Gestational Diabetes Mellitus and Its Correlation in the Development of Pancreatic Cancer: A 10-Year Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Definition and Epidemiology of Pancreatic Cancer
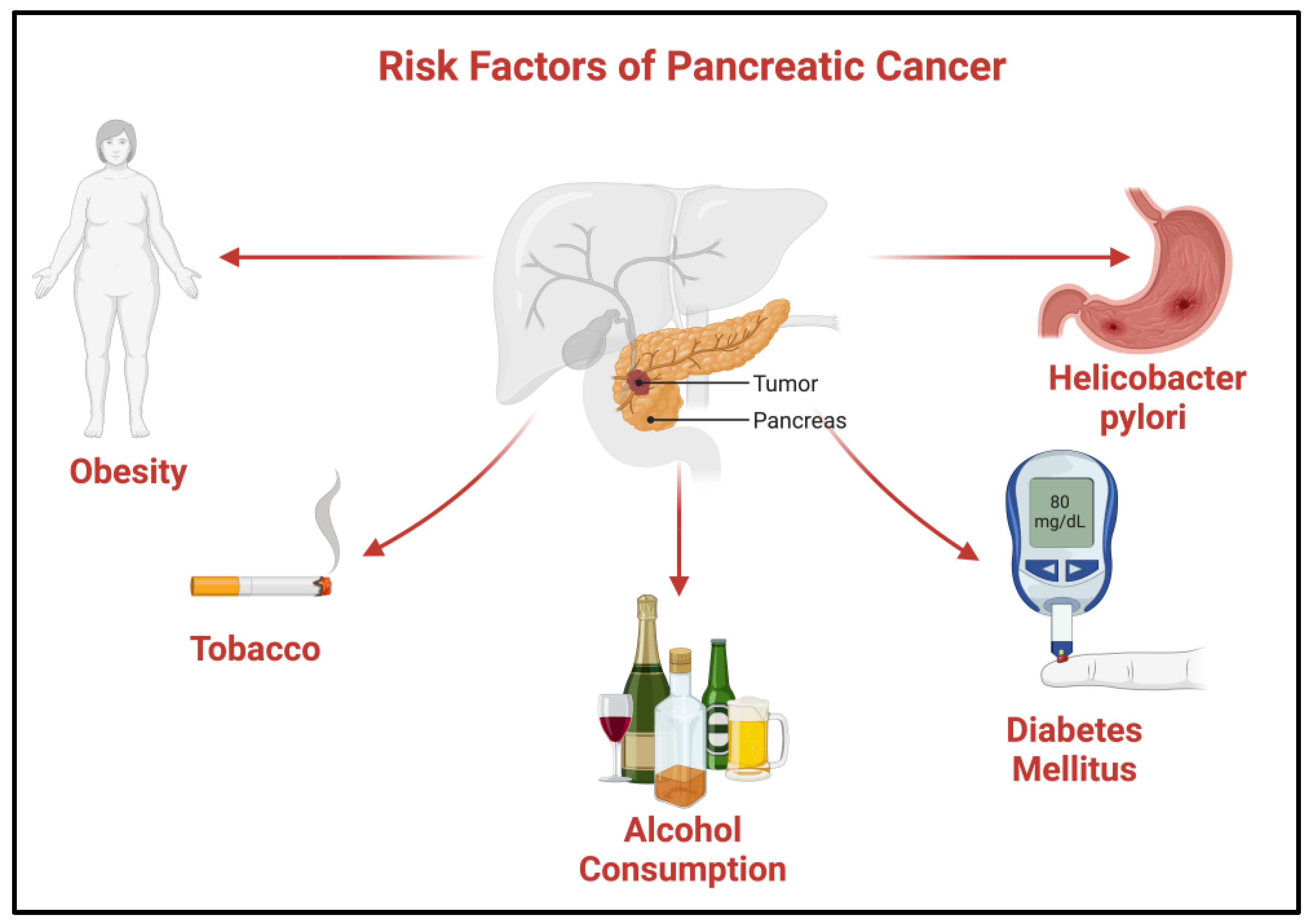
Risk Factors of Pancreatic Cancer
1.2. Diabetes Mellitus
1.2.1. Diabetes Mellitus and Its Classification
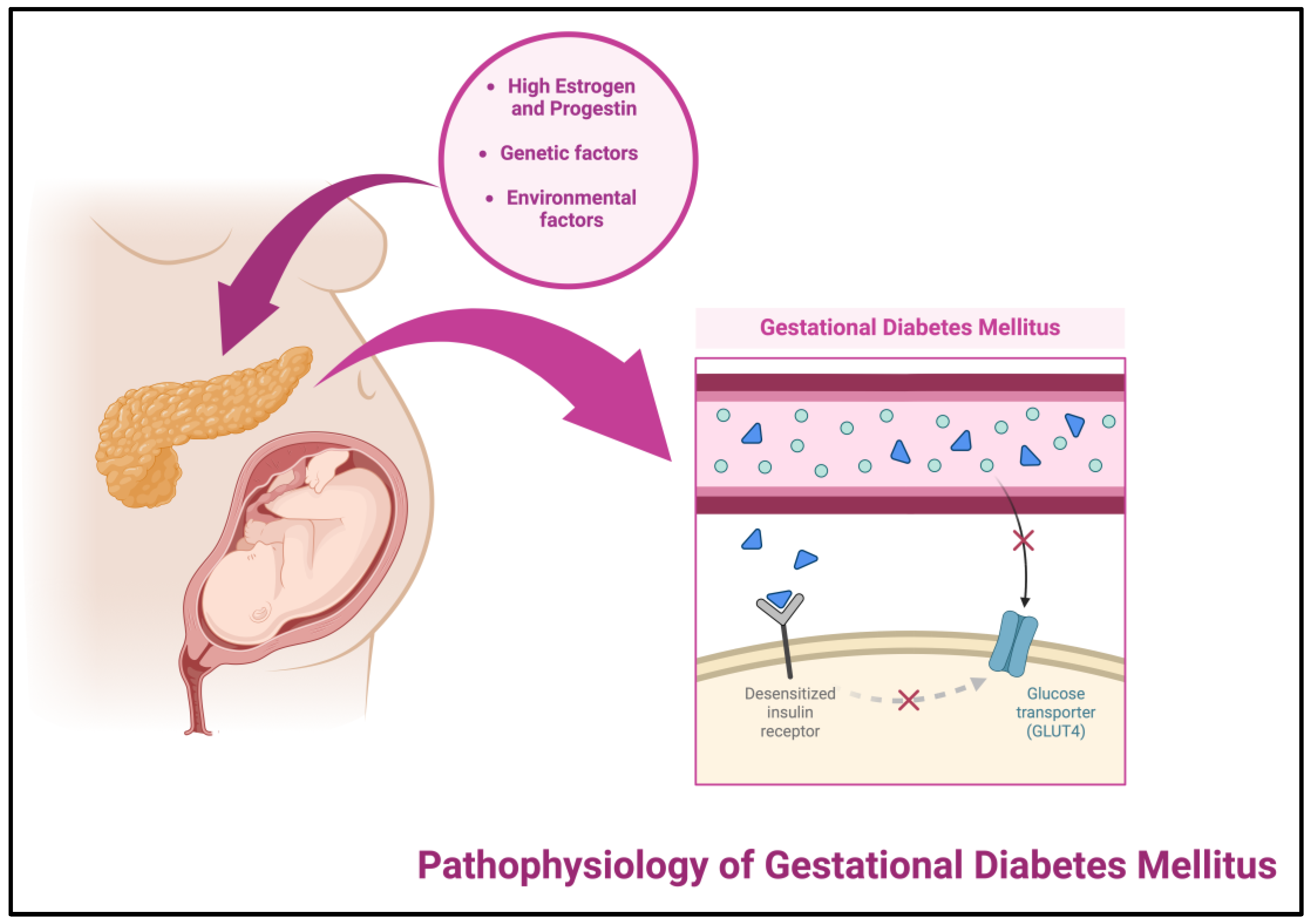
1.2.2. Pathophysiology of Gestational Diabetes Mellitus
1.2.3. Gestational Diabetes Mellitus Diagnosis
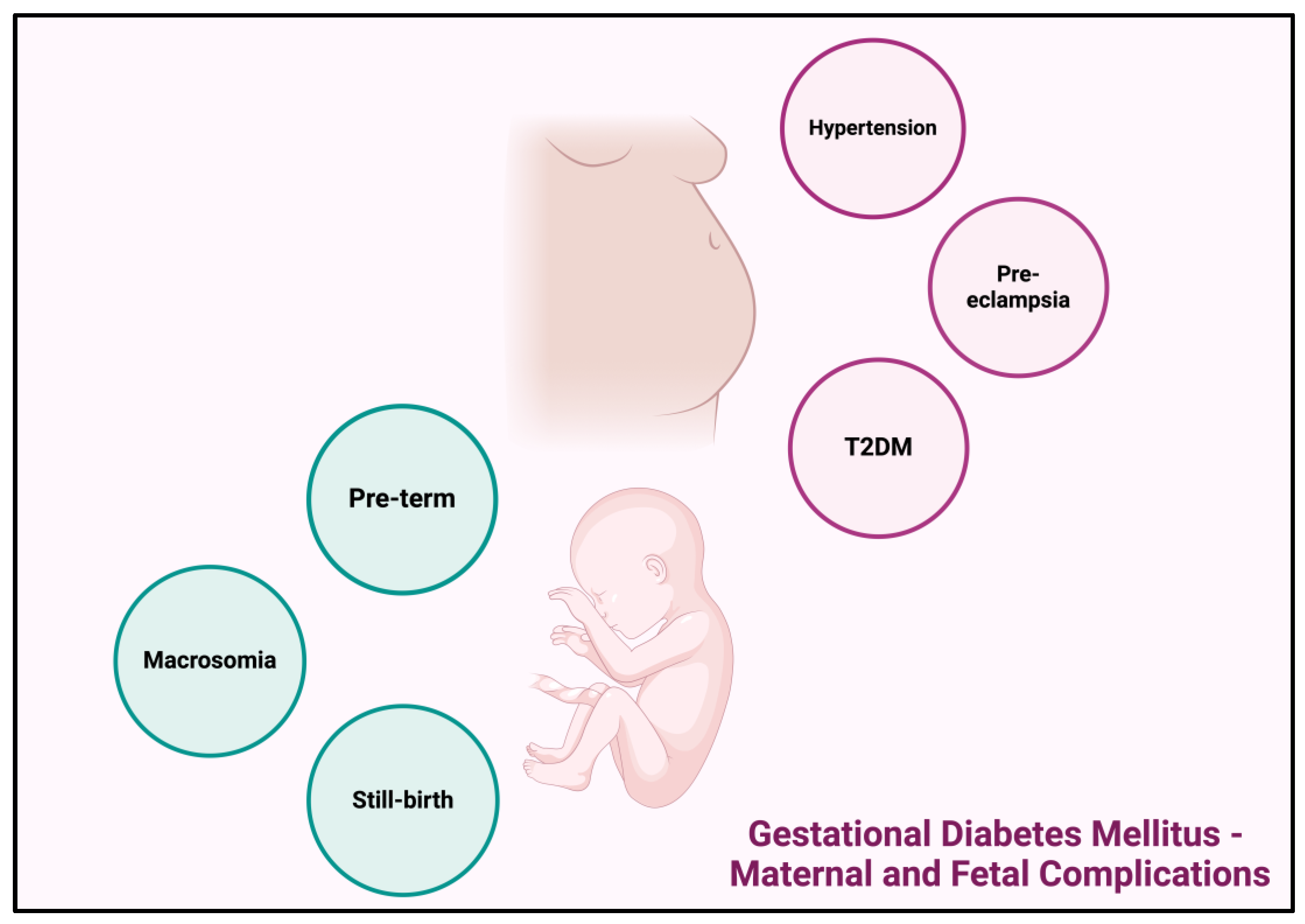
1.2.4. Gestational Diabetes Mellitus—Maternal and Fetal Complications
2. Materials and Methods
2.1. PICO Model
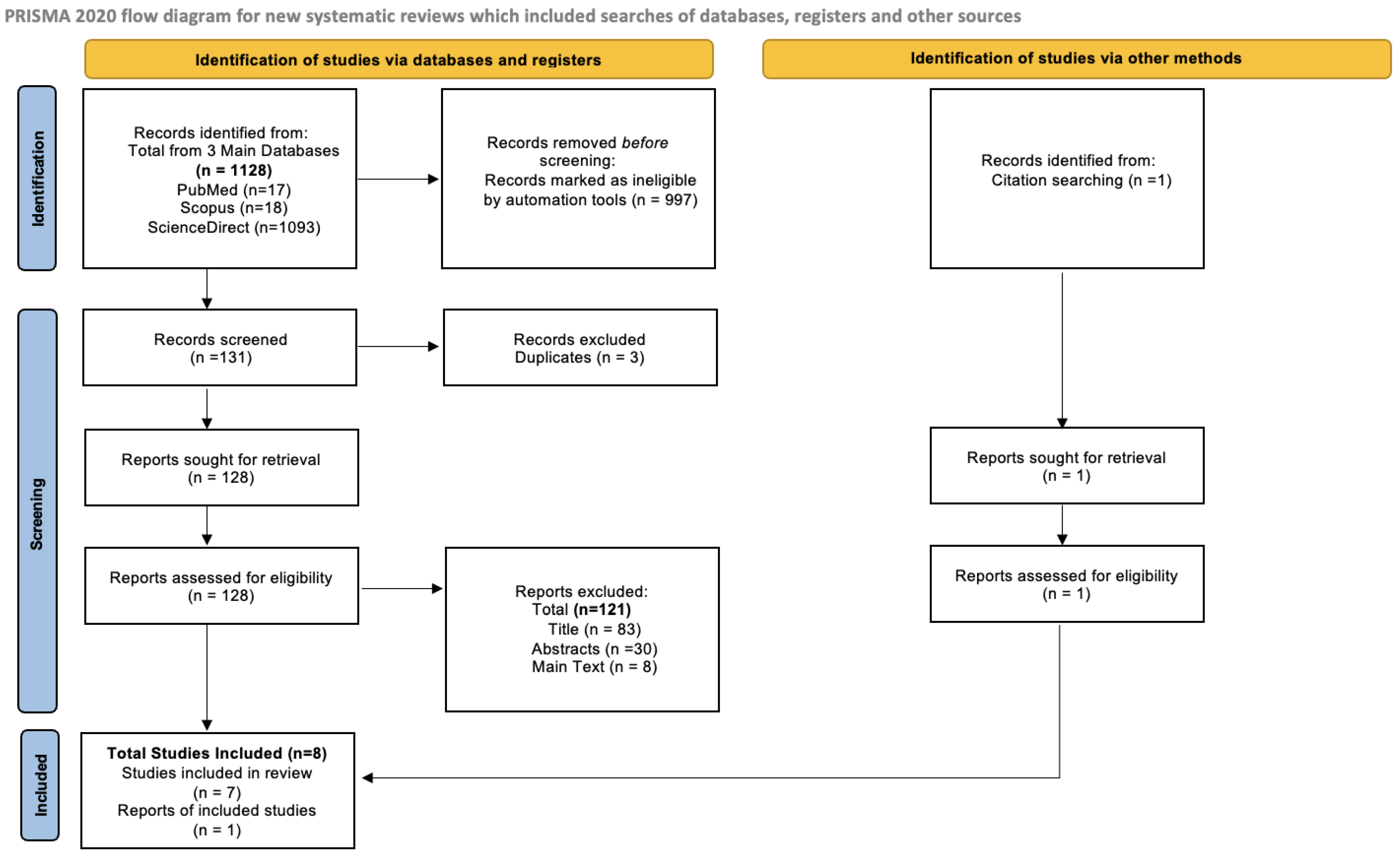
2.2. PRISMA Flow Diagram
2.3. Methodological Quality Assessment
3. Results
3.1. PRISMA Flow Diagram
3.2. Methodological Quality Assessment
3.2.1. Newcastle Ottawa Scale (NOS)
3.2.2. NIH Quality Assessment Tool
3.2.3. Cochrane Risk Bias Tool
4. Discussion
4.1. Risk Factors for the Development of Gestational Diabetes Mellitus
4.2. Risk Factors for the Development of Pancreatic Cancer
4.3. Association of Gestational Diabetes Mellitus and Development of Pancreatic Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harris, S. Europe Is Facing a Pancreatic Cancer Emergency. Medscape, 25 January 2024. [Google Scholar]
- Perrin, M.; Terry, M.; Kleinhaus, K.; Deutsch, L.; Yanetz, R.; Tiram, E.; Calderon, R.; Friedlander, Y.; Paltiel, O.; Harlap, S. Gestational diabetes as a risk factor for pancreatic cancer: A prospective cohort study. BMC Med. 2007, 5, 25. [Google Scholar] [CrossRef]
- Stoffel, E.M.; Brand, R.E.; Goggins, M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology 2023, 164, 752–765. [Google Scholar] [CrossRef]
- Gagliardi, J. Mortality Rate of Pancreatic Cancer in Europe in 2022, by Country and Gender; Health Pharma Medtech: Washington, DC, USA, 2024. [Google Scholar]
- Cancer Stat Facts: Pancreatic Cancer; NIH, National Cancer Institute: Bethesda, MD, USA, 2024.
- Sarantis, P.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020, 12, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Goueslard, K.; Arveux, P.; Bechraoui-Quantin, S.; Petit, J.-M.; Quantin, C. Increased Risk of Hospitalization for Pancreatic Cancer in the First 8 Years after a Gestational Diabetes Mellitus regardless of Subsequent Type 2 Diabetes: A Nationwide Population-Based Study. Cancers 2021, 13, 308. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment, and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef]
- Roy, A.; Sahoo, J.; Kamalanathan, S.; Naik, D.; Mohan, P.; Kalayarasan, R. Diabetes and pancreatic cancer: Exploring the two-way traffic. World J. Gastroenterol. 2021, 27, 4939–4962. [Google Scholar] [CrossRef] [PubMed]
- BioRender n.d. Available online: https://app.biorender.com/illustrations/65ff040d4603630b1370a481 (accessed on 25 March 2024).
- Sapra, A.; Bhandari, P. Diabetes. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Kerner, W.; Brückel, J. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2014, 122, 384–386. [Google Scholar] [CrossRef]
- Types of Diabetes; Diabetes UK: London, UK, 2019.
- Lucier, J.; Weinstock, R.S. Type 1 Diabetes. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Goyal, R.; Singhal, M.; Jialal, I. Type 2 Diabetes. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Blogger, G. Keeping Mom and Child Healthy after Gestational Diabetes Mellitus; American Diabetes Association: Arlington, VA, USA, 2024. [Google Scholar]
- IDF Diabetes Atlas, 10th ed.; n.d.; Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 7 May 2024).
- Rph, C.L.I.; Tinong, R.T. The Incidence and Management of Type 2 Diabetes Mellitus after Gestational Diabetes Mellitus. Cureus 2023, 15, e44468. [Google Scholar] [CrossRef]
- Alfadhli, E.M. Gestational diabetes mellitus. Saudi Med. J. 2015, 36, 399–406. [Google Scholar] [CrossRef]
- Reece, E.A.; Leguizamón, G.; Wiznitzer, A. Gestational diabetes: The need for a common ground. Lancet 2009, 373, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, T.A.; Xiang, A.H.; Page, K.A. Gestational diabetes mellitus: Risks and management during and after pregnancy. Nat. Rev. Endocrinol. 2012, 8, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, M. Gestational Diabetes Mellitus. Malays. J. Med. Sci. 2000, 7, 4–9. [Google Scholar] [PubMed]
- Naik, R.R.; Pednekar, G.; Cacodcar, J. Incidence and risk factors of gestational diabetes mellitus in antenatal mothers in Goa, India. Int. J. Reprod. Contracept. Obstet. Gynecol. 2019, 8, 586. [Google Scholar] [CrossRef]
- By Mayo Clinic Staff. Fetal Macrosomia; Mayo Clinic: Rochester, MN, USA, 2022. [Google Scholar]
- By Mayo Clinic Staff. Gestational Diabetes; Mayo Clinic: Rochester, MN, USA, 2022. [Google Scholar]
- Senat, M.-V.; Deruelle, P. Le diabète gestationnel. Gynecol. Obstet. Fertil. 2016, 44, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). 2024. Available online: https://www.prisma-statement.org/ (accessed on 7 May 2024).
- Tong, G.-X.; Cheng, J.; Chai, J.; Geng, Q.-Q.; Chen, P.-L.; Shen, X.-R.; Liang, H.; Wang, D.-B. Association Between Gestational Diabetes Mellitus and Subsequent Risk of Cancer: A Systematic Review of Epidemiological Studies. Asian Pac. J. Cancer Prev. 2014, 15, 4265–4269. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.-W.; Shen, X.-F.; Ding, H.-J.; Liu, Y.-Q.; Meng, L.; Kalionis, B. Pancreatic carcinoma underlying a complex presentation in late pregnancy: A case report. J. Med. Case Rep. 2018, 12, 369. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-S.; Lin, J.-R.; Cheng, B.-H.; Ho, C.; Lin, Y.-H.; Shen, C.-H.; Tsai, M.-H. Incidence and relative risk for developing cancers in women with gestational diabetes mellitus: A nationwide cohort study in Taiwan. BMJ Open 2019, 9, e024583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, P.; Fu, T.; Yuan, J.; Yang, G.; Liu, Y.; Zhang, Z.-J. The association between gestational diabetes mellitus and cancer in women: A systematic review and meta-analysis of observational studies. Diabetes Metab. 2020, 46, 461–471. [Google Scholar] [CrossRef]
- Quaresima, P.; Saccone, G.; Pellegrino, R.; Vaccarisi, S.; Taranto, L.; Mazzulla, R.; Bernardo, S.; Venturella, R.; Di Carlo, C.; Morelli, M. Incidental diagnosis of a pancreatic adenocarcinoma in a woman affected by gestational diabetes mellitus: Case report and literature review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100471. [Google Scholar] [CrossRef]
- Choudhury, A.A.; Devi Rajeswari, V. Gestational diabetes mellitus—A metabolic and reproductive disorder. Biomed. Pharmacother. 2021, 143, 112183. [Google Scholar] [CrossRef] [PubMed]
- Slouha, E.; Gates, K.M.; Al-Geizi, H.; Baah, E.; Clunes, L.A.; Kollias, T.F. The Relationship Between Gestational Diabetes and the Risk of Cancer: A Systematic Review. Cureus 2024, 16, e53328. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Luo, M.; Wang, T.; Wei, J.; Zhang, S.; Shu, J.; Zhong, T.; Liu, Y.; Chen, Q.; Zhu, P.; et al. Effect of the interaction between advanced maternal age and pre-pregnancy BMI on pre-eclampsia and GDM in Central China. BMJ Open Diabetes Res. Care 2023, 11, e003324. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, L. What Is Hellp Syndrome? Pre-Eclampsia Foundation, 2023. Available online: https://www.preeclampsia.org/hellp-syndrome (accessed on 7 May 2024).
| Plasma Glucose Values (mg/dL) | |
|---|---|
| Fasting | 95 |
| 1 h | 180 |
| 2 h | 150 |
| 3 h | 140 |
| PICO | |
|---|---|
| P | Pregnant women of no specific age or number of past pregnancies |
| I | Gestational diabetes mellitus |
| C | Pancreatic cancer |
| O | Gestational diabetes mellitus as a risk factor for the development of pancreatic cancer |
| # | Authors | Type of Study | Risk Factor for GDM | Risk Factor for PC | Identification of GDM | Association between PC and GDM |
|---|---|---|---|---|---|---|
| 1 | Tong, G.-X., 2014 [29] | Systematic Review | Obesity | - | The unexpectedly high prevalence of hyperglycemia among cancer patients led investigators to suggest the use of blood glucose measurements as a new screening or diagnostic method for cancer. | Similar to DM, GDM seems to be related to pancreatic cancer. Five new pancreatic cancer cases were found in women with GDM history and forty-nine without history; the hazard ratio of GDM history was 7.1. Similar results observed in two studies. |
| 2 | Shi AW, 2018 [30] | Case report | GDM is reported to be increasing and is more common among African Americans, Hispanics, Asians and Native Americans than among non-Hispanic whites | GDM, HELLP syndrome, pulmonary hypertension and DIC in late pregnancy. | Known GDM before admitted to hospital. | Women with a history of gestational diabetes showed a relative risk of pancreatic cancer of 7.1. Studies showed that gestational diabetes mellitus could be one of the important risk factors for pancreatic cancer. |
| 3 | Peng et al., 2019 [31] | Cohort Study | Phenomenon increases with maternal age, obesity issues and decreases with daily physical activity | - | - | Women with GDM had a higher risk of developing PC in comparison with women who did not suffer from GDM, but the overall association was still low. |
| 4 | Wang Y, 2020 [32] | Systematic review and Meta-analysis | PCOS, maternal obesity or overweight, family history of type 2 diabetes mellitus (T2DM), prediabetes, previous history of fetal death and increased maternal age | - | Mixed identification methods for GDM from studies retrieved. Either self-reported, OGTT or glucose challenge test. | Two cohort studies found a significant positive association between GDM and pancreatic cancer risk, whereas another cohort study did not. Evidence of severe heterogeneity. |
| 5 | Quaresima P, 2021 [33] | Case report and Literature review | - | Obesity, heavy smoking, alcohol intake, history of diabetes, chronic pancreatitis, chronic cirrhosis, previous cholecystectomy and genetic predisposition, with approximately 5–10% of patients diagnosed with a pancreatic cancer having a family history of the disease. Presented with HELLP syndrome. | - | GDM shows a 7-fold increase in the risk of pancreatic cancer over the course of their lives. |
| 6 | Simon J, 2021 [7] | Cohort study | - | Risk of PC increases by 80% in case of type 2 diabetes. | Before 2010, GDM screening was based on a two-step procedure for all women: the first test was on venous blood glucose 1 h after ingestion of 50 g of glucose, and in the event of a positive result, the second screening test was performed for oral glucose tolerance. Since 2010, recommended screening for these women is fasting blood glucose at the first prenatal consultation, and if it is not performed, an oral glucose tolerance test in the second trimester. | Over the eight years of follow-up, GDM was significantly associated with a higher risk of hospitalization with PC in the first and second Cox regression models adjusted for age and subsequent type 2 diabetes. |
| 7 | Choudhury AA, 2021 [34] | Literature Review | Insulin resistance, decreased chemerin levels, hereditary, reduced pancreatic insulin production similar to T2DM, obesity | - | Increased levels of glucose and C-reactive protein, lower levels of sex hormone-binding globulin and an increased chance of hyperinsulinemia when compared to pregnant women who do not have GDM. | It is reported that patients who have been diagnosed with DM are more likely to develop cancers such as pancreatic, colon, liver, kidney, bladder or BC. High linkage of GDM and development of cancer. |
| 8 | Slouha E, 2024 [35] | Systematic Review | Age, gestation, obesity and PCOS | - | Afamine, 1,5-anhydroglucitol and adiponectin markers. | Women with GDM have higher chances of developing breast, ovarian, cervical and uterine cancer and cancer in non-reproductive organs, like thyroid and pancreas. |
| References | Selection | Comparability | Outcome | Total Quality Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of Exposed Cohort | Sample Size | Ascertainment of Exposure | Non-Respondents | Adjust for the Most Important Risk Factors | Adjust for Other Risk Factors | Assessment of Outcome | Statistical Test | ||
| Peng et al., 2019 [31] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Simon J, 2021 [7] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Reference | NIH Quality Assessment Tool-Criteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q’1 | Q’2 | Q’3 | Q’4 | Q’5 | Q’6 | Q’7 | Q’8 | Q’9 | Total Quality Score | |
| Quaresima P, 2021 [33] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 4 |
| Shi AW, 2018 [30] | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 5 |
| Author and Year | Random Sequence Generation | Allocation Concealment | Participant and Personnel Blinding | Outcome Assessment Blinding | Incomplete Outcome Data and Biased Reporting | Further Biased Sources | Total Score |
|---|---|---|---|---|---|---|---|
| Tong, G.-X., 2014 [29] | + | + | + | + | + | + | 6 |
| Wang Y., 2020 [32] | + | - | ? | ? | + | - | 2 |
| Slouha E., 2024 [35] | + | + | ? | + | + | + | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsokkou, S.; Konstantinidis, I.; Georgaki, M.-N.; Kavvadas, D.; Papadopoulou, K.; Keramas, A.; Sioga, A.; Papamitsou, T.; Karachrysafi, S. Gestational Diabetes Mellitus and Its Correlation in the Development of Pancreatic Cancer: A 10-Year Systematic Review. Cancers 2024, 16, 1840. https://doi.org/10.3390/cancers16101840
Tsokkou S, Konstantinidis I, Georgaki M-N, Kavvadas D, Papadopoulou K, Keramas A, Sioga A, Papamitsou T, Karachrysafi S. Gestational Diabetes Mellitus and Its Correlation in the Development of Pancreatic Cancer: A 10-Year Systematic Review. Cancers. 2024; 16(10):1840. https://doi.org/10.3390/cancers16101840
Chicago/Turabian StyleTsokkou, Sophia, Ioannis Konstantinidis, Maria-Nefeli Georgaki, Dimitrios Kavvadas, Kyriaki Papadopoulou, Antonios Keramas, Antonia Sioga, Theodora Papamitsou, and Sofia Karachrysafi. 2024. "Gestational Diabetes Mellitus and Its Correlation in the Development of Pancreatic Cancer: A 10-Year Systematic Review" Cancers 16, no. 10: 1840. https://doi.org/10.3390/cancers16101840
APA StyleTsokkou, S., Konstantinidis, I., Georgaki, M.-N., Kavvadas, D., Papadopoulou, K., Keramas, A., Sioga, A., Papamitsou, T., & Karachrysafi, S. (2024). Gestational Diabetes Mellitus and Its Correlation in the Development of Pancreatic Cancer: A 10-Year Systematic Review. Cancers, 16(10), 1840. https://doi.org/10.3390/cancers16101840









