Combined Regional Approach of Talimogene laherparepvec and Radiotherapy in the Treatment of Advanced Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
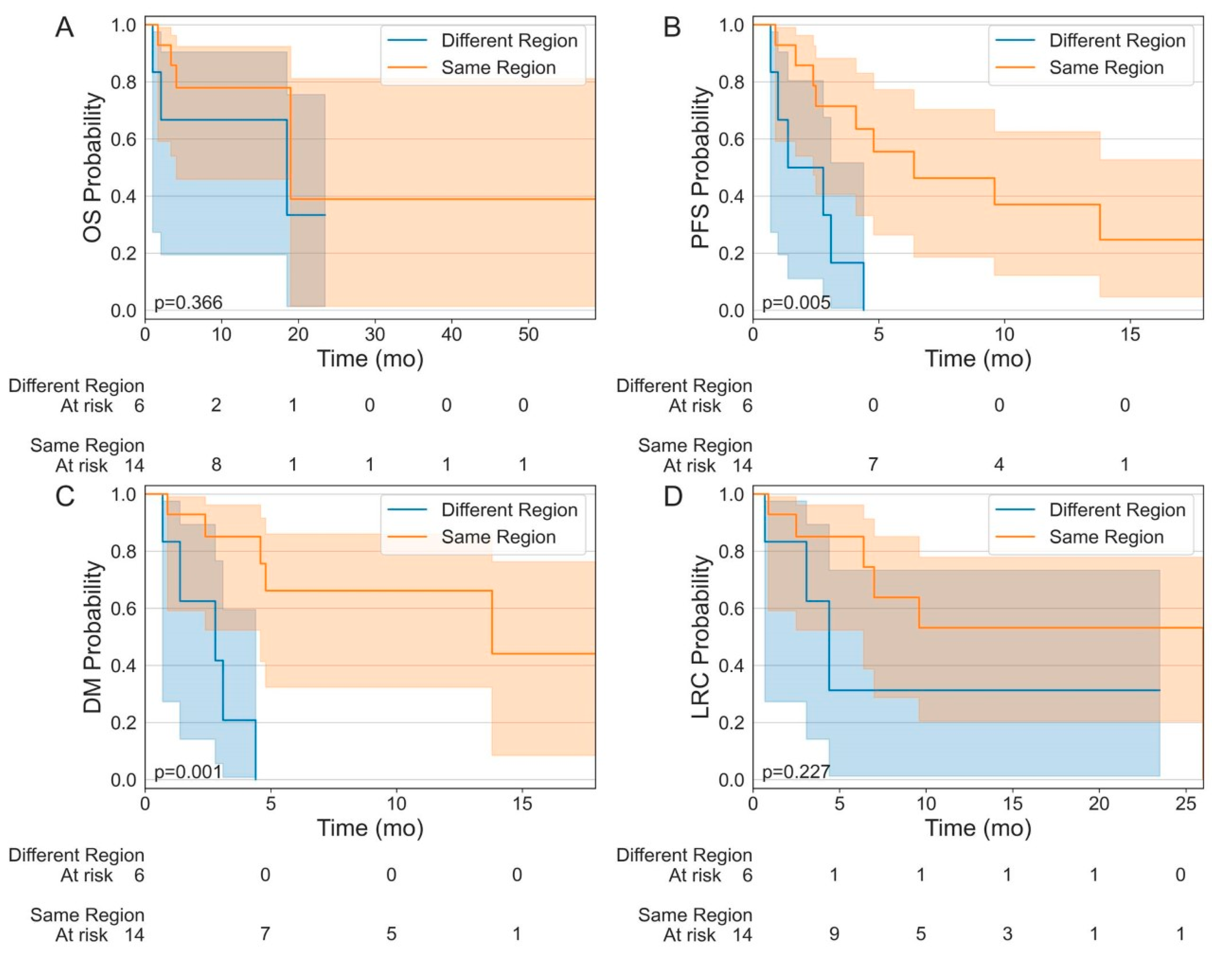
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.I.; Collichio, F.; Harrington, K.J.; Middleton, M.R.; Downey, G.; Ӧhrling, K.; Kaufman, H.L. Final Analyses of OPTiM: A Randomized Phase III Trial of Talimogene Laherparepvec versus Granulocyte-Macrophage Colony-Stimulating Factor in Unresectable Stage III–IV Melanoma. J. Immunother. Cancer 2019, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Kohlhapp, F.J.; Kaufman, H.L. Molecular Pathways: Mechanism of Action for Talimogene Laherparepvec, a New Oncolytic Virus Immunotherapy. Clin. Cancer Res. 2016, 22, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.; Puzanov, I.; Collichio, F.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination with Ipilimumab Versus Ipilimumab Alone in Patients with Advanced, Unresectable Melanoma. J. Clin. Oncol. 2018, 36, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Gómez, V.; Mustapha, R.; Ng, K.; Ng, T. Radiation Therapy and the Innate Immune Response: Clinical Implications for Immunotherapy Approaches. Br. J. Clin. Pharmacol. 2020, 86, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- McGee, H.M.; Marciscano, A.E.; Campbell, A.M.; Monjazeb, A.M.; Kaech, S.M.; Teijaro, J.R. Parallels Between the Antiviral State and the Irradiated State. J. Natl. Cancer Inst. 2021, 113, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, M.A.; Reddy, G.K. Systemic Antitumor Effects and Abscopal Responses in Melanoma Patients Receiving Radiation Therapy. Oncology 2020, 98, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.A.; D’Angelo, S.P.; Steckler, A.M.; Lian, M.; Wasilewski, G.; Lacouture, M.E.; Chapman, P.B.; Shoushtari, A.N.; Ariyan, C.E. A Phase II Randomized Trial of Talimogene Laherparepvec (T-VEC) Oncolytic Immunotherapy with or without Radiotherapy for Patients with Cutaneous Metastases from Solid Tumors. J. Clin. Oncol. 2023, 41, 2639. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 2: Treatment—Update 2022. Eur. J. Cancer 2022, 170, 256–284. [Google Scholar] [CrossRef]
- Coit, D.G.; Thompson, J.A.; Albertini, M.R.; Barker, C.; Carson, W.E.; Contreras, C.; Daniels, G.A.; DiMaio, D.; Fields, R.C.; Fleming, M.D.; et al. Cutaneous Melanoma, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 367–402. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.W.; Michielin, O.; Bastiaannet, E.; Beishon, M.; Catalano, O.; Del Marmol, V.; Delgado-Bolton, R.; Dendale, R.; Trill, M.D.; Ferrari, A.; et al. ECCO Essential Requirements for Quality Cancer Care: Melanoma. Crit. Rev. Oncol. Hematol. 2018, 122, 164–178. [Google Scholar] [CrossRef]
- Thomas, D.L.; Kranz, D.M.; Roy, E.J. Experimental Manipulations of Afferent Immune Responses Influence Efferent Immune Responses to Brain Tumors. Cancer Immunol. Immunother. 2008, 57, 1323–1333. [Google Scholar] [CrossRef]
- Memorial Sloan Kettering Cancer Center A Phase II Randomized Trial of Intralesional Talimogene Laherparepvec (TALIMOGENE LAHERPAREPVEC) with or without Radiotherapy for Cutaneous Melanoma, Merkel Cell Carcinoma, or Other Solid Tumors. 2023. Available online: https://clinicaltrials.gov (accessed on 7 February 2024).
- Kim, M.-S.; Kim, W.; Park, I.H.; Kim, H.J.; Lee, E.; Jung, J.-H.; Cho, L.C.; Song, C.W. Radiobiological Mechanisms of Stereotactic Body Radiation Therapy and Stereotactic Radiation Surgery. Radiat. Oncol. J. 2015, 33, 265–275. [Google Scholar] [CrossRef]
- Galluzzi, L.; Maiuri, M.C.; Vitale, I.; Zischka, H.; Castedo, M.; Zitvogel, L.; Kroemer, G. Cell Death Modalities: Classification and Pathophysiological Implications. Cell Death Differ. 2007, 14, 1237–1243. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Combining Radiotherapy and Cancer Immunotherapy: A Paradigm Shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor Microenvironment as A “Game Changer” in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef]
- Schaue, D.; Ratikan, J.A.; Iwamoto, K.S.; McBride, W.H. Maximizing Tumor Immunity with Fractionated Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Reijmen, E.; De Mey, S.; De Mey, W.; Gevaert, T.; De Ridder, K.; Locy, H.; Martens, S.; De Blay, E.; Bouwens, L.; Debie, P.; et al. Fractionated Radiation Severely Reduces the Number of CD8+ T Cells and Mature Antigen Presenting Cells within Lung Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.W.; Tang, C.; de Groot, P.; Naing, A.; Hess, K.R.; Heymach, J.V.; Papadimitrakopoulou, V.A.; Cushman, T.R.; Subbiah, V.; Chang, J.Y.; et al. Phase II Trial of Ipilimumab with Stereotactic Radiation Therapy for Metastatic Disease: Outcomes, Toxicities, and Low-Dose Radiation–Related Abscopal Responses. Cancer Immunol. Res. 2019, 7, 1903–1909. [Google Scholar] [CrossRef]
- Youland, R.S.; Blanchard, M.L.; Dronca, R.; Kottschade, L.; Markovic, S.N.; Olivier, K.R.; Park, S.S. Role of Radiotherapy in Extracranial Metastatic Malignant Melanoma in the Modern Era. Clin. Transl. Radiat. Oncol. 2017, 6, 25–30. [Google Scholar] [CrossRef] [PubMed]
- IMLYGIC. FDA 2023. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/imlygic (accessed on 18 January 2024).
- Zhu, S.; Wang, Y.; Tang, J.; Cao, M. Radiotherapy Induced Immunogenic Cell Death by Remodeling Tumor Immune Microenvironment. Front. Immunol. 2022, 13, 1074477. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, M.E.; Vitale, I.; Harrington, K.J.; Melero, I.; Galluzzi, L. Immunological Impact of Cell Death Signaling Driven by Radiation on the Tumor Microenvironment. Nat. Immunol. 2020, 21, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Teitz-Tennenbaum, S.; Li, Q.; Rynkiewicz, S.; Ito, F.; Davis, M.A.; McGinn, C.J.; Chang, A.E. Radiotherapy Potentiates the Therapeutic Efficacy of Intratumoral Dendritic Cell Administration. Cancer Res. 2003, 63, 8466–8475. [Google Scholar] [PubMed]
- Daguenet, E.; Louati, S.; Wozny, A.-S.; Vial, N.; Gras, M.; Guy, J.-B.; Vallard, A.; Rodriguez-Lafrasse, C.; Magné, N. Radiation-Induced Bystander and Abscopal Effects: Important Lessons from Preclinical Models. Br. J. Cancer 2020, 123, 339–348. [Google Scholar] [CrossRef]
- Charpentier, M.; Spada, S.; Van Nest, S.J.; Demaria, S. Radiation Therapy-Induced Remodeling of the Tumor Immune Microenvironment. Semin. Cancer Biol. 2022, 86, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Monga, V.; Miller, B.J.; Tanas, M.; Boukhar, S.; Allen, B.; Anderson, C.; Stephens, L.; Hartwig, S.; Varga, S.; Houtman, J.; et al. Intratumoral Talimogene Laherparepvec Injection with Concurrent Preoperative Radiation in Patients with Locally Advanced Soft-Tissue Sarcoma of the Trunk and Extremities: Phase IB/II Trial. J. Immunother. Cancer 2021, 9, e003119. [Google Scholar] [CrossRef] [PubMed]
- Young, K.H.; Baird, J.R.; Savage, T.; Cottam, B.; Friedman, D.; Bambina, S.; Messenheimer, D.J.; Fox, B.; Newell, P.; Bahjat, K.S.; et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS ONE 2016, 11, e0157164. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef]
- Buchwald, Z.S.; Wynne, J.; Nasti, T.H.; Zhu, S.; Mourad, W.F.; Yan, W.; Gupta, S.; Khleif, S.N.; Khan, M.K. Radiation, Immune Checkpoint Blockade and the Abscopal Effect: A Critical Review on Timing, Dose and Fractionation. Front. Oncol. 2018, 8, 612. [Google Scholar] [CrossRef]
- Roccuzzo, G.; Bongiovanni, E.; Tonella, L.; Pala, V.; Marchisio, S.; Ricci, A.; Senetta, R.; Bertero, L.; Ribero, S.; Berrino, E.; et al. Emerging Prognostic Biomarkers in Advanced Cutaneous Melanoma: A Literature Update. Expert Rev. Mol. Diagn. 2024, 24, 49–66. [Google Scholar] [CrossRef] [PubMed]
| TVEC + RT to Same Region (n = 14) | TVEC + RT to Different Regions (n = 6) | p Value | |
|---|---|---|---|
| Age, median (range) | 75.5 (44 to 90) years | 67.3 (59 to 79) years | 0.099 |
| Sex, n (%) | |||
| Female | 7 (50.0) | 3 (50.0) | 1.000 |
| Male | 7 (50.0) | 3 (50.0) | |
| Race, n (%) | |||
| Asian/Pacific Islander | 1 (7.1) | 0 (0.0) | 0.679 |
| Black/African American | 1 (7.1) | 0 (0.0) | |
| White | 11 (78.6) | 6 (100.0) | |
| Other/Unknown | 1 (7.1) | 0 (0.0) | |
| Ethnicity, n (%) | |||
| Hispanic/Latino | 6 (42.9) | 2 (33.3) | 0.692 |
| Non-Hispanic/Latino | 7 (50.0) | 4 (66.7) | |
| Unknown | 1 (7.1) | 0 (0.0) | |
| Stage, n (%) | |||
| Stage III | 10 (71.4) | 4 (66.7) | 0.831 |
| Stage IV | 4 (28.6) | 2 (33.3) | |
| Had SLNBx as part of staging?, n (%) | |||
| Yes | [Stage III] 10 (100.0) [Stage IV] 2 (50.0) | [Stage III] 3 (75.0) [Stage IV] 2 (100.0) | 0.101 0.221 |
| Had CLND for staging?, n (%) | |||
| Yes | [Stage III] 8 (80.0) [Stage IV] 0 (0.0) | [Stage III] 4 (100.0) [Stage IV] 1 (50.0) | 0.334 0.121 |
| BRAF-mutation, n (%) | |||
| Positive | 1 (7.1) | 1 (16.7) | 0.281 |
| Negative | 10 (71.4) | 2 (33.3) | |
| Unknown | 3 (21.4) | 3 (50.0) | |
| PD-L1 expression ≥1, n (%) | |||
| Positive | 7 (50.0) | 4 (66.7) | 0.339 |
| Negative | 4 (28.6) | 0 (0.0) | |
| Unknown | 3 (21.4) | 2 (33.3) | |
| Treatment sequence, n (%) | |||
| TVEC first | 9 (64.3) | 3 (50.0) | 0.550 |
| RT first | 5 (35.7) | 3 (50.0) | |
| Time Interval between TVEC + RT, median (range) | 6.6 (0.6 to 39.7) months | 10.5 (1.2 to 46.7) months | 0.458 |
| Regions treated by TVEC + RT | left leg: 5 (35.7), right leg: 5 (35.7), torso: 1 (7.1), left arm: 1 (7.1), right arm: 1 (7.1), scalp: 1 (7.1) | n/a | n/a |
| RT regimen | |||
| RT dose and fractionation | [to the region treated by TVEC + RT] 6 (42.9) [Conventional/Hypofractionated; 48–70 Gy/20–35 fx] 10 (71.4) [SBRT, 27–40 Gy/3–5 fx] | [to the regions not treated by TVEC] 1 (16.7) [Hypofractionated; 48 Gy/20 fx] 5 (83.3) [SBRT, 14.8–40 Gy/3–5 fx] | n/a |
| Other treatments | |||
| Immunotherapy | 14 (100.0) | 6 (100.0) | 1.000 |
| Chemotherapy | 4 (28.6) | 1 (16.7) | 0.573 |
| Other (i.e., BRAF inhibitors and CMP-001) | 5 (35.7) | 4 (66.7) | 0.202 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tam, A.; Ladbury, C.; Kassardjian, A.; Modi, B.; McGee, H.; Melstrom, L.; Margolin, K.; Xing, Y.; Amini, A. Combined Regional Approach of Talimogene laherparepvec and Radiotherapy in the Treatment of Advanced Melanoma. Cancers 2024, 16, 1951. https://doi.org/10.3390/cancers16111951
Tam A, Ladbury C, Kassardjian A, Modi B, McGee H, Melstrom L, Margolin K, Xing Y, Amini A. Combined Regional Approach of Talimogene laherparepvec and Radiotherapy in the Treatment of Advanced Melanoma. Cancers. 2024; 16(11):1951. https://doi.org/10.3390/cancers16111951
Chicago/Turabian StyleTam, Andrew, Colton Ladbury, Ari Kassardjian, Badri Modi, Heather McGee, Laleh Melstrom, Kim Margolin, Yan Xing, and Arya Amini. 2024. "Combined Regional Approach of Talimogene laherparepvec and Radiotherapy in the Treatment of Advanced Melanoma" Cancers 16, no. 11: 1951. https://doi.org/10.3390/cancers16111951
APA StyleTam, A., Ladbury, C., Kassardjian, A., Modi, B., McGee, H., Melstrom, L., Margolin, K., Xing, Y., & Amini, A. (2024). Combined Regional Approach of Talimogene laherparepvec and Radiotherapy in the Treatment of Advanced Melanoma. Cancers, 16(11), 1951. https://doi.org/10.3390/cancers16111951






