Clinical Characteristics, Patterns of Care, and Treatment Outcomes of Radiation-Associated Sarcomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Patient Cohort
2.2. Clinical Demographics and Radiation History
2.3. Histopathologic and Molecular Analysis of RAS
2.4. Dosimetric Analysis of Index Radiation Dose Received at RAS Tumor Site
2.5. Outcome Data
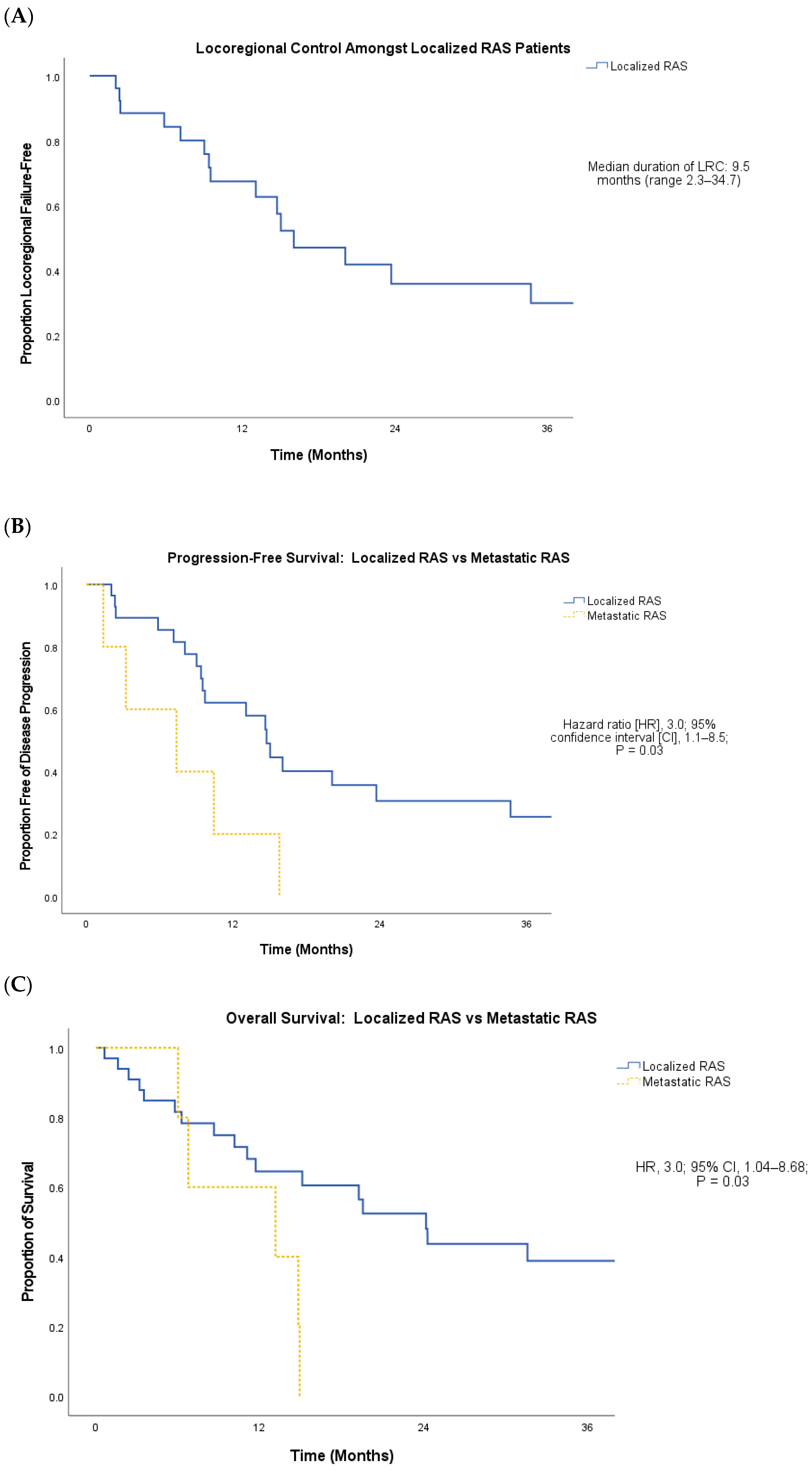
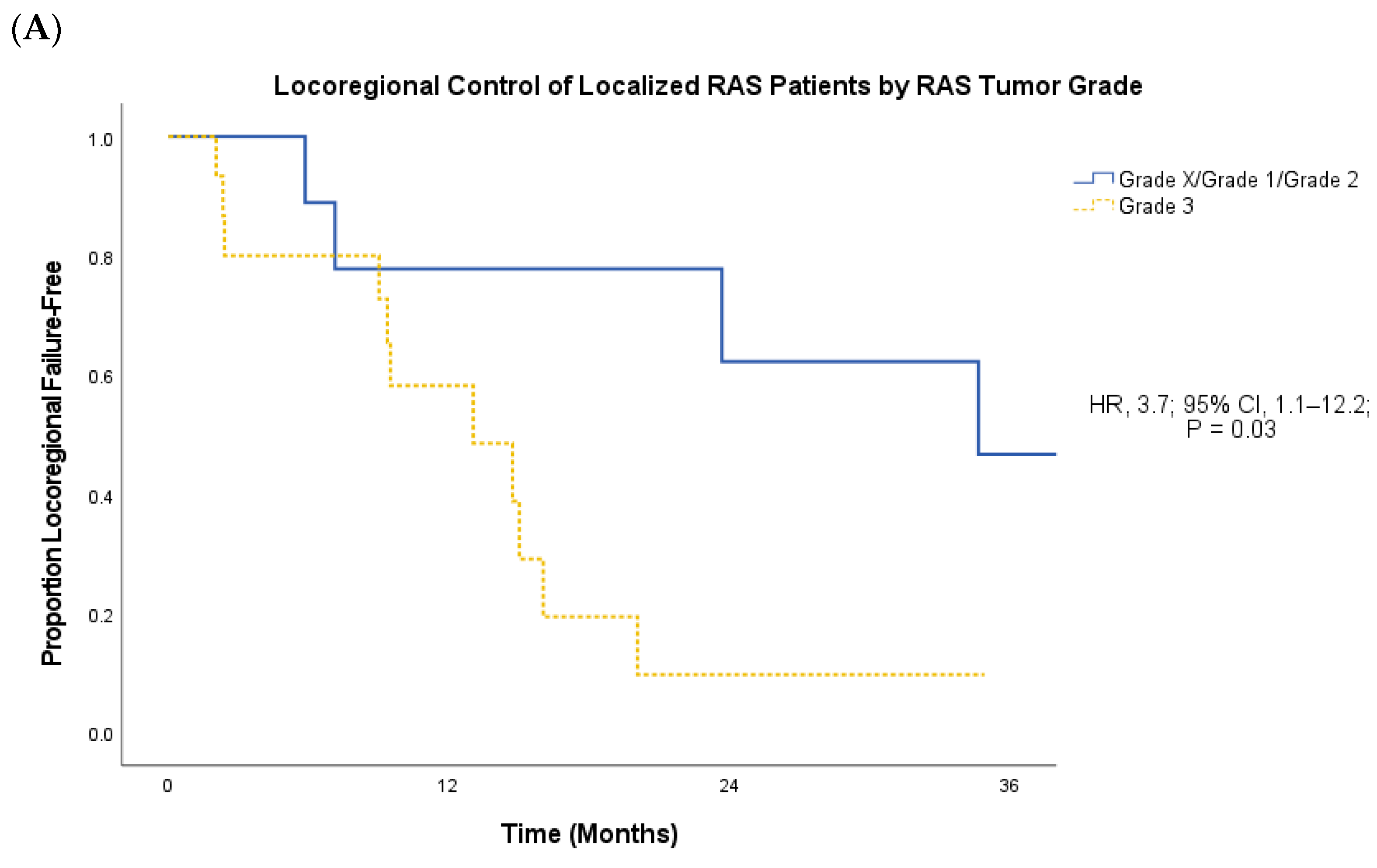
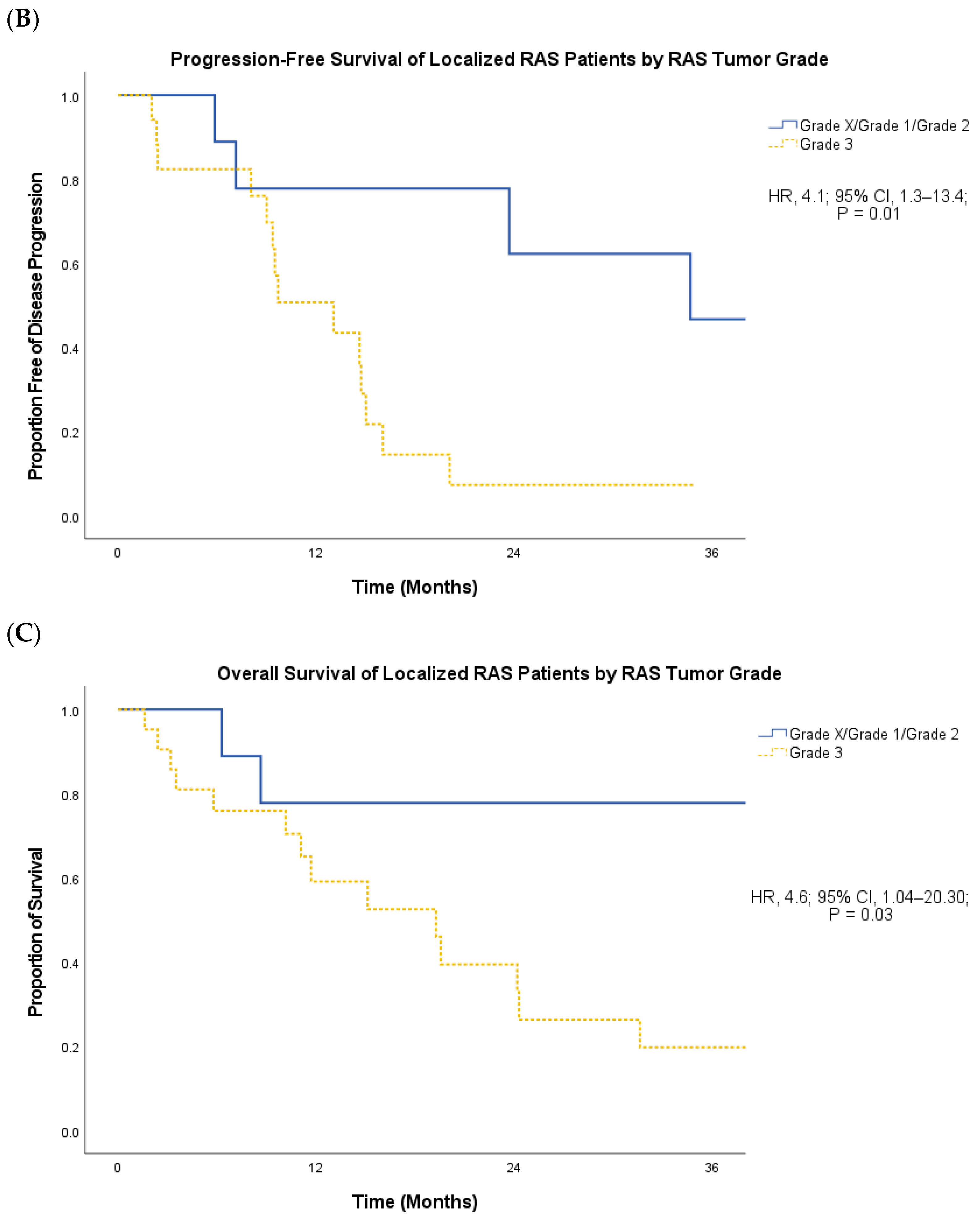
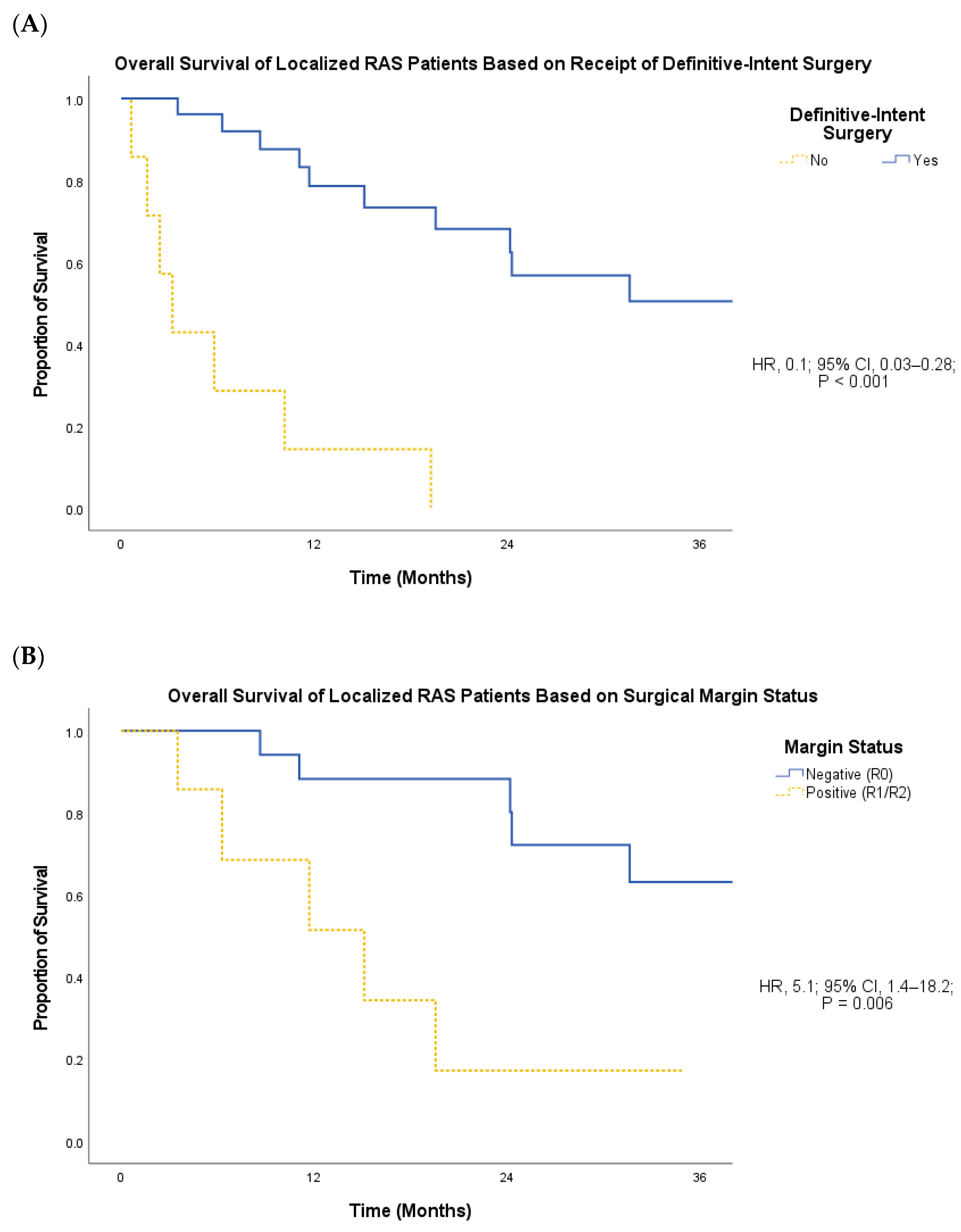
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer Treatment and Survivorship Statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Mito, J.K.; Mitra, D.; Doyle, L.A. Radiation-Associated Sarcomas: An Update on Clinical, Histologic, and Molecular Features. Surg. Pathol. Clin. 2019, 12, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Gladdy, R.A.; Qin, L.-X.; Moraco, N.; Edgar, M.A.; Antonescu, C.R.; Alektiar, K.M.; Brennan, M.F.; Singer, S. Do Radiation-Associated Soft Tissue Sarcomas Have the Same Prognosis as Sporadic Soft Tissue Sarcomas? J. Clin. Oncol. 2010, 28, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Bjerkehagen, B.; Smeland, S.; Walberg, L.; Skjeldal, S.; Hall, K.S.; Nesland, J.M.; Småstuen, M.C.; Fosså, S.D.; Saeter, G. Radiation-Induced Sarcoma: 25-Year Experience from the Norwegian Radium Hospital. Acta Oncol. 2008, 47, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Riad, S.; Biau, D.; Holt, G.E.; Werier, J.; Turcotte, R.E.; Ferguson, P.C.; Griffin, A.M.; Dickie, C.I.; Chung, P.W.; Catton, C.N.; et al. The Clinical and Functional Outcome for Patients with Radiation-Induced Soft Tissue Sarcoma. Cancer 2012, 118, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Pala, E.; Guerra, G.; Ruggieri, P. Post-Radiation Sarcomas. Clinical Outcome of 52 Patients. J. Surg. Oncol. 2012, 105, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, S.J.; Pinnock, N.; Giblin, V.; Fisher, C.; Thway, K.; Thomas, J.M.; Hayes, A.J. Treatment and Outcome of Radiation-Induced Soft-Tissue Sarcomas at a Specialist Institution. Eur. J. Surg. Oncol. 2009, 35, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.K.; Banegas, M.P.; Martinez, M.E.; Mell, L.K.; Murphy, J.D. Trends in Radiation Therapy among Cancer Survivors in the United States, 2000–2030. Cancer Epidemiol Biomark. Prev. 2017, 26, 963–970. [Google Scholar] [CrossRef]
- Dineen, S.P.; Roland, C.L.; Feig, R.; May, C.; Zhou, S.; Demicco, E.; Sannaa, G.A.; Ingram, D.; Wang, W.-L.; Ravi, V.; et al. Radiation-Associated Undifferentiated Pleomorphic Sarcoma Is Associated with Worse Clinical Outcomes than Sporadic Lesions. Ann. Surg. Oncol. 2015, 22, 3913–3920. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wang, H.; Miao, R.; Jacobson, A.; Choy, E.; Cote, G.; Hornicek, F.J.; DeLaney, T.F. Outcomes of a Large Single Institutional Series of Radiation Associated Sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, S79. [Google Scholar] [CrossRef]
- Behjati, S.; Gundem, G.; Wedge, D.C.; Roberts, N.D.; Tarpey, P.S.; Cooke, S.L.; Van Loo, P.; Alexandrov, L.B.; Ramakrishna, M.; Davies, H.; et al. Mutational Signatures of Ionizing Radiation in Second Malignancies. Nat. Commun. 2016, 7, 12605. [Google Scholar] [CrossRef] [PubMed]
- Hadj-Hamou, N.-S.; Ugolin, N.; Ory, C.; Britzen-Laurent, N.; Sastre-Garau, X.; Chevillard, S.; Malfoy, B. A Transcriptome Signature Distinguished Sporadic from Postradiotherapy Radiation-Induced Sarcomas. Carcinogenesis 2011, 32, 929–934. [Google Scholar] [CrossRef]
- Kim, E.; Han, D.-J.; Kim, B.H.; Yoo, J.; Kim, H.J.; Wu, H.-G.; Kim, K.S.; Kim, H.-S.; Han, I.; Moon, K.C.; et al. Whole-Genome Sequencing Reveals Mutational Signatures Related to Radiation-Induced Sarcomas and DNA-Damage-Repair Pathways. Mod. Pathol. 2023, 36, 100004. [Google Scholar] [CrossRef] [PubMed]
- Cahan, W.G.; Woodard, H.Q. Sarcoma Arising in Irradiated Bone; Report of 11 Cases. Cancer 1948, 1, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Laurino, S.; Omer, L.C.; Albano, F.; Marino, G.; Bianculli, A.; Solazzo, A.P.; Sgambato, A.; Falco, G.; Russi, S.; Bochicchio, A.M. Radiation-Induced Sarcomas: A Single Referral Cancer Center Experience and Literature Review. Front. Oncol. 2022, 12, 986123. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Yadav, V.; Singh, P.; Bhowmik, K.T. Radiation-Induced Malignancies Making Radiotherapy a “Two-Edged Sword”: A Review of Literature. World J. Oncol. 2017, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Laskin, W.B.; Silverman, T.A.; Enzinger, F.M. Postradiation Soft Tissue Sarcomas. An Analysis of 53 Cases. Cancer 1988, 62, 2330–2340. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.; Westbury, G.; Harmer, C.L. Radiation-Induced Soft-Tissue Sarcoma. Br. J. Surg. 1986, 73, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Mito, J.K.; Mitra, D.; Barysauskas, C.M.; Mariño-Enriquez, A.; Morgan, E.A.; Fletcher, C.D.M.; Raut, C.P.; Baldini, E.H.; Doyle, L.A. A Comparison of Outcomes and Prognostic Features for Radiation-Associated Angiosarcoma of the Breast and Other Radiation-Associated Sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 425–435. [Google Scholar] [CrossRef]
- Cha, C.; Antonescu, C.R.; Quan, M.L.; Maru, S.; Brennan, M.F. Long-Term Results with Resection of Radiation-Induced Soft Tissue Sarcomas. Ann. Surg. 2004, 239, 903–909; discussion 909–910. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.F.; Antonescu, C.R.; Moraco, N.; Singer, S. Lessons Learned from the Study of 10,000 Patients with Soft Tissue Sarcoma. Ann. Surg. 2014, 260, 416–421; discussion 421–422. [Google Scholar] [CrossRef] [PubMed]
- Gonin-Laurent, N.; Hadj-Hamou, N.S.; Vogt, N.; Houdayer, C.; Gauthiers-Villars, M.; Dehainault, C.; Sastre-Garau, X.; Chevillard, S.; Malfoy, B. RB1 and TP53 Pathways in Radiation-Induced Sarcomas. Oncogene 2007, 26, 6106–6112. [Google Scholar] [CrossRef] [PubMed]
- Mentzel, T.; Schildhaus, H.U.; Palmedo, G.; Büttner, R.; Kutzner, H. Postradiation Cutaneous Angiosarcoma after Treatment of Breast Carcinoma Is Characterized by MYC Amplification in Contrast to Atypical Vascular Lesions after Radiotherapy and Control Cases: Clinicopathological, Immunohistochemical and Molecular Analysis of 66 Cases. Mod. Pathol. 2012, 25, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, L.; Chang, N.-E.; Singer, S.; Maki, R.G.; Antonescu, C.R. Consistent MYC and FLT4 Gene Amplification in Radiation-Induced Angiosarcoma but Not in Other Radiation-Associated Atypical Vascular Lesions. Genes Chromosomes Cancer 2011, 50, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Manner, J.; Radlwimmer, B.; Hohenberger, P.; Mössinger, K.; Küffer, S.; Sauer, C.; Belharazem, D.; Zettl, A.; Coindre, J.-M.; Hallermann, C.; et al. MYC High Level Gene Amplification Is a Distinctive Feature of Angiosarcomas after Irradiation or Chronic Lymphedema. Am. J. Pathol. 2010, 176, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Yoshida, A.; Guo, T.; Chang, N.-E.; Zhang, L.; Agaram, N.P.; Qin, L.-X.; Brennan, M.F.; Singer, S.; Maki, R.G. KDR Activating Mutations in Human Angiosarcomas Are Sensitive to Specific Kinase Inhibitors. Cancer Res. 2009, 69, 7175–7179. [Google Scholar] [CrossRef] [PubMed]
- Kokkali, S.; Moreno, J.D.; Klijanienko, J.; Theocharis, S. Clinical and Molecular Insights of Radiation-Induced Breast Sarcomas: Is There Hope on the Horizon for Effective Treatment of This Aggressive Disease? Int. J. Mol. Sci. 2022, 23, 4125. [Google Scholar] [CrossRef] [PubMed]
- Lahat, G.; Dhuka, A.R.; Hallevi, H.; Xiao, L.; Zou, C.; Smith, K.D.; Phung, T.L.; Pollock, R.E.; Benjamin, R.; Hunt, K.K.; et al. Angiosarcoma: Clinical and Molecular Insights. Ann. Surg. 2010, 251, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Chen, C.-L.; Thomas, R.; Breen, M.; Bonnet, F.; Sevenet, N.; Longy, M.; Maki, R.G.; Coindre, J.-M.; Antonescu, C.R. Alterations of the P53 and PIK3CA/AKT/mTOR Pathways in Angiosarcomas: A Pattern Distinct from Other Sarcomas with Complex Genomics. Cancer 2012, 118, 5878–5887. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.-C.; Yang, J.; Sun, C.; Liu, Y.-T.; Shen, L.-J.; Xu, B.-S.; Que, Y.; Xia, X.; Zhang, X. Genomic Profiling of Radiation-Induced Sarcomas Reveals the Immunologic Characteristics and Its Response to Immune Checkpoint Blockade. Clin. Cancer Res. 2023, 29, 2869–2884. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total (n = 38) |
|---|---|
| Median age at RT for index malignancy (range), years | 55.3 (0.2–77.5) |
| Diagnosis, n (%) | |
| Breast carcinoma | 9 (24) |
| Prostate adenocarcinoma | 8 (21) |
| Head and neck squamous cell carcinoma | 5 (13) |
| Sarcoma | 5 (13) |
| Rectal adenocarcinoma | 2 (5) |
| Lymphoma | 2 (5) |
| Other | 7 (18) |
| Treatment characteristics for primary malignancy | |
| Median radiation dose (range), Gy | 50.4 (36.0–77.4) |
| Radiation field, n (%) | |
| Pelvis/groin | 14 (37) |
| Head and neck | 12 (32) |
| Breast | 8 (21) |
| Trunk/chest wall | 3 (8) |
| Spine | 1 (3) |
| Upper extremity | 1 (3) |
| Chemotherapy use, n (%) | 21 (55) |
| Characteristics | Total (n = 38) |
|---|---|
| Median age at RAS diagnosis (range), years Median latency after index RT (range), years | 68.4 (27.9–85.4) 9.1 (3.7–46.3) |
| Location, n (%) | |
| Abdomen/pelvis | 15 (40) |
| Head and neck | 10 (26) |
| Thorax | 10 (26) |
| Extremity | 2 (5) |
| Other | 1 (3) |
| Histology, n (%) | |
| Angiosarcoma | 10 (26) |
| Undifferentiated pleomorphic sarcoma | 8 (21) |
| Osteosarcoma | 7 (18) |
| Leiomyosarcoma | 4 (11) |
| Spindle cell sarcoma | 3 (8) |
| Other undifferentiated sarcoma | 2 (5) |
| Myxoinflammatory myofibroblastic sarcoma | 1 (3) |
| Fibrosarcoma | 1 (3) |
| Malignant biphasic neoplasm | 1 (3) |
| Hemangiopericytoma | 1 (3) |
| Histologic grade, n (%) | |
| Not assessed | 5 |
| Low | 5 (15) |
| Intermediate | 3 (9) |
| High | 25 (76) |
| Next-generation sequencing data, n (%) | |
| TP53 mutation (missense, nonsense, frameshift) | 12 (44) |
| CDKN2A/B copy number loss | 7 (26) |
| MYC amplification/copy number gain | 6 (22) |
| Localized RAS | |
|---|---|
| Surgery, n (%) | |
| No | 7 (21) |
| Yes | 26 (79) |
| Surgical margin status, n (%) | |
| Negative (R0) | 19 (73) |
| Microscopic disease (R1) | 5 (19) |
| Macroscopic disease (R2) | 2 (8) |
| Chemotherapy, n (%) | 18 (55) |
| Radiotherapy, n (%) | 8 (24) |
| Median radiation dose, Gy (range; n = 8) | 50.2 (44.0–57.5) |
| Metastatic RAS | |
| Palliative-intent surgery, n (%) | 1 (20) |
| Chemotherapy, n (%) | |
| No | 0 (0) |
| Yes | 5 (100) |
| Palliative-intent radiotherapy, n (%) | 3 (60) |
| Median radiation dose, Gy (range; n = 3) | 39.0 (20.0–44.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, R.; Kim, H.G.; Xu, M.; Roach, T.; Liebner, D.; Konieczkowski, D.; Tinoco, G. Clinical Characteristics, Patterns of Care, and Treatment Outcomes of Radiation-Associated Sarcomas. Cancers 2024, 16, 1918. https://doi.org/10.3390/cancers16101918
Raj R, Kim HG, Xu M, Roach T, Liebner D, Konieczkowski D, Tinoco G. Clinical Characteristics, Patterns of Care, and Treatment Outcomes of Radiation-Associated Sarcomas. Cancers. 2024; 16(10):1918. https://doi.org/10.3390/cancers16101918
Chicago/Turabian StyleRaj, Rohit, Han Gil Kim, Menglin Xu, Tyler Roach, David Liebner, David Konieczkowski, and Gabriel Tinoco. 2024. "Clinical Characteristics, Patterns of Care, and Treatment Outcomes of Radiation-Associated Sarcomas" Cancers 16, no. 10: 1918. https://doi.org/10.3390/cancers16101918
APA StyleRaj, R., Kim, H. G., Xu, M., Roach, T., Liebner, D., Konieczkowski, D., & Tinoco, G. (2024). Clinical Characteristics, Patterns of Care, and Treatment Outcomes of Radiation-Associated Sarcomas. Cancers, 16(10), 1918. https://doi.org/10.3390/cancers16101918






