Angiogenesis Still Plays a Crucial Role in Human Melanoma Progression
Abstract
Simple Summary
Abstract
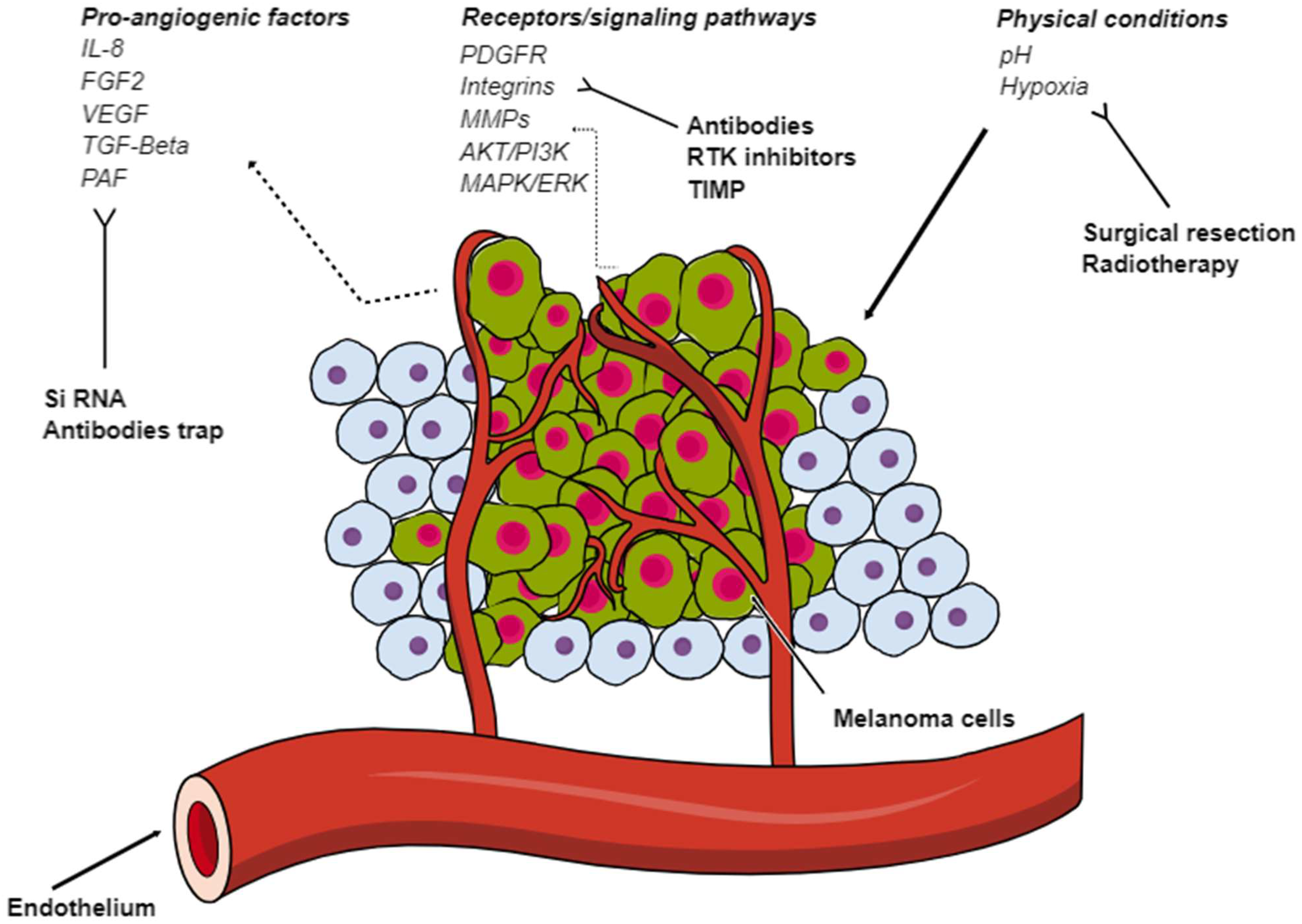
1. Introduction
2. Pro-Angiogenic Factors
2.1. Vascular Endothelial Growth Factor (VEGF)
2.2. Placental Growth Factor (PlGF)
2.3. Fibroblast Growth Factor-2 (FGF-2)
2.4. Interleukin-8 (IL-8)
2.5. Angiopoietin (ANG)
2.6. Transforming Growth Factor-Beta (TGF-β)
2.7. Platelet-Derived Growth Factor (PDGF)
3. Integrin Signaling and Extracellular Matrix Enzymes
3.1. Integrins
3.2. Matrix Metalloproteinases (MMPs)
3.3. Platelet-Activating Factor (PAF)
4. Cells in the TME
4.1. Cancer-Associated Fibroblasts (CAFs)
4.2. Mast Cells
4.3. Melanoma-Associated Macrophages (MAs)
4.4. External Conditions
5. Data from Next-Generation Sequencing (NGS) and Single-Cell RNA Sequencing (scRNAseq)
6. Anti-Angiogenesis in Melanoma
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jeong, J.H.; Ojha, U.; Lee, Y.M. Pathological angiogenesis and inflammation in tissues. Arch. Pharm. Res. 2021, 44, 1–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eelen, G.; de Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramasamy, S.K.; Kusumbe, A.P.; Adams, R.H. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 2015, 25, 148–157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribatti, D.; Vacca, A.; Dammacco, F. The role of the vascular phase in solid tumor growth: A historical review. Neoplasia 1999, 1, 293–302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waseh, S.; Lee, J.B. Advances in melanoma: Epidemiology, diagnosis, and prognosis. Front. Med. 2023, 10, 1268479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, P.; Kim, H.J.; Schwartz, R.A. Superficial spreading melanoma: An analysis of 97 702 cases using the SEER database. Melanoma Res. 2016, 26, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Maiques, O.; Sanz-Moreno, V. Location, location, location: Melanoma cells “living at the edge”. Exp. Dermatol. 2022, 31, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Bobos, M. Histopathologic classification and prognostic factors of melanoma: A 2021 update. Ital. J. Dermatol. Venerol. 2021, 156, 300–321. [Google Scholar] [CrossRef] [PubMed]
- Maibach, F.; Sadozai, H.; Seyed Jafari, S.M.; Hunger, R.E.; Schenk, M. Tumor-Infiltrating Lymphocytes and Their Prognostic Value in Cutaneous Melanoma. Front. Immunol. 2020, 11, 2105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribatti, D.; Annese, T.; Longo, V. Angiogenesis and melanoma. Cancers 2010, 2, 114–132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Srivastava, A.; Laidler, P.; Davies, R.P.; Horgan, K.; Hughes, L.E. The prognostic significance of tumor vascularity in intermediate-thickness (0.76–4.0 mm thick) skin melanoma. A quantitative histologic study. Am. J. Pathol. 1988, 133, 419–423. [Google Scholar] [PubMed] [PubMed Central]
- Srivastava, A.; Hughes, L.E.; Woodcock, J.P.; Laidler, P. Vascularity in cutaneous melanoma detected by Doppler sonography and histology: Correlation with tumour behaviour. Br. J. Cancer 1989, 59, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Fallowfield, M.E.; Cook, M.G. The vascularity of primary cutaneous melanoma. J. Pathol. 1991, 164, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Straume, O.; Salvesen, H.B.; Akslen, L.A. Angiogenesis is prognostically important in vertical growth phase melanomas. Int. J. Oncol. 1999, 15, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Kashani-Sabet, M.; Sagebiel, R.W.; Ferreira, C.M.; Nosrati, M.; Miller, J.R., 3rd. Tumor vascularity in the prognostic assessment of primary cutaneous melanoma. J. Clin. Oncol. 2002, 20, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Vacca, A.; Ribatti, D.; Roncali, L.; Lospalluti, M.; Serio, G.; Carrel, S.; Dammacco, F. Melanocyte tumor progression is associated with changes in angiogenesis and expression of the 67-kilodalton laminin receptor. Cancer 1993, 72, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Ria, R.; Reale, A.; Castrovilli, A.; Mangialardi, G.; Dammacco, F.; Ribatti, D.; Vacca, A. Angiogenesis and progression in human melanoma. Dermatol. Res. Pract. 2010, 2010, 185687. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skobe, M.; Hamberg, L.M.; Hawighorst, T.; Schirner, M.; Wolf, G.L.; Alitalo, K.; Detmar, M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growt factor-C in melanoma. Am. J. Pathol. 2001, 159, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P.; Ghebremariam, Y.T. Endothelial nicotinic acetylcholine receptors and angiogenesis. Trends Cardiovasc. Med. 2008, 18, 247–253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Shan, Y.; Pan, X.; He, L. Recent advances in antiangiogenic agents with VEGFR as target. Mini Rev. Med. Chem. 2011, 11, 920–946. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, M.; Ghodsi, R. Structure-activity relationship studies of indolin-2-one derivatives as vascular endothelial growth factor receptor inhibitors and anticancer agents. Arch. Pharm. 2020, 353, e2000022. [Google Scholar] [CrossRef] [PubMed]
- Rofstad, E.K.; Danielsen, T. Hypoxia-induced angiogenesis and vascular endothelial growth factor secretion in human melanoma. Br. J. Cancer 1998, 77, 897–902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rofstad, E.K.; Halsør, E.F. Vascular endothelial growth factor, interleukin 8, platelet-derived endothelial cell growth factor, and basic fibroblast growth factor promote angiogenesis and metastasis in human melanoma xenografts. Cancer Res. 2000, 60, 4932–4938. [Google Scholar] [PubMed]
- Ribatti, D.; Nico, B.; Floris, C.; Mangieri, D.; Piras, F.; Ennas, M.G.; Vacca, A.; Sirigu, P. Microvascular density, vascular endothelial growth factor immunoreactivity in tumor cells, vessel diameter and intussusceptive microvascular growth in primary melanoma. Oncol. Rep. 2005, 14, 81–84. [Google Scholar] [PubMed]
- Yu, J.L.; Rak, J.W.; Klement, G.; Kerbel, R.S. Vascular endothelial growth factor isoform expression as a determinant of blood vessel patterning in human melanoma xenografts. Cancer Res. 2002, 62, 1838–1846. [Google Scholar]
- Salven, P.; Heikkilä, P.; Joensuu, H. Enhanced expression of vascular endothelial growth factor in metastatic melanoma. Br. J. Cancer 1997, 76, 930–934. [Google Scholar] [CrossRef]
- Sala, R.; Jefferies, W.A.; Walker, B.; Yang, J.; Tiong, J.; Law, S.K.; Carlevaro, M.F.; Di Marco, E.; Vacca, A.; Cancedda, R.; et al. The human melanoma associated protein melanotransferrin promotes endothelial cell migration and angiogenesis in vivo. Eur. J. Cell Biol. 2002, 81, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Erhard, H.; Rietveld, F.J.; van Altena, M.C.; Bröcker, E.B.; Ruiter, D.J.; de Waal, R.M. Transition of horizontal to vertical growth phase melanoma is accompanied by induction of vascular endothelial growth factor expression and angiogenesis. Melanoma Res. 1997, 2, S19–S26. [Google Scholar] [CrossRef]
- Marcoval, J.; Moreno, A.; Graells, J.; Vidal, A.; Escribà, J.M.; Garcia-Ramírez, M.; Fabra, A. Angiogenesis and malignant melanoma. Angiogenesis is related to the development of vertical (tumorigenic) growth phase. J. Cutan. Pathol. 1997, 24, 212–218. [Google Scholar] [CrossRef]
- Gorski, D.H.; Leal, A.D.; Goydos, J.S. Differential expression of vascular endothelial growth factor-A isoforms at different stages of melanoma progression. J. Am. Coll. Surg. 2003, 197, 408–418. [Google Scholar] [CrossRef]
- Claffey, K.P.; Brown, L.F.; del Aguila, L.F.; Tognazzi, K.; Yeo, K.T.; Manseau, E.J.; Dvorak, H.F. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996, 56, 172–181. [Google Scholar]
- Küsters, B.; Leenders, W.P.; Wesseling, P.; Smits, D.; Verrijp, K.; Ruiter, D.J.; Peters, J.P.; van Der Kogel, A.J.; de Waal, R.M. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 2002, 62, 341–345. [Google Scholar]
- Pagani, E.; Ruffini, F.; Antonini Cappellini, G.C.; Scoppola, A.; Fortes, C.; Marchetti, P.; Graziani, G.; D’Atri, S.; Lacal, P.M. Placenta growth factor and neuropilin-1 collaborate in promoting melanoma aggressiveness. Int. J. Oncol. 2016, 48, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.G.; Ceci, C.; Ruffini, F.; Scimeca, M.; Cicconi, R.; Mattei, M.; Lacal, P.M.; Graziani, G. The Anti-Vascular Endothelial Growth Factor Receptor 1 (VEGFR-1) D16F7 Monoclonal Antibody Inhibits Melanoma Adhesion to Soluble VEGFR-1 and Tissue Invasion in Response to Placenta Growth Factor. Cancers 2022, 14, 5578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nurmi, H.; Saharinen, P.; Zarkada, G.; Zheng, W.; Robciuc, M.R.; Alitalo, K. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol. Med. 2015, 7, 1418–1425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004, 5, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Salven, P.; Lymbousaki, A.; Heikkila, P.; Jääskela-Saari, H.; Enholm, B.; Aase, K.; von Euler, G.; Eriksson, U.; Alitalo, K.; Joensuu, H. Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am. J. Pathol. 1998, 153, 103–108. [Google Scholar] [CrossRef]
- Dadras, S.S.; Paul, T.; Bertoncini, J.; Brown, L.F.; Muzikansky, A.; Jackson, D.G.; Ellwanger, U.; Garbe, C.; Mihm, M.C.; Detmar, M. Tumor lymphangiogenesis: A novel prognostic indicator for cutaneous melanoma metastasis and survival. Am. J. Pathol. 2003, 162, 1951–1960. [Google Scholar] [CrossRef]
- Wobser, M.; Siedel, C.; Schrama, D.; Bröcker, E.B.; Becker, J.C.; Vetter-Kauczok, C.S. Expression pattern of the lymphatic and vascular markers VEGFR-3 and CD31 does not predict regional lymph node metastasis in cutaneous melanoma. Arch. Dermatol. Res. 2006, 297, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Massi, D.; Puig, S.; Franchi, A.; Malvehy, J.; Vidal-Sicart, S.; González-Cao, M.; Baroni, G.; Ketabchi, S.; Palou, J.; Santucci, M. Tumour lymphangiogenesis is a possible predictor of sentinel lymph node status in cutaneous melanoma: A case-control study. J. Clin. Pathol. 2006, 59, 166–173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boone, B.; Blokx, W.; De Bacquer, D.; Lambert, J.; Ruiter, D.; Brochez, L. The role of VEGF-C staining in predicting regional metastasis in melanoma. Virchows Arch. 2008, 453, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Conejero, C.; Carapeto, F.J. Lymphangiogenesis: Implications for diagnosis, treatment, and prognosis in patients with melanoma. Actas Dermosifiliogr. 2015, 106, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Pötgens, A.J.; Lubsen, N.H.; van Altena, M.C.; Schoenmakers, J.G.; Ruiter, D.J.; de Waal, R.M. Vascular permeability factor expression influences tumor angiogenesis in human melanoma lines xenografted to nude mice. Am. J. Pathol. 1995, 146, 197–209. [Google Scholar] [PubMed]
- Pötgens, A.J.; van Altena, M.C.; Lubsen, N.H.; Ruiter, D.J.; de Waal, R.M. Analysis of the tumor vasculature and metastatic behavior of xenografts of human melanoma cell lines transfected with vascular permeability factor. Am. J. Pathol. 1996, 148, 1203–1217. [Google Scholar] [PubMed]
- Oku, T.; Tjuvajev, J.G.; Miyagawa, T.; Sasajima, T.; Joshi, A.; Joshi, R.; Finn, R.; Claffey, K.P.; Blasberg, R.G. Tumor growth modulation by sense and antisense vascular endothelial growth factor gene expression: Effects on angiogenesis, vascular permeability, blood volume, blood flow, fluorodeoxyglucose uptake, and proliferation of human melanoma intracerebral xenografts. Cancer Res. 1998, 58, 4185–4192. [Google Scholar] [PubMed]
- Marcellini, M.; De Luca, N.; Riccioni, T.; Ciucci, A.; Orecchia, A.; Lacal, P.M.; Ruffini, F.; Pesce, M.; Cianfarani, F.; Zambruno, G.; et al. Increased melanoma growth and metastasis spreading in mice overexpressing placenta growth factor. Am. J. Pathol. 2006, 169, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Presta, M.; Dell’Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 179–186. [Google Scholar] [CrossRef]
- Czyz, M. Fibroblast Growth Factor Receptor Signaling in Skin Cancers. Cells 2019, 8, 540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Streit, S.; Mestel, D.S.; Schmidt, M.; Ullrich, A.; Berking, C. FGFR4 Arg388 allele correlates with tumour thickness and FGFR4 protein expression with survival of melanoma patients. Br. J. Cancer 2006, 94, 1879–1886. [Google Scholar] [CrossRef]
- Kurschat, P.; Eming, S.; Nashan, D.; Krieg, T.; Mauch, C. Early increase in serum levels of the angiogenesis-inhibitor endostatin and of basic fibroblast growth factor in melanoma patients during disease progression. Br. J. Dermatol. 2007, 156, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Dréau, D.; Foster, M.; Hogg, M.; Swiggett, J.; Holder, W.D.; White, R.L. Angiogenic and immune parameters during recombinant interferon-alpha2b adjuvant treatment in patients with melanoma. Oncol. Res. 2000, 12, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Vacca, A.; Ria, R.; Marzullo, A.; Nico, B.; Filotico, R.; Roncali, L.; Dammacco, F. Neovascularisation, expression of fibroblast growth factor-2, and mast cells with tryptase activity increase simultaneously with pathological progression in human malignant melanoma. Eur. J. Cancer 2003, 39, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Bar-Eli, M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology 1999, 67, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Varney, M.; Singh, R.K. Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Res. 2009, 69, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Rofstad, E.K.; Halsør, E.F. Hypoxia-associated spontaneous pulmonary metastasis in human melanoma xenografts: Involvement of microvascular hot spots induced in hypoxic foci by interleukin 8. Br. J. Cancer 2002, 86, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, F.; Lee, J.; Dong, Z. Selective induction of interleukin-8 expression in metastatic melanoma cells by transforming growth factor-β1. Cytokine 2005, 31, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G. Role of angiopietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 2003, 314, 61–68. [Google Scholar] [CrossRef]
- Lin, P.; Buxton, J.A.; Acheson, A.; Radziejewski, C.; Maisonpierre, P.C.; Yancopoulos, G.D.; Channon, K.M.; Hale, L.P.; Dewhirst, M.W.; George, S.E.; et al. Antiangiogenic gene therapy targeting the endothelium-specific receptor tyrosine kinase Tie2. Proc. Natl. Acad. Sci. USA 1998, 95, 8829–8834. [Google Scholar] [CrossRef]
- Siemeister, G.; Schirner, M.; Weindel, K.; Reusch, P.; Menrad, A.; Marmé, D.; Martiny-Baron, G. Two independent mechanisms essential for tumor angiogenesis: Inhibition of human melanoma xenograft growth by interfering with either the vascular endothelial growth factor receptor pathway or the Tie-2 pathway. Cancer Res. 1999, 59, 3185–3191. [Google Scholar]
- Jendreyko, N.; Popkov, M.; Rader, C.; Barbas, C.F., 3rd. Phenotypic knockout of VEGF-R2 and Tie-2 with an intraantibody reduces tumor growth and angiogenesis in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 8293–8298. [Google Scholar] [CrossRef] [PubMed]
- Nasarre, P.; Thomas, M.; Kruse, K.; Helfrich, I.; Wolter, V.; Deppermann, C.; Schadendorf, D.; Thurston, G.; Fiedler, U.; Augustin, H.G. Host-derived angiopoietin-2 affects early stages of tumor development and vessel maturation but is dispensable for later stages of tumor growth. Cancer Res. 2009, 69, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Wiguna, A.P.; Walden, P. Role of IL-10 and TGF-β in melanoma. Exp. Dermatol. 2015, 24, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Busse, A.; Keilholz, U. Role of TGF-β in melanoma. Curr. Pharm. Biotechnol. 2011, 12, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J. Endothelial-to-Mesenchymal Transition. Circ. Res. 2019, 124, 1163–1165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, L.; Pang, Y.; Moses, H.L. TGF-beta and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010, 31, 220–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, K. Signaling Cross Talk between TGF-β/Smad and Other Signaling Pathways. Cold Spring Harb. Perspect. Biol. 2017, 9, a022137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cesati, M.; Scatozza, F.; D’Arcangelo, D.; Antonini-Cappellini, G.C.; Rossi, S.; Tabolacci, C.; Nudo, M.; Palese, E.; Lembo, L.; Di Lella, G.; et al. Investigating Serum and Tissue Expression Identified a Cytokine/Chemokine Signature as a Highly Effective Melanoma Marker. Cancers 2020, 12, 3680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruffini, F.; Levati, L.; Graziani, G.; Caporali, S.; Atzori, M.G.; D’Atri, S.; Lacal, P.M. Platelet-derived growth factor-C promotes human melanoma aggressiveness through activation of neuropilin-1. Oncotarget 2017, 8, 66833–66848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robinson, S.P.; Ludwig, C.; Paulsson, J.; Ostman, A. The effects of tumor-derived platelet-derived growth factor on vascular morphology and function in vivo revealed by susceptibility MRI. Int. J. Cancer 2008, 122, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Heldin, C.H.; Heuchel, R.L. Platelet-derived growth factor receptor-beta, carrying the activating mutation D849N, accelerates the establishment of B16 melanoma. BMC Cancer 2007, 7, 224. [Google Scholar] [CrossRef]
- Faraone, D.; Aguzzi, M.S.; Toietta, G.; Facchiano, A.M.; Facchiano, F.; Magenta, A.; Martelli, F.; Truffa, S.; Cesareo, E.; Ribatti, D.; et al. Platelet-derived growth factor-receptor alpha strongly inhibits melanoma growth in vitro and in vivo. Neoplasia 2009, 11, 732–742. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Q.; Dong, Z.; Liu, K. Integrins in cancer: Emerging mechanisms and therapeutic opportunities. Pharmacol. Ther. 2023, 247, 108458. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef] [PubMed]
- McClelland, L.; Chen, Y.; Soong, J.; Kuo, I.; Scott, G. Plexin B1 inhibits integrin-dependent pp125FAK and Rho activity in melanoma. Pigment. Cell Melanoma Res. 2011, 24, 165–174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naffa, R.; Vogel, L.; Hegedűs, L.; Pászty, K.; Tóth, S.; Kelemen, K.; Singh, N.; Reményi, A.; Kállay, E.; Cserepes, M.; et al. P38 MAPK Promotes Migration and Metastatic Activity of BRAF Mutant Melanoma Cells by Inducing Degradation of PMCA4b. Cells 2020, 9, 1209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meier, F.; Schittek, B.; Busch, S.; Garbe, C.; Smalley, K.; Satyamoorthy, K.; Li, G.; Herlyn, M. The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front. Biosci. 2005, 10, 2986–3001. [Google Scholar] [CrossRef] [PubMed]
- Bosserhoff, A.K. Novel biomarkers in malignant melanoma. Clin. Chim. Acta 2006, 367, 28–35. [Google Scholar] [CrossRef]
- Kuphal, S.; Bauer, R.; Bosserhoff, A.-K. Integrin signaling in malignant melanoma. Cancer Metastasis Rev. 2005, 24, 195–222. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, Y.; Gao, M.; Lin, D.; Du, G.; Cai, Y. Prognostic and Immunological Roles of MMP-9 in Pan-Cancer. BioMed Res. Int. 2022, 2022, 2592962. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.B.; Westphal, J.R.; Van Muijen, G.N.P.; Ruiter, D.J. Matrix metalloproteinases in human melanoma. J. Investig. Dermatol. 2000, 115, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Schnaeker, E.-M.; Ossig, R.; Ludwig, T.; Dreier, R.; Oberleithner, H.; Wilhelmi, M.; Schneider, S.W. Microtubule-dependent matrix metalloproteinase-2/matrix metalloproteinase-9 exocytosis: Prerequisite in human melanoma cell invasion. Cancer Res. 2004, 64, 8924–8931. [Google Scholar] [CrossRef] [PubMed]
- Zamolo, G.; Grahovac, M.; Žauhar, G.; Vučinić, D.; Kovač, L.; Brajenić, N.; Grahovac, B. Matrix metalloproteinases MMP-1, MMP-2, and MMP-13 are overexpressed in primary nodular melanoma. J. Cutan. Pathol. 2020, 47, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kerkela, E.; Saarialho-Kere, U. Matrix metalloproteinases in tumor progression: Focus on basal and squamous cell skin cancer. Exp. Dermatol. 2003, 12, 109–125. [Google Scholar] [CrossRef]
- Napoli, S.; Scuderi, C.; Gattuso, G.; Bella, V.D.; Candido, S.; Basile, M.S.; Libra, M.; Falzone, L. Functional Roles of Matrix Metalloproteinases and Their Inhibitors in Melanoma. Cells 2020, 9, 1151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melnikova, V.; Bar-Eli, M. Inflammation and melanoma growth and metastasis: The role of platelet-activating factor (PAF) and its receptor. Cancer Metastasis Rev. 2007, 26, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.P. Expression of the platelet-activating factor receptor enhances benzyl isothiocyanate-induced apoptosis in murine and human melanoma cells. Mol. Med. Rep. 2015, 12, 394–400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The implication of platelet activating factor in cancer growth and metastasis: Potent beneficial role of PAF-inhibitors and antioxidants. Infect. Disord. Drug Targets 2009, 9, 390–399. [Google Scholar] [CrossRef]
- Melnikova, V.O.; Villares, G.J.; Bar-Eli, M. Emerging roles of PAR-1 and PAFR in melanoma metastasis. Cancer Microenviron. 2008, 1, 103–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Villares, G.J.; Zigler, M.; Wang, H.; Melnikova, V.O.; Wu, H.; Friedman, R.; Leslie, M.C.; Vivas-Mejia, P.E.; Lopez-Berestein, G.; Sood, A.K.; et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 2008, 68, 9078–9086. [Google Scholar] [CrossRef] [PubMed]
- Glabman, R.A.; Choyke, P.L.; Sato, N. Cancer-Associated Fibroblasts: Tumorigenicity and Targeting for Cancer Therapy. Cancers 2022, 14, 3906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galbo, P.M., Jr.; Zang, X.; Zheng, D. Molecular Features of Cancer-associated Fibroblast Subtypes and their Implication on Cancer Pathogenesis, Prognosis, and Immunotherapy Resistance. Clin. Cancer Res. 2021, 27, 2636–2647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, G.; Ji, P.; Xia, P.; Song, H.; Guo, Z.; Hu, X.; Guo, Y.; Yuan, X.; Song, Y.; Shen, R.; et al. Identification and targeting of cancer-associated fibroblast signature genes for prognosis and therapy in Cutaneous melanoma. Comput. Biol. Med. 2023, 167, 107597. [Google Scholar] [CrossRef] [PubMed]
- Hutchenreuther, J.; Nguyen, J.; Quesnel, K.; Vincent, K.M.; Petitjean, L.; Bourgeois, S.; Boyd, M.; Bou-Gharios, G.; Postovit, L.M.; Leask, A. Cancer-associated Fibroblast-specific Expression of the Matricellular Protein CCN1 Coordinates Neovascularization and Stroma Deposition in Melanoma Metastasis. Cancer Res. Commun. 2024, 4, 556–570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jobe, N.P.; Rösel, D.; Dvořánková, B.; Kodet, O.; Lacina, L.; Mateu, R.; Smetana, K.; Brábek, J. Simultaneous blocking of IL-6 and IL-8 is sufficient to fully inhibit CAF-induced human melanoma cell invasiveness. Histochem. Cell Biol. 2016, 146, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; Kovacs, D.; Bellei, B.; Caputo, S.; Migliano, E.; Cota, C.; Picardo, M. Profiling Cancer-Associated Fibroblasts in Melanoma. Int. J. Mol. Sci. 2021, 22, 7255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duncan, L.M.; Richards, L.A.; Mihm, M.C., Jr. Increased mast cell density in invasive melanoma. J. Cutan. Pathol. 1998, 25, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Ennas, M.G.; Vacca, A.; Ferreli, F.; Nico, B.; Orru, S.; Sirigu, P. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur. J. Clin. Investig. 2003, 33, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Crivellato, E.; Nico, B.; Andreis, P.G.; Nussdorfer, G.G.; Ribatti, D. An image analysis of the spatial distribution of perivascular mast cells in human melanoma. Int. J. Mol. Med. 2006, 17, 981–987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tóth-Jakatics, R.; Jimi, S.; Takebayashi, S.; Kawamoto, N. Cutaneous malignant melanoma: Correlation between neovascularization and peritumor accumulation of mast cells overexpressing vascular endothelial growth factor. Hum. Pathol. 2000, 31, 955–960. [Google Scholar] [CrossRef]
- Rajabi, P.; Bagheri, A.; Hani, M. Intratumoral and Peritumoral Mast Cells in Malignant Melanoma: An Immunohistochemical Study. Adv. Biomed. Res. 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kohl, L.M.; Sumpter, T.L. Melanomas and mast cells: An ambiguous relationship. Melanoma Res. 2024, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chatziioannou, E.; Aydin, S.A.; Forchhammer, S.; Sinnberg, T.; Eigentler, T. Makrophagen im Melanom—Von molekularen Signalen zur therapeutischen Anwendung [Melanoma-associated macrophages-from molecular signals to therapeutic application]. Dermatologie 2022, 73, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Qi, M.; Stoeger, T.; Zhang, J.; Chen, S. The role of tumor-associated macrophages and soluble mediators in pulmonary metastatic melanoma. Front. Immunol. 2022, 13, 1000927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adams, R.; Osborn, G.; Mukhia, B.; Laddach, R.; Willsmore, Z.; Chenoweth, A.; Geh, J.L.C.; MacKenzie Ross, A.D.; Healy, C.; Barber, L.; et al. Influencing tumor-associated macrophages in malignant melanoma with monoclonal antibodies. Oncoimmunology 2022, 11, 2127284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hussein, M.R. Tumour-associated macrophages and melanoma tumourigenesis: Integrating the complexity. Int. J. Exp. Pathol. 2006, 87, 163–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, T.; Liu, G.; Wang, R. The Intercellular Metabolic Interplay between Tumor and Immune Cells. Front. Immunol. 2014, 5, 358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Altenberg, B.; Greulich, K.O. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics 2004, 84, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Hjelmeland, A.B.; Wu, Q.; Heddleston, J.M.; Choudhary, G.S.; MacSwords, J.; Lathia, J.D.; McLendon, R.; Lindner, D.; Sloan, A.; Rich, J.N. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011, 18, 829–840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Lin, Y.; Gillies, R.J. Tumor pH and its measurement. J. Nucl. Med. 2010, 51, 1167–1170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Justus, C.R.; Sanderlin, E.J.; Yang, L.V. Molecular Connections between Cancer Cell Metabolism and the Tumor Microenvironment. Int. J. Mol. Sci. 2015, 16, 11055–11086. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Böhme, I.; Bosserhoff, A.K. Acidic tumor microenvironment in human melanoma. Pigment. Cell Melanoma Res. 2016, 29, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Olbryt, M.; Rajczykowski, M.; Bal, W.; Fiszer-Kierzkowska, A.; Cortez, A.J.; Mazur, M.; Suwiński, R.; Widłak, W. NGS Analysis of Liquid Biopsy (LB) and Formalin-Fixed Paraffin-Embedded (FFPE) Melanoma Samples Using Oncomine™ Pan-Cancer Cell-Free Assay. Genes 2021, 12, 1080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- King, A.D.; Deirawan, H.; Klein, P.A.; Dasgeb, B.; Dumur, C.I.; Mehregan, D.R. Next-generation sequencing in dermatology. Front. Med. 2023, 10, 1218404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tirosh, I.; Izar, B.; Prakadan, S.M.; Wadsworth, M.H., 2nd; Treacy, D.; Trombetta, J.J.; Rotem, A.; Rodman, C.; Lian, C.; Murphy, G.; et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016, 352, 189–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loeffler-Wirth, H.; Binder, H.; Willscher, E.; Gerber, T.; Kunz, M. Pseudotime Dynamics in Melanoma Single-Cell Transcriptomes Reveals Different Mechanisms of Tumor Progression. Biology 2018, 7, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gerber, T.; Willscher, E.; Loeffler-Wirth, H.; Hopp, L.; Schadendorf, D.; Schartl, M.; Anderegg, U.; Camp, G.; Treutlein, B.; Binder, H.; et al. Mapping heterogeneity in patient-derived melanoma cultures by single-cell RNA-seq. Oncotarget 2017, 8, 846–862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kiss, M.; Van Gassen, S.; Movahedi, K.; Saeys, Y.; Laoui, D. Myeloid cell heterogeneity in cancer: Not a single cell alike. Cell Immunol. 2018, 330, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chhabra, Y.; Weeraratna, A.T. Fibroblasts in cancer: Unity in heterogeneity. Cell 2023, 186, 1580–1609. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Rizos, H. Single-cell RNA sequencing in melanoma: What have we learned so far? eBioMedicine 2024, 100, 104969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corrie, P.G.; Marshall, A.; Nathan, P.D.; Lorigan, P.; Gore, M.; Tahir, S.; Faust, G.; Kelly, C.G.; Marples, M.; Danson, S.J.; et al. Adjuvant bevacizumab for melanoma patients at high risk of recurrence: Survival analysis of the AVAST-M trial. Ann. Oncol. 2018, 29, 1843–1852, Erratum in Ann. Oncol. 2019, 30, 2013–2014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, X.; Ge, P.; Liu, S.; Yang, D.; Zhang, J.; Wang, X.; Liang, W. Efficacy and safety of bevacizumab in patients with malignant melanoma: A systematic review and PRISMA-compliant meta-analysis of randomized controlled trials and non-comparative clinical studies. Front. Pharmacol. 2023, 14, 1163805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carvajal, R.D.; Wong, M.K.; Thompson, J.A.; Gordon, M.S.; Lewis, K.D.; Pavlick, A.C.; Wolchok, J.D.; Rojas, P.B.; Schwartz, J.D.; Bedikian, A.Y.; et al. A phase 2 randomised study of ramucirumab (IMC-1121B) with or without dacarbazine in patients with metastatic melanoma. Eur. J. Cancer 2014, 50, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Rahma, O.E.; Tyan, K.; Giobbie-Hurder, A.; Brohl, A.S.; Bedard, P.L.; Renouf, D.J.; Sharon, E.; Streicher, H.; Hathaway, E.; Cunningham, R.; et al. Phase IB study of ziv-aflibercept plus pembrolizumab in patients with advanced solid tumors. J. Immunother. Cancer 2022, 10, e003569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baginska, J.; Nau, A.; Gomez Diaz, I.; Giobbie-Hurder, A.; Weirather, J.; Vergara, J.; Abrecht, C.; Hallisey, M.; Dennis, J.; Severgnini, M.; et al. Ziv-aflibercept plus pembrolizumab in patients with advanced melanoma resistant to anti-PD-1 treatment. Cancer Immunol. Immunother. 2024, 73, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mouriaux, F.; Servois, V.; Parienti, J.J.; Lesimple, T.; Thyss, A.; Dutriaux, C.; Neidhart-Berard, E.M.; Penel, N.; Delcambre, C.; Peyro Saint Paul, L.; et al. Sorafenib in metastatic uveal melanoma: Efficacy, toxicity and health-related quality of life in a multicentre phase II study. Br. J. Cancer 2016, 115, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Tsubaki, M.; Kato, N.; Genno, S.; Ichimura, E.; Enomoto, A.; Imano, M.; Satou, T.; Nishida, S. Sorafenib treatment of metastatic melanoma with c-Kit aberration reduces tumor growth and promotes survival. Oncol. Lett. 2021, 22, 827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, D.S.; Kurzrock, R.; Wheler, J.J.; Naing, A.; Falchook, G.S.; Fu, S.; Kim, K.B.; Davies, M.A.; Nguyen, L.M.; George, G.C.; et al. Phase I Dose-Escalation Study of the Multikinase Inhibitor Lenvatinib in Patients with Advanced Solid Tumors and in an Expanded Cohort of Patients with Melanoma. Clin. Cancer Res. 2015, 21, 4801–4810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tran, T.T.; Caulfield, J.; Zhang, L.; Schoenfeld, D.; Djureinovic, D.; Chiang, V.L.; Oria, V.; Weiss, S.A.; Olino, K.; Jilaveanu, L.B.; et al. Lenvatinib or anti-VEGF in combination with anti-PD-1 differentially augments antitumor activity in melanoma. JCI Insight 2023, 8, e157347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeramian, A.; Sorolla, A.; Velasco, A.; Santacana, M.; Dolcet, X.; Valls, J.; Abal, L.; Moreno, S.; Egido, R.; Casanova, J.M.; et al. Inhibition of activated receptor tyrosine kinases by Sunitinib induces growth arrest and sensitizes melanoma cells to Bortezomib by blocking Akt pathway. Int. J. Cancer 2012, 130, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.D.M.; Guhan, S.; Tsao, H. KIT and Melanoma: Biological Insights and Clinical Implications. Yonsei Med. J. 2020, 61, 562–571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, F.; Li, Y.; Meng, Y.; Sun, H.; He, Y.; Yin, M.; Chen, X.; Deng, G. BET inhibitors synergize with sunitinib in melanoma through GDF15 suppression. Exp. Mol. Med. 2023, 55, 364–376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzato, G.; Ingravallo, G.; Ribatti, D. Angiogenesis Still Plays a Crucial Role in Human Melanoma Progression. Cancers 2024, 16, 1794. https://doi.org/10.3390/cancers16101794
Cazzato G, Ingravallo G, Ribatti D. Angiogenesis Still Plays a Crucial Role in Human Melanoma Progression. Cancers. 2024; 16(10):1794. https://doi.org/10.3390/cancers16101794
Chicago/Turabian StyleCazzato, Gerardo, Giuseppe Ingravallo, and Domenico Ribatti. 2024. "Angiogenesis Still Plays a Crucial Role in Human Melanoma Progression" Cancers 16, no. 10: 1794. https://doi.org/10.3390/cancers16101794
APA StyleCazzato, G., Ingravallo, G., & Ribatti, D. (2024). Angiogenesis Still Plays a Crucial Role in Human Melanoma Progression. Cancers, 16(10), 1794. https://doi.org/10.3390/cancers16101794







