Association between Environmental Temperature and Survival in Gastroesophageal Cancers: A Population Based Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Population
2.2. Statistical Analysis
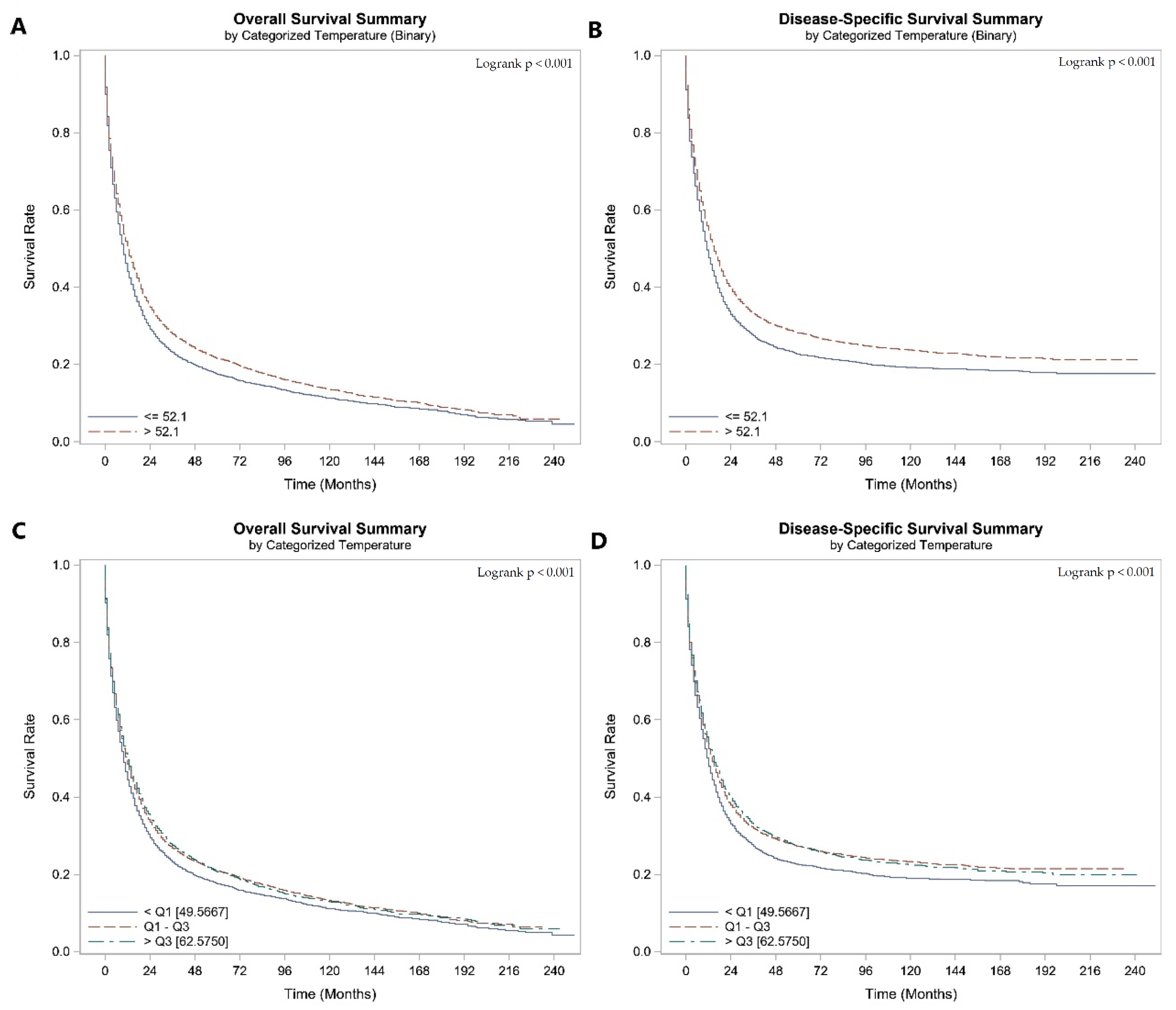
3. Results
- A.
- Patients with esophageal cancer
- 1.
- Characteristics of the included patients
- 2.
- Impact of AAT at diagnosis on survival outcomes
- B.
- Gastric cancer patients
- 1.
- Characteristics of the included patients
- 2.
- Impact of AAT at Diagnosis on survival outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Stomach (Gastric) Cancer Survival Rates. Available online: https://www.cancer.org/cancer/stomach-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 24 December 2022).
- Survival Rates for Esophageal Cancer|Esophageal Cancer Outlook. Available online: https://www.cancer.org/cancer/esophagus-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 24 December 2022).
- Jim, M.A.; Pinheiro, P.S.; Carreira, H.; Espey, D.K.; Wiggins, C.L.; Weir, H.K. Stomach Cancer Survival in the United States by Race and Stage (2001–2009): Findings from the CONCORD-2 Study. Cancer 2017, 123 (Suppl. 24), 4994. [Google Scholar] [CrossRef] [PubMed]
- Napier, K.J.; Scheerer, M.; Misra, S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J. Gastrointest. Oncol. 2014, 6, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, J.; Kim, T.; Jeong, W.; Park, E.C. Association between gastric cancer and the risk of depression among South Korean adults. BMC Psychiatry 2022, 22, 207. [Google Scholar] [CrossRef]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal Cancer. Nat. Rev. Dis. Prim. 2017, 3, 17048. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713. [Google Scholar] [CrossRef]
- Bae, J.M.; Kim, E.H. Epstein-Barr Virus and Gastric Cancer Risk: A Meta-analysis With Meta-regression of Case-control Studies. J. Prev. Med. Public Health 2016, 49, 97. [Google Scholar] [CrossRef]
- Park, J.-M.; Ryu, W.-S.; Kim, J.-H.; Park, S.-S.; Kim, S.-J.; Kim, C.-S.; Mok, Y.-J. Prognostic Factors for Advanced Gastric Cancer: Stage-stratified Analysis of Patients who Underwent Curative Resection. Cancer Res. Treat. 2006, 38, 13. [Google Scholar] [CrossRef]
- Jiang, N.; Ge, X.-L.; Zhang, Z.-Y.; Liu, J.; Wang, P.-P.; Sun, X.-C.; Yang, M. Prognostic factors for patients with esophageal cancer receiving definitive radiotherapy alone: A retrospective analysis. Cancer Manag. Res. 2021, 13, 3229–3234. [Google Scholar] [CrossRef]
- Kulig, P.; Nowakowski, P.; Sierzęga, M.; Pach, R.; Majewska, O.; Markiewicz, A.; Kołodziejczyk, P.; Kulig, J.; Richter, P. Analysis of Prognostic Factors Affecting Short-term and Long-term Outcomes of Gastric Cancer Resection. Anticancer Res. 2021, 41, 3523–3534. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.L.; Yu, S.J. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J. Surg. 2018, 41, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Chitti, B.; Pham, A.; Marcott, S.; Wang, X.; Potters, L.; Wernicke, A.G.; Parashar, B. Temporal Changes in Esophageal Cancer Mortality by Geographic Region: A Population-based Analysis. Cureus 2018, 10, e3596. [Google Scholar] [CrossRef] [PubMed]
- Rana, N.; Gosain, R.; Lemini, R.; Wang, C.; Gabriel, E.; Mohammed, T.; Siromoni, B.; Mukherjee, S. Socio-Demographic Disparities in Gastric Adenocarcinoma: A Population-Based Study. Cancers 2020, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, S.; Ning, B.; Huang, T.; Li, Y.; Wei, Y. Stress and cancer: The mechanisms of immune dysregulation and management. Front. Immunol. 2022, 13, 1032294. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.W.-L.; Reed, C.B.; Kokolus, K.M.; Pitoniak, R.; Utley, A.; Bucsek, M.J.; Ma, W.W.; Repasky, E.A.; Hylander, B.L. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β2-adrenergic receptor activation. Nat. Commun. 2015, 6, 6426. [Google Scholar] [CrossRef]
- MacDonald, C.; Ministero, S.; Pandey, M.; Robinson, D.; Hong, E.F.; Hylander, B.; McCarthy, P.; Gordon, C.; Repasky, E.; Mohammadpour, H. Comparing thermal stress reduction strategies that influence MDSC accumulation in tumor bearing mice. Cell Immunol. 2021, 361, 104285. [Google Scholar] [CrossRef]
- Vialard, F.; Olivier, M. Thermoneutrality and Immunity: How Does Cold Stress. Affect. Disease? Front. Immunol. 2020, 11, 3031. [Google Scholar] [CrossRef]
- Messmer, M.N.; Kokolus, K.M.; Eng, J.W.L.; Abrams, S.I.; Repasky, E.A. Mild cold-stress depresses immune responses: Implications for cancer models involving laboratory mice. Bioessays 2014, 36, 884. [Google Scholar] [CrossRef]
- Eckerling, A.; Ricon-Becker, I.; Sorski, L.; Sandbank, E.; Ben-Eliyahu, S. Stress and cancer: Mechanisms, significance and future directions. Nat. Rev. Cancer 2021, 21, 767–785. [Google Scholar] [CrossRef]
- Kokolus, K.M.; Capitano, M.L.; Lee, C.T.; Eng, J.W.; Waight, J.D.; Hylander, B.L.; Sexton, S.; Hong, C.C.; Gordon, C.J.; Abrams, S.I.; et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc. Natl. Acad. Sci. USA 2013, 110, 20176–20181. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, H.; MacDonald, C.R.; McCarthy, P.L.; Abrams, S.I.; Repasky, E.A. β2-adrenergic receptor signaling regulates metabolic pathways critical to myeloid-derived suppressor cell function within the TME. Cell Rep. 2021, 37, 109883. [Google Scholar] [CrossRef] [PubMed]
- Voskarides, K. The “cancer–cold” hypothesis and possible extensions for the Nordic populations. Scand. J. Public Health 2019, 47, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Rieger, R.H.; Pan, L.X. Precipitation and Climate Zone Explains the Geographical Disparity in the Invasive Cancer Incidence Rates in the United States. Environ. Eng. Sci. 2022, 36, 1452–1458. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, T.; Panwar, M.S.; Sharma, D.; Bundel, R.; Hamilton, R.T.; A Radosevich, J.; Mandal, C.C. Colder environments are associated with a greater cancer incidence in the female population of the United States. Tumour Biol. 2017, 39, 1010428317724784. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, H.K.; Joshi, S.; Panwar, M.S.; Mandal, C.C. A link between cold environment and cancer. Tumour Biol. 2015, 36, 5953–5964. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Attwood, K.; Gupta, K.; Gandhi, A.; Edge, S.; Takabe, K.; Gandhi, S. CLO22-051: Influence of Environmental Temperature on Pathological Complete Response and Overall Survival in Breast Cancer: A National Cancer Database Population-Based Study. J. Natl. Compr. Cancer Netw. 2022, 20, CLO22-051. [Google Scholar] [CrossRef]
- NCEI. National Centers for Environmental Information (NCEI). Monthly Climate Tables. 2019. Available online: https://www.ncei.noaa.gov/ (accessed on 28 June 2022).
- Gandhi, S.; Oshi, M.; Murthy, V.; Repasky, E.A.; Takabe, K. Enhanced thermogenesis in triple-negative breast cancer is associated with pro-tumor immune microenvironment. Cancers 2021, 13, 2559. [Google Scholar] [CrossRef]
- Wang, L.; Cai, M.; Song, Y.; Bai, J.; Sun, W.; Yu, J.; Du, S.; Lu, J.; Fu, S. Multidimensional difference analysis in gastric cancer patients between high and low latitude. Front. Genet. 2022, 13, 944492. [Google Scholar] [CrossRef]
- Saini, R.; Singh, A.K.; Dhanapal, S.; Saeed, T.H.; Hyde, G.J.; Baskar, R. Brief temperature stress during reproductive stages alters meiotic recombination and somatic mutation rates in the progeny of Arabidopsis. BMC Plant Biol. 2017, 17, 103. [Google Scholar] [CrossRef]
- Kach, J.; Conzen, S.D.; Szmulewitz, R.Z. Glucocorticoid receptor signaling in breast and prostate cancers: Emergence as a therapeutic target. Sci. Transl. Med. 2015, 7, 305ps19. [Google Scholar] [CrossRef] [PubMed]
- Bucsek, M.J.; Qiao, G.; MacDonald, C.R.; Giridharan, T.; Evans, L.; Niedzwecki, B.; Liu, H.; Kokolus, K.M.; Eng, J.W.-L.; Messmer, M.N.; et al. β-Adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017, 77, 5639–5651. [Google Scholar] [CrossRef]
- Chang, A.; Le, C.P.; Walker, A.K.; Creed, S.J.; Pon, C.K.; Albold, S.; Carroll, D.; Halls, M.L.; Lane, J.R.; Riedel, B.; et al. β2-Adrenoceptors on tumor cells play a critical role in stress-enhanced metastasis in a mouse model of breast cancer. Brain Behav. Immun. 2016, 57, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat. Protoc. 2012, 7, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.G.; Morizono, K.; Karanikolas, B.D.W.; Wu, L.; et al. Sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Pandey, M.R.; Attwood, K.; Ji, W.; Witkiewicz, A.K.; Knudsen, E.S.; Allen, C.; Tario, J.D.; Wallace, P.K.; Cedeno, C.D.; et al. Phase I Clinical Trial of Combination Propranolol and Pembrolizumab in Locally Advanced and Metastatic Melanoma: Safety, Tolerability, and Preliminary Evidence of Antitumor Activity. Clin. Cancer Res. 2021, 27, 87–95. [Google Scholar] [CrossRef]
- Hiller, J.G.; Cole, S.W.; Crone, E.M.; Byrne, D.J.; Shackleford, D.M.; Pang, J.M.B.; Henderson, M.A.; Nightingale, S.S.; Ho, K.M.; Myles, P.S.; et al. Preoperative β-Blockade with Propranolol Reduces Biomarkers of Metastasis in Breast Cancer: A Phase II Randomized Trial. Clin. Cancer Res. 2020, 26, 1803–1811. [Google Scholar] [CrossRef]
- Li, J.; Bates, K.A.; Hoang, K.L.; Hector, T.E.; Knowles, S.C.L.; King, K.C. Experimental temperatures shape host microbiome diversity and composition. Glob. Change Biol. 2023, 29, 41–56. [Google Scholar] [CrossRef]
- Sepulveda, J.; Moeller, A.H. The Effects of Temperature on Animal Gut Microbiomes. Front. Microbiol. 2020, 11, 384. [Google Scholar] [CrossRef]

| AAT ≤ 53.5 °F | AAT > 53.5 °F | p-Value | ||
|---|---|---|---|---|
| N | 7637 (43.9) | 9771 (56.1) | ||
| Age | Mean/Std/N | 64.27/11.52/7637 | 65.68/11.62/9771 | <0.001 |
| Sex | Male | 6099 (79.9%) | 7719 (79.0%) | 0.163 |
| Female | 1538 (20.1%) | 2052 (21.0%) | ||
| Race | Non-Hispanic White | 6111 (80.0%) | 7809 (79.9%) | <0.001 |
| Non-Hispanic Black | 1286 (16.8%) | 1420 (14.5%) | ||
| Hispanic | 240 (3.1%) | 542 (5.5%) | ||
| Marital Status | Married | 4183 (54.8%) | 5299 (54.2%) | 0.477 |
| Single | 3454 (45.2%) | 4472 (45.8%) | ||
| Insurance Status | Insured | 3959 (51.8%) | 5239 (53.6%) | 0.037 |
| Uninsured | 231 (3.0%) | 259 (2.7%) | ||
| Unknown | 3447 (45.1%) | 4273 (43.7%) | ||
| Primary Site | C150 Cervical esophagus | 144 (1.9%) | 196 (2.0%) | <0.001 |
| C151 Thoracic esophagus | 264 (3.5%) | 353 (3.6%) | ||
| C152 Abdominal esophagus | 47 (0.6%) | 82 (0.8%) | ||
| C153 Upper third of the esophagus | 397 (5.2%) | 463 (4.7%) | ||
| C154 Middle third of esophagus | 1282 (16.8%) | 1593 (16.3%) | ||
| C155 Lower third of esophagus | 4573 (59.9%) | 5642 (57.7%) | ||
| C158 Overlapping lesion of the esophagus | 299 (3.9%) | 405 (4.1%) | ||
| C159 Esophagus, NOS | 631 (8.3%) | 1037 (10.6%) | ||
| Histology | Adenocarcinoma | 4768 (62.4%) | 5957 (61.0%) | 0.104 |
| Squamous | 2811 (36.8%) | 3726 (38.1%) | ||
| Adenosquamous | 58 (0.8%) | 88 (0.9%) | ||
| Stage | Localized | 1931 (25.3%) | 2325 (23.8%) | 0.076 |
| Regional | 2716 (35.6%) | 3539 (36.2%) | ||
| Distant | 2990 (39.2%) | 3907 (40.0%) | ||
| Grade | I/II | 3195 (41.8%) | 3914 (40.1%) | <0.001 |
| III/IV | 3046 (39.9%) | 4223 (43.2%) | ||
| Unknown | 1396 (18.3%) | 1634 (16.7%) | ||
| AAT at Diagnosis | OS | DSS | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |||
| Overall population | Every 5-degree increment | 0.964 (0.954–0.973) | <0.0001 | 0.959 (0.948–0.969) | <0.0001 | |
| Temperature at Diagnosis | <Q1 [48.59] | Ref. | Ref. | |||
| Q1–Q3 | 0.909 (0.873–0.945) | <0.0001 | 0.902 (0.865–0.942) | <0.0001 | ||
| >Q3 [62.69] | 0.873 (0.834–0.914) | <0.0001 | 0.854 (0.813–0.897) | <0.0001 | ||
| Temperature at Diagnosis | ≤53.5 | Ref. | Ref. | |||
| >53.5 | 0.889 (0.860–0.918) | <0.0001 | 0.873 (0.843–0.904) | <0.0001 | ||
| Adenocarcinoma Subgroup | Every 5-degree increment | 0.968 (0.955–0.980) | <0.0001 | 0.960 (0.947–0.974) | <0.0001 | |
| Temperature at Diagnosis | <Q1 [48.59] | Ref. | Ref. | |||
| Q1–Q3 | 0.924 (0.879–0.972) | 0.0023 | 0.917 (0.869–0.968) | 0.0018 | ||
| >Q3 [62.69] | 0.893 (0.842–0.947) | 0.0002 | 0.867 (0.814–0.924) | <0.0001 | ||
| Temperature at Diagnosis | ≤53.5 | Ref. | Ref. | |||
| >53.5 | 0.902 (0.864–0.941) | <0.0001 | 0.879 (0.841–0.920) | <0.0001 | ||
| Squamous Subgroup | Every 5-degree increment | 0.959 (0.944–0.975) | <0.0001 | 0.958 (0.941–0.975) | <0.0001 | |
| Temperature at Diagnosis | <Q1 [48.59] | Ref. | Ref. | |||
| Q1–Q3 | 0.903 (0.845–0.964) | 0.0023 | 0.897 (0.836–0.963) | 0.0026 | ||
| >Q3 [62.69] | 0.852 (0.791–0.918) | <0.0001 | 0.843 (0.778–0.913) | <0.0001 | ||
| Temperature at Diagnosis | ≤53.5 | Ref. | Ref. | |||
| >53.5 | 0.877 (0.832–0.924) | <0.0001 | 0.871 (0.824–0.922) | <0.0001 | ||
| ≤53.5 | >53.5 | Overall | p-Value | ||
|---|---|---|---|---|---|
| N | 8025 (39.1) | 12,508 (60.9) | 20,533 (100%) | ||
| Age | Mean/Std/N | 66.87/13.29/8025 | 68.51/13.31/12,508 | 67.87/13.33/20,533 | <0.001 |
| Sex | Male | 5399 (67.3%) | 8272 (66.1%) | 13,671 (66.6%) | 0.090 |
| Female | 2626 (32.7%) | 4236 (33.9%) | 6862 (33.4%) | ||
| Race | Non-Hispanic White | 4510 (56.2%) | 7366 (58.9%) | 11,876 (57.8%) | <0.001 |
| Non-Hispanic Black | 1612 (20.1%) | 2221 (17.8%) | 3833 (18.7%) | ||
| Hispanic | 1041 (13.0%) | 1186 (9.5%) | 2227 (10.8%) | ||
| Other | 862 (10.7%) | 1735 (13.9%) | 2597 (12.6%) | ||
| Marital Status | Married | 4517 (56.3%) | 6919 (55.3%) | 11,436 (55.7%) | 0.172 |
| Single | 3508 (43.7%) | 5589 (44.7%) | 9097 (44.3%) | ||
| Insurance Status | Insured | 3966 (49.4%) | 6144 (49.1%) | 10,110 (49.2%) | 0.844 |
| Uninsured | 212 (2.6%) | 344 (2.8%) | 556 (2.7%) | ||
| Unknown | 3847 (47.9%) | 6020 (48.1%) | 9867 (48.1%) | ||
| Primary Site | C160 Cardia, NOS | 2795 (34.8%) | 4212 (33.7%) | 7007 (34.1%) | 0.003 |
| C161 Fundus of stomach | 309 (3.9%) | 446 (3.6%) | 755 (3.7%) | ||
| C162 Body of stomach | 605 (7.5%) | 979 (7.8%) | 1584 (7.7%) | ||
| C163 Gastric antrum | 1504 (18.7%) | 2487 (19.9%) | 3991 (19.4%) | ||
| C164 Pylorus | 252 (3.1%) | 325 (2.6%) | 577 (2.8%) | ||
| C165 Lesser curvature of the stomach, NOS | 585 (7.3%) | 1066 (8.5%) | 1651 (8.0%) | ||
| C166 Greater curvature of the stomach, NOS | 321 (4.0%) | 460 (3.7%) | 781 (3.8%) | ||
| C168 Overlapping lesion of the stomach | 516 (6.4%) | 769 (6.1%) | 1285 (6.3%) | ||
| C169 Stomach, NOS | 1138 (14.2%) | 1764 (14.1%) | 2902 (14.1%) | ||
| Site | Stomach | 5198 (64.8%) | 8264 (66.1%) | 13,462 (65.6%) | 0.056 |
| Esophagus GE Junction | 2827 (35.2%) | 4244 (33.9%) | 7071 (34.4%) | ||
| Stage | Localized | 2136 (26.6%) | 3472 (27.8%) | 5608 (27.3%) | 0.003 |
| Regional | 2537 (31.6%) | 4112 (32.9%) | 6649 (32.4%) | ||
| Distant | 3352 (41.8%) | 4924 (39.4%) | 8276 (40.3%) | ||
| Grade | I/II | 2682 (33.4%) | 4327 (34.6%) | 7009 (34.1%) | <0.001 |
| III/IV | 4160 (51.8%) | 6644 (53.1%) | 10,804 (52.6%) | ||
| Unknown | 1183 (14.7%) | 1537 (12.3%) | 2720 (13.2%) | ||
| Temperature at Diagnosis | OS | DSS | |||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | ||
| Every 5-degree increment | 0.961 (0.952–0.971) | <0.0001 | 0.955 (0.945–0.965) | <0.0001 | |
| AAT at Diagnosis (Categorical) | <Q1 [49.5667] | Ref. | Ref. | ||
| Q1–Q3 | 0.904 (0.871–0.938) | <0.0001 | 0.886 (0.851–0.923) | <0.0001 | |
| >Q3 [62.5750] | 0.869 (0.833–0.907) | <0.0001 | 0.855 (0.816–0.895) | <0.0001 | |
| The temperature at Diagnosis (Categorical—Binary) | ≤53.5 | Ref. | Ref. | ||
| >53.5 | 0.875 (0.848–0.903) | <0.0001 | 0.858 (0.829–0.887) | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, K.; George, A.; Attwood, K.; Gupta, A.; Roy, A.M.; Gandhi, S.; Siromoni, B.; Singh, A.; Repasky, E.; Mukherjee, S. Association between Environmental Temperature and Survival in Gastroesophageal Cancers: A Population Based Study. Cancers 2024, 16, 74. https://doi.org/10.3390/cancers16010074
Gupta K, George A, Attwood K, Gupta A, Roy AM, Gandhi S, Siromoni B, Singh A, Repasky E, Mukherjee S. Association between Environmental Temperature and Survival in Gastroesophageal Cancers: A Population Based Study. Cancers. 2024; 16(1):74. https://doi.org/10.3390/cancers16010074
Chicago/Turabian StyleGupta, Kush, Anthony George, Kristopher Attwood, Ashish Gupta, Arya Mariam Roy, Shipra Gandhi, Beas Siromoni, Anurag Singh, Elizabeth Repasky, and Sarbajit Mukherjee. 2024. "Association between Environmental Temperature and Survival in Gastroesophageal Cancers: A Population Based Study" Cancers 16, no. 1: 74. https://doi.org/10.3390/cancers16010074
APA StyleGupta, K., George, A., Attwood, K., Gupta, A., Roy, A. M., Gandhi, S., Siromoni, B., Singh, A., Repasky, E., & Mukherjee, S. (2024). Association between Environmental Temperature and Survival in Gastroesophageal Cancers: A Population Based Study. Cancers, 16(1), 74. https://doi.org/10.3390/cancers16010074







