Influence of Amino Acids and Exercise on Muscle Protein Turnover, Particularly in Cancer Cachexia
Abstract
Simple Summary
Abstract
1. Introduction
2. Metabolic Dysfunction in Cancer Cachexia
2.1. Muscle Wasting in Cancer Cachexia
2.2. Oxidative Stress in Cancer Cachexia
2.3. Altered Energy Balance in Cancer Cachexia
2.4. Insulin Resistance in Cancer Cachexia
2.5. Adipose Tissue Wasting and Lipid and Fat Burning in Cancer Cachexia
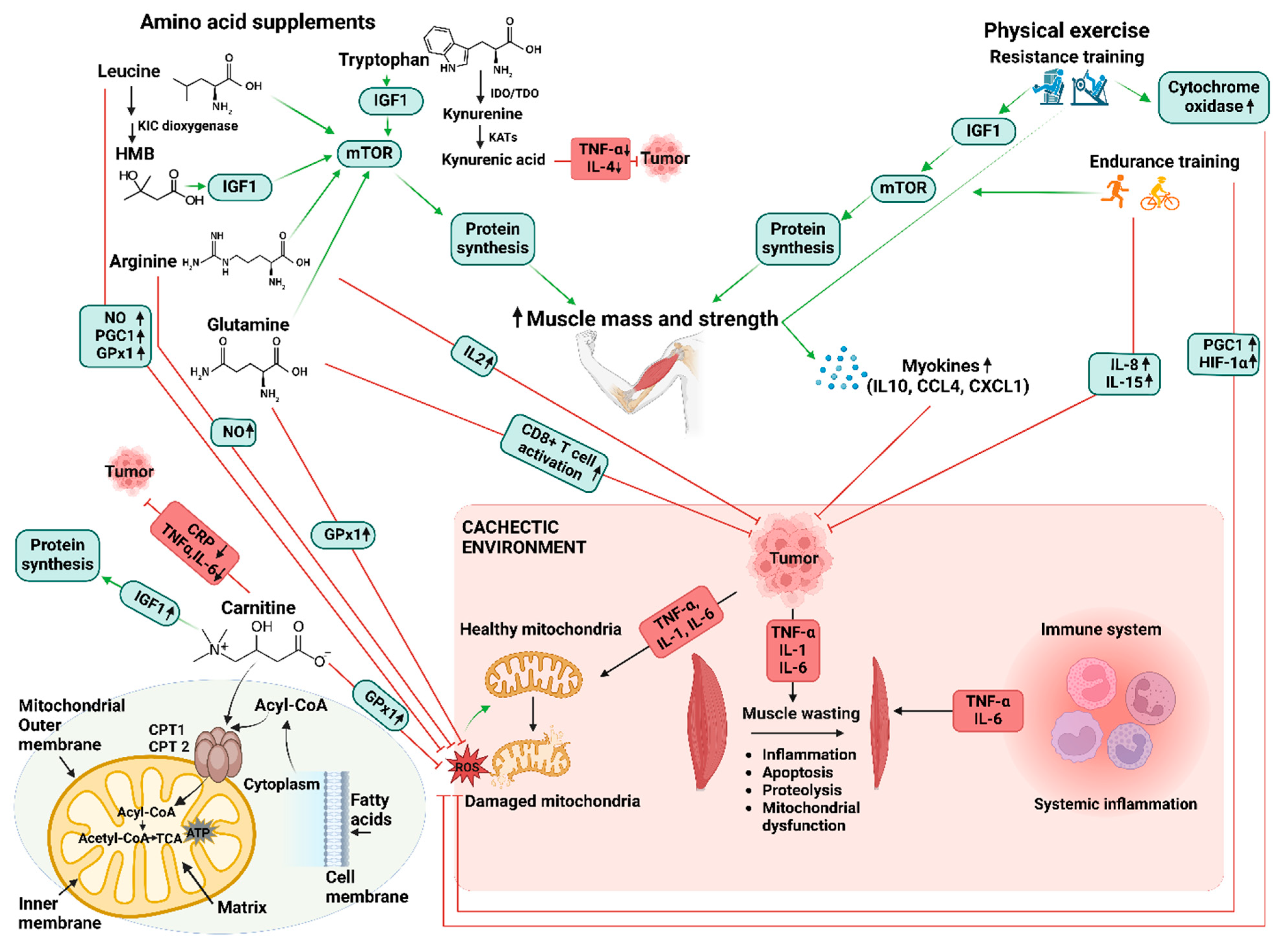
3. Role of Amino Acids in Combatting Cancer Cachexia-Induced Inflammation and Mitochondrial Dysfunction
4. The Application of Exercise and Amino Acid Supplementation in Managing Cancer Cachexia
5. Exercise and Amino Acids in Healthy Individuals
5.1. Resistance Training and Amino Acids
5.2. Endurance Training and Amino Acids
5.3. Exercise in Managing Cancer Cachexia
6. Synergistic Effects of Combined Application in Managing Cancer Cachexia
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Law, M.L. Cancer cachexia: Pathophysiology and association with cancer-related pain. Front. Pain Res. 2022, 3, 971295. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.D.P.; Howell, S.L.; Teixeira, F.J.; Pimentel, G.D. Dietary Amino Acids and Immunonutrition Supplementation in Cancer-Induced Skeletal Muscle Mass Depletion: A Mini-Review. Curr. Pharm. Des. 2020, 26, 970–978. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.; Dolan, R.D.; Skipworth, R.J.; Laird, B.J.; McMillan, D.C. Cancer cachexia: A nutritional or a systemic inflammatory syndrome? Br. J. Cancer 2022, 127, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Ábrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of Oxidative Stress as Key Regulator of Muscle Wasting during Cachexia. Oxidative Med. Cell. Longev. 2018, 2018, 2063179. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Rubio-Zarapuz, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F. Nutritional and Exercise Interventions in Cancer-Related Cachexia: An Extensive Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 4604. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.; Deutz, N.; Erickson, N.; Laviano, A.; Lisanti, M.; Lobo, D. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Butler, M.; van der Meer, L.T.; van Leeuwen, F.N. Amino Acid Depletion Therapies: Starving Cancer Cells to Death. Trends Endocrinol. Metab. 2021, 32, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Schink, K.; Herrmann, H.J.; Schwappacher, R.; Meyer, J.; Orlemann, T.; Waldmann, E.; Wullich, B.; Kahlmeyer, A.; Fietkau, R.; Lubgan, D.; et al. Effects of whole-body electromyostimulation combined with individualized nutritional support on body composition in patients with advanced cancer: A controlled pilot trial. BMC Cancer 2018, 18, 886. [Google Scholar] [CrossRef]
- Reljic, D.; Herrmann, H.J.; Jakobs, B.; Dieterich, W.; Mougiakakos, D.; Neurath, M.F.; Zopf, Y. Feasibility, Safety, and Preliminary Efficacy of Very Low-Volume Interval Training in Advanced Cancer Patients. Med. Sci. Sports Exerc. 2022, 54, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, G.W.P.d.; Farkas, J.; Dora, E.; von Haehling, S.; Lainscak, M. Cancer cachexia and related metabolic dysfunction. Int. J. Mol. Sci. 2020, 21, 2321. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Hanna, D.L.; Zhang, W.; Baba, H.; Lenz, H.-J. Molecular pathways: Cachexia signaling—A targeted approach to cancer treatment. Clin. Cancer Res. 2016, 22, 3999–4004. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.J. Signaling pathways perturbing muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; López-Soriano, F.J. The ubiquitin-dependent proteolytic pathway in skeletal muscle: Its role in pathological states. Trends Pharmacol. Sci. 1996, 17, 223–226. [Google Scholar] [CrossRef]
- Carnac, G.; Vernus, B.; Bonnieu, A. Myostatin in the pathophysiology of skeletal muscle. Curr. Genom. 2007, 8, 415–422. [Google Scholar]
- Freire, P.P.; Fernandez, G.J.; Cury, S.S.; De Moraes, D.; Oliveira, J.S.; De Oliveira, G.; Dal-Pai-Silva, M.; Dos Reis, P.P.; Carvalho, R.F. The pathway to cancer cachexia: MicroRNA-regulated networks in muscle wasting based on integrative meta-analysis. Int. J. Mol. Sci. 2019, 20, 1962. [Google Scholar] [CrossRef]
- Piccirillo, R.; Demontis, F.; Perrimon, N.; Goldberg, A.L. Mechanisms of muscle growth and atrophy in mammals and Drosophila. Dev. Dyn. 2014, 243, 201–215. [Google Scholar] [CrossRef]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI (3) K/Akt/mTOR and PI (3) K/Akt/GSK3 pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Luo, L.; Eash, J.; Ibebunjo, C.; Glass, D.J. The SCF-Fbxo40 complex induces IRS1 ubiquitination in skeletal muscle, limiting IGF1 signaling. Dev. Cell 2011, 21, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Dupont-Versteegden, E.E. Apoptosis in skeletal muscle and its relevance to atrophy. World J. Gastroenterol. WJG 2006, 12, 7463. [Google Scholar] [CrossRef] [PubMed]
- Hasselgren, P.-O.; Wray, C.; Mammen, J. Molecular regulation of muscle cachexia: It may be more than the proteasome. Biochem. Biophys. Res. Commun. 2002, 290, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.J.; Aversa, Z.; Hasselgren, P.O.; Pacelli, F.; Rosa, F.; Doglietto, G.B.; Bossola, M. Calpain activity is increased in skeletal muscle from gastric cancer patients with no or minimal weight loss. Muscle Nerve 2011, 43, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Tardif, N.; Klaude, M.; Lundell, L.; Thorell, A.; Rooyackers, O. Autophagic-lysosomal pathway is the main proteolytic system modified in the skeletal muscle of esophageal cancer patients. Am. J. Clin. Nutr. 2013, 98, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, J.; Simon, F.; Cabrera, D.; Vilos, C.; Cabello-Verrugio, C. Mitochondrial dysfunction in skeletal muscle pathologies. Curr. Protein Pept. Sci. 2019, 20, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Le Bricon, T.; Gugins, S.; Cynober, L.; Baracos, V. Negative impact of cancer chemotherapy on protein metabolism in healthy and tumor-bearing rats. Metabolism 1995, 44, 1340–1348. [Google Scholar] [CrossRef]
- Penna, F.; Baccino, F.M.; Costelli, P. Coming back: Autophagy in cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 241–246. [Google Scholar] [CrossRef]
- Johnston, A.J.; Murphy, K.T.; Jenkinson, L.; Laine, D.; Emmrich, K.; Faou, P.; Weston, R.; Jayatilleke, K.M.; Schloegel, J.; Talbo, G. Targeting of Fn14 prevents cancer-induced cachexia and prolongs survival. Cell 2015, 162, 1365–1378. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Ye, Z.-Y.; Qian, Z.-Y.; Xu, X.-D.; Hu, J.-F. Expression of TRAF6 and ubiquitin mRNA in skeletal muscle of gastric cancer patients. J. Exp. Clin. Cancer Res. 2012, 31, 81. [Google Scholar] [CrossRef] [PubMed]
- Kandarian, S.C.; Nosacka, R.L.; Delitto, A.E.; Judge, A.R.; Judge, S.M.; Ganey, J.D.; Moreira, J.D.; Jackman, R.W. Tumour-derived leukaemia inhibitory factor is a major driver of cancer cachexia and morbidity in C26 tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2018, 9, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yoneda, J.; Ohmori, H.; Sasaki, T.; Shimbo, K.; Eto, S.; Kato, Y.; Miyano, H.; Kobayashi, T.; Sasahira, T. Cancer usurps skeletal muscle as an energy repository. Cancer Res. 2014, 74, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Hitachi, K.; Tsuchida, K. Role of microRNAs in skeletal muscle hypertrophy. Front. Physiol. 2014, 4, 408. [Google Scholar] [CrossRef] [PubMed]
- He, W.A.; Calore, F.; Londhe, P.; Canella, A.; Guttridge, D.C.; Croce, C.M. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc. Natl. Acad. Sci. USA 2014, 111, 4525–4529. [Google Scholar] [CrossRef] [PubMed]
- Sanchís, D.; Busquets, S.l.; Alvarez, B.; Ricquier, D.; López-Soriano, F.J.; Argilés, J.M. Skeletal muscle UCP2 and UCP3 gene expression in a rat cancer cachexia model. FEBS Lett. 1998, 436, 415–418. [Google Scholar] [CrossRef]
- Collins, P.; Bing, C.; McCulloch, P.; Williams, G. Muscle UCP-3 mRNA levels are elevated in weight loss associated with gastrointestinal adenocarcinoma in humans. Br. J. Cancer 2002, 86, 372–375. [Google Scholar] [CrossRef]
- Penna, F.; Ballarò, R.; Beltrà, M.; De Lucia, S.; García Castillo, L.; Costelli, P. The skeletal muscle as an active player against cancer cachexia. Front. Physiol. 2019, 10, 41. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Becana, M. Molecular responses of legumes to abiotic stress: Post-translational modifications of proteins and redox signaling. J. Exp. Bot. 2021, 72, 5876–5892. [Google Scholar] [CrossRef]
- Masi, T.; Patel, B.M. Altered glucose metabolism and insulin resistance in cancer-induced cachexia: A sweet poison. Pharmacol. Rep. 2021, 73, 17–30. [Google Scholar] [CrossRef]
- Tijerina, A.J. The Biochemical Basis of Metabolism in Cancer Cachexia. Dimens. Crit. Care Nurs. 2004, 23, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Habold, C.; Le Maho, Y.; Oudart, H.; Foltzer-Jourdainne, C.; Lignot, J.-H. Intestinal gluconeogenesis and glucose transport according to body fuel availability in rats. J. Physiol. 2005, 566, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; An, Z.-Y.; Lin, D.-H.; Jin, W.-L. Targeting cancer cachexia: Molecular mechanisms and clinical study. Medcomm 2022, 3, e164. [Google Scholar] [CrossRef] [PubMed]
- Busquets, S.; Almendro, V.; Barreiro, E.; Figueras, M.; Argilés, J.M.; López-Soriano, F.J. Activation of UCPs gene expression in skeletal muscle can be independent on both circulating fatty acids and food intake: Involvement of ROS in a model of mouse cancer cachexia. FEBS Lett. 2005, 579, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, M.J. Wasting in cancer. J. Nutr. 1999, 129, 243S–246S. [Google Scholar] [CrossRef] [PubMed]
- Honors, M.A.; Kinzig, K.P. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J. Cachexia Sarcopenia Muscle 2012, 3, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Murray, D.L.; Choy, L.N.; Spiegelman, B.M. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 4854–4858. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Petruzzelli, M. A waste of insulin interference. Nature 2015, 521, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Miyazaki, M.; Sawada, A.; Sawamura, D.; Yoshida, S. Decreased insulin-like growth factor-1 expression in response to mechanical loading is associated with skeletal muscle anabolic resistance in cancer cachexia. Growth Horm. IGF Res. 2023, 69–70, 101536. [Google Scholar] [CrossRef]
- Figueroa-Clarevega, A.; Bilder, D. Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev. Cell 2015, 33, 47–55. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, J.; Almendro, V.; Busquets, S.; López-Soriano, F.J. Cross-talk between skeletal muscle and adipose tissue: A link with obesity? Med. Res. Rev. 2005, 25, 49–65. [Google Scholar] [CrossRef]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef]
- Kir, S.; White, J.P.; Kleiner, S.; Kazak, L.; Cohen, P.; Baracos, V.E.; Spiegelman, B.M. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 2014, 513, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Shellock, F.G.; Riedinger, M.S.; Fishbein, M.C. Brown adipose tissue in cancer patients: Possible cause of cancer-induced cachexia. J. Cancer Res. Clin. Oncol. 1986, 111, 82–85. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Luan, Y.; Dong, R.; Abazarikia, A.; Kim, S.-Y. Adipose Tissue Wasting as a Determinant of Pancreatic Cancer-Related Cachexia. Cancers 2022, 14, 4754. [Google Scholar] [CrossRef]
- Kliewer, K.L.; Ke, J.-Y.; Tian, M.; Cole, R.M.; Andridge, R.R.; Belury, M.A. Adipose tissue lipolysis and energy metabolism in early cancer cachexia in mice. Cancer Biol. Ther. 2014, 16, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Romani, M.; Berger, M.M.; D’amelio, P. From the Bench to the Bedside: Branched Amino Acid and Micronutrient Strategies to Improve Mitochondrial Dysfunction Leading to Sarcopenia. Nutrients 2022, 14, 483. [Google Scholar] [CrossRef]
- van der Meij, B.S.; De Groot, L.M.; Deutz, N.E.P.; Engelen, M. Effects of acute oral feeding on protein metabolism and muscle protein synthesis in individuals with cancer. Nutrition 2019, 67–68, 110531. [Google Scholar] [CrossRef]
- Gomes-Marcondes, M.; Ventrucci, G.; Toledo, M.; Cury, L.; Cooper, J. A leucine-supplemented diet improved protein content of skeletal muscle in young tumor-bearing rats. Braz. J. Med. Biol. Res. 2003, 36, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Storck, L.J.; Ruehlin, M.; Gaeumann, S.; Gisi, D.; Schmocker, M.; Meffert, P.J.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effect of a leucine-rich supplement in combination with nutrition and physical exercise in advanced cancer patients: A randomized controlled intervention trial. Clin. Nutr. 2020, 39, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Son, S.M.; Park, S.J.; Lee, H.; Siddiqi, F.; Lee, J.E.; Menzies, F.M.; Rubinsztein, D.C. Leucine Signals to mTORC1 via Its Metabolite Acetyl-Coenzyme A. Cell Metab. 2019, 29, 192–201.e7. [Google Scholar] [CrossRef] [PubMed]
- van Norren, K.; Peters, S.J.; van Helvoort, A.; Kegler, D.; Argilès, J.M.; Luiking, Y.C.; Laviano, A.; van Bergenhenegouwen, J.; Deutz, N.E.; Haagsman, H.P.; et al. Dose-dependent effects of leucine supplementation on preservation of muscle mass in cancer cachectic mice. Oncol. Rep. 2011, 26, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Eley, H.L.; Russell, S.T.; Tisdale, M.J. Effect of branched-chain amino acids on muscle atrophy in cancer cachexia. Biochem. J. 2007, 407, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Huang, Y.; Shen, Y.; et al. Mitochondrial dysfunction: Roles in skeletal muscle atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, C.; Segala, A.; Valerio, A.; Nisoli, E. Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress. Curr. Opin. Clin. Nutr. Metab. Care 2020, 24, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sánchez, M.S.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free. Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef]
- Aversa, Z.; Bonetto, A.; Costelli, P.; Minero, V.G.; Penna, F.; Baccino, F.M.; Lucia, S.; Fanelli, F.R.; Muscaritoli, M. β-hydroxy-β-methylbutyrate (HMB) attenuates muscle and body weight loss in experimental cancer cachexia. Int. J. Oncol. 2011, 38, 713–720. [Google Scholar]
- Mirza, K.A.; Pereira, S.L.; Voss, A.C.; Tisdale, M.J. Comparison of the anticatabolic effects of leucine and Ca-β-hydroxy-β-methylbutyrate in experimental models of cancer cachexia. Nutrition 2014, 30, 807–813. [Google Scholar] [CrossRef] [PubMed]
- May, P.E.; Barber, A.; D’Olimpio, J.T.; Hourihane, A.; Abumrad, N.N. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am. J. Surg. 2002, 183, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Orsso, C.E.; Pereira, S.L.; Atherton, P.J.; Deutz, N.E.P. Effects of β-hydroxy β-methylbutyrate (HMB) supplementation on muscle mass, function, and other outcomes in patients with cancer: A systematic review. J. Cachexia Sarcopenia Muscle 2022, 13, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Berk, L.; James, J.; Schwartz, A.; Hug, E.; Mahadevan, A.; Samuels, M.; Kachnic, L. A randomized, double-blind, placebo-controlled trial of a β-hydroxyl β-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support. Care Cancer 2008, 16, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; You, Z.; Shi, H.; Sun, Y.; Du, X.; Palacios, G.; Guy, C.; Yuan, S.; Chapman, N.M.; Lim, S.A.; et al. SLC38A2 and glutamine signalling in cDC1s dictate anti-tumour immunity. Nature 2023, 620, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kaibara, A.; Ishibashi, N.; Shirouzu, K. Glutamine supplementation in cancer patients. Nutrition 2001, 17, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Martins, H.A.; Bazotte, R.B.; Vicentini, G.E.; Lima, M.M.; Guarnier, F.A.; Hermes-Uliana, C.; Frez, F.C.V.; Bossolani, G.D.P.; Fracaro, L.; Fávaro, L.d.S.; et al. l-Glutamine supplementation promotes an improved energetic balance in Walker-256 tumor–bearing rats. Tumor Biol. 2017, 39, 1010428317695960. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Rhoads, J.M.; Satterfield, M.C.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Pinzon-Guzman, C.; Barbul, A. Arginine—Dual roles as an onconutrient and immunonutrient. J. Surg. Oncol. 2016, 115, 273–280. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, Y.; Zhang, Y.; Zhu, X.; Jin, F. L-Arginine supplementation inhibits the growth of breast cancer by enhancing innate and adaptive immune responses mediated by suppression of MDSCs in vivo. BMC Cancer 2016, 16, 343. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Long, Y.C. Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Sci. Rep. 2016, 6, 30033. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Clanchy, F.I.L.; Huang, Y.-S.; Chiang, N.-Y.; Darlington, L.G.; Williams, R.O. An integrated cytokine and kynurenine network as the basis of neuroimmune communication. Front. Neurosci. 2022, 16, 1002004. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Kink, P.; Egger, E.M.; Willenbacher, W.; Fuchs, D.; Weiss, G.; Kurz, K. Inflammation-Induced Tryptophan Breakdown is Related With Anemia, Fatigue, and Depression in Cancer. Front. Immunol. 2020, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Agulló-Ortuño, M.T.; Mancebo, E.; Grau, M.; Sobrino, J.A.N.; Paz-Ares, L.; López-Martín, J.A.; Flández, M. Tryptophan Modulation in Cancer-Associated Cachexia Mouse Models. Int. J. Mol. Sci. 2023, 24, 13005. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Lv, T.; Zhang, T.; Feng, D.; Zhu, F.; Xu, Y.; Zhang, L.; Gu, L.; Guo, Z.; Ding, C.; et al. Interleukin-6 promotes skeletal muscle catabolism by activating tryptophan-indoleamine 2,3-dioxygenase 1-kynurenine pathway during intra-abdominal sepsis. J. Cachexia Sarcopenia Muscle 2023, 14, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Cabral, R.E.L.; Mendes, T.B.; Vendramini, V.; Miraglia, S.M. Carnitine partially improves oxidative stress, acrosome integrity, and reproductive competence in doxorubicin-treated rats. Andrology 2018, 6, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Fathizadeh, H.; Milajerdi, A.; Reiner, Ž.; Amirani, E.; Asemi, Z.; Mansournia, M.A.; Hallajzadeh, J. The effects of L-carnitine supplementation on indicators of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2020, 19, 1879–1894. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Couturier, A.; Haferkamp, M.; Most, E.; Eder, K. Supplementation of carnitine leads to an activation of the IGF-1/PI3K/Akt signalling pathway and down regulates the E3 ligase MuRF1 in skeletal muscle of rats. Nutr. Metab. 2013, 10, 28. [Google Scholar] [CrossRef]
- Gualano, B.; Roschel, H.; Lancha, A.H., Jr.; Brightbill, C.E.; Rawson, E.S. In sickness and in health: The widespread application of creatine supplementation. Amino Acids 2012, 43, 519–529. [Google Scholar] [CrossRef]
- Esfahani, M.; Sahafi, S.; Derakhshandeh, A.; Moghaddas, A. The anti-wasting effects of L-carnitine supplementation on cancer: Experimental data and clinical studies. Asia Pac. J. Clin. Nutr. 2018, 27, 503–511. [Google Scholar] [PubMed]
- Kraft, M.; Kraft, K.; Gärtner, S.; Mayerle, J.; Simon, P.; Weber, E.; Schütte, K.; Stieler, J.; Koula-Jenik, H.; Holzhauer, P.; et al. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)–A randomized multicentre trial. Nutr. J. 2012, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Gramignano, G.; Lusso, M.R.; Madeddu, C.; Massa, E.; Serpe, R.; Deiana, L.; Lamonica, G.; Dessì, M.; Spiga, C.; Astara, G.; et al. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition 2006, 22, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, H.-J.; Zhang, Z.-Q.; Chen, Q.; Liu, B.; Wu, J.-P.; Zhu, L. L-carnitine ameliorates cancer cachexia in mice by regulating the expression and activity of carnitine palmityl transferase. Cancer Biol. Ther. 2011, 12, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Busquets, S.; Pérez-Peiró, M.; Salazar-Degracia, A.; Argilés, J.M.; Serpe, R.; Rojano-Toimil, A.; López-Soriano, F.J.; Barreiro, E. Differential structural features in soleus and gastrocnemius of carnitine-treated cancer cachectic rats. J. Cell. Physiol. 2020, 235, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Fernández-García, B.; Lehmann, H.I.; Li, G.; Kroemer, G.; López-Otín, C.; Xiao, J. Exercise sustains the hallmarks of health. J. Sport. Health Sci. 2023, 12, 8–35. [Google Scholar] [CrossRef]
- Aguirre, N.; van Loon, L.J.; Baar, K. The role of amino acids in skeletal muscle adaptation to exercise. Nestle Nutr. Inst. Workshop Ser. 2013, 76, 85–102. [Google Scholar] [PubMed]
- Furuichi, Y.; Manabe, Y.; Takagi, M.; Aoki, M.; Fujii, N.L. Evidence for acute contraction-induced myokine secretion by C2C12 myotubes. PLoS ONE 2018, 13, e0206146. [Google Scholar] [CrossRef] [PubMed]
- Eckel, J. Myokines in metabolic homeostasis and diabetes. Diabetologia 2019, 62, 1523–1528. [Google Scholar] [CrossRef]
- McKendry, J.; Currier, B.S.; Lim, C.; Mcleod, J.C.; Thomas, A.C.; Phillips, S.M. Nutritional supplements to support resistance exercise in countering the sarcopenia of aging. Nutrients 2020, 12, 2057. [Google Scholar] [CrossRef]
- Gontzea, I.; Sutzesco, P.; Dumitrache, S. Research on the protein requirement of man during muscular activity. Arch. Sci. Physiol. 1962, 16, 97–120. [Google Scholar]
- Biolo, G.; Tipton, K.D.; Klein, S.; Wolfe, R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. -Endocrinol. Metab. 1997, 273, E122–E129. [Google Scholar] [CrossRef]
- Kato, H.; Suzuki, K.; Bannai, M.; Moore, D.R. Protein requirements are elevated in endurance athletes after exercise as determined by the indicator amino acid oxidation method. PLoS ONE 2016, 11, e0157406. [Google Scholar] [CrossRef]
- Lemon, P.W. Do athletes need more dietary protein and amino acids? Int. J. Sport. Nutr. Exerc. Metab. 1995, 5, S39–S61. [Google Scholar] [CrossRef] [PubMed]
- Lemon, P.W. Protein and amino acid needs of the strength athlete. Int. J. Sport. Nutr. Exerc. Metab. 1991, 1, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.R.; Lira, F.S.d.; De Mello, M.; Santos, R.V.T. Importance of exercise immunology in health promotion. Amino Acids 2011, 41, 1165–1172. [Google Scholar] [CrossRef]
- Hatazawa, Y.; Tadaishi, M.; Nagaike, Y.; Morita, A.; Ogawa, Y.; Ezaki, O.; Takai-Igarashi, T.; Kitaura, Y.; Shimomura, Y.; Kamei, Y. PGC-1α-mediated branched-chain amino acid metabolism in the skeletal muscle. PLoS ONE 2014, 9, e91006. [Google Scholar] [CrossRef] [PubMed]
- Hatazawa, Y.; Senoo, N.; Tadaishi, M.; Ogawa, Y.; Ezaki, O.; Kamei, Y.; Miura, S. Metabolomic analysis of the skeletal muscle of mice overexpressing PGC-1α. PLoS ONE 2015, 10, e0129084. [Google Scholar] [CrossRef]
- Kim, H.K.; Suzuki, T.; Saito, K.; Yoshida, H.; Kobayashi, H.; Kato, H.; Katayama, M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J. Am. Geriatr. Soc. 2012, 60, 16–23. [Google Scholar] [CrossRef]
- Osmond, A.D.; Directo, D.J.; Elam, M.L.; Juache, G.; Kreipke, V.C.; Saralegui, D.E.; Wildman, R.; Wong, M.; Jo, E. The effects of leucine-enriched branched-chain amino acid supplementation on recovery after high-intensity resistance exercise. Int. J. Sports Physiol. Perform. 2019, 14, 1081–1088. [Google Scholar] [CrossRef]
- Karlsson, H.K.; Nilsson, P.-A.; Nilsson, J.; Chibalin, A.V.; Zierath, J.R.; Blomstrand, E. Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am. J. Physiol.-Endocrinol. Metab. 2004, 287, E1–E7. [Google Scholar] [CrossRef]
- Blomstrand, E.; Eliasson, J.; Karlsson, H.K.; Köhnke, R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J. Nutr. 2006, 136, 269S–273S. [Google Scholar] [CrossRef]
- Jackman, S.R.; Witard, O.C.; Philp, A.; Wallis, G.A.; Baar, K.; Tipton, K.D. Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans. Front. Physiol. 2017, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Reule, C.A.; Scholz, C.; Schoen, C.; Brown, N.; Siepelmeyer, A.; Alt, W.W. Reduced muscular fatigue after a 12-week leucine-rich amino acid supplementation combined with moderate training in elderly: A randomised, placebo-controlled, double-blind trial. BMJ Open Sport. Exerc. Med. 2017, 2, e000156. [Google Scholar] [CrossRef]
- Brooks, N.; Cloutier, G.J.; Cadena, S.M.; Layne, J.E.; Nelsen, C.A.; Freed, A.M.; Roubenoff, R.; Castaneda-Sceppa, C. Resistance training and timed essential amino acids protect against the loss of muscle mass and strength during 28 days of bed rest and energy deficit. J. Appl. Physiol. 2008, 105, 241–248. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Lockwood, C.M.; Stout, J.R. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: A case for whey protein. Nutr. Metab. 2010, 7, 51. [Google Scholar] [CrossRef]
- Jakubowski, J.S.; Nunes, E.A.; Teixeira, F.J.; Vescio, V.; Morton, R.W.; Banfield, L.; Phillips, S.M. Supplementation with the Leucine Metabolite β-hydroxy-β-methylbutyrate (HMB) does not Improve Resistance Exercise-Induced Changes in Body Composition or Strength in Young Subjects: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1523. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Stout, J.; Wilborn, C. Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids 2007, 32, 467–477. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, J.A., Jr.; Falavigna, G.; Rogero, M.M.; Pires, I.S.; Pedrosa, R.G.; Castro, I.A.; Donato, J., Jr.; Tirapegui, J. Effect of chronic supplementation with branched-chain amino acids on the performance and hepatic and muscle glycogen content in trained rats. Life Sci. 2006, 79, 1343–1348. [Google Scholar] [CrossRef]
- Hargreaves, M.; Snow, R. Amino acids and endurance exercise. Int. J. Sport. Nutr. Exerc. Metab. 2001, 11, 133–145. [Google Scholar] [CrossRef]
- Watson, P.; Shirreffs, S.M.; Maughan, R.J. The effect of acute branched-chain amino acid supplementation on prolonged exercise capacity in a warm environment. Eur. J. Appl. Physiol. 2004, 93, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Cheuvront, S.N.; Carter, R., III; Kolka, M.A.; Lieberman, H.R.; Kellogg, M.D.; Sawka, M.N. Branched-chain amino acid supplementation and human performance when hypohydrated in the heat. J. Appl. Physiol. 2004, 97, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Spillane, M.; Emerson, C.; Willoughby, D.S. The effects of 8 weeks of heavy resistance training and branched-chain amino acid supplementation on body composition and muscle performance. Nutr. Health 2012, 21, 263–273. [Google Scholar] [CrossRef]
- Munroe, M.; Pincu, Y.; Merritt, J.; Cobert, A.; Brander, R.; Jensen, T.; Rhodes, J.; Boppart, M.D. Impact of β-hydroxy β-methylbutyrate (HMB) on age-related functional deficits in mice. Exp. Gerontol. 2017, 87, 57–66. [Google Scholar] [CrossRef]
- Silva, E., Jr.; Borges, L.d.S.; Mendes-da-Silva, C.; Hirabara, S.M.; Lambertucci, R.H. l-Arginine supplementation improves rats’ antioxidant system and exercise performance. Free Radic. Res. 2017, 51, 281–293. [Google Scholar] [CrossRef]
- Stefani, G.P.; Marmett, B.; Alves, J.P.; Möller, G.B.; Heck, T.G.; Frizzo, M.N.; Di Domenico, M.; Motta, G.A.; Dal Lago, P.; Nunes, R.B. Resistance training and L-arginine supplementation are determinant in genomic stability, cardiac contractility and muscle mass development in rats. PLoS ONE 2018, 13, e0204858. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, A.Y.; Raizel, R.; Bonvini, A.; Hypólito, T.; Godois, A.d.M.; Pereira, J.R.R.; Garcia, A.B.d.O.; Lara, R.d.S.B.; Rogero, M.M.; Tirapegui, J. Effects of glutamine and alanine supplementation on central fatigue markers in rats submitted to resistance training. Nutrients 2018, 10, 119. [Google Scholar] [CrossRef]
- Knuiman, P.; Hopman, M.T.E.; Verbruggen, C.; Mensink, M. Protein and the Adaptive Response With Endurance Training: Wishful Thinking or a Competitive Edge? Front. Physiol. 2018, 9, 598. [Google Scholar] [CrossRef]
- Konopka, A.R.; Harber, M.P. Skeletal muscle hypertrophy after aerobic exercise training. Exerc. Sport. Sci. Rev. 2014, 42, 53. [Google Scholar] [CrossRef]
- Konopka, A.R.; Suer, M.K.; Wolff, C.A.; Harber, M.P. Markers of human skeletal muscle mitochondrial biogenesis and quality control: Effects of age and aerobic exercise training. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 371–378. [Google Scholar] [CrossRef]
- Coffey, V.G.; Moore, D.R.; Burd, N.A.; Rerecich, T.; Stellingwerff, T.; Garnham, A.P.; Phillips, S.M.; Hawley, J.A. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur. J. Appl. Physiol. 2011, 111, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Pannemans, D.L.E.; Jeukendrup, A.E.; Gijsen, A.P.; Senden, J.M.G.; Halliday, D.; Saris, W.H.M.; Loon, L.J.C.v.; Wagenmakers, A.J.M. Combined ingestion of protein and carbohydrate improves protein balance during ultra-endurance exercise. Am. J. Physiol.-Endocrinol. Metab. 2004, 287, E712–E720. [Google Scholar] [CrossRef]
- Hansen, M.; Bangsbo, J.; Jensen, J.; Krause-Jensen, M.; Bibby, B.M.; Sollie, O.; Hall, U.A.; Madsen, K. Protein intake during training sessions has no effect on performance and recovery during a strenuous training camp for elite cyclists. J. Int. Soc. Sports Nutr. 2016, 13, 9. [Google Scholar] [CrossRef]
- Hill, K.M.; Stathis, C.G.; Grinfeld, E.; Hayes, A.; McAinch, A.J. Co-ingestion of carbohydrate and whey protein isolates enhance PGC-1α mRNA expression: A randomised, single blind, cross over study. J. Int. Soc. Sports Nutr. 2013, 10, 1–8. [Google Scholar] [CrossRef]
- Randolph, A.C.; Markofski, M.M.; Rasmussen, B.B.; Volpi, E. Effect of essential amino acid supplementation and aerobic exercise on insulin sensitivity in healthy older adults: A randomized clinical trial. Clin. Nutr. 2020, 39, 1371–1378. [Google Scholar] [CrossRef]
- Markofski, M.M.; Jennings, K.; Timmerman, K.L.; Dickinson, J.M.; Fry, C.S.; Borack, M.S.; Reidy, P.T.; Deer, R.R.; Randolph, A.; Rasmussen, B.B. Effect of aerobic exercise training and essential amino acid supplementation for 24 weeks on physical function, body composition, and muscle metabolism in healthy, independent older adults: A randomized clinical trial. J. Gerontol. Ser. A 2019, 74, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Bassit, R.A.; Sawada, L.c.A.; Bacurau, R.F.; Navarro, F.; Martins, E., Jr.; Santos, R.V.; Caperuto, E.C.; Rogeri, P.; Rosa, L.F.C. Branched-chain amino acid supplementation and the immune response of long-distance athletes. Nutrition 2002, 18, 376–379. [Google Scholar] [CrossRef]
- Shimomura, Y.; Inaguma, A.; Watanabe, S.; Yamamoto, Y.; Muramatsu, Y.; Bajotto, G.; Sato, J.; Shimomura, N.; Kobayashi, H.; Mawatari, K. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int. J. Sport. Nutr. Exerc. Metab. 2010, 20, 236–244. [Google Scholar] [CrossRef]
- Blomstrand, E.; Saltin, B. BCAA intake affects protein metabolism in muscle after but not during exercise in humans. Am. J. Physiol.-Endocrinol. Metab. 2001, 281, E365–E374. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Landa, J.; Todorovic, N.; Santibañez-Gutierrez, A.; Ostojic, S.M.; Calleja-González, J.; Sekulic, D.; Mielgo-Ayuso, J. Effects of HMB on Endurance Performance in a Healthy Population: A Systematic Review and Meta-Analysis. J. Strength Cond. Res. 2023, 38, e202–e210. [Google Scholar] [CrossRef]
- Coqueiro, A.Y.; Rogero, M.M.; Tirapegui, J. Glutamine as an Anti-Fatigue Amino Acid in Sports Nutrition. Nutrients 2019, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, N.; Pedersen, B.K. Exercise-induced immunodepression- plasma glutamine is not the link. J. Appl. Physiol. 2002, 93, 813–822. [Google Scholar] [CrossRef]
- Gleeson, M. Dosing and efficacy of glutamine supplementation in human exercise and sport training. J. Nutr. 2008, 138, 2045s–2049s. [Google Scholar] [CrossRef]
- Sato, S.; Gao, S.; Puppa, M.J.; Kostek, M.C.; Wilson, L.B.; Carson, J.A. High-Frequency Stimulation on Skeletal Muscle Maintenance in Female Cachectic Mice. Med. Sci. Sports Exerc. 2019, 51, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Deboer, M.D. Animal models of anorexia and cachexia. Expert. Opin. Drug Discov. 2009, 4, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, N.; Görgens, S.W.; Thoresen, G.H.; Aas, V.; Eckel, J.; Eckardt, K. Electrical pulse stimulation of cultured skeletal muscle cells as a model for in vitro exercise—Possibilities and limitations. Acta Physiol. 2017, 220, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.; Görgens, S.W.; Raschke, S.; Eckel, J. Myokines in insulin resistance and type 2 diabetes. Diabetologia 2014, 57, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Görgens, S.W.; Eckardt, K.; Jensen, J.; Drevon, C.A.; Eckel, J. Exercise and regulation of adipokine and myokine production. Prog. Mol. Biol. Transl. Sci. 2015, 135, 313–336. [Google Scholar] [PubMed]
- Aas, V.; Torblå, S.; Andersen, M.H.; Jensen, J.; Rustan, A.C. Electrical stimulation improves insulin responses in a human skeletal muscle cell model of hyperglycemia. Ann. N. Y. Acad. Sci. 2002, 967, 506–515. [Google Scholar] [CrossRef]
- Nikolić, N.; Aas, V. Electrical pulse stimulation of primary human skeletal muscle cells. In Myogenesis; Humana Press: New York, NY, USA, 2019; pp. 17–24. [Google Scholar]
- Nikolić, N.; Skaret Bakke, S.; Tranheim Kase, E.; Rudberg, I.; Flo Halle, I.; Rustan, A.C.; Thoresen, G.H.; Aas, V. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS ONE 2012, 7, e33203. [Google Scholar] [CrossRef]
- Lambernd, S.; Taube, A.; Schober, A.; Platzbecker, B.; Görgens, S.; Schlich, R.; Jeruschke, K.; Weiss, J.; Eckardt, K.; Eckel, J. Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signalling pathways. Diabetologia 2012, 55, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Nintou, E.; Karligiotou, E.; Vliora, M.; Ioannou, L.G.; Flouris, A.D. Characteristics of the Protocols Used in Electrical Pulse Stimulation of Cultured Cells for Mimicking In Vivo Exercise: A Systematic Review, Meta-Analysis, and Meta-Regression. Int. J. Mol. Sci. 2022, 23, 13446. [Google Scholar] [CrossRef] [PubMed]
- Jee, H.; Chang, J.-E.; Yang, E.J. Positive prehabilitative effect of intense treadmill exercise for ameliorating cancer cachexia symptoms in a mouse model. J. Cancer 2016, 7, 2378. [Google Scholar] [CrossRef] [PubMed]
- Puppa, M.J.; White, J.P.; Velázquez, K.T.; Baltgalvis, K.A.; Sato, S.; Baynes, J.W.; Carson, J.A. The effect of exercise on IL-6-induced cachexia in the Apc Min/+ mouse. J. Cachexia Sarcopenia Muscle 2012, 3, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sugimoto, K.; Fujimoto, T.; Xie, K.; Takahashi, T.; Akasaka, H.; Kurinami, H.; Yasunobe, Y.; Matsumoto, T.; Fujino, H. Preventive effects of low-intensity exercise on cancer cachexia–induced muscle atrophy. FASEB J. 2019, 33, 7852–7862. [Google Scholar] [CrossRef] [PubMed]
- Pigna, E.; Berardi, E.; Aulino, P.; Rizzuto, E.; Zampieri, S.; Carraro, U.; Kern, H.; Merigliano, S.; Gruppo, M.; Mericskay, M. Aerobic exercise and pharmacological treatments counteract cachexia by modulating autophagy in colon cancer. Sci. Rep. 2016, 6, 26991. [Google Scholar] [CrossRef]
- Coletti, D.; Aulino, P.; Pigna, E.; Barteri, F.; Moresi, V.; Annibali, D.; Adamo, S.; Berardi, E. Spontaneous physical activity downregulates Pax7 in cancer cachexia. Stem Cells Int. 2016, 2016, 6729268. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Miyazaki, M.; Kikuchi, S. Voluntary exercise prevents abnormal muscle mitochondrial morphology in cancer cachexia mice. Physiol. Rep. 2021, 9, e15016. [Google Scholar] [CrossRef] [PubMed]
- Hiroux, C.; Dalle, S.; Koppo, K.; Hespel, P. Voluntary exercise does not improve muscular properties or functional capacity during C26-induced cancer cachexia in mice. J. Muscle Res. Cell Motil. 2021, 42, 169–181. [Google Scholar] [CrossRef]
- Morinaga, M.; Sako, N.; Isobe, M.; Lee-Hotta, S.; Sugiura, H.; Kametaka, S. Aerobic Exercise Ameliorates Cancer Cachexia-Induced Muscle Wasting through Adiponectin Signaling. Int. J. Mol. Sci. 2021, 22, 3110. [Google Scholar] [CrossRef]
- Ranjbar, K.; Ballarò, R.; Bover, Q.; Pin, F.; Beltrà, M.; Penna, F.; Costelli, P. Combined Exercise Training Positively Affects Muscle Wasting in Tumor-Bearing Mice. Med. Sci. Sports Exerc. 2019, 51, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Wood, N.R.; Garritson, J.; Mathias, A.; Haughian, J.M.; Hayward, R. Moderate Intensity Endurance and Resistance Exercise Attenuates Cachexia in Tumor-bearing Mice. Anticancer. Res. 2022, 42, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Ballarò, R.; Beltrà, M.; De Lucia, S.; Pin, F.; Ranjbar, K.; Hulmi, J.J.; Costelli, P.; Penna, F. Moderate exercise in mice improves cancer plus chemotherapy-induced muscle wasting and mitochondrial alterations. FASEB J. 2019, 33, 5482–5494. [Google Scholar] [CrossRef] [PubMed]
- Assi, M.; Rébillard, A. The Janus-faced role of antioxidants in cancer cachexia: New insights on the established concepts. Oxidative Med. Cell. Longev. 2016, 2016, 9579868. [Google Scholar] [CrossRef] [PubMed]
- Penna, F.; Ballarò, R.; Costelli, P. The redox balance: A target for interventions against muscle wasting in cancer cachexia? Antioxid. Redox Signal. 2020, 33, 542–558. [Google Scholar] [CrossRef] [PubMed]
- Dimeo, F.; Rumberger, B.G.; Keul, J. Aerobic exercise as therapy for cancer fatigue. Med. Sci. Sports Exerc. 1998, 30, 475–478. [Google Scholar] [CrossRef]
- Mock, V.; Dow, K.H.; Meares, C.J.; Grimm, P.M.; Dienemann, J.A.; Haisfield-Wolfe, M.E.; Quitasol, W.; Mitchell, S.; Chakravarthy, A.; Gage, I. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol. Nurs. Forum 1997, 24, 991–1000. [Google Scholar] [PubMed]
- Kolden, G.G.; Strauman, T.J.; Ward, A.; Kuta, J.; Woods, T.E.; Schneider, K.L.; Heerey, E.; Sanborn, L.; Burt, C.; Millbrandt, L. A pilot study of group exercise training (GET) for women with primary breast cancer: Feasibility and health benefits. Psycho-Oncol. J. Psychol. Soc. Behav. Dimens. Cancer 2002, 11, 447–456. [Google Scholar] [CrossRef]
- Na, Y.-M.; Kim, M.-Y.; Kim, Y.-K.; Ha, Y.-R.; Yoon, D.S. Exercise therapy effect on natural killer cell cytotoxic activity in stomach cancer patients after curative surgery. Arch. Phys. Med. Rehabil. 2000, 81, 777–779. [Google Scholar] [CrossRef]
- Dimeo, F.; Schwartz, S.; Fietz, T.; Wanjura, T.; Böning, D.; Thiel, E. Effects of endurance training on the physical performance of patients with hematological malignancies during chemotherapy. Support. Care Cancer 2003, 11, 623–628. [Google Scholar] [CrossRef]
- Courneya, K.; Friedenreich, C.; Quinney, H.; Fields, A.; Jones, L.; Fairey, A. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur. J. Cancer Care 2003, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Ciuró, A.; Fernández-Sánchez, M.; Martín-Núñez, J.; Calvache-Mateo, A.; Rodríguez-Torres, J.; López-López, L.; Valenza, M.C. High-intensity interval training effects in cardiorespiratory fitness of lung cancer survivors: A systematic review and meta-analysis. Support. Care Cancer 2021, 30, 3017–3027. [Google Scholar] [CrossRef] [PubMed]
- Porock, D.; Kristjanson, L.J.; Tinnelly, K.; Duke, T.; Blight, J. An exercise intervention for advanced cancer patients experiencing fatigue: A pilot study. J. Palliat. Care 2000, 16, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Lötzerich, H.; Niemeier, B.; Schüle, K.; Uhlenbruck, G. Influence of a moderate exercise training on natural killer cytotoxicity and personality traits in cancer patients. Anticancer. Res. 1994, 14, 1033–1036. [Google Scholar] [PubMed]
- Machado, P.; Pimenta, S.; Oliveiros, B.; Ferreira, J.P.; Martins, R.A.; Cruz, J. Effect of Exercise Training on Quality of Life after Colorectal and Lung Cancer Surgery: A Meta-Analysis. Cancers 2021, 13, 4975. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Thomas, S.M.; Herndon, J.E.; Douglas, P.S.; Yu, A.F.; Rusch, V.; Huang, J.; Capaci, C.; Harrison, J.N.; Stoeckel, K.J.; et al. Effects and tolerability of exercise therapy modality on cardiorespiratory fitness in lung cancer: A randomized controlled trial. J. Cachex- Sarcopenia Muscle 2021, 12, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Repka, C.P.; Hayward, R. Effects of an exercise intervention on cancer-related fatigue and its relationship to markers of oxidative stress. Integr. Cancer Ther. 2018, 17, 503–510. [Google Scholar] [CrossRef]
- Goh, J.; Niksirat, N.; Campbell, K.L. Exercise training and immune crosstalk in breast cancer microenvironment: Exploring the paradigms of exercise-induced immune modulation and exercise-induced myokines. Am. J. Transl. Res. 2014, 6, 422–438. [Google Scholar] [PubMed]
- Repka, C.P.; Hayward, R. Oxidative stress and fitness changes in cancer patients after exercise training. Med. Sci. Sports Exerc. 2016, 48, 607–614. [Google Scholar] [CrossRef]
- Padilha, C.S.; Cella, P.S.; Chimin, P.; Voltarelli, F.A.; Marinello, P.C.; Testa, M.T.D.J.; Guirro, P.B.; Duarte, J.A.R.; Cecchini, R.; Guarnier, F.A.; et al. Resistance Training’s Ability to Prevent Cancer-induced Muscle Atrophy Extends Anabolic Stimulus. Med. Sci. Sports Exerc. 2021, 53, 1572–1582. [Google Scholar] [CrossRef]
- Johnston, A.P.W.; Lisio, M.D.; Parise, G. Resistance training, sarcopenia, and the mitochondrial theory of aging. Appl. Physiol. Nutr. Metab. 2008, 33, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Schwappacher, R.; Dieterich, W.; Reljic, D.; Pilarsky, C.; Mukhopadhyay, D.; Chang, D.K.; Biankin, A.V.; Siebler, J.; Herrmann, H.J.; Neurath, M.F.; et al. Muscle-Derived Cytokines Reduce Growth, Viability and Migratory Activity of Pancreatic Cancer Cells. Cancers 2021, 13, 3820. [Google Scholar] [CrossRef] [PubMed]
- Wiskemann, J.; Clauss, D.; Tjaden, C.; Hackert, T.; Schneider, L.; Ulrich, C.M.; Steindorf, K. Progressive resistance training to impact physical fitness and body weight in pancreatic cancer patients: A randomized controlled trial. Pancreas 2019, 48, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Rosebrock, K.; Sinn, M.; Uzunoglu, F.G.; Bokemeyer, C.; Jensen, W.; Salchow, J. Effects of Exercise Training on Patient-Specific Outcomes in Pancreatic Cancer Patients: A Scoping Review. Cancers 2023, 15, 5899. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.J.; Reid, R.D.; Courneya, K.S.; Sigal, R.J.; Kenny, G.P.; Prud’Homme, D.G.; Malone, S.C.; Wells, G.A.; Scott, C.G.; Slovinec D’Angelo, M.E. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J. Clin. Oncol. 2009, 27, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Segal, R.J.; Mackey, J.R.; Gelmon, K.; Reid, R.D.; Friedenreich, C.M.; Ladha, A.B.; Proulx, C.; Vallance, J.; Lane, K. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J. Clin. Oncol. 2007, 25, 4396–4404. [Google Scholar] [CrossRef] [PubMed]
- Grote, M.; Maihöfer, C.; Weigl, M.; Davies-Knorr, P.; Belka, C. Progressive resistance training in cachectic head and neck cancer patients undergoing radiotherapy: A randomized controlled pilot feasibility trial. Radiat. Oncol. 2018, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Aquila, G.; Re Cecconi, A.D.; Brault, J.J.; Corli, O.; Piccirillo, R. Nutraceuticals and exercise against muscle wasting during cancer cachexia. Cells 2020, 9, 2536. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J. Cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9, S39–S50. [Google Scholar] [CrossRef]
- Koshimoto, S.; Arimoto, M.; Saitou, K.; Uchibori, M.; Hashizume, A.; Honda, A.; Amano, K.; Nakajima, Y.; Uetake, H.; Matsushima, E. Need and demand for nutritional counselling and their association with quality of life, nutritional status and eating-related distress among patients with cancer receiving outpatient chemotherapy: A cross-sectional study. Support. Care Cancer 2019, 27, 3385–3394. [Google Scholar] [CrossRef]
- Bauer, J. Nutritional management and dietary guidelines for cancer cachexia. J.-Nutr. Manag. Diet. Guidel. Cancer Cachexia 2007, 1, 12–14. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Blanc-Bisson, C.; Doussau, A.; Germain, C.; Blanc, J.-F.; Dauba, J.; Lahmar, C.; Terrebonne, E.; Lecaille, C.; Ceccaldi, J. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: A two-year randomized controlled trial. PLoS ONE 2014, 9, e108687. [Google Scholar]
- Zhang, F.; Jin, Y.; Qiang, W. The effects of dietary advice on malnutrition in Cancer patients: A systematic review and meta-analysis. Support. Care Cancer 2020, 28, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Burden, S.; Jones, D.J.; Sremanakova, J.; Sowerbutts, A.M.; Lal, S.; Pilling, M.; Todd, C. Dietary interventions for adult cancer survivors. Cochrane Database Syst. Rev. 2019, 2019, CD011287. [Google Scholar] [CrossRef]
- Di Girolamo, F.G.; Guadagni, M.; Fiotti, N.; Situlin, R.; Biolo, G. Contraction and nutrition interaction promotes anabolism in cachectic muscle. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Del Fabbro, E. Combination therapy in cachexia. Ann. Palliat. Med. 2018, 8, 59–66. [Google Scholar] [CrossRef]
- Kim, A.J.; Hong, D.S.; George, G.C. Diet-related interventions for cancer-associated cachexia. J. Cancer Res. Clin. Oncol. 2021, 147, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.C.; Cook, J.; Maddocks, M.; Skipworth, R.J.E.; Fallon, M.; Laird, B.J. Combined exercise and nutritional rehabilitation in outpatients with incurable cancer: A systematic review. Support. Care Cancer 2019, 27, 2371–2384. [Google Scholar] [CrossRef]
- Fry, C.S.; B Rasmussen, B. Skeletal muscle protein balance and metabolism in the elderly. Curr. Aging Sci. 2011, 4, 260–268. [Google Scholar] [CrossRef]
- Morton, R.W.; Traylor, D.A.; Weijs, P.J.; Phillips, S.M. Defining anabolic resistance: Implications for delivery of clinical care nutrition. Curr. Opin. Crit. Care 2018, 24, 124–130. [Google Scholar] [CrossRef]
- Sawada, A.; Yoneta, K.; Togashi, E.; Asaka, S.; Tada, R.; Asada, T.; Son, S.; Tayama, M.; Kimura, M.; Fujita, S. The effects of resistance exercise and leucine-enriched essential amino acid supplementation on muscle mass and physical function in post-gastrectomy patients: A pilot randomized controlled trial. J. Phys. Ther. Sci. 2024, 36, 218–225. [Google Scholar] [CrossRef]
- Antoun, S.; Raynard, B. Muscle protein anabolism in advanced cancer patients: Response to protein and amino acids support, and to physical activity. Ann. Oncol. 2018, 29 (Suppl. S2), ii10–ii17. [Google Scholar] [CrossRef]
- Payne, C.; Larkin, P.J.; McIlfatrick, S.; Dunwoody, L.; Gracey, J.H. Exercise and nutrition interventions in advanced lung cancer: A systematic review. Curr. Oncol. 2013, 20, e321–e337. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.P., Jr.; Borges, L.; Bachi, A.L.L.; Hirabara, S.M.; Lambertucci, R.H. L-arginine Improves Plasma Lipid Profile and Muscle Inflammatory Response in Trained Rats After High-Intense Exercise. Res. Q. Exerc. Sport. 2021, 92, 82–90. [Google Scholar] [CrossRef]
- Alves, C.R.; das Neves, W.; Tobias, G.C.; de Almeida, N.R.; Barreto, R.F.; Melo, C.M.; Carneiro, C.d.G.; Garcez, A.T.; Faria, D.d.P.; Chammas, R. High-intensity interval training slows down tumor progression in mice bearing Lewis lung carcinoma. JCSM Rapid Commun. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Patel, D.I.; Abuchowski, K.; Sheikh, B.; Rivas, P.; Musi, N.; Kumar, A.P. Exercise preserves muscle mass and force in a prostate cancer mouse model. Eur. J. Transl. Myol. 2019, 29, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Padilha, C.S.; Borges, F.H.; Costa Mendes da Silva, L.E.; Frajacomo, F.T.T.; Jordao, A.A.; Duarte, J.A.; Cecchini, R.; Guarnier, F.A.; Deminice, R. Resistance exercise attenuates skeletal muscle oxidative stress, systemic pro-inflammatory state, and cachexia in Walker-256 tumor-bearing rats. Appl. Physiol. Nutr. Metab. 2017, 42, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Tweed, T.T.; Sier, M.A.; Van Bodegraven, A.A.; Van Nie, N.C.; Sipers, W.M.; Boerma, E.-J.G.; Stoot, J.H. Feasibility and Efficiency of the BEFORE (Better Exercise and Food, Better Recovery) Prehabilitation Program. Nutrients 2021, 13, 3493. [Google Scholar] [CrossRef]
- Schink, K.; Gaßner, H.; Reljic, D.; Herrmann, H.J.; Kemmler, W.; Schwappacher, R.; Meyer, J.; Eskofier, B.M.; Winkler, J.; Neurath, M.F.; et al. Assessment of gait parameters and physical function in patients with advanced cancer participating in a 12-week exercise and nutrition programme: A controlled clinical trial. Eur. J. Cancer Care 2020, 29, e13199. [Google Scholar] [CrossRef]
- Reid, J.; Blair, C.; Dempster, M.; McKeaveney, C.; Slee, A.; Fitzsimons, D. Multimodal interventions for cachexia management. Emergencias 2023, 2023, CD015749. [Google Scholar] [CrossRef]
- Wheelwright, S.J.; Johnson, C.D. Patient-reported outcomes in cancer cachexia clinical trials. Curr. Opin. Support. Palliat. Care 2015, 9, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.P.; Vanderbyl, B.L.; Kanbalian, M.; Windholz, T.Y.; Tran, A.-T.; Jagoe, R.T. A multidisciplinary rehabilitation programme for cancer cachexia improves quality of life. BMJ Support. Palliat. Care 2017, 7, 441–449. [Google Scholar] [CrossRef] [PubMed]
| Experimental Models and Sex | Exercise Types | Treatment (Dosage, Duration, Route) | Result | Reference |
|---|---|---|---|---|
| (M) Wistar rats | Moderate intense training @ 5 d/wks for 8 wks | 300 mg/kg L-Arg in 30 mL DW daily for 8 wks | ↓ plasma cholesterol, VEGF, CINC, triglycerides | [206] |
| (M) Wistar rats | Swimming for 6 wks @ 60 min/day | 3.57% and 4.76% BCAAs in two groups for 6 wks | ↑ hepatic and muscle glycogen | [119] |
| (M) Mice | Treadmill running @ 5 days/wk for 4 wks (1st wk, 30 min at 10 m/min; 2nd wk, 60 min at 10 m/min; 3rd and 4th wk, 60 min at 12 m/min) | BCAAs @ 1.5 mg/g body weight/d in drinking water | ↑ SOD, CAT, GSH-PX | [67] |
| (M) LLC mice model | HIIT; each session: five intervals of 3 min of treadmill running @ 18 m/min, then 4 min of running @ 25 m/min; 16 days | No additional treatment | ↓ tumor progression, ↑ survival rate, running capacity, skeletal muscle contractility | [207] |
| (M) C-26 mice model | Moderate exercise: 0.5 km/h, 70% maxHR; severe exercise: 1 km/h, 90% maxHR; Both 45 min/d, once every two days for 4 wks | No additional treatment | ↓ muscle atrophy, ↑ QoL, survival rate | [154] |
| (F/M) ApcMin/+ mice model | Moderate exercise: 18 m/min, 1 h, 6 days/wk | No additional treatment | ↓ IL-6-dependent cachexia status ↑ insulin sensitivity, muscle metabolism, oxidative capacity | [155] |
| (M) AH130-induced rat model | Low-intense exercise: 15 m/min, 30 min/session, 1 wk | No additional treatment | ↓ ubiquitin-proteasome pathway, cachexia-induced muscle atrophy ↑ HIF-1α, phospho-AMPK, mTOR pathway | [156] |
| (F/M) TRAMP mice model | Voluntary wheel running, 20 wks | No additional treatment | ↓ myostatin level ↑ muscle mass, forelimb grip force | [208] |
| (F) C-26 mice model | Voluntary wheel running, 19 days | No additional treatment | ↓ atrogene induction, autophagic flux, cachexia ↑ muscle mass, muscle homeostasis | [157] |
| (F) C-26 mice model | Voluntary wheel running, 19 days | No additional treatment | ↓ Pax7 overexpression, NF-κB activation, cachexia ↑ muscle mass, fiber size | [158] |
| (M) C-26 mice model | Combined training; RT: climbing 1 m ladder inclined at 85°, ET: wheel running, 25 min, 5–9 m/min for 5 days | No additional treatment | ↓ autophagy (LC3B-I/II ratio), cachexia ↑ muscle mass, strength | [162] |
| (M) Walker-256 rat model | RT, voluntary ladder climbing, 12 days | No additional treatment | ↓ muscle wasting, oxidative stress, inflammation | [209] |
| Participants and Sex | Exercise Types and Duration | Study Types | Result | Reference |
|---|---|---|---|---|
| 42 [135] or 50 [136] healthy adults (F/M) | For 22/24 weeks; 15 g EAA or placebo daily, ET (progressive vigorous treadmill walking 3 times/wk) | Randomized controlled trial | ET improved insulin sensitivity [135]; ↑ muscle protein synthesis [136] | [135,136] |
| 12 healthy adults (F) | BCAAs (Ile:Leu:Val = 1:2.3:1.2), seven sets of 20 squats/set with 3 min intervals between sets | Randomized controlled trial | ↑ serum myoglobulin by exercise but not BCAA; BCAA suppressed muscle damage | [138] |
| 7 healthy adults (M) | Ergometer cycle exercise, 60 min on cycle ergometer, semirecumbent position, work rate 164 ± 7 W, ~75% VO2max | Non-randomized controlled trial | After exercise, the protein-sparing effect | [139] |
| 65 pancreatic cancer patients (F/M) | Supervised progressive RT (RT1), home-based RT (RT2), and control; two times RT/wk, 6 months | Randomized controlled trial | RT1 improved elbow flexor/extensor and knee extensor muscle strength | [184] |
| 121 prostate cancer patients (F/M) | RT or ET, RT: two sets of 8–12 repetitions of 10 different exercises, three times/wk for 24 wks; ET: 50–60% VO2 peak for 1–4 wks, then 70–75% for 5–24 wks | Randomized controlled trial | Both RT and ET reduced fatigue; RT ameliorated muscle strength, triglycerides, and body fat | [186] |
| 242 breast cancer patients (F/M) | RT or ET, adjuvant chemotherapy to usual care (n = 82), supervised RT (n = 82) or supervised ET (n = 78); 17 wks | Randomized controlled trial | No improvement in QoL; both RT and ET improved self-esteem, physical fitness, and body composition | [187] |
| 20 head–neck cancer patients (F/M) | Progressive RT (n = 10), usual care (n = 10); 3 × 30 min/week; 7–8 wks post-radiotherapy | Randomized controlled trial | RT improved fatigue and QoL | [188] |
| 131 advanced cancer patients (F/M) | Usual care control with individualized nutrition (n = 35), intervention (n = 96); RT (20 min WB-EMS session, bipolar, 85 Hz, 2×/wk, 12 wks) | Non-randomized controlled trial | ↑ skeletal muscle mass, body weight, physical function, and performance status by RT | [12] |
| 9 colorectal cancer patients (F/M) | RT or ET, patients were given protein-rich meals. RT: consisted of 20 repetitions (60–65% 1-RM) followed by two sets of six repetitions (80–85% 1-RM); ET: consisted of 30 s and 60 s intervals with a 1:3 work–recovery ratio. Sessions were 60–75 min long, 3 times/wk for 4 wks. | Non-randomized controlled trial | Patients demonstrated a compliance rate of ≥80% with the exercise training program and nutritional intervention | [210] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, R.; Dieterich, W.; Natarajan, A.; Schwappacher, R.; Reljic, D.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Influence of Amino Acids and Exercise on Muscle Protein Turnover, Particularly in Cancer Cachexia. Cancers 2024, 16, 1921. https://doi.org/10.3390/cancers16101921
Pradhan R, Dieterich W, Natarajan A, Schwappacher R, Reljic D, Herrmann HJ, Neurath MF, Zopf Y. Influence of Amino Acids and Exercise on Muscle Protein Turnover, Particularly in Cancer Cachexia. Cancers. 2024; 16(10):1921. https://doi.org/10.3390/cancers16101921
Chicago/Turabian StylePradhan, Rashmita, Walburga Dieterich, Anirudh Natarajan, Raphaela Schwappacher, Dejan Reljic, Hans J. Herrmann, Markus F. Neurath, and Yurdagül Zopf. 2024. "Influence of Amino Acids and Exercise on Muscle Protein Turnover, Particularly in Cancer Cachexia" Cancers 16, no. 10: 1921. https://doi.org/10.3390/cancers16101921
APA StylePradhan, R., Dieterich, W., Natarajan, A., Schwappacher, R., Reljic, D., Herrmann, H. J., Neurath, M. F., & Zopf, Y. (2024). Influence of Amino Acids and Exercise on Muscle Protein Turnover, Particularly in Cancer Cachexia. Cancers, 16(10), 1921. https://doi.org/10.3390/cancers16101921








