Simple Summary
Head and neck cancer patients are frequently treated with primary chemoradiation, but response to therapy is hard to predict. In this study, we identified the expression of the SEC62 gene as a significant and independent predictor of patient outcome in a cohort of 127 head and neck cancer patients undergoing primary chemoradiation. Further significant prognostic factors indicating a significantly shortened overall and progression-free survival included response to therapy (RECIST1.1), lymph node metastases, distant metastases, tobacco consumption, recurrence of disease, and advanced clinical stage of disease. Together, SEC62 represents a promising and valid prognostic biomarker in this treatment setting of head and neck cancer. Its role in tumor cell biology and potential therapeutic strategies targeting SEC62 should be further investigated.
Abstract
Primary chemoradiotherapy (CRT) is an established treatment option for locally advanced head and neck squamous cell carcinomas (HNSCC) usually combining intensity modified radiotherapy with concurrent platinum-based chemotherapy. Though the majority of patients can be cured with this regimen, treatment response is highly heterogeneous and can hardly be predicted. SEC62 represents a metastasis stimulating oncogene that is frequently overexpressed in various cancer entities and is associated with poor outcome. Its role in HNSCC patients undergoing CRT has not been investigated so far. A total of 127 HNSCC patients treated with primary CRT were included in this study. The median follow-up was 5.4 years. Pretherapeutic tissue samples of the primary tumors were used for immunohistochemistry targeting SEC62. SEC62 expression, clinical and histopathological parameters, as well as patient outcome, were correlated in univariate and multivariate survival analyses. High SEC62 expression correlated with a significantly shorter overall survival (p = 0.015) and advanced lymph node metastases (p = 0.024). Further significant predictors of poor overall and progression-free survival included response to therapy (RECIST1.1), nodal status, distant metastases, tobacco consumption, recurrence of disease, and UICC stage. In a multivariate Cox hazard proportional regression analysis, only SEC62 expression (p = 0.046) and response to therapy (p < 0.0001) maintained statistical significance as independent predictors of the patients’ overall survival. This study identified SEC62 as an independent prognostic biomarker in HNSCC patients treated with primary CRT. The role of SEC62 as a potential therapeutic target and its interaction with radiation-induced molecular alterations in head and neck cancer cells should further be investigated.
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) represents the 8th most common cancer entity worldwide, accounting for more than 878,000 new cases and 444,000 deaths in 2020 [1]. Most patients are diagnosed in advanced stages of the disease, which limits therapeutic options and leads to a persistently poor five-year overall survival of approximately 60% [2]. For locally advanced (LA) HNSCC patients, primary chemoradiation is an established and frequently used therapeutic option, especially in advanced T stages and with other considerations that make surgery less preferable [2,3,4,5,6,7]. Here, intensity-modulated radiation therapy (IMRT) is combined with cisplatin, which has been shown to achieve three-year locoregional control rates of 55–80% in large-scale, prospective clinical trials [8,9,10,11,12]. However, the response to therapy is highly heterogeneous [5] with only a few established predictive biomarkers, including tumor human papillomavirus (HPV) status [13,14,15], tobacco consumption [13,15], tumor stage [13], nodal stage [13], and tumor hypoxia [16]. Hence, additional predictive and prognostic biomarkers are urgently needed for a more effective and individualized therapeutic management of LA-HNSCC patients.
The SEC62 gene, located on chromosomal region 3q26.2, encodes for an endoplasmic reticulum (ER) transmembrane protein that regulates the intracellular transport of secretory and transmembrane proteins [17,18], intracellular calcium homeostasis [19], and recovery from ER stress conditions via so called recovER-phagy [20]. Over the past several years, increasing evidence has suggested a relevant role of SEC62 in human cancer [21]. High SEC62 expression levels were associated with unfavorable prognosis in HNSCC [22], melanoma [23], breast cancer [24], and non-small cell lung cancer (NSCLC [25,26]), advanced lymph node metastasis in NSCLC [25], melanoma [23], gastric cancer [27], colorectal cancer [28], and HNSCC [22], as well as distant metastasis in breast cancer [24] and melanoma [23]. In hepatocellular carcinoma, high SEC62 expression correlated with a higher risk of tumor recurrence [29]. Recently, a pan-cancer analysis using DNA sequencing data from over 40,000 patients showed that SEC62 gene amplification represents a highly significant indicator of poor overall survival [21]. As potentially underlying molecular mechanisms, in vitro studies showed that SEC62 drives cancer metastasis by activating the MAPK/ATF2/UCA1 axis [28], mediating UPR-induced autophagy activation [27], and limiting calcium efflux across the ER membrane [19,26]. Furthermore, cancer cells benefit from high SEC62 expression levels through improved recovery from ER stress conditions which are not only induced by a high protein turnover due to elevated proliferation rate, but also by radiotherapy [30,31,32,33]. Together, these observations raise the question if SEC62-mediated ER stress tolerance also leads to an increased resistance to chemoradiotherapy (CRT). So far, SEC62 expression and its correlation with clinical parameters including therapy outcome were only investigated in HNSCC cohorts that were predominately treated with surgery [22] or in very small cohorts treated with CRT [34].
This study aimed to evaluate the influence of SEC62 expression and further clinical and histopathological parameters on treatment response and patient outcome in HNSCC patients treated with definitive chemoradiotherapy. SEC62 expression was further correlated with the patients’ clinical and histological characteristics to identify the potential effects on HNSCC biology.
2. Materials and Methods
2.1. Patients and Tissue Samples
In total, 127 HNSCC patients treated at the Department of Otorhinolaryngology, Head and Neck surgery and the Department of Radiation Oncology at the Saarland University Medical Center (Homburg, Germany) between October 2005 and August 2016 were included in this study. All the patients received primary concurrent chemoradiation (cisplatin 40 mg/m2 weekly or 100 mg/m2 three weekly + radiation with 63 to 70 Gy in 30 daily fractions). The median follow-up of the patients included in our study was 64.8 months (5.4 years). All patients gave their informed consent for their participation in our study. The study was conducted in accordance with the Declaration of Helsinki and all other relevant national ethical standards. The details on the patients’ clinical and histopathological data are shown in Table 1. The study was approved by the Saarland Medical Association ethics review board (reference number 280/10).

Table 1.
Clinical data of the included patients.
For immunohistochemical analyses, formalin-fixed paraffin-embedded (FFPE) tissue samples of the primary tumor that were taken during diagnostic panendoscopy prior to the start of treatment were obtained for all patients. Alcohol abuse was defined as the consumption of ≥200 g alcohol per week. Smoking behavior was categorized into four groups for further statistical analyses: lifelong non-smoker (group 1), current smoker (group 2), current reformed smoker for >15 years (group 3), and current reformed smoker for ≤15 years (group 4). The TNM and UICC stages were determined according to the 7th edition of the AJCC/UICC TNM staging system for head and neck cancers as all patients included in this study were treated before 2017.
2.2. Immunohistochemistry
For immunohistochemical staining targeting the proteins Sec62 and p16, FFPE tissue sections were used. First, 4 μm sections were prepared using a Leica RM 2235 microtome (Leica Microsystems, Wetzlar, Germany), transferred onto Superfrost Ultra Plus glass slides (Menzel-Gläser, Braunschweig, Germany), and dried overnight at 37 °C. After deparaffinization, the epitopes were unmasked by incubating the slides in a rice cooker with Tris-EDTA retrieval buffer (10 mM TRIS, I mM EDTA, pH 9). Afterwards, the unspecific protein binding sites were blocked by incubating the slides in PBS (phosphate-buffered saline, Sigma Aldrich, St. Louis, MO, USA) and bovine serum albumin (3% w/v; BSA, Sigma Aldrich, St. Louis, MO, USA) at pH 7.2 for 30 min at room temperature. In the next step, the slides were incubated with the primary antibody (1:1500 dilution for SEC62 and 1:4000 for p16 in 1% BSA/PBS; Anti-SEC62, clone EPR9213; ab140644, Abcam, Cambridge, UK; Anti-p16, clone 1D7D2A1; ab201980, Abcam, Cambridge, UK) for one hour at room temperature. For visualization, we used the Dako REAL detection system Alkaline Phosphatase (Dako Agilent Technologies, Glostrup, Denmark) according to the manufacturer’s instructions. Finally, the slides were counterstained with hematoxylin (Sigma Aldrich, St. Louis, MO, USA). Each immunohistochemical staining series included negative controls by omission of the primary antibody and positive controls by staining FFPE slides from a patient with an HPV-associated, SEC62 positive tonsil squamous cell carcinoma. SEC62-immunoreactivity was evaluated using the well-established immunoreactive score (IRS) by Remmele and Stegner [35] with IRS values ranging from a minimum of 0 (weak) to a maximum of 12 (strong). P16-immunoreactivity was valued as either negative or positive. All immunohistochemical stainings were valued by three experienced examiners, including one board certified histopathologist, and the mean IRS scores were finally used for statistical analyses.
2.3. Statistical Analysis
For statistical analyses, Prism 9 software (GraphPad Software, Boston, MA, USA) was used. To check the acquired data for Gaussian distribution, the Anderson–Darling test, D’Agostino and Pearson test, Shapiro–Wilk test, and Kolmogorov–Smirnov test were used. If the data passed ≥2 of the normality tests, parametric tests were used for statistical testing (unpaired t test with Welch’s correction). If the data did not pass ≥2 of the aforementioned normality tests, non-parametric tests were used (Mann–Whitney U test). Univariate survival analyses were performed using the Kaplan–Meier method and a log-rank test. For multivariate survival analyses, the Cox proportional hazard regression model was used. The fitting between the regression model and the analyzed data was evaluated using Akaike’s information criterion (AIC) and the Wald test. p values < 0.05 were considered statistically significant (α = 0.05) and are indicated in the figures. The tests that were used for statistical testing are indicated in the figure legends or the text, respectively.
3. Results
3.1. SEC62 Expression in HNSCC Patients and Correlation with Clinical Data as Well as Patient Outcome
First, the expression of 3q oncogene SEC62 was analyzed for all patients included in this study using immunohistochemical staining of pre-therapeutic FFPE tissue samples and quantified by an immunoreactive score (IRS, see Figure 1A,B). Thereby, 98 cases had a positive staining with a median IRS of 6.93 (2 patients with an IRS 2–3 resp. weak expression; 40 patients with an IRS 4–8 resp. moderate expression; 56 patients with an IRS 9–12 resp. strong expression) while only 29 patients were negative for SEC62 (IRS 0–1).
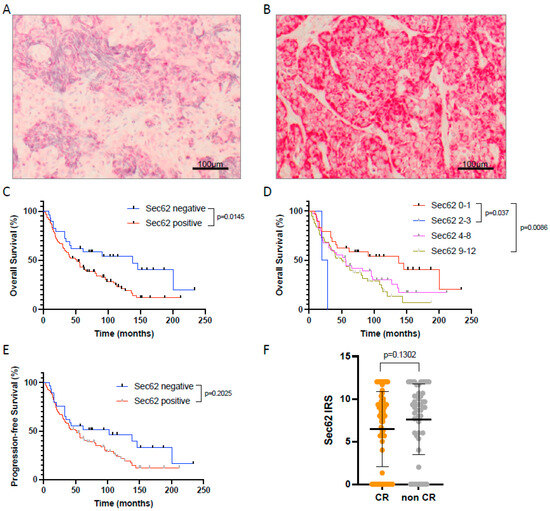
Figure 1.
SEC62 expression in HNSCC tissue samples and impact on patient outcome. (A,B) Examples of HNSCC tissue samples with weak (A) and strong (B) SEC62 expression. (C) Overall survival of patients with SEC62 positive (blue) vs. SEC62 negative (red) tumors. (D) Overall survival of patients with no (IRS 0–1, red), weak (IRS 2–3, blue), moderate (IRS 4–8, magenta), and strong SEC62 expression (IRS 9-12, green). (E) Progression-free survival (PFS) of patients with SEC62 positive (red) vs. SEC62 negative tumors (blue). (F) Comparison of SEC62 expression in patients with complete response (CR) to CRT vs. patients without complete response (non CR; median ± standard deviation is indicated by black lines). In (D), only p-values < 0.05 are shown. IRS—immunoreactive score.
When correlating SEC62 expression with the patients’ overall (OS) and progression-free survival (PFS), we found a significantly longer OS (p = 0.0145) and a trend towards prolonged PFS in Sec62 negative compared to SEC62 positive patients (Figure 1C,E). No difference in OS and PFS was observed between patients with weak, moderate, and strong SEC62 expression (Figure 1D). Regarding a potential correlation between SEC62 expression and response to treatment according to RECIST 1.1 criteria, no significant difference in SEC62 levels was seen between patients with complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD), with only a slight trend towards lower SEC62 levels in CR patients compared to non-CR patients (PR + SD + PD; Figure 1F).
In the next step, we correlated SEC62 expression levels on tumor cells with the patients’ clinical and histopathological data, including tumor size, nodal status, distant metastasis, tumor differentiation, tumor localization, p16 status, nicotine consumption, patient age, and gender (Figure 2). Here, higher SEC62 expression levels correlated significantly with advanced nodal metastasis (p = 0.0237 for N0 vs. N3; p = 0.0491 for N2 vs. N3), p16 positivity (p = 0.0004), and chronic tobacco exposure (p = 0.0285 for smoking group 2 vs. 3; p = 0.0259 for smoking group 3 vs. 4). When comparing clinical and histopathological characteristics between SEC62 positive (n = 98) and SEC62 negative patients (n = 29), we found that only SEC62 positive patients showed distant metastases (n = 10) as well as UICC stage IV disease (n = 13). Additionally, only SEC62 positive patients showed p16 positive tumor cells in 20.4% of cases, while no SEC62 negative patient was p16 positive (see Supplementary Table S1).
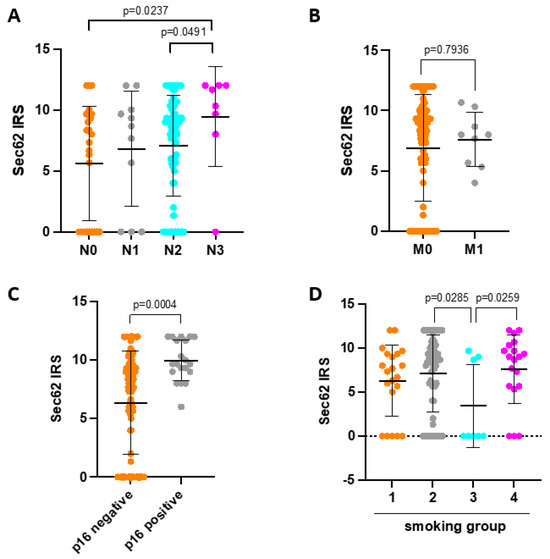
Figure 2.
Correlation of SEC62 expression with clinical and histopathological data. (A) SEC62 expression in patients with N0, N1, N2, and N3 nodal status. (B) SEC62 expression in patients with (M1) and without (M0) distant metastasis. (C) SEC62 expression in patients with p16 negative vs. p16 positive tumors. (D) SEC62 expression depending on nicotine exposure (smoking category 1—never smoker; smoking category 2—current smoker; smoking category 3—reformed smoker ≥ 15 years; smoking category 4—reformed smoker < 15 years). In (A–D), the median ± standard deviation are indicated by black horizontal lines. In (A,D), only p-values < 0.05 are indicated. IRS—immunoreactive score.
3.2. Prognostic Factors Influencing Overall Survival of HNSCC Patients Undergoing Primary CRT
To identify further factors that have a significant influence on patient outcome in our cohort, we first performed univariate survival analyses using the Kaplan–Meier Method. Thereby, the treatment response according to RECIST 1.1 criteria (p < 0.0001 for CR vs. non-CR), absence of tumor recurrence (p = 0.0079), chronic tobacco exposure (p = 0.0148 for smoking group 1 + 3 vs. smoking group 2 + 4), advanced UICC stages (p = 0.0106 for UICC 1 + 2 vs. UICC3 + 4), negative nodal status (p = 0.0079), and absence of distant metastasis (p = 0.0029) significantly correlated with a prolonged overall survival (Figure 3).
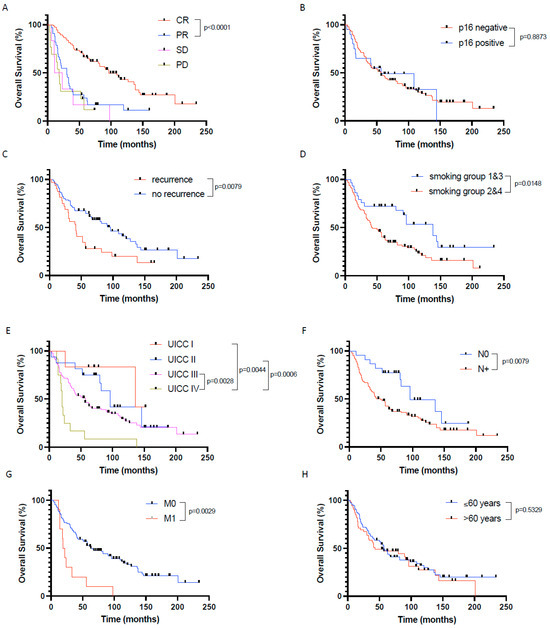
Figure 3.
Prognostic factors significantly influencing patients’ overall survival in the investigated primary CRT cohort of HNSCC patients. Overall survival depending on (A) response to chemoradiation defined by RECIST1.1, (B) p16 expression, (C) recurrence vs. no recurrence, (D) nicotine exposure, (E) UICC stages, (F) nodal status, (G) distant metastasis, and (H) age. CR—complete remission, PR—partial remission, SD—stable disease, PD—progressive disease. In (A,E), only p-values < 0.05 are indicated.
Overall survival was not affected by p16 status (neither in the whole patient cohort (n = 127) nor in the oropharyngeal cancer only patient cohort (n = 55)), chronic alcohol consumption, tumor size, tumor localization, tumor differentiation, and patient age and gender.
3.3. Prognostic Factors influencing Progression-Free Survival of HNSCC Patients Undergoing Primary Crt
When correlating the patients’ clinical and histopathological data with progression-free survival (PFS), we identified the presence of distant metastases (p = 0.0112), chronic tobacco exposure (p = 0.0231), positive nodal status (p = 0.0018), tumor recurrence (p = 0.0038), poor response to therapy (p < 0.0001 for non-CR vs. CR), and advanced UICC stages (p = 0.0128 for UICC 3 + 4 vs. UICC 1 + 2) as indicators for a significantly shortened PFS (Figure 4).
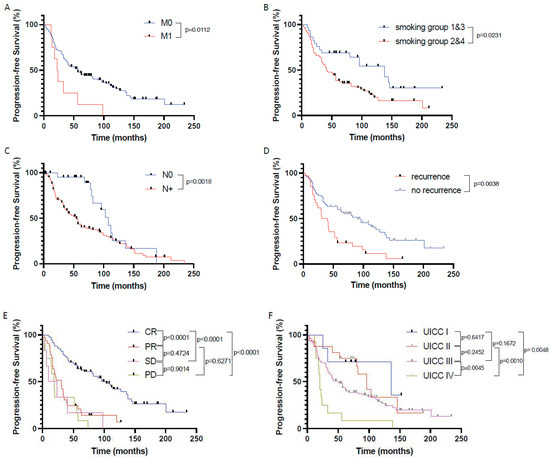
Figure 4.
Prognostic factors significantly influencing patients’ progression-free survival in the investigated primary CRT cohort of HNSCC patients. Progression-free survival in patients (A) with (M1) and without (M0) distant metastasis, (B) depending on nicotine exposure, (C) depending on nodal status, (D) depending on tumor recurrence, (E) depending on response to CRT according to RECIST1.1, and (F) depending on UICC stages. CR—complete remission, PR—partial remission, SD—stable disease, PD—progressive disease.
PFS was not influenced by p16 status (neither in the whole HNSCC cohort nor in the oropharyngeal cancer only cohort), patient age and gender, chronic alcohol consumption, tumor size, tumor localization, and tumor differentiation.
3.4. Identification of Independent Prognostic Factors in the Primary CRT Cohort
In a next step, we performed multivariate statistical testing using the Cox proportional hazard regression method to delineate the independence of those prognostic factors that significantly correlated with overall survival in univariate log-rank testing (see Table 2): N stage (N+ vs. N0), M stage (M1 vs. M0), UICC stage (1 + 2 vs. 3 + 4), response to therapy according to RECIST1.1 (CR vs. non-CR), tumor recurrence (yes vs. no), SEC62 expression (positive vs. negative), and chronic tobacco exposure (group 2 + 4 vs. group 1 + 3). An AIC analysis (666.5 vs. 695.9) and Wald test (p < 0.0001) showed that our regression model was appropriate to describe the observed data.

Table 2.
Multivariate analysis of prognostic factors influencing overall survival using Cox proportional hazard regression.
Here, only treatment response (non-CR vs. CR; HR 2.723; 95% CI 1.709–4.354; p < 0.0001) and SEC62 expression (positive vs. negative; HR 1.79; 95% CI 1.035–3.276; p = 0.0462) proved to be independent prognostic factors in our study cohort, as shown in Table 2 As p16 is already known from the literature to represent a clinically established prognostic biomarker in HNSCC treated with primary CRT, we also included p16 expression (positive vs. negative) as covariate in our regression model but, again, achieved no statistically significant prognostic relevance (HR 1.044; 95% CI 0.523–1.926; p = 0.8964).
4. Discussion
In our study, we used FFPE tissue samples from n = 127 HNSCC patients treated with primary chemoradiation in order to investigate the potential relevance of 3q oncogene SEC62 as an indicator of patient outcome and response to therapy. We found that SEC62 positivity of tumor cells in pre-therapeutic samples was a significant and independent prognostic factor indicating a shorter overall survival and significantly correlated with advanced lymph node metastases, p16 positivity, and chronic tobacco exposure. The treatment response according to RECISTS1.1 proved to be the only other significant and independent predictor of overall survival in our study cohort. Further significant, but not independent, prognostic factors included nodal status, distant metastasis, UICC stage, tumor recurrence, and tobacco exposure. Together, this study underlines the high prognostic relevance of the SEC62 oncogene in HNSCC undergoing definitive chemoradiotherapy.
Regarding its role as an adverse prognostic biomarker, the results of our study are in line with previous reports that showed a correlation of high tumoral SEC62 expression with significantly shorter overall survival in HNSCC [22], melanoma [23], breast cancer [24], and NSCLC [25,26]. Comparably, a correlation between elevated SEC62 expression and the presence of lymph node metastases, as shown in our study (see Figure 2A), has also been reported in an earlier study of our group that investigated SEC62 and SOX2 expression in a cohort of 65 HNSCC patients and 29 cancer of unknown primary (CUP) patients undergoing surgery [22]. Here, higher SEC62 expression levels were observed in lymph node metastases compared with the primary tumor in HNSCC patients, as well as in lymph node metastases from CUP patients compared to lymph node metastases from HNSCC patients. Additionally, SEC62 expression showed a stepwise increase from N1 to N3 metastases. A correlation between higher SEC62 expression and advanced lymph node metastases has also been reported for NSCLC [25], melanoma [23], colorectal cancer [28], and gastric cancer [27]. Together, these data emphasize the metastasis stimulating effect of high SEC62 expression levels that has mechanistically been linked to the MAPK/ATF2/UCA1 axis [28], cellular calcium homeostasis [19], and UPR-induced autophagy activation [27]. Importantly, when comparing SEC62 positive with SEC62 negative patients in our study, we found that only SEC62 positive patients showed distant metastases, as well as UICC stage IV disease, which might have contributed to the worse outcome of the SEC62 positive group and underlines the aforementioned relevance of SEC62 for the molecular process of metastasis formation. Additionally, high SEC62 expression correlated with increased tobacco exposure as well as p16 positivity in our study cohort, which can hardly be explained from a molecular background and necessitates further hypothesis-generating investigations.
With respect to potential molecular mechanisms that can explain how elevated SEC62 expression mediates resistance to chemoradiation associated with poor prognosis, one can hypothesize that an increased ER stress tolerance mediated by SEC62 might counteract the therapeutic effects of CRT in head and neck cancer cells. Several studies in recent years reported that radiotherapy induces cellular ER stress, which represents one minor of the many molecular mechanisms how radiation exerts its therapeutic effects [30,31,32,33]. As it was shown that high SEC62 expression levels allow human cancer cells to better compensate ER stress conditions, HNSCCs might tolerate higher radiation doses once they overexpress SEC62 [26]. Additionally, it was shown that SEC62 promotes stemness and chemoresistance of human colorectal cancer through activating the Wnt/ß-catenin pathway, which represents a potential mechanism of SEC62-mediated resistance in human cancer cells to chemotherapy [36]. Regarding a potential use of SEC62 as a therapeutic target in HNSCC, Körner et al. have shown that antagonizing SEC62 function by a combined treatment with Calmodulin antagonist Trifluoperazin (TFP) and SERCA inhibitor Thapsigargin (TG) can sufficiently suppress lymphatic metastasis of HNSCC in vivo using an orthotopic xenograft head and neck cancer model [19]. In another study, a combination of TFP and TG markedly suppressed the growth of subcutaneously injected HNSCC cells in vivo [37]. The first clinical trials that investigated comparable therapeutic strategies in humans showed promising results that need to be confirmed by phase II and III trials [38]. Other potential strategies to inhibit SEC62 function in human cancer cells include the use of autophagy inhibitors such as hydroxychloroquine [27].
From a critical point of view, one has to wonder why p16 as an already established prognostic and predictive biomarker in oropharyngeal cancer did not show any significant correlation with patient outcome or with treatment response. Furthermore, after adjusting TNM and UICC stages to the 8th version of the AJCC staging system, no significant differences in outcome were seen. It would have been expected that a positive HPV status is associated with prolonged overall and progression-free survival, as well as improved treatment response, as it was shown in large-scale, prospective, randomized clinical trials [13,14]. One potential explanation for this discrepancy might be the comparatively low number of only 20 cases with p16 positive tumors, which hampers valid conclusions on the prognostic relevance of p16 status. Furthermore, it has to be mentioned that 90% (18/20) of the p16 positive HNSCC cases were diagnosed at UICC stages III and IV, in contrast to only 81% (87/107) of the p16 negative cases, which represents another potential bias. Additionally, p16 positive cases showed significantly higher SEC62 expression levels compared to p16 negative samples in our study cohort, which might have counteracted the well-known beneficial effects of HPV-associated tumor cell biology in terms of treatment response. Nonetheless, when adjusting survival analyses for UICC stages, as well as SEC62 expression as covariates, p16 did still not correlate with patient outcome; therefore, eventually we can only speculate why we found no prognostic significance of p16. Additionally, therapeutic protocols did not exactly match between all included cases with small differences regarding radiation doses (63 to 70 Gy in the primary tumor region) and cisplatin regimen (100 m2 every three weeks vs. 40 mg/m2 weekly), which might impair comparability between study subjects. However, we decided to exclude all patients that received a cumulative radiation dose of less than 60 Gy in the primary tumor region to delimitate insufficient radiation dose as a potential bias for the survival analyses. Furthermore, the data from clinical trials have shown that both of the aforementioned cisplatin regimens do not significantly differ in terms of patient outcome when combined with radiotherapy for primary or adjuvant treatment of locally advanced HNSCC [39,40]. Finally, the fact that we used pretherapeutic tumor biopsies for immunohistochemical SEC62 staining might lead to a sampling bias as we could not take into account a potential intra-tumoral heterogeneity of SEC62 expression. Nonetheless, SEC62 expression showed a very homogeneous expression pattern within tumors in previous studies of our group, including also HNSCC samples [22,41].
5. Conclusions
Taken together, we have shown in our study that SEC62 represents an independent prognostic biomarker in HNSCC patients treated with primary chemoradiotherapy with SEC62 overexpression correlating with a significantly shorter overall survival and advanced lymph node metastases. These results motivate further investigations focusing on the role of SEC62 as a potential therapeutic target in head and neck cancer and its interaction with radiation-induced molecular alterations in order to overcome treatment resistance to definitive CRT.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16010098/s1, Table S1: Clinical and histopathological characteristics of SEC62 positive vs. SEC62 negative patients.
Author Contributions
Conceptualization, M.L., M.H., J.P.K. and B.S.; methodology, M.L, M.S., S.K. and M.K.; software, M.L., L.A.B. and J.P.K.; validation, F.L.B., S.W. and M.W.; formal analysis, M.L. and M.K.; investigation, M.S.; resources, M.L.; data curation, M.L, M.S. and J.P.K.; writing—original draft preparation, M.L. and J.P.K.; writing—review and editing, M.L., M.S., S.K., M.K., L.A.B., F.L.B., S.W., M.W., M.H., B.S. and J.P.K.; visualization, M.L. and J.P.K.; supervision, M.L., M.H. and B.S.; project administration, M.L.; funding acquisition, M.L. and J.P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by an Else Kröner-Fresenius-Stiftung (EKFS) grant to ML (2022EKEA.21) and a HOMFOR grant to JPK.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Saarland Medical Association ethics review board (index number 280/10).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
The excellent technical assistance of Carolin Bick, Ulrike Bechtel, and Barbara Linxweiler is gratefully acknowledged. We acknowledge support by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) and Saarland University within the Open Access Publication Funding program.
Conflicts of Interest
M.L. reports a conflict of interest with MSD (advisory role, speakers’ bureau, honoraria, travel expenses) and Karl Storz SE and Co. KG (advisory role, speakers’ bureau, honoraria, travel expenses). M.H. reports a conflict of interest with Merck (advisory role, speakers’ bureau, honoraria, travel expenses, research funding); MSD (advisory role, speakers’ bureau, honoraria, travel expenses, research funding); AstraZeneca (speakers’ bureau, honoraria, travel expenses, research funding); Sanofi (advisory role, speakers’ bureau, honoraria); Novartis (research funding); BMS (advisory role, honoraria, speakers’ bureau); Teva (travel expenses). All other authors declare no competing interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Bauml, J.M.; Vinnakota, R.; Park, Y.H.A.; Bates, S.E.; Fojo, T.; Aggarwal, C.; Limaye, S.; Damjanov, N.; Di Stefano, J.; Ciunci, C.; et al. Cisplatin Every 3 Weeks Versus Weekly with Definitive Concurrent Radiotherapy for Squamous Cell Carcinoma of the Head and Neck. J. Natl. Cancer Inst. 2019, 111, 490–497. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, I.; Nardone, V.; Reginelli, A.; Cappabianca, S. Chemoradiotherapy for Head and Neck Cancer. Cancers 2023, 15, 2820. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; le Maître, A.; Maillard, E.; Bourhis, J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Lacas, B.; Carmel, A.; Landais, C.; Wong, S.J.; Licitra, L.; Tobias, J.S.; Burtness, B.; Ghi, M.G.; Cohen, E.E.W.; Grau, C.; et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother. Oncol. 2021, 156, 281–293. [Google Scholar] [CrossRef]
- Schüttrumpf, L.; Marschner, S.; Scheu, K.; Hess, J.; Rietzler, S.; Walch, A.; Baumeister, P.; Kirchner, T.; Ganswindt, U.; Zitzelsberger, H.; et al. Definitive chemoradiotherapy in patients with squamous cell cancers of the head and neck—Results from an unselected cohort of the clinical cooperation group "Personalized Radiotherapy in Head and Neck Cancer". Radiat. Oncol. 2020, 15, 7. [Google Scholar] [CrossRef]
- Bourhis, J.; Sire, C.; Graff, P.; Grégoire, V.; Maingon, P.; Calais, G.; Gery, B.; Martin, L.; Alfonsi, M.; Desprez, P.; et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): An open-label phase 3 randomised trial. Lancet Oncol. 2012, 13, 145–153. [Google Scholar] [CrossRef]
- Budach, V.; Stuschke, M.; Budach, W.; Baumann, M.; Geismar, D.; Grabenbauer, G.; Lammert, I.; Jahnke, K.; Stueben, G.; Herrmann, T.; et al. Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: Final results of the radiotherapy cooperative clinical trials group of the German Cancer Society 95-06 Prospective Randomized Trial. J. Clin. Oncol. 2005, 23, 1125–1135. [Google Scholar] [CrossRef]
- Noronha, V.; Joshi, A.; Patil, V.M.; Agarwal, J.; Ghosh-Laskar, S.; Budrukkar, A.; Murthy, V.; Gupta, T.; D’Cruz, A.K.; Banavali, S.; et al. Once-a-Week Versus Once-Every-3-Weeks Cisplatin Chemoradiation for Locally Advanced Head and Neck Cancer: A Phase III Randomized Noninferiority Trial. J. Clin. Oncol. 2018, 36, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Fietkau, R.; Hecht, M.; Hofner, B.; Lubgan, D.; Iro, H.; Gefeller, O.; Rödel, C.; Hautmann, M.G.; Kölbl, O.; Salay, A.; et al. Randomized phase-III-trial of concurrent chemoradiation for locally advanced head and neck cancer comparing dose reduced radiotherapy with paclitaxel/cisplatin to standard radiotherapy with fluorouracil/cisplatin: The PacCis-trial. Radiother. Oncol. 2020, 144, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Huang, S.H.; Siu, L.L.; Waldron, J.; Zhao, H.; Perez-Ordonez, B.; Weinreb, I.; Kim, J.; Ringash, J.; Bayley, A.; et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J. Clin. Oncol. 2013, 31, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Rühle, A.; Grosu, A.L.; Wiedenmann, N.; Stoian, R.; Haehl, E.; Zamboglou, C.; Baltas, D.; Werner, M.; Kayser, G.; Nicolay, N.H. Immunohistochemistry-based hypoxia-immune prognostic classifier for head-and-neck cancer patients undergoing chemoradiation—Post-hoc analysis from a prospective imaging trial. Radiother. Oncol. 2021, 159, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Lakkaraju, A.K.; Thankappan, R.; Mary, C.; Garrison, J.L.; Taunton, J.; Strub, K. Efficient secretion of small proteins in mammalian cells relies on Sec62-dependent posttranslational translocation. Mol. Biol. Cell 2012, 23, 2712–2722. [Google Scholar] [CrossRef]
- Lang, S.; Benedix, J.; Fedeles, S.V.; Schorr, S.; Schirra, C.; Schäuble, N.; Jalal, C.; Greiner, M.; Hassdenteufel, S.; Tatzelt, J.; et al. Different effects of Sec61α, Sec62 and Sec63 depletion on transport of polypeptides into the endoplasmic reticulum of mammalian cells. J. Cell Sci. 2012, 125, 1958–1969. [Google Scholar] [CrossRef]
- Körner, S.; Pick, T.; Bochen, F.; Wemmert, S.; Körbel, C.; Menger, M.D.; Cavalié, A.; Kühn, J.P.; Schick, B.; Linxweiler, M. Antagonizing Sec62 function in intracellular Ca(2+) homeostasis represents a novel therapeutic strategy for head and neck cancer. Front. Physiol. 2022, 13, 880004. [Google Scholar] [CrossRef]
- Fumagalli, F.; Noack, J.; Bergmann, T.J.; Cebollero, E.; Pisoni, G.B.; Fasana, E.; Fregno, I.; Galli, C.; Loi, M.; Soldà, T.; et al. Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat. Cell Biol. 2016, 18, 1173–1184. [Google Scholar] [CrossRef]
- Sicking, M.; Lang, S.; Bochen, F.; Roos, A.; Drenth, J.P.H.; Zakaria, M.; Zimmermann, R.; Linxweiler, M. Complexity and Specificity of Sec61-Channelopathies: Human Diseases Affecting Gating of the Sec61 Complex. Cells 2021, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Bochen, F.; Adisurya, H.; Wemmert, S.; Lerner, C.; Greiner, M.; Zimmermann, R.; Hasenfus, A.; Wagner, M.; Smola, S.; Pfuhl, T.; et al. Effect of 3q oncogenes SEC62 and SOX2 on lymphatic metastasis and clinical outcome of head and neck squamous cell carcinomas. Oncotarget 2017, 8, 4922–4934. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.S.L.; Pföhler, C.; Wahl, M.; Bochen, F.; Körner, S.; Kühn, J.P.; Bozzato, A.; Schick, B.; Linxweiler, M. Expression of SEC62 Oncogene in Benign, Malignant and Borderline Melanocytic Tumors-Unmasking the Wolf in Sheep’s Clothing? Cancers 2021, 13, 1645. [Google Scholar] [CrossRef] [PubMed]
- Takacs, F.Z.; Radosa, J.C.; Linxweiler, M.; Kasoha, M.; Bohle, R.M.; Bochen, F.; Unger, C.; Solomayer, E.F.; Schick, B.; Juhasz-Böss, I. Identification of 3q oncogene SEC62 as a marker for distant metastasis and poor clinical outcome in invasive ductal breast cancer. Arch. Gynecol. Obstet. 2019, 299, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Linxweiler, M.; Linxweiler, J.; Barth, M.; Benedix, J.; Jung, V.; Kim, Y.J.; Bohle, R.M.; Zimmermann, R.; Greiner, M. Sec62 bridges the gap from 3q amplification to molecular cell biology in non-small cell lung cancer. Am. J. Pathol. 2012, 180, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Linxweiler, M.; Schorr, S.; Schäuble, N.; Jung, M.; Linxweiler, J.; Langer, F.; Schäfers, H.J.; Cavalié, A.; Zimmermann, R.; Greiner, M. Targeting cell migration and the endoplasmic reticulum stress response with calmodulin antagonists: A clinically tested small molecule phenocopy of SEC62 gene silencing in human tumor cells. BMC Cancer 2013, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Shi, Y.T.; Chu, Y.; Jiang, M.Z.; Wu, N.; Xu, B.; Zhou, H.; Lin, J.C.; Jin, Y.R.; Li, X.F.; et al. Sec62 promotes gastric cancer metastasis through mediating UPR-induced autophagy activation. Cell Mol. Life Sci. 2022, 79, 133. [Google Scholar] [CrossRef]
- Jin, Y.; Han, Y.; Yang, S.; Cao, J.; Jiang, M.; Liang, J. Endoplasmic reticulum-resident protein Sec62 drives colorectal cancer metastasis via MAPK/ATF2/UCA1 axis. Cell Prolif. 2022, 55, e13253. [Google Scholar] [CrossRef]
- Weng, L.; Du, J.; Zhou, Q.; Cheng, B.; Li, J.; Zhang, D.; Ling, C. Identification of cyclin B1 and Sec62 as biomarkers for recurrence in patients with HBV-related hepatocellular carcinoma after surgical resection. Mol. Cancer 2012, 11, 39. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef]
- Dadey, D.Y.; Kapoor, V.; Khudanyan, A.; Urano, F.; Kim, A.H.; Thotala, D.; Hallahan, D.E. The ATF6 pathway of the ER stress response contributes to enhanced viability in glioblastoma. Oncotarget 2016, 7, 2080–2092. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, E.; Kim, W.; Seong, K.M.; Youn, H.; Kim, J.W.; Kim, J.; Youn, B. Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines. J. Biol. Chem. 2013, 288, 27343–27357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Pang, X.; Su, Y.; Ai, G.; Wang, T. ER stress induced by ionising radiation in IEC-6 cells. Int. J. Radiat. Biol. 2010, 86, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Wemmert, S.; Lindner, Y.; Linxweiler, J.; Wagenpfeil, S.; Bohle, R.; Niewald, M.; Schick, B. Initial evidence for Sec62 as a prognostic marker in advanced head and neck squamous cell carcinoma. Oncol. Lett. 2016, 11, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]
- Liu, X.; Su, K.; Sun, X.; Jiang, Y.; Wang, L.; Hu, C.; Zhang, C.; Lu, M.; Du, X.; Xing, B. Sec62 promotes stemness and chemoresistance of human colorectal cancer through activating Wnt/β-catenin pathway. J. Exp. Clin. Cancer Res. 2021, 40, 132. [Google Scholar] [CrossRef]
- Körbel, C.; Linxweiler, M.; Bochen, F.; Wemmert, S.; Schick, B.; Meyer, M.; Maurer, H.; Menger, M.D.; Zimmermann, R.; Greiner, M. Treatment of SEC62 over-expressing tumors by Thapsigargin and Trifluoperazine. Biomol. Concepts 2018, 9, 53–63. [Google Scholar] [CrossRef]
- Mahalingam, D.; Wilding, G.; Denmeade, S.; Sarantopoulas, J.; Cosgrove, D.; Cetnar, J.; Azad, N.; Bruce, J.; Kurman, M.; Allgood, V.E.; et al. Mipsagargin, a novel thapsigargin-based PSMA-activated prodrug: Results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumours. Br. J. Cancer 2016, 114, 986–994. [Google Scholar] [CrossRef]
- Gupta, T.; Kannan, S.; Ghosh-Laskar, S.; Agarwal, J.P. Concurrent chemoradiotherapy with cisplatin given once-a-week versus every-three weekly in head and neck squamous cell carcinoma: Non-inferior, equivalent, or superior? Oral. Oncol. 2022, 134, 106130. [Google Scholar] [CrossRef]
- Kiyota, N.; Tahara, M.; Mizusawa, J.; Kodaira, T.; Fujii, H.; Yamazaki, T.; Mitani, H.; Iwae, S.; Fujimoto, Y.; Onozawa, Y.; et al. Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. J. Clin. Oncol. 2022, 40, 1980–1990. [Google Scholar] [CrossRef]
- Kühn, J.P.; Speicher, S.; Linxweiler, B.; Körner, S.; Rimbach, H.; Wagner, M.; Solomayer, E.F.; Schick, B.; Linxweiler, M. Dual Sec62/Ki67 immunocytochemistry of liquid-based cytological preparations represents a highly valid biomarker for non-invasive detection of head and neck squamous cell carcinomas. Cytopathology 2023, 35, 113–121. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).