.S.; Williams, C.L.; Kozono, D.E.; Baldini, E.H.; Chen, A.B.; Nguyen, P.L.; et al. Cardiac Radiation Dose, Cardiac Disease, and Mortality in Patients with Lung Cancer. J. Am. Coll. Cardiol. 2019, 73, 2976–2987. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantifi-cation by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Associ-ation of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Shu, F.; Zhang, C.; Song, F.; Xu, Y.; Guo, Y.; Xue, K.; Lin, J.; Shu, X.; Hsi, D.H.; et al. Early Detection and Prediction of Anthracycline-Induced Right Ventricular Cardiotoxicity by 3-Dimensional Echocardiography. JACC CardioOncol. 2020, 2, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Laufer-Perl, M.; Perelman-Gvili, M.; Dorfman, S.S.; Baruch, G.; Rothschild, E.; Beer, G.; Arbel, Y.; Arnold, J.H.; Rozenbaum, Z.; Banai, S.; et al. Prevalence of Right Ventricle Strain Changes following Anthracycline Therapy. Life 2022, 12, 291. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Duane, F.; Aznar, M.C.; Bartlett, F.; Cutter, D.J.; Darby, S.C.; Jagsi, R.; Lorenzen, E.L.; McArdle, O.; McGale, P.; Myerson, S.; et al. A cardiac contouring atlas for radiotherapy. Radiother. Oncol. 2017, 122, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, J.; Wu, W.; Ta, S.; Xie, X. The impact of right ventricular function on prognosis in patients with stage III non-small cell lung cancer after concurrent chemoradiotherapy. Int. J. Cardiovasc. Imaging 2019, 35, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Smolarek, D.; Gruchała, M.; Sobiczewski, W. Echocardiographic evaluation of right ventricular systolic function: The traditional and innovative approach. Cardiol. J. 2017, 24, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.-C.; Takeuchi, M. Echocardiographic assessment of right ventricular systolic function. Cardiovasc. Diagn. Ther. 2018, 8, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Soloperto, R.; Casciano, O.; Esposito, R.; Lembo, M.; Canonico, M.; Arpino, G.; Giuliano, M.; De Placido, S.; Esposito, G. Right ventricular dysfunction parallels left ven-tricular functional involvement in women with breast cancer experiencing subclinical cardiotoxicity. Eur. Heart J. Cardiovasc. Imaging 2021, 22 (Suppl. S1), jeaa356-187. [Google Scholar] [CrossRef]
- Christiansen, J.R.; Massey, R.; Dalen, H.; Kanellopoulos, A.; Hamre, H.; Ruud, E.; Kiserud, C.E.; Fosså, S.D.; Aakhus, S. Right ventricular function in long-term adult survivors of childhood lymphoma and acute lymphoblastic leukaemia. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Murbraech, K.; Holte, E.; Broch, K.; Smeland, K.B.; Holte, H.; Rösner, A.; Lund, M.B.; Dalen, H.; Kiserud, C.; Aakhus, S. Impaired Right Ventricular Function in Long-Term Lymphoma Survivors. J. Am. Soc. Echocardiogr. 2016, 29, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Keramida, K.; Farmakis, D.; Bingcang, J.; Sulemane, S.; Sutherland, S.; Bingcang, R.A.; Ramachandran, K.; Tzavara, C.; Charalampopoulos, G.; Filippiadis, D.; et al. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur. J. Heart Fail. 2019, 21, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Shih, J.-Y.; Feng, Y.-H.; Chiang, C.-Y.; Kuo, Y.H.; Chen, W.-Y.; Wu, H.-C.; Cheng, J.-T.; Wang, J.-J.; Chen, Z.-C. The Early Predictive Value of Right Ventricular Strain in Epirubicin-Induced Cardiotoxicity in Patients with Breast Cancer. Acta Cardiol. Sin. 2016, 32, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Boczar, K.E.; Aseyev, O.; Sulpher, J.; Johnson, C.; Burwash, I.G.; Turek, M.; Dent, S.; Dwivedi, G. Right heart function deteriorates in breast cancer patients undergoing anthracycline-based chemotherapy. Echo Res. Pract. 2016, 3, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Calleja, A.; Poulin, F.; Khorolsky, C.; Shariat, M.; Bedard, P.L.; Amir, E.; Rakowski, H.; McDonald, M.; Delgado, D.; Thavendiranathan, P. Right Ventricular Dysfunction in Patients Experiencing Cardiotoxicity during Breast Cancer Therapy. J. Oncol. 2015, 2015, 609194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, P.; Liu, K.; Zhang, J.; Ma, X.; Li, L.; Li, M.; Liu, J. Evaluation of changes in right ventricular myocardial mechanical properties in breast cancer patients receiving pirarubicin using three-dimensional speckle tracking imaging. Nan Fang Yi Ke Da Xue Xue Bao 2018, 38, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Mao, L.; Liu, H.; Zhang, Y.; Yang, J. Assessment of Subclinical Deterioration of Right Ventricular Function by Three-Dimensional Speckle Tracking Echocardiography in Breast Cancer Patients Undergoing Anthracycline-Based Chemo-therapy. Int. J. Gen. Med. 2021, 14, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, H.; Zhang, Q.; Zhang, B.; Ni, Y.; Zhao, R.; Hsi, D.H.; Cheng, L. Right Ventricular Ejection Fraction Assessed by Three-Dimensional Echocardiography Is Associated with Long-Term Adverse Clinical Cardiac Events in Patients with Anthracycline-Induced Cardiotoxicity. J. Am. Soc. Echocardiogr. 2022, 35, 600–608. [Google Scholar] [CrossRef] [PubMed]


| Variable | All Patients (n = 43) | Study Group (n = 23) | Control Group (n = 20) | p Value |
|---|---|---|---|---|
| Age, years | 64.9 ± 8.1 | 64.4 ± 8.6 | 65.5 ± 7.5 | 0.68 |
| Males, n (%) | 25 (58.1%) | 13 (56.5%) | 12 (60.0%) | 0.82 |
| Comorbidities, n (%): | ||||
| Hypertension | 26 (60.5%) | 14 (60.1%) | 12 (60.0%) | 1.00 |
| Coronary artery disease | 7 (16.3%) | 3 (13.0%) | 4 (20.0%) | 0.69 |
| Heart failure | 6 (13.9%) | 3 (13.0%) | 3 (15.0%) | 0.32 |
| Atrial fibrillation | 14 (32.6%) | 6 (26.1%) | 8 (40.0%) | 0.10 |
| Hypercholesterolemia | 7 (16.3%) | 6 (26.1%) | 1 (5.0%) | 0.52 |
| Diabetes | 1 (2.3%) | 1 (4.3%) | 0 | 0.10 |
| Chronic kidney disease | 5 (11.6%) | 2 (8.7%) | 3 (15.0%) | 1.00 |
| COPD | 5 (11.6%) | 2 (8.7%) | 3 (15.0%) | 1.00 |
| Charlson Comorbidity Index, median | 2 (0–6) | 1 (0–3) | 6 (2–7) | 0.029 |
| Smoking history, n (%): | 1.00 | |||
| Current | 12 (27.9%) | 6 (26.1%) | 6 (30%) | |
| Former | 27 (62.8%) | 15 (65.2%) | 12 (60%) | |
| Never | 4 (9.3%) | 2 (8.7%) | 2 (10%) | |
| Number of pack-years, n | 37.5 ± 20.2 | 38.5 ± 20.6 | 36.3 ± 20.2 | 0.73 |
| Histological type of lung cancer, n (%): | 0.077 | |||
| Adenocarcinoma | 20 (46.5%) | 9 (39.1%) | 11 (55.0%) | |
| Squamous cell carcinoma | 20 (46.5%) | 14 (60.1%) | 6 (30.0%) | |
| Small cell lung cancer | 2 (4.7%) | 0 | 2 (10.0%) | |
| Carcinoma not otherwise specified | 1 (2.3%) | 0 | 1 (5.0%) | |
| Death, n (%): | 9 (20.9%) | 5 (21.7%) | 4 (20%) | 1.00 |
| All Patients (n = 23) | Left-Sided Lung Cancer (n = 11) | Right-Sided Lung Cancer (n = 12) | p Value | |
|---|---|---|---|---|
| PTV, mL | 610.94 ± 357.67 | 617.99 ± 422.66 | 601.77 ± 272.69 | 0.83 |
| PTV-heart, mL | 11.74 ± 12.60 | 12.04 ± 13.64 | 11.35 ± 11.82 | 0.88 |
| Mean heart dose, Gy | 9.71 ± 5.43 | 9.65 ± 5.81 | 9.79 ± 5.19 | 0.95 |
| Heart V5 Gy, % | 40.46 ± 23.22 | 39.22 ± 24.26 | 42.06 ± 22.98 | 0.78 |
| Heart V30 Gy, % | 9.47 ± 7.72 | 9.58 ± 8.26 | 9.33 ± 7.39 | 0.94 |
| Mean pericardium dose, Gy | 17.75 ± 5.81 | 18.65 ± 6.72 | 16.58 ± 4.42 | 0.38 |
| Mean RV dose, Gy | 4.38 ± 3.18 | 4.19 ± 3.78 | 4.61 ± 2.36 | 0.75 |
| RV V5 Gy, % | 29.77 ± 27.17 | 28.53 ± 31.77 | 31.37 ± 21.29 | 0.44 |
| RV V30 Gy, % | 0.22 ± 0.77 | 0.05 ± 0.14 | 0.45 ± 1.14 | 0.67 |
| Mean LV dose, Gy | 4.61 ± 4.14 | 5.68 ± 4.88 | 3.22 ± 2.50 | 0.13 |
| LV V5 Gy, % | 23.79 ± 25.50 | 25.59 ± 23.97 | 21.44 ± 28.50 | 0.53 |
| LV V30 Gy, % | 1.91 ± 4.91 | 3.32 ± 6.25 | 0.07 ± 0.14 | 0.061 |
| Mean RA dose, Gy | 9.93 ± 10.01 | 6.05 ± 5.45 | 14.98 ± 12.46 | 0.032 |
| RA V5 Gy, % | 39.33 ± 31.02 | 29.42 ± 29.99 | 42.30 ± 27.51 | 0.11 |
| RA V30 Gy, % | 10.45 ± 18.66 | 3.05 ± 6.16 | 20.08 ± 24.87 | 0.005 |
| Mean LA dose, Gy | 17.45 ± 10.36 | 18.05 ± 11.78 | 16.67 ± 8.72 | 0.75 |
| LA V5 Gy, % | 64.45 ± 30.05 | 60.41 ± 34.45 | 59.79 ± 29.73 | 0.45 |
| LA V30 Gy, % | 21.26 ± 19.28 | 24.6 ± 22.36 | 16.92 ± 14.31 | 0.33 |
| Mean LAD dose, Gy | 9.6 ± 5.39 | 11.63 ± 6.18 | 6.97 ± 2.53 | 0.025 |
| Mean Cx dose, Gy | 12.56 ± 12.01 | 17.7 ± 13.77 | 5.87 ± 3.42 | 0.010 |
| Mean RCA dose, Gy | 6.01 ± 7.28 | 4.51 ± 3.88 | 7.97 ± 10.10 | 0.37 |
| All Patients (n = 43) | |||||
|---|---|---|---|---|---|
| Variable | Baseline | Immediately after Treatment | p Value | Three Months after Treatment | p Value (vs. Baseline) |
| 3DRVEF, % | 52.0 ± 7.0 | 51.1 ± 6.6 | 0.34 | 50.4 ± 6.0 | 0.56 |
| RV FWLS, % | −22.5 ± 5.6 | −23.2 ± 5.2 | 0.60 | −22.2 ± 5.9 | 0.27 |
| RV GLS, % | −20.0 ± 5.3 | −19.0 ± 3.6 | 0.09 | −20.6 ± 4.5 | 0.34 |
| RV S’, cm/s | 13.7 ± 3.3 | 13.4 ± 2.2 | 0.56 | 13.7 ± 2.8 | 0.79 |
| TAPSE, mm | 22.4 ± 3.4 | 21.5 ± 3.9 | 0.30 | 21.1 ± 3.4 | 0.09 |
| RVOT, mm | 32.9 ± 4.6 | 32.5 ± 3.7 | 0.19 | 33.3 ± 3.9 | 0.88 |
| RVIT, mm | 33.4 ± 3.4 | 33.4 ± 3.4 | 0.91 | 34.5 ± 4.4 | 0.36 |
| RA area, cm2 | 14.1 ± 3.3 | 14.4 ± 2.8 | 0.43 | 14.5 ± 3.4 | 0.75 |
| Study group (n = 23) | |||||
| 3DRVEF, % | 52.3 ± 6.3 | 49.7 ± 5.7 | 0.08 | 49.4 ± 4.2 | 0.40 |
| RV FWLS, % | −23.8 ± 4.5 | −22.9 ± 5.3 | 0.38 | −21.8 ± 5.8 | 0.046 |
| RV GLS, % | −21.1 ± 4.0 | −18.4 ± 4.1 | 0.001 | −19.1 ± 4.3 | 0.016 |
| RV S’, cm/s | 13.5 ± 3.5 | 12.4 ± 1.9 | 0.41 | 12.8 ± 2.3 | 0.91 |
| TAPSE, mm | 22.1 ± 3.3 | 20.3 ± 3.2 | 0.021 | 20.8 ± 3.3 | 0.051 |
| RVOT, mm | 33.2 ± 5.4 | 32.9 ± 2.2 | 0.07 | 33.1 ± 3.4 | 0.09 |
| RVIT, mm | 34.3 ± 3.1 | 33.7 ± 3.3 | 0.24 | 36.0 ± 3.2 | 0.18 |
| RA area, cm2 | 15.2 ± 4.2 | 14.4 ± 3.3 | 0.37 | 16.2 ± 3.7 | 0.60 |
| Control group (n = 20) | |||||
| 3DRVEF, % | 51.8 ± 7.9 | 52.5 ± 7.2 | 0.73 | 51.7 ± 7.9 | 0.92 |
| RV FWLS, % | −21.2 ± 6.4 | −23.6 ± 5.2 | 0.22 | −22.8 ± 6.4 | 0.82 |
| RV GLS, % | −18.8 ± 6.2 | −19.8 ± 2.9 | 0.80 | −22.8 ± 4.1 | 0.36 |
| RV S’, cm/s | 14.0 ± 3.0 | 14.5 ± 2.0 | 0.81 | 14.8 ± 3.1 | 0.81 |
| TAPSE, mm | 22.8 ± 3.7 | 22.8 ± 4.2 | 0.31 | 21.6 ± 3.6 | 0.55 |
| RVOT, mm | 32.6 ± 3.6 | 32.2 ± 4.8 | 0.14 | 33.5 ± 4.6 | 0.05 |
| RVIT, mm | 32.5 ± 3.5 | 32.9 ± 3.6 | 0.94 | 32.7 ± 5.0 | 0.95 |
| RA area, cm2 | 13.0 ± 1.5 | 14.3 ± 2.2 | 0.03 | 12.5 ± 1.5 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławiński, G.; Hawryszko, M.; Lasocka-Koriat, Z.; Romanowska, A.; Myszczyński, K.; Wrona, A.; Daniłowicz-Szymanowicz, L.; Lewicka, E. Effect of Radiotherapy on the Right Ventricular Function in Lung Cancer Patients. Cancers 2024, 16, 1979. https://doi.org/10.3390/cancers16111979
Sławiński G, Hawryszko M, Lasocka-Koriat Z, Romanowska A, Myszczyński K, Wrona A, Daniłowicz-Szymanowicz L, Lewicka E. Effect of Radiotherapy on the Right Ventricular Function in Lung Cancer Patients. Cancers. 2024; 16(11):1979. https://doi.org/10.3390/cancers16111979
Chicago/Turabian StyleSławiński, Grzegorz, Maja Hawryszko, Zofia Lasocka-Koriat, Anna Romanowska, Kamil Myszczyński, Anna Wrona, Ludmiła Daniłowicz-Szymanowicz, and Ewa Lewicka. 2024. "Effect of Radiotherapy on the Right Ventricular Function in Lung Cancer Patients" Cancers 16, no. 11: 1979. https://doi.org/10.3390/cancers16111979
APA StyleSławiński, G., Hawryszko, M., Lasocka-Koriat, Z., Romanowska, A., Myszczyński, K., Wrona, A., Daniłowicz-Szymanowicz, L., & Lewicka, E. (2024). Effect of Radiotherapy on the Right Ventricular Function in Lung Cancer Patients. Cancers, 16(11), 1979. https://doi.org/10.3390/cancers16111979






