Osteosarcoma Arising as a Secondary Malignancy following Treatment for Hematologic Cancer: A Report of 33 Affected Patients from the Cooperative Osteosarcoma Study Group (COSS)
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Selection and Data Collection
2.2. Statistical Analyses
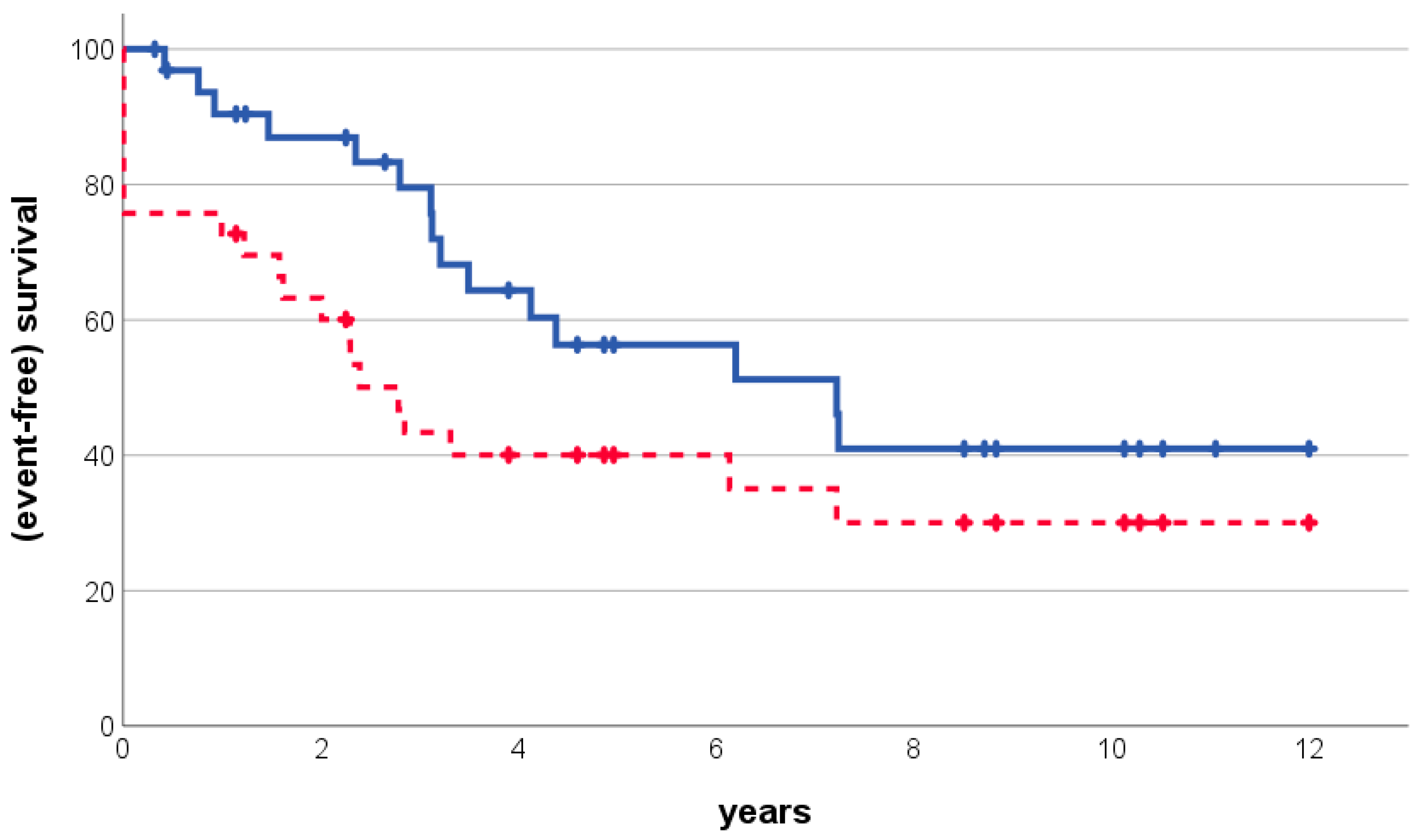
3. Results
Patients
- -
- 20 (61%) lymphomas (13 Hodgkin (HL), 7 non-Hodgkin (NHL), 3 diffuse large B-cell (DLBCL), 1 anaplastic large-cell (ALCL), 1 lymphoblastic, 1 mucosa-associated lymphoid tissue (MALT), and 1 unspecified NHL). Among the 20 lymphomas, 16 (80%) were known to have involved the trunk, 4 (20%) the head and neck (incl. 1 with involvement of both regions), and 1 (5%) an extremity.
- -
- 13 (29%) leukemias (12 acute lymphoblastic (ALL)—7 B-precursor, 4 T-, 1 unspecified; 1 acute myeloid leukemia (AML)).
- -
- 1 benign tumor (adenoma of the thyroid treated by surgery);
- -
- 3 borderline tumors (2 basal cell carcinomas of the skin, 1 phylloides tumors of the breast, all treated by surgery only);
- -
- 1 solid malignancy (1 breast cancer treated by surgery and local radiotherapy);
- -
- 2 hematologic malignancies (1 untreated large-cell NHL, 1 chronic lymphocytic leukemia treated by chemotherapy).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pui, C.-H.; Yang, J.J.; Hunger, S.P.; Pieters, R.; Schrappe, M.; Biondi, A.; Vora, A.; Baruchel, A.; Silverman, L.B.; Schmiegelow, K.; et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. J. Clin. Oncol. 2015, 33, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F. Non-Hodgkin lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef]
- Connors, J.M.; Cozen, W.; Steidl, C.; Carbone, A.; Hoppe, R.T.; Flechtner, H.-H.; Bartlett, N.L. Hodgkin lymphoma. Nat. Rev. Dis. Prim. 2020, 6, 1–25. [Google Scholar] [CrossRef]
- Cortes, J.; Pavlovsky, C.; Saußele, S. Chronic myeloid leukaemia. Lancet 2021, 398, 1914–1926. [Google Scholar] [CrossRef] [PubMed]
- Shimony, S.; Stahl, M.; Stone, R.M. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 502–526. [Google Scholar] [CrossRef]
- Fulbright, J.M.; Raman, S.; McClellan, W.S.; August, K.J. Late effects of childhood leukemia therapy. Curr. Hematol. Malign- Rep. 2011, 6, 195–205. [Google Scholar] [CrossRef]
- Langer, T.; Grabow, D.; Steinmann, D.; Wörmann, B.; Calaminus, G. Late Effects and Long-Term Follow-Up after Cancer in Childhood. Oncol. Res. Treat. 2017, 40, 746–750. [Google Scholar] [CrossRef]
- Henderson, T.O.; Oeffinger, K.C. Paediatrics: Addressing the health burden of childhood cancer survivors-improvements are needed. Nat. Rev. Clin. Oncol. 2018, 15, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Meadows, A.T.; Friedman, D.L.; Neglia, J.P.; Mertens, A.C.; Donaldson, S.S.; Stovall, M.; Hammond, S.; Yasui, Y.; Inskip, P.D. Second neoplasms in survivors of childhood cancer: Findings from the Childhood Cancer Survivor Study cohort. J. Clin. Oncol. 2009, 27, 2356–2362. [Google Scholar] [CrossRef]
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Prim. 2022, 8, 77. [Google Scholar] [CrossRef]
- Bielack, S.S.; Kempf-Bielack, B.; Heise, U.; Schwenzer, D.; Winkler, K. Combined modality treatment for osteosarcoma occurring as a second malignant disease. Cooperative German-Austrian-Swiss Osteosarcoma Study Group. J. Clin. Oncol. 1999, 17, 1164. [Google Scholar] [CrossRef] [PubMed]
- Tabone, M.-D.; Terrier, P.; Pacquement, H.; Brunat-Mentigny, M.; Schmitt, C.; Babin-Boilletot, A.; Mahmoud, H.H.; Kalifa, C. Outcome of radiation-related osteosarcoma after treatment of childhood and adolescent cancer: A study of 23 cases. J. Clin. Oncol. 1999, 17, 2789. [Google Scholar] [CrossRef]
- Kratz, C.P.; Achatz, M.I.; Brugières, L.; Frebourg, T.; Garber, J.E.; Greer, M.-L.C.; Hansford, J.R.; Janeway, K.A.; Kohlmann, W.K.; McGee, R.; et al. Cancer Screening Recommendations for Individuals with Li-Fraumeni Syndrome. Clin. Cancer Res. 2017, 23, e38–e45. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Zhu, B.; Koster, R.; Karlins, E.; Dean, M.; Yeager, M.; Gianferante, M.; Spector, L.G.; Morton, L.M.; Karyadi, D.; et al. Frequency of Pathogenic Germline Variants in Cancer-Susceptibility Genes in Patients with Osteosarcoma. JAMA Oncol. 2020, 6, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kager, L.; Kühne, T.; Langer, T.; Reichardt, P.; Blattmann, C.; Kevric, M.; Mettmann, V.; Sorg, B.; Hecker-Nolting, S. Establishment, Maintenance, and Performance of the Cooperative Osteosarcoma Study Group (COSS). Cancers 2023, 15, 1520. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.; Jürgens, H.; Jundt, G.; Kevric, M.; Kühne, T.; Reichardt, P.; Zoubek, A.; Werner, M.; Winkelmann, W.; Kotz, R. Osteosarcoma: The COSS experience. Cancer Treat Res. 2009, 152, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Bielack, S.S.; Smeland, S.; Longhi, A.; Egerer, G.; Hall, K.S.; Donati, D.; Kevric, M.; Brosjö, O.; Comandone, A.; et al. EURO-B.O.S.S.: A European study on chemotherapy in bone-sarcoma patients aged over 40: Outcome in primary high-grade osteosarcoma. Tumori J. 2018, 104, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and prognosis with osteosarcoma: Outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. A system for the surgical staging of musculoskeletal sarcoma. Clin. Orthop. Relat. Res. 1980, 153, 106–120. [Google Scholar] [CrossRef]
- Salzer-Kuntschik, M.; Brand, G.; Delling, G. Bestimmung des morphologischen Regressionsgrades nach Chemotherapie bei malignen Knochentumoren. Pathologe 1983, 4, 135–141. [Google Scholar]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Mantel, M. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966, 50, 163–170. [Google Scholar] [PubMed]
- PDQ Pediatric Treatment Editorial Board. Osteosarcoma and Undifferentiated Pleomorphic Sarcoma of Bone Treatment (PDQ®): Health Professional Version. 2023 Apr 5. In PDQ Cancer Information Summaries [Internet]; Bethesda, M.D., Ed.; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Bhagat, A.; Kleinerman, E.S. Anthracycline-induced cardiotoxicity: Causes, mechanisms, and prevention. Adv. Exp. Med. Biol. 2020, 1257, 181–192. [Google Scholar] [CrossRef] [PubMed]

| No. | Gender | Hematological Malignancy | Intercurrent | |||
|---|---|---|---|---|---|---|
| Age | Type | Radiotherapy | Chemotherapy | Neoplasm | ||
| 1 | male | 14.2 | HL | yes | none | none |
| 2 | male | 2.9 | NHL (NFS) | yes | yes | none |
| 3 | male | 29.7 | HL | yes | none | none |
| 4 | male | 12.6 | HL | yes | yes | none |
| 5 | male | 16.6 | HL | yes | yes | none |
| 6 | male | 10.8 | ALL (B-precursor) | yes (TBI) | yes | none |
| 7 | female | 20.8 | HL | yes | none | none |
| 8 | male | 15.1 | HL | yes | yes | none |
| 9 | male | 5.6 | ALL (B-precursor) | none | yes | none |
| 10 | male | 7.4 | ALL (NFS) | yes (TBI) | yes | none |
| 11 1 | male | 9.4 | NHL (ALCL) | none | yes | NHL (B-large-cell) |
| 12 | male | 5.8 | HL | yes | yes | none |
| 13 | female | 10.5 | ALL (T-) | yes (TBI) | yes | none |
| 14 | male | 12.9 | ALL (T-) | yes | yes | none |
| 15 | male | 33.4 | HL | yes | none | basal-cell carcinoma |
| 16 | male | 22.4 | HL | yes | yes | none |
| 17 | female | 49.8 | NHL (DLBCL) | yes | yes | none |
| 18 | male | 37.1 | HL | yes | none | none |
| 19 | male | 0.8 | ALL (B-precursor) | yes (TBI) | yes | none |
| 20 | female | 22.6 | HL | yes | yes | none |
| 21 | male | 58.2 | NHL (MALT) | yes | none | CLL |
| 22 | female | 38.1 | NHL (DLBCL) | yes | yes | none |
| 23 | male | 3.3 | ALL (T-) | yes (TBI) | yes | none |
| 24 | male | 15.0 | HL | none | yes | none |
| 25 | male | 1.4 | ALL (B-precursor) | yes (TBI) | yes | thyroid adenoma |
| 26 | female | 1.2 | ALL (B-precursor) | yes (TBI) | yes | none |
| 27 | male | 9.4 | ALL (T-) | yes (TBI) | yes | none |
| 28 | female | 45.7 | NHL (DLBC) | yes | none | breast cancer |
| 29 | male | 11.1 | NHL (lymphoblastic) | yes | yes | basal-cell carcinoma |
| 30 2 | female | 5.4 | ALL (B-precursor) | none | yes | none |
| 31 2 | female | 12.4 | ALL (B-precursor) | yes (TBI) | yes | phylloides tumor |
| 32 | female | 4.0 | AML | ND | yes | none |
| 33 | m | 15.3 | HL | yes | yes | none |
| No. | Inter- | Osteosarcoma | Follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| val | Age | Type | RX-rel | Prim mets | Surg | Response | Rx | Chem | Years | Further neopl | Outcome | |
| 1 | 5.5 | 19.7 | HGC | yes | none | yes | good | none | yes | 4.1 | none | DOD 2nd rec |
| 2 | 9.8 | 12.7 | EOS | yes | none | no | NOP | none | yes | 0.8 | none | DOD no CR |
| 3 | 18.0 | 47.7 | EOS | yes | none | yes | POP | none | yes | 3.1 | none | DOD 1st rec |
| 4 | 4.6 | 17.2 | HGC | yes | none | yes | good | none | yes | 4.6 | none | alive CR1 |
| 5 | 19.0 | 35.6 | HGC | yes | none | yes | NFS | none | yes | 3.1 | none | DOD 1st rec |
| 6 | 8.2 | 19.0 | HGC | yes | none | no | NOP | yes | yes | 7.2 | MDS | DOD SMD 1 |
| 7 | 17.7 | 38.5 | HGC | yes | none | yes | poor | none | yes | 1.5 | none | DOD 1st rec |
| 8 | 3.9 | 18.9 | HGC | yes | none | yes | good | none | yes | 11.0 | none | alive CR2 |
| 9 | 7.5 | 13.1 | HGC | no | lung | yes | good | none | yes | 6.2 | none | DOD 2nd rec |
| 10 | 8.8 | 16.1 | POS | yes | bone | yes | poor | none | yes | 4.4 | none | DOD 1st rec |
| 11 | 5.3 | 14.7 | HGC | no | none | yes | good | none | yes | 7.2 | T-ALL | DOC SMD 2 |
| 12 | 13.3 | 19.1 | HGC | yes | none | yes | poor | none | yes | 8.7 | none | alive CR3 |
| 13 | 3.2 | 13.6 | HGC | yes | lung | yes | poor | none | yes | 2.4 | none | DOD 1st rec |
| 14 | 7.0 | 19.8 | HGC | no | lung | yes | NFS | none | yes | 3.5 | none | DOD 2nd rec |
| 15 | 27.1 | 60.5 | HGC | yes | none | yes | poor | none | yes | 12.0 | SCC | alive CR1 3 |
| 16 | 16.1 | 38.6 | UPS | no | none | yes | good | none | yes | 8.8 | BCC | alive CR1 3 |
| 17 | 6.9 | 56.7 | UPS | yes | none | yes | POP | yes | yes | 10.5 | none | alive CR1 |
| 18 | 24.4 | 61.5 | HGC | yes | lung, bone | yes | POP | none | yes | 0.9 | none | DOD no CR |
| 19 | 13.7 | 14.5 | HGC | yes | none | yes | good | none | yes | 10.1 | none | alive CR1 |
| 20 | 7.7 | 30.3 | HGC | yes | none | no | NFS | NFS | NFS | 0.4 | none | alive no CR |
| 21 | 11.4 | 69.6 | HGC | yes | lung, bone | no | NFS | NFS | NFS | 0.4 | B-NHL 4 | DUC no CR |
| 22 | 11.2 | 49.3 | HGC | yes | none | yes | POP | none | yes | 1.1 | none | alive CR1 |
| 23 | 13.9 | 17.2 | HGC | yes | none | yes | poor | none | yes | 2.8 | none | DOD 2nd rec |
| 24 | 0.5 | 15.5 | HGC | no | lung | yes | good | none | yes | 10.3 | none | alive CR1 |
| 25 | 16.6 | 18.1 | POS | yes | none | yes | poor | none | yes | 3.9 | none | alive CR1 |
| 26 | 5.6 | 6.8 | HGC | yes | none | yes | poor | none | yes | 8.5 | none | alive CR1 |
| 27 | 9.5 | 18.9 | HGC | yes | none | yes | NFS | none | yes | 5.0 | none | alive CR1 |
| 28 | 19.3 | 65.0 | HGC | yes | none | yes | poor | none | yes | 2.3 | none | alive CR1 |
| 29 | 41.6 | 52.7 | HGC | yes | lung | yes | NFS | yes | yes | 2.6 | none | alive no CR |
| 30 | 9.3 | 14.8 | HGC | no | none | yes | NFS | none | yes | 4.9 | none | alive CR1 |
| 31 | 9.4 | 21.9 | HGC | yes | none | no | NOP | none | yes | 1.2 | none | alive no CR |
| 32 | 6.0 | 9.9 | HGC | NFS | none | yes | good | none | yes | 3.2 | none | DOD 1st rec |
| 33 | 7.8 | 23.1 | HGC | yes | none | no | NOP | none | yes | 0.3 | none | alive no CR |
| Patients (%) | 5-Year Overall Survival | 5-Year Event-Free Survival | |||
|---|---|---|---|---|---|
| Estimate (SE) | p 1 | Estimate (SE) | p 1 | ||
| all | |||||
| all eligible patients | 33 (100%) | 56% (10%) | - | 40% (9%) | - |
| gender | |||||
| male | 23 (70%) | 57% (11%) | 0.831 | 39% (10%) | 0.71 |
| female | 10 (30%) | 51% (20%) | 44% (18%) | ||
| age at leukemia/lymphoma | |||||
| below 12 years | 15 (45%) | 64% (13%) | 0.991 | 40% (13%) | 0.983 |
| 12 years and above | 18 (55%) | 48% (14%) | 40% (12%) | ||
| type of hematological malignancy | |||||
| lymphoma | 20 (61%) | 57% (13%) | 0.856 | 42% (12%) | 0.735 |
| leukemia | 13 (39%) | 57% (15%) | 39% (14%) | ||
| chemotherapy for hematological malignancy | |||||
| yes | 26 (79%) | 67% (10%) | 0.024 | 45% (10%) | 0.477 |
| no | 7 (21%) | 19% (17%) | 19% (17%) | ||
| interval hematological malignancy to osteosarcoma | |||||
| below 10 years | 19 (58%) | 63% (12%) | 0.736 | 42% (11%) | 0.967 |
| 10 years or more | 14 (42%) | 49% (15%) | 36% (14%) | ||
| age at osteosarcoma | |||||
| below 18 years | 12 (36%) | 58% (14%) | 0.797 | 50% (14%) | 0.265 |
| 18 years and above | 21 (64%) | 55% (13%) | 34% (11%) | ||
| osteosarcoma in radiation-field 2,3 | |||||
| yes | 26 (81%) | 51% (11%) | 0.596 | 35% (10%) | 0.273 |
| no | 6 (19%) | 82% (15%) | 67% (19%) | ||
| osteosarcoma site | |||||
| extremity | 19 (58%) | 56% (11%) | 0.225 | 56% (12%) | 0.011 |
| trunk or head and neck | 14 (42%) | 40% (17%) | 18% (11%) | ||
| primary osteosarcoma metastases | |||||
| absent | 25 (76%) | 65% (11%) | 0.05 | 49% (10%) | 0.063 |
| present | 8 (24%) | 31% (18%) | 13% (12%) | ||
| macroscopic surgical remission | |||||
| achieved | 25 (76%) | 60% (10%) | 0.017 | NA | |
| not achieved | 8 (24%) | 51% (20%) | |||
| additional malignancies 4 | |||||
| no | 28 (85%) | 52% (11%) | 0.807 | 38% (9%) | 0.893 |
| yes | 5 (15%) | 80% (18%) | 60% (22%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielack, S.S.; Mettmann, V.; Baumhoer, D.; Blattmann, C.; Burkhardt, B.; Deinzer, C.K.W.; Kager, L.; Kevric, M.; Mauz-Körholz, C.; Müller-Abt, P.; et al. Osteosarcoma Arising as a Secondary Malignancy following Treatment for Hematologic Cancer: A Report of 33 Affected Patients from the Cooperative Osteosarcoma Study Group (COSS). Cancers 2024, 16, 1836. https://doi.org/10.3390/cancers16101836
Bielack SS, Mettmann V, Baumhoer D, Blattmann C, Burkhardt B, Deinzer CKW, Kager L, Kevric M, Mauz-Körholz C, Müller-Abt P, et al. Osteosarcoma Arising as a Secondary Malignancy following Treatment for Hematologic Cancer: A Report of 33 Affected Patients from the Cooperative Osteosarcoma Study Group (COSS). Cancers. 2024; 16(10):1836. https://doi.org/10.3390/cancers16101836
Chicago/Turabian StyleBielack, Stefan S., Vanessa Mettmann, Daniel Baumhoer, Claudia Blattmann, Birgit Burkhardt, Christoph K. W. Deinzer, Leo Kager, Matthias Kevric, Christine Mauz-Körholz, Peter Müller-Abt, and et al. 2024. "Osteosarcoma Arising as a Secondary Malignancy following Treatment for Hematologic Cancer: A Report of 33 Affected Patients from the Cooperative Osteosarcoma Study Group (COSS)" Cancers 16, no. 10: 1836. https://doi.org/10.3390/cancers16101836
APA StyleBielack, S. S., Mettmann, V., Baumhoer, D., Blattmann, C., Burkhardt, B., Deinzer, C. K. W., Kager, L., Kevric, M., Mauz-Körholz, C., Müller-Abt, P., Reinhardt, D., Sabo, A.-A., Schrappe, M., Sorg, B., Windhager, R., & Hecker-Nolting, S. (2024). Osteosarcoma Arising as a Secondary Malignancy following Treatment for Hematologic Cancer: A Report of 33 Affected Patients from the Cooperative Osteosarcoma Study Group (COSS). Cancers, 16(10), 1836. https://doi.org/10.3390/cancers16101836






