1. Introduction
All tissues require a constant supply of nutrients and oxygen with concomitant waste disposal by the vascular supply. Tumors also require these functions for growth and dissemination in case of malignancy. The physiological properties of organs, such as the kidney or liver, depend on a structurally highly organized vascular architecture [
1,
2,
3]. Furthermore, the study of the vascular tree of malignancies has, during recent decades, received increased attention, since the ever-expanding therapeutic arsenal ultimately depends on vascular access to the tissue. Today, anti-angiogenesis by receptor tyrosine kinase inhibition directly targets tumor vasculature, and cytostatics and radiation treatment protocols highly depend on vascular integrity [
4,
5].
Another valid example is immune checkpoint inhibition, which strongly depends on vascular factors for efficacy. Vascular structure and organization have traditionally been studied via light and electron microscopy. The three-dimensional vascular architecture has been studied via, for example, resin vascular corrosion casts [
2,
6,
7] and, more recently, via confocal microscopy and micro-CT [
8,
9,
10]. The dynamics of blood flow distribution in specific parts of a complex organ, like the kidney, has been studied, e.g., via the labeled microsphere technique [
11,
12,
13]. However, most studies have been performed in animals, and studies on human material are rare, as are studies combining flow measurements with structural investigation. In this study, we used an ex vivo perfusion protocol of human kidneys nephrectomized due to tumors, combined with a variety of morphological techniques and with flow physiological measurements to characterize the vascular tree of both normal and cancerous kidney tissue. We finally discuss the strengths and limitations of the different techniques.
2. Materials and Methods
2.1. Ethics
The Swedish Ethics Authority approved the study (Dnr 2021-00477).
2.2. Procedures
We performed the perfusion study from 1979 to 1984. The kidneys were immediately taken care of in the operating room following a nephrectomy due to a diagnosed renal tumor. The renal vessels were divided, and the artery was cannulated. Subsequently, the kidney was flushed with 5% low-molecular-weight dextran (Perfadex; Pharmacia, Uppsala, Sweden, presently RescueFlow
®; Øresund Pharma ApS, Copenhagen, Denmark) at room temperature until the venous effluent was clear. The vessels were connected to a perfusion system schematically depicted in
Figure 1 [
14]. The set-up allowed for the injection of labelled microspheres, 15 µm in diameter as well as withdrawal of reference perfusate volumes, enabling the quantitative estimation of perfusate flow.
2.3. Perfusion Technique
Via an arterial cannula, the nephrectomies were connected to rubber tubing (
Figure 1) and a peristaltic constant flow pump (Ismatec MP4, Ismatec SA, Glattbrugg, Switzerland). The specimens were perfused at 37 °C with oxygenated perfusate consisting of 4% dextran (70 kD; Macrodex; Pharmacia, Uppsala, Sweden) and 100 mL of horse serum in 1 L of a salt solution with 143 mM Na
+, 4.3 mM K
+, 2.5 mM Ca
2+, 0.83 mM Mg
2+, 141 mM Cl
−, 13.3 mM HCO
3−, 0.46 mM H
2PO
4 and 5.6 mM glucose. The preparation was immersed in perfusate to prevent gravitational pressure artifacts. The venous effluent was drained into the perfusate. The afferent tubing also accommodated two thin catheters (PE 50) introduced via a T-connection, ending approximately 1 cm within the renal artery. One catheter was used to draw a reference perfusate sample during microsphere injection, while the other was used for pressure recording. Papaverine was given as a bolus dose (median dose 120 mg; range 80 to 160 mg) to induce maximal vascular relaxation. The pump flow was gradually increased to produce a pressure of approximately 30 to 40 mm Hg in the renal artery, and the flow was set at that rate for the rest of the experiment. Microspheres (New England Nuclear, Boston, MA, USA; 15 µm) labeled with I
125, approximately 300,000 spheres in 1 mL of saline with Tween, were injected with a fine needle through the latex tubing for 45 s to avoid sphere aggregation. Fifteen seconds before, during and 30 s after the microsphere injection, a reference sample was drawn at 2 mL/min (Model 351 Sage infusion pump). Vascular reactivity was determined after norepinephrine injection using another two sets of differently labeled 15 µm microspheres [
14]. This part of the experiments will not be further described. Most experiments were concluded with manual perfusion at a higher pressure of a micronized (<1 µm) barium sulphate suspension (Barosperse; Mallinckrodt, Inc., St. Louis, MO, USA) in perfusate medium (0.45 g/mL) with 10 percent formaldehyde [
15].
2.4. Post-Experimental Handling of the Perfused Kidneys
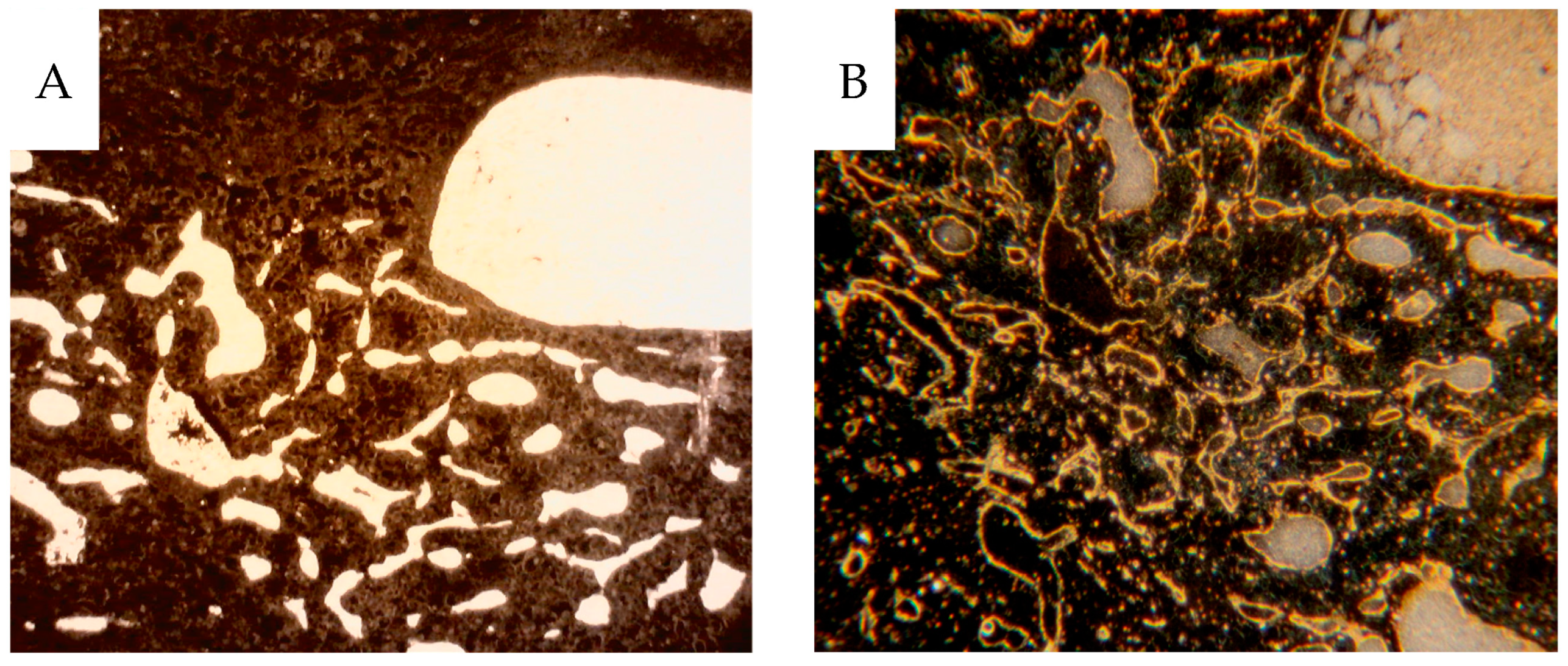
The kidneys were immersed in formaldehyde solution for a few days, after which perirenal tissue was removed, and the kidney with the tumor was sliced sagitally into approximately 1 to 4 mm thick slices using a kitchen food slicer. The slices were prepared for further experiments in the following way: (a) the thinnest section, 1 mm, was used for contact X-ray microangiography. The sections were placed between plastic films and applied to the envelopes of a Kodak X-omatic film (VWR-Avantor, Kista, Sweden). Exposure was made at 27 kV, 63 mA in a CGR mammograph with conventional film development. The result provided information on the vascular architecture and density and whether any part of the specimen had been inadequately perfused. (b) A slightly thicker section, 2 mm, was used for autoradiography and placed between envelopes of Kodak X-omatic films on either side of the section. One film was exposed for 2 to 3 weeks and, depending upon the degree of blackening, the other was exposed for up to 2 to 3 months. The result provided information on the gross perfusate flow distribution of the microspheres. (c) A thicker section, approximately 3 to 4 mm, was used to take 0.1 to 1 g pieces for quantitative radioactivity measurement to provide perfusate flow data. Five to 20 pieces were taken from cortical and medullary tissues, and 10 to 20 pieces from tumor tissue; each sample was weighed and placed in a tube with 4% formaldehyde. After the radioactivity measurements, these pieces were prepared for routine histology and immunohistochemistry (CD31 for endothelium) to ensure tissue representation and make possible correlation between morphology and physiology. The 4 µm paraffin sections with a surface area of 0.5–1 cm2, were macrophotographed at low-angle illumination from below providing a dark-field image of the contrast-filled blood vessels. On top of the paraffin sections, a drop of immersion oil was applied to avoid light scattering from the crystalline paraffin. Before deparaffination, the 4 µm sections were studied via dark-field microscopy, see below, to identify contrast-filled vessels. After that, the same sections were processed for CD31 endothelial immunohistochemistry. The fraction of perfused vessels could thus be estimated despite the loss of contrast during the IHC procedure. An ”England finder” (Labtech, Heathfield, UK), an object glass slide etched with a minute X–Y graticule to enable the location of a selected area in the specimen microscopic slide, was used to identify the same area of the section documented with darkfield microscopy before and after CD31 IHC.
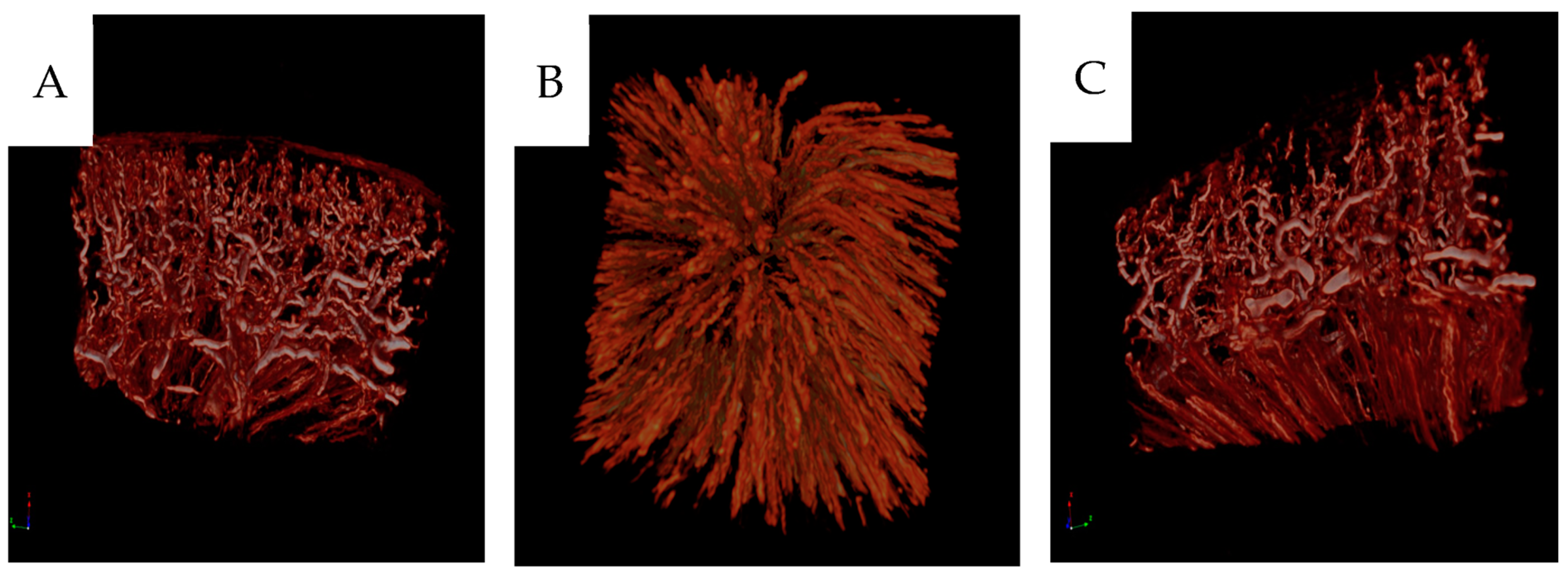
2.5. Micro-CT Analysis
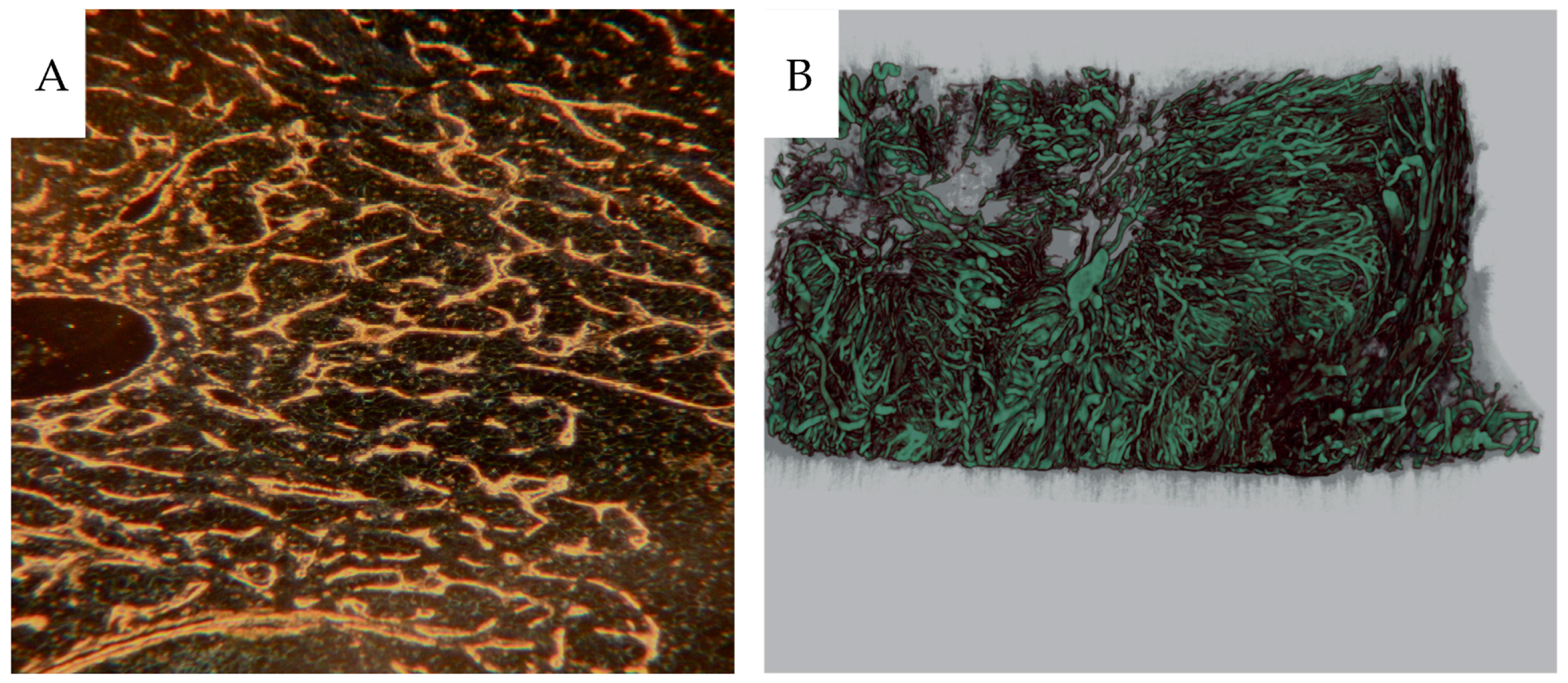
The paraffin blocks were scanned in a preclinical micro-CT scanner, SkyScan 1176 micro-CT (Bruker, Antwerp, Belgium). The scanning was conducted at 55 kV and 455 mA with a 0.2 mm aluminum filter. The exposure time was 815 ms. The X-ray projections were obtained at 0.36° intervals with a scanning angular rotation of 180° at a resolution of 9 µm. Data were reconstructed into a three-dimensional (3D) structure using NRECON software (version 1.6.9.8; Bruker) with a beam hardening correction of 30%. The projection images were visualized as three-dimensional images using the software CTVox (version 2.7, Bruker) [
16].
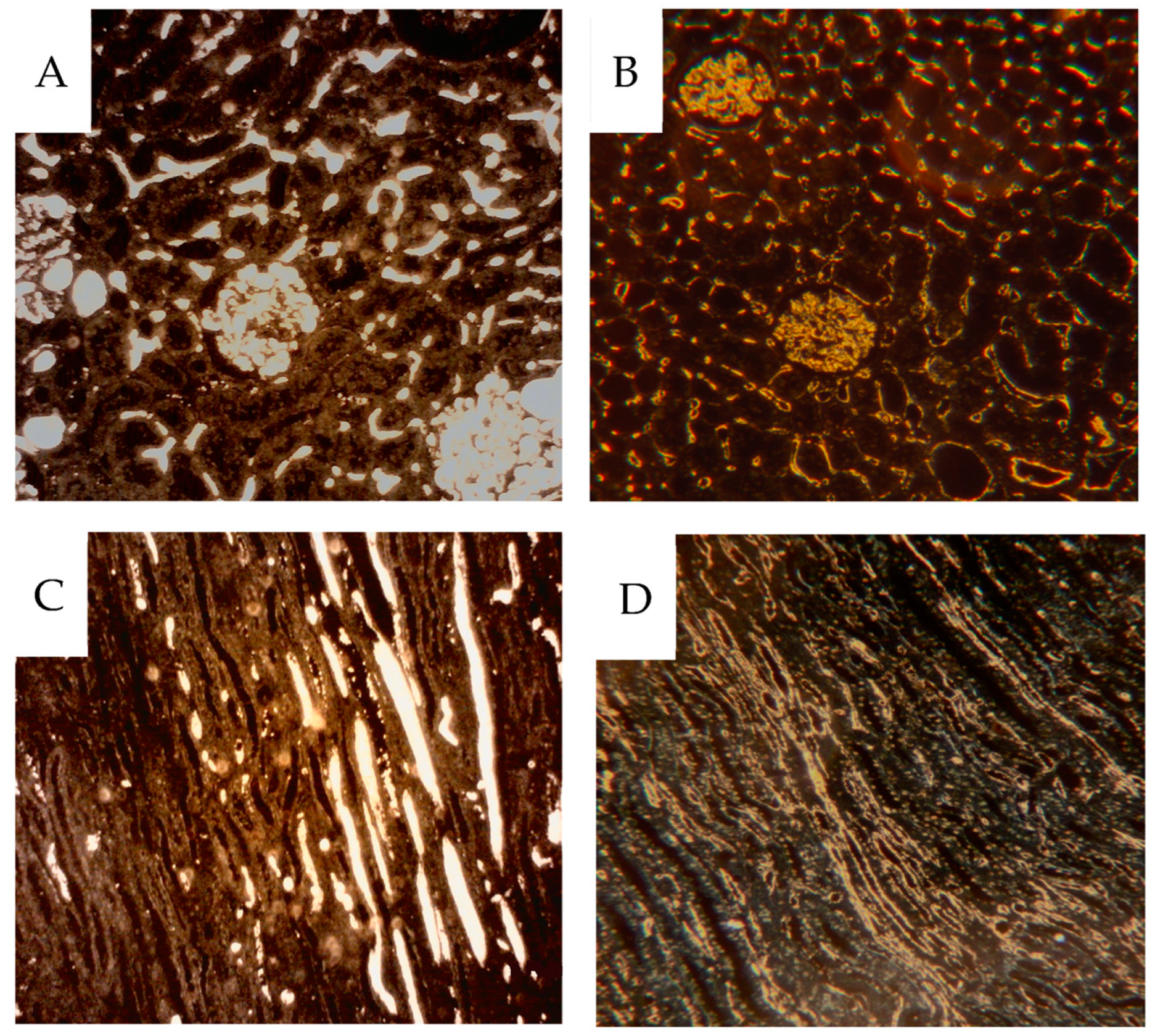
2.6. CD31 Immunohistochemistry
Four micrometer-thick sections of the paraffin-embedded samples were cut and mounted on Superfrost® Plus glasses (Gerhard Menzel GmbH & Co.KG, Braunschweig, Germany). The sections were then deparaffinized, hydrated and treated with 3% hydrogen peroxide to quench endogenous peroxidase activity. Heat-induced antigen retrieval was performed using an EDTA buffer with pH 8.0. The sections were incubated with a rabbit monoclonal antibody against CD 31 (ab182981, Abcam, Cambridge, UK) at 1/4500 dilution, followed by an anti-rabbit Impress-HRP reagent (Vector laboratories, Inc., Newark, CA, USA). The immunoreactions were visualized using a Liquid DAB + substrate chromogen system (Agilent, Santa Clara, CA, USA), resulting in a brown reaction product.
2.7. Dark-Field Microscopy
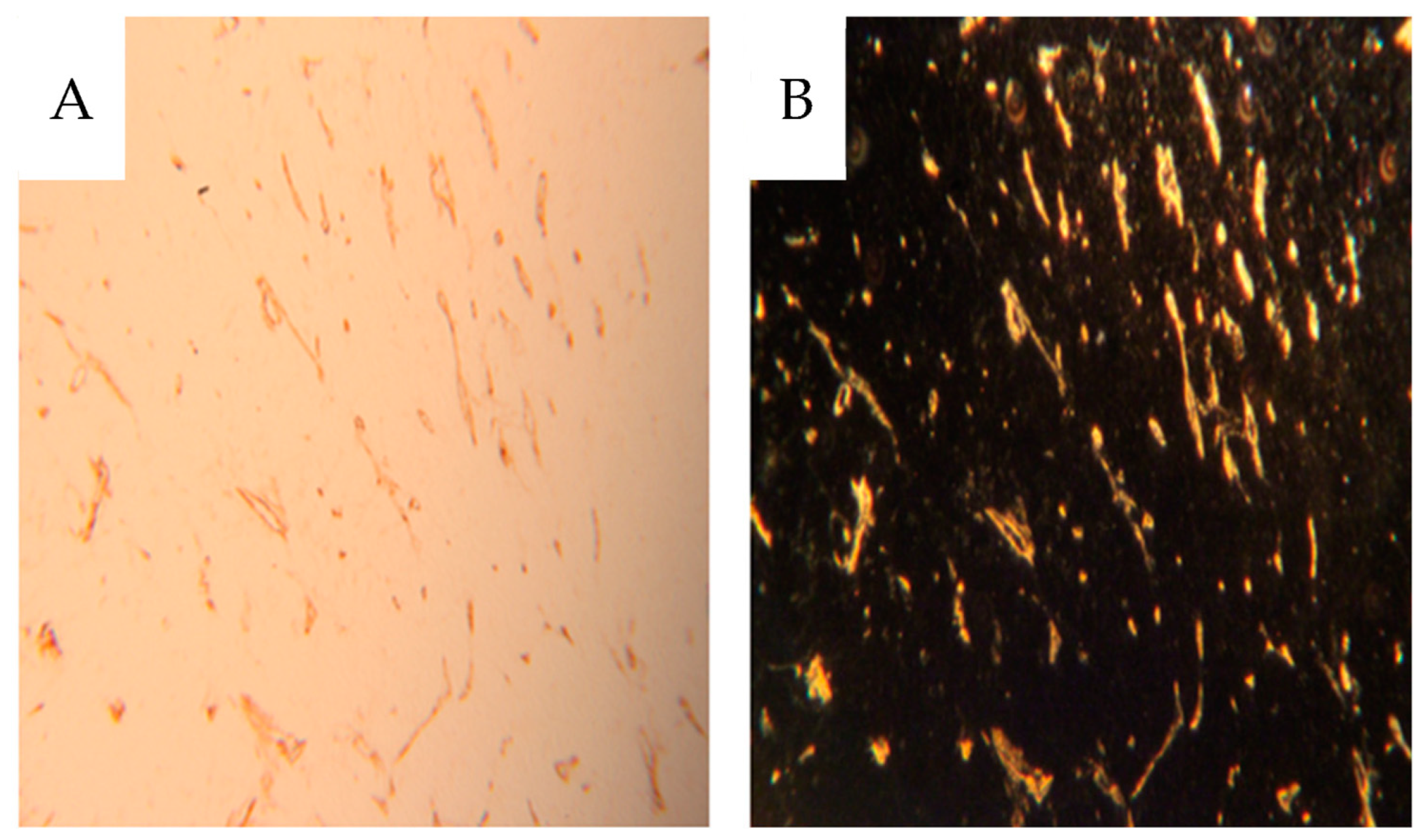
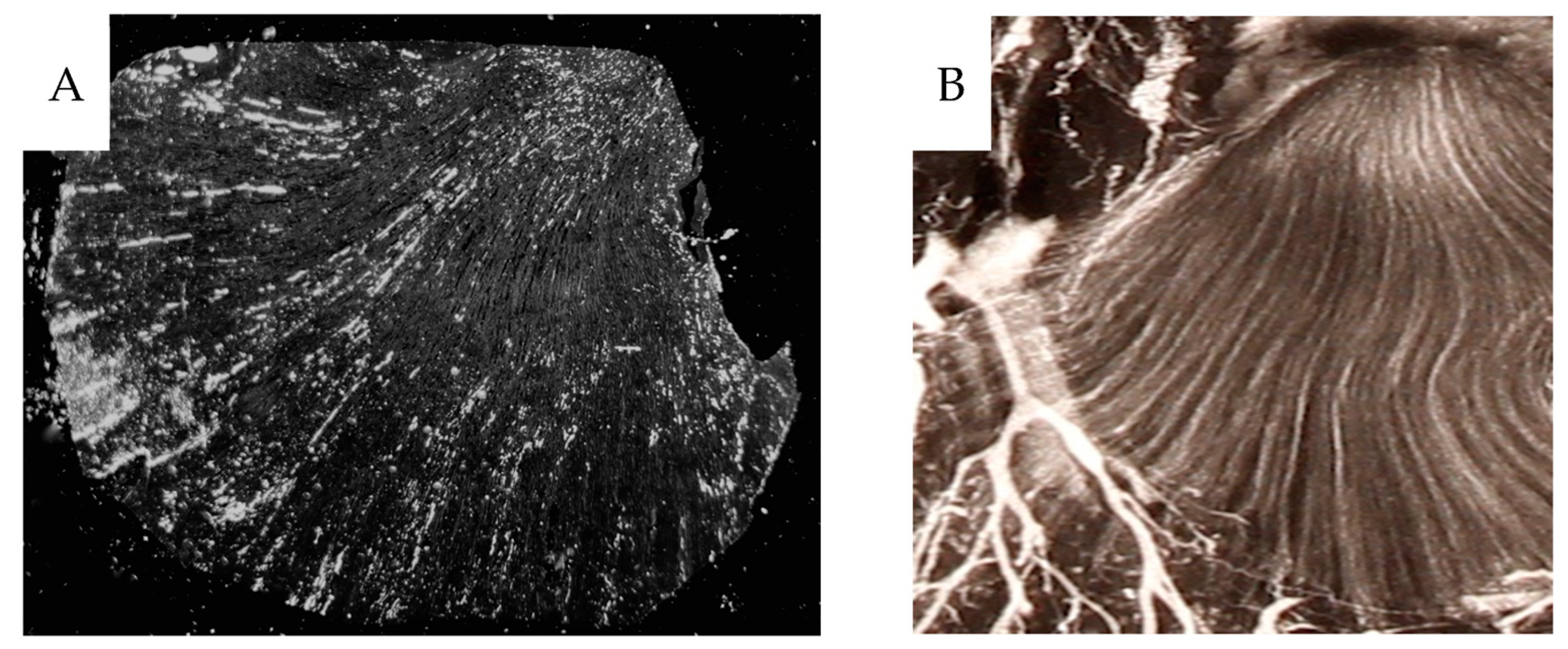
Dark-field microscopy utilizes the scattered light from structures in the object and prevents direct light from reaching the objective, providing bright scattering structures against a dark background. A Zeiss microscope (Standard 16, Carl Zeiss AG, Oberkochen, Germany) with a phase contrast condenser was used. The condenser was set for high power objectives, but the low power 10x objective excluded direct light from the condenser phase ring to pass to the objective. For high power objectives, a dedicated dark-field Zeiss condenser, including use of substage oil immersion, was used. The micronized barium sulphate particles (<1 µm) scatter the light incident to the specimen much more than the soft tissue, augmenting the visibility of vessels with contrast.
The same sections, 4 µm thick, were then stained for CD31 immunohistochemistry, also best visualized using dark-field according to Jennische et al., allowing for analysis of contrast-filled vessels [
17]. Dark-field macro photography (Canon EOS700D, Canon-Sweden, Solna, Sweden) of the sections, 0.5–1 cm
2, was also performed, see above.
2.8. Flow Analysis
Capillary, <15 µm, perfusate flow analysis: Only pieces with more than 50 spheres, as indicated from activity measurement, were included in the analyses. Calculations: Blood flow (Q mL × min
−1 × 100 g
−1) estimated by measurement of Iodine
125 in tissue samples was calculated as follows:
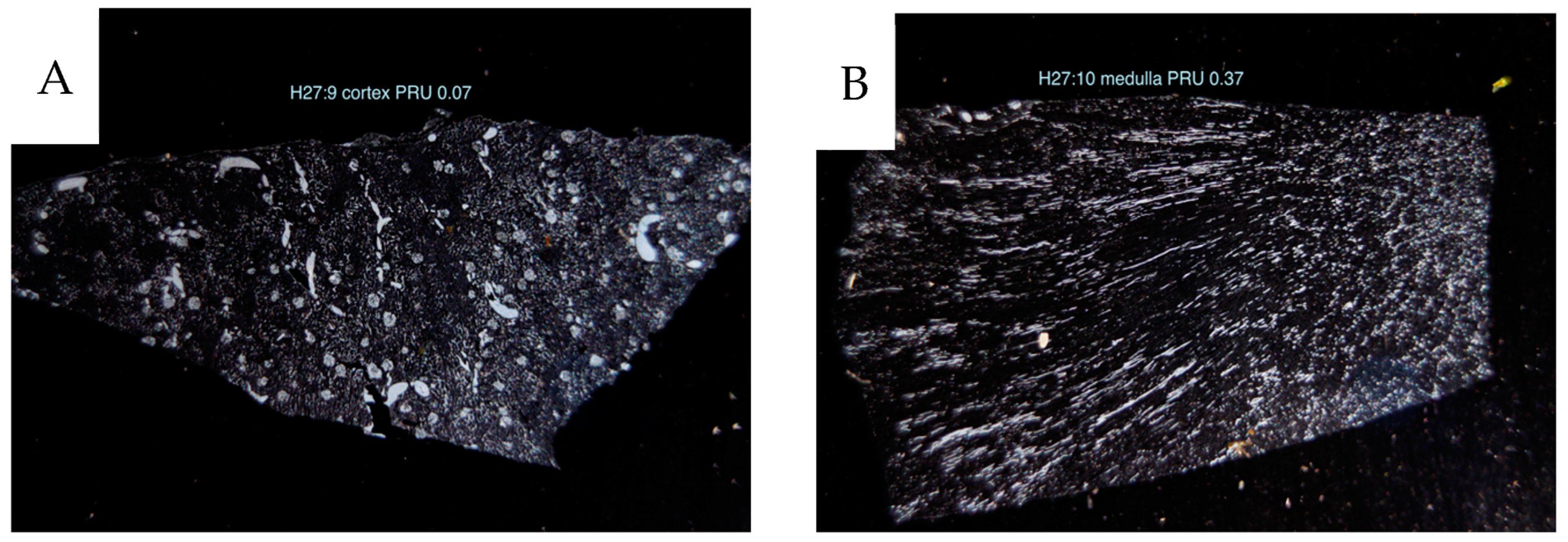
The reference withdrawal flow rate was 2.0 mL × min−1. Peripheral resistance units (PRU) were obtained by dividing the perfusate pressure by the flow and weight for each sample (perfusion pressure mmHg/mL perfusate flow/100 g tissue).
Tumor volume was measured on the fresh sections or contact angiograms by multiplying the longest diameter by the perpendicular and the mean of the two using the formula 4/3 × π × D3/8.
2.9. Histopathology
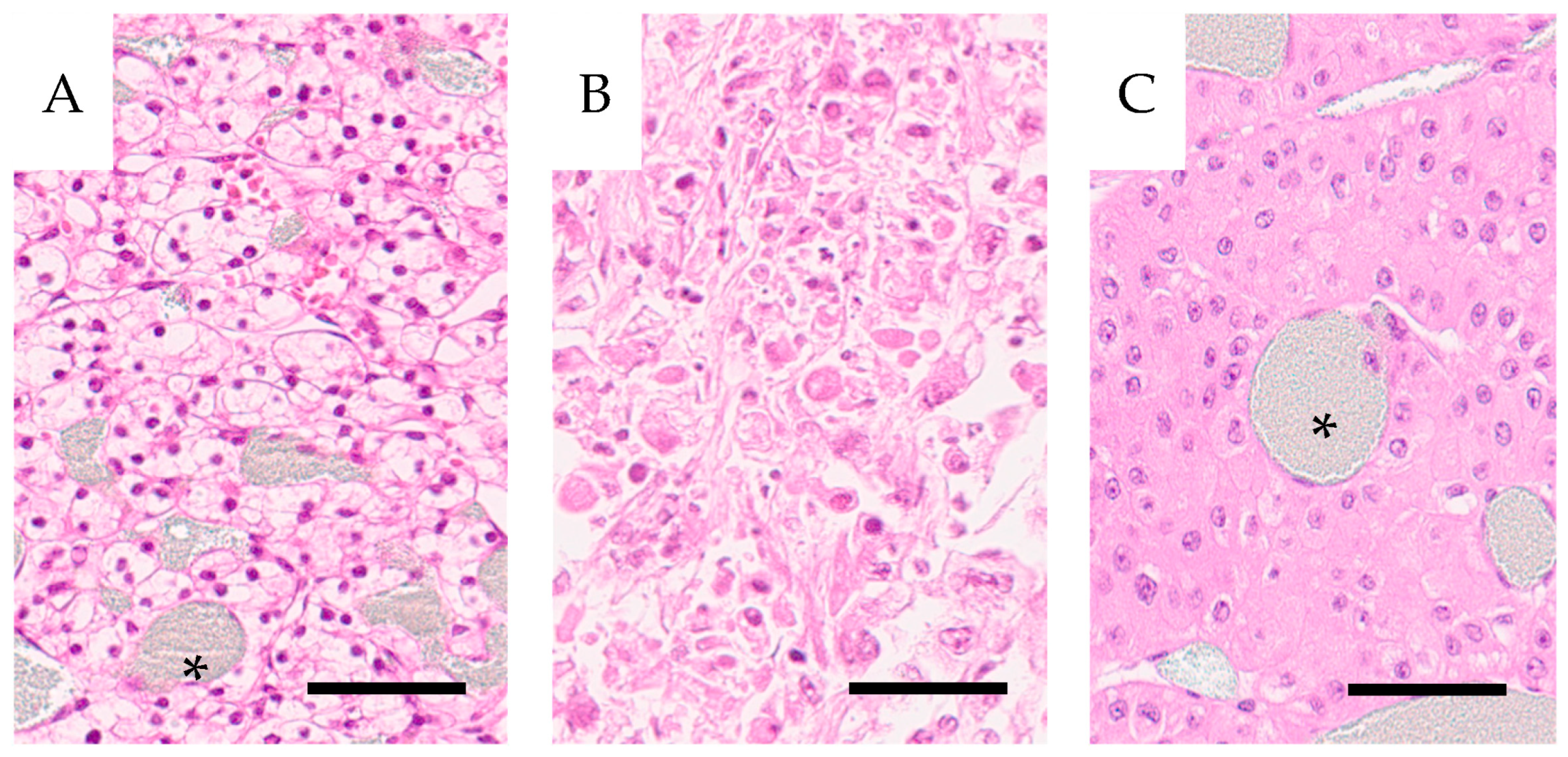
Hematoxylin-eosin stained 4 µm sections of tumor tissue were scanned in a Hamamatsu slide scanner and classified for tumor type, clear cell renal cell carcinoma, (CCRCC) and oncocytoma) and for malignancy grade according to ISUP (International Society for Urological Pathology) grading.
2.10. Clinical Outcome
Postnephrectomy survival time and cause of death were correlated to tumor type and grade.
2.11. Statistical Analysis
Linear regression analysis was performed using the SPSS software (IBM SPSS Statistics for Windows, version 28.0 IBM Corp. As cut off for significance a p-value < 0.05 was used.
4. Discussion
This work presents techniques to characterize complex vascular networks that apply to normal kidney or kidney cancer tissue and other tissues/organ, postmortem or excised at surgery [
6]. The organ perfusion set-up did not include collection of perfusate from the renal veins and shunting of spheres could therefore not be analyzed. Further, only 15 µm spheres were used, limiting analysis of vascular dimensions. Microscopy of the thin 4 µm sections disclosed few 15 µm spheres, almost exclusively located within glomeruli, which indicated that most spheres were trapped in this primary capillary network. At this section thickness, many spheres are probably lost. We believe this fact does not prohibit us from obtaining a crude estimate of the location of the spheres in the renal vascular tree. However, as measured via sample radioactivity, approximately 10% of the 15 µm spheres reached and were trapped in the medulla. This result could be explained by shunting through arteriovenous passages > 15 µm in the juxtamedullary cortical tissue. McNay et al. found approximately 1% of injected 19 µm spheres in the medulla and 0.5% passing through ex vivo perfused porcine kidneys [
12]. The contrast perfusions at relatively high pressures widen the vessels compared to CD31-positive vessels in specimens not perfused by contrast. Some microspheres may have been displaced from the cortical tissue into the medulla,
Figure 4. The tumors in our specimens have a high PRU regarding nutritive flow (arteriovenous passages < 15 µm). This tissue might allow an unrecorded volume to pass through arterio-venous passages larger than 15 µm, represented by the densely contrast-filled vasculature in parts of the tumors. Preoperative angiography in some of the patients in this material demonstrated hyper-vascularized tumors corresponding to our findings. Tumors with a rapid contrast passage indicate the presence of arteriovenous shunts. A clinical study comparing a rapid radiographic arterio-venous passage with measuring
99mTc-MAA demonstrated a 15–57% shunting [
18]. Also significant shunting has been reported in similar tumors such as hepatic adenocarcinoma (PMID: 2813766).
The angiograms demonstrate a heterogeneous distribution of contrast-filled vessels, which makes a vascular/capillary density or area classification difficult. Most tumors included a dense vascular network and parts devoid of vessels in necrotic areas. Only two small tumors showed a dense network throughout (H11 and H25). Also, these tumors had a high vascular resistance as measured with 15 µm spheres. Thus, it was difficult to classify them into well-defined categories concerning the vascular pattern. In conclusion, the vascular density does not translate into degree of perfusion. Tumor areas with an extreme vascular density are often poorly perfused compared to cortical tissue with a less dense network. Tumors develop an increased interstitial fluid pressure, compressing the thin-walled tumor vessels, which results in reduced circulation, explaining our findings [
19]. Further, the haphazard network predisposes to a disorganized flow direction described in vivo [
20].
The material was initially collected to study norepinephrine sensitivity in renal and tumor vessels [
14]. The vessels of the specimens were maximally relaxed using papaverine, then perfused with increasing doses of norepinephrine. At each level, an injection of approximately <300,000 15 µm spheres, with different radioactive labeling, was done. Thus, capillary blocking from previously injected 15 µm spheres could prevent contrast filling. However, there are 0.8 to 1 million glomeruli in a human kidney and the afferent arteriolus splits into 6 to 8 loops. Thus, 5 to 8 million passages are present. At maximum, approximately 3 × 300,000 spheres were injected, i.e., a minority of passages were blocked. After injection of the microspheres, no increase in perfusion pressure despite constant flow was found, which indicates no significant vascular blocking. Since the manual contrast infusion time and pressure were not standardized, this could add to the varying degrees of contrast filling. The incomplete contrast filling is likely due to the viscosity of the suspension; however, it has a rheology similar to blood with a normal hematocrit of 0.45 [
15]. All three differently labeled spheres will inevitably add to the autoradiogram blackening. The initial injection at vascular relaxation was done with I
125 which has a low energy gamma emission (35 keV) with a higher absorption in the film than the higher energies from Ce
141 and Ru
103 used in the following injections. Thus, the autoradiograms represent essentially the perfusion at maximal vascular relaxation.
In 1896, a postmortem kidney was first injected with a suspension of red lead particles [
21]. The infusion of micronized Barium sulphate [
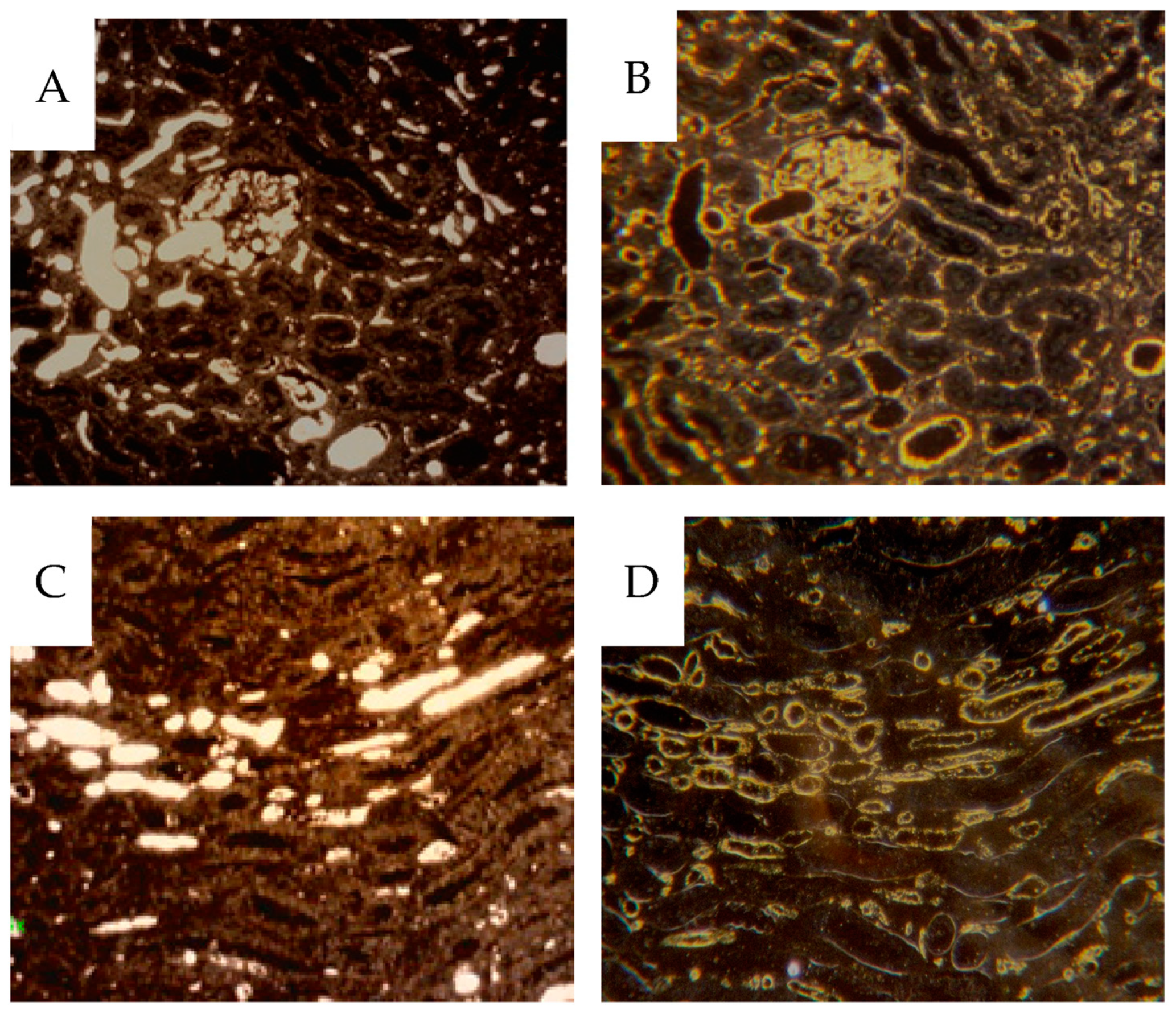
22] has a drawback of higher viscosity than liquid iodine-containing contrast media, necessitating the relatively high infusion pressure. However, it will result in high X-ray absorption and high contrast. Further, it will not diffuse from the vascular volume, resulting in good definition. The tumor vessels permit protein leakage, but extravascular micronized particles could not be observed. More confluent lakes of contrast could be observed, probably representing ruptured sinusoids. Liquid contrast media prevents further material handling, i.e., fixation and histological preparation, which would eliminate the contrast medium. The Barium sulphate will withstand handling, but as stated above, immunohistochemistry will, to some degree, rinse the contrast from the 4 µm sections. Therefore, we studied the vascular contrast in paraffin sections using dark-field microscopy before immunohistochemistry. This procedure allowed analysis of the fraction of vessels being contrast-filled by relocalizing the same area after CD31 immunostaining, which showed that only a fraction of the CD31-positive vessels were contrast-filled.
Some glomeruli totally or partially lacked contrast, as did most peritubular cortical capillaries. These findings agree with the micro-CT results where only the vascular network, including the glomeruli, are seen, but not the efferent and peritubular capillaries. In some sagittal angiograms and autoradiograms, it is clear that parts of the cortex are neither contrast-filled nor perfused with microspheres. This finding probably represents an unperfused auxiliary renal artery or occlusion caused by tumor growth nearby. Mixing of microspheres is crucial for representative distribution. In our perfusion setup the spheres were injected into the tubing to the renal artery. A low variation between different cortical samples within each specimen indicated sufficient mixing.
Unexpectedly, a minority of vasa recta were contrast-filled. Since few peritubular capillaries were contrast-filled, the filled vasa recta likely originated from juxtamedullary glomeruli, where the efferent arterioli pass directly into the medulla. Gross vascularity was visualized via contrast infusion but was also seen in more detail after immunohistochemical staining of the CD31 epitope. We chose CD31 as endothelial marker rather than CD34, since CD31 also labels immature vessels, whereas CD34 is more selective towards mature vasculature [
23,
24]. We used darkfield microscopy for visualization of the contrast resulting in intense light scatter, as shown above, and for enhanced visualization of the denatured immunoprotein precipitates at IHC [
17]. The low-angle illumination principle was also used to visualize entire microslides with sections 0.5–1 cm
2, giving a better overview of the vascular network. As seen in illustrations of vascularity, including X-ray angiograms, micro-CT and darkfield microscopy, the contrast produced is high. Presumably, a lower concentration of Barium sulphate with lower viscosity would provide sufficient contrast. A lower perfusion pressure would be needed and possibly a better vascular filling would result.