Treatment Outcomes after Dose-Escalated Moderately Hypofractionated Radiotherapy for Frail Patients with High-Grade Glioma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Treatment
2.3. Endpoints and Statistical Analysis
3. Results
3.1. Baseline Characteristics
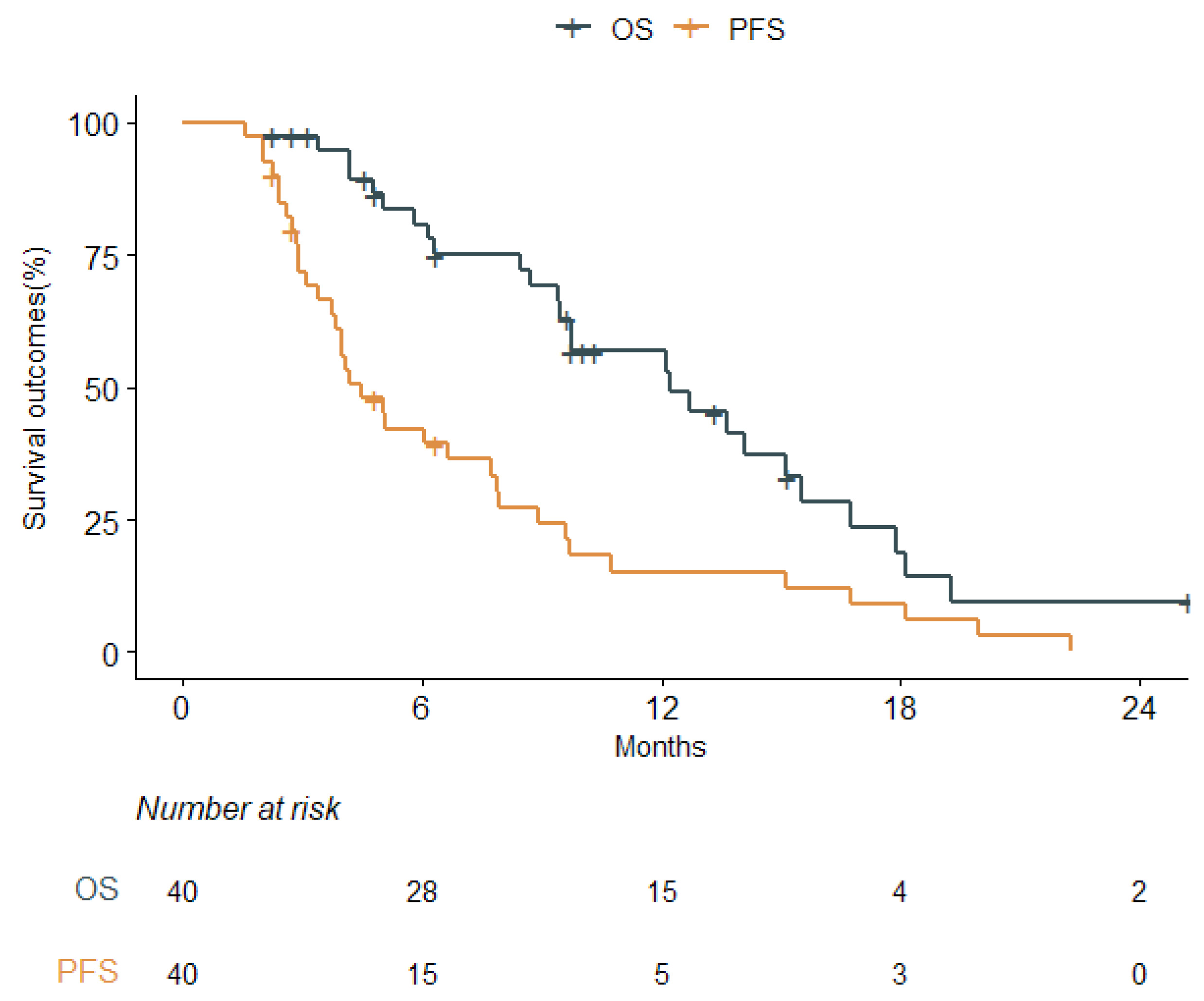
3.2. Clinical Outcomes
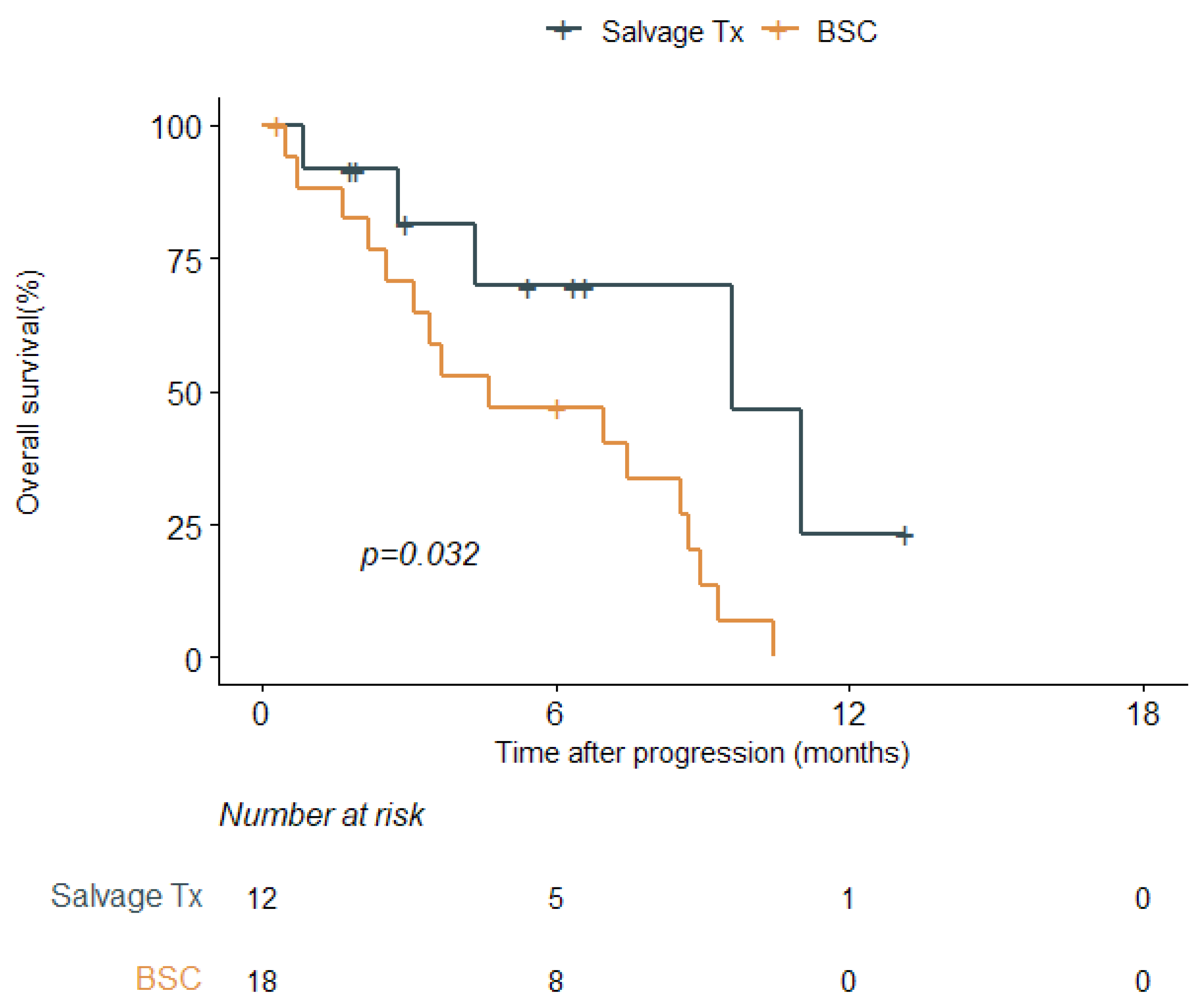
3.3. Salvage Treatment after Disease Progression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 who classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Central Nervous System Cancers (Version 1.2023). Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (accessed on 12 July 2023).
- Ohgaki, H.; Dessen, P.; Jourde, B.; Horstmann, S.; Nishikawa, T.; Di Patre, P.L.; Burkhard, C.; Schüler, D.; Probst-Hensch, N.M.; Maiorka, P.C.; et al. Genetic pathways to glioblastoma: A population-based study. Cancer Res. 2004, 64, 6892–6899. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Wagner, J.; Bischof, M.; Welzel, T.; Wagner, F.; Debus, J.; Schulz-Ertner, D. Postoperative treatment of primary glioblastoma multiforme with radiation and concomitant temozolomide in elderly patients. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.W. Radiotherapy for newly diagnosed glioblastoma in the elderly: What is the standard? Brain Tumor Res. Treat. 2022, 10, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Keime-Guibert, F.; Chinot, O.; Taillandier, L.; Cartalat-Carel, S.; Frenay, M.; Kantor, G.; Guillamo, J.S.; Jadaud, E.; Colin, P.; Bondiau, P.Y.; et al. Radiotherapy for glioblastoma in the elderly. N. Engl. J. Med. 2007, 356, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Pellerino, A.; Palmiero, R.; Bertero, L.; Mantovani, C.; Garbossa, D.; Soffietti, R.; Rudà, R. Glioblastoma in the elderly: Review of molecular and therapeutic aspects. Biomedicines 2022, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Malmström, A.; Grønberg, B.H.; Marosi, C.; Stupp, R.; Frappaz, D.; Schultz, H.; Abacioglu, U.; Tavelin, B.; Lhermitte, B.; Hegi, M.E.; et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The nordic randomised, phase 3 trial. Lancet Oncol. 2012, 13, 916–926. [Google Scholar] [CrossRef]
- Minniti, G.; Lanzetta, G.; Scaringi, C.; Caporello, P.; Salvati, M.; Arcella, A.; De Sanctis, V.; Giangaspero, F.; Enrici, R.M. Phase ii study of short-course radiotherapy plus concomitant and adjuvant temozolomide in elderly patients with glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 93–99. [Google Scholar] [CrossRef]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef]
- Roa, W.; Brasher, P.M.; Bauman, G.; Anthes, M.; Bruera, E.; Chan, A.; Fisher, B.; Fulton, D.; Gulavita, S.; Hao, C.; et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: A prospective randomized clinical trial. J. Clin. Oncol. 2004, 22, 1583–1588. [Google Scholar] [CrossRef]
- Roa, W.; Kepka, L.; Kumar, N.; Sinaika, V.; Matiello, J.; Lomidze, D.; Hentati, D.; Guedes de Castro, D.; Dyttus-Cebulok, K.; Drodge, S.; et al. International atomic energy agency randomized phase iii study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 2015, 33, 4145–4150. [Google Scholar] [CrossRef] [PubMed]
- Mohile, N.A.; Messersmith, H.; Gatson, N.T.; Hottinger, A.F.; Lassman, A.; Morton, J.; Ney, D.; Nghiemphu, P.L.; Olar, A.; Olson, J.; et al. Therapy for diffuse astrocytic and oligodendroglial tumors in adults: Asco-sno guideline. J. Clin. Oncol. 2022, 40, 403–426. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. Eano guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lehrer, E.J.; Wang, M.; Perlow, H.K.; Zaorsky, N.G.; Trifiletti, D.M.; Bovi, J.; Navarria, P.; Scoccianti, S.; Gondi, V.; et al. Dose escalated radiation therapy for glioblastoma multiforme: An international systematic review and meta-analysis of 22 prospective trials. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 371–384. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Radiographic patterns of relapse in glioblastoma. J. Neurooncol. 2011, 101, 319–323. [Google Scholar] [CrossRef]
- Salloum, R.; DeWire, M.; Lane, A.; Goldman, S.; Hummel, T.; Chow, L.; Miles, L.; Sutton, M.; Stevenson, C.; Fouladi, M.; et al. Patterns of progression in pediatric patients with high-grade glioma or diffuse intrinsic pontine glioma treated with bevacizumab-based therapy at diagnosis. J. Neurooncol. 2015, 121, 591–598. [Google Scholar] [CrossRef]
- Wick, A.; Kessler, T.; Platten, M.; Meisner, C.; Bamberg, M.; Herrlinger, U.; Felsberg, J.; Weyerbrock, A.; Papsdorf, K.; Steinbach, J.P.; et al. Superiority of temozolomide over radiotherapy for elderly patients with rtk ii methylation class, mgmt promoter methylated malignant astrocytoma. Neuro. Oncol. 2020, 22, 1162–1172. [Google Scholar] [CrossRef]
- Wee, C.W.; Kim, I.H.; Park, C.K.; Kim, N.; Suh, C.O.; Chang, J.H.; Lim, D.H.; Nam, D.H.; Kim, I.A.; Kim, C.Y.; et al. Chemoradiation in elderly patients with glioblastoma from the multi-institutional gbm-molrpa cohort: Is short-course radiotherapy enough or is it a matter of selection? J. Neurooncol. 2020, 148, 57–65. [Google Scholar] [CrossRef]
- Mak, K.S.; Agarwal, A.; Qureshi, M.M.; Truong, M.T. Hypofractionated short-course radiotherapy in elderly patients with glioblastoma multiforme: An analysis of the national cancer database. Cancer Med. 2017, 6, 1192–1200. [Google Scholar] [CrossRef]
- Mallick, S.; Gupta, S.; Amariyil, A.; Kunhiparambath, H.; Laviraj, M.A.; Sharma, S.; Sagiraju, H.K.R.; Julka, P.K.; Sharma, D.; Rath, G.K. Hypo-fractionated accelerated radiotherapy with concurrent and maintenance temozolomide in newly diagnosed glioblastoma: Updated results from phase ii hart-gbm trial. J. Neurooncol. 2023, 164, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Zanello, M.; Roux, A.; Ursu, R.; Peeters, S.; Bauchet, L.; Noel, G.; Guyotat, J.; Le Reste, P.J.; Faillot, T.; Litre, F.; et al. Recurrent glioblastomas in the elderly after maximal first-line treatment: Does preserved overall condition warrant a maximal second-line treatment? J. Neurooncol. 2017, 135, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Socha, J.; Kepka, L.; Ghosh, S.; Roa, W.; Kumar, N.; Sinaika, V.; Matiello, J.; Lomidze, D.; de Castro, D.G.; Hentati, D.; et al. Outcome of treatment of recurrent glioblastoma multiforme in elderly and/or frail patients. J. Neurooncol. 2016, 126, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Fariña Nuñez, M.T.; Franco, P.; Cipriani, D.; Neidert, N.; Behringer, S.P.; Mader, I.; Delev, D.; Fung, C.; Beck, J.; Sankowski, R.; et al. Resection of recurrent glioblastoma multiforme in elderly patients: A pseudo-randomized analysis revealed clinical benefit. J. Neurooncol. 2020, 146, 381–387. [Google Scholar] [CrossRef]
- Prajapati, H.P.; Singh, D.K. Recurrent glioblastoma in elderly: Options and decision for the treatment. Surg. Neurol. Int. 2022, 13, 397. [Google Scholar] [CrossRef]
| Characteristics | N (%) |
|---|---|
| Age, years | |
| Median [IQR] | 74 [68–81] |
| ≤80 | 28 (70.0) |
| >80 | 12 (30.0) |
| Sex | |
| Female | 15 (37.5) |
| Male | 25 (62.5) |
| KPS | |
| Median [IQR] | 70 [60–80] |
| <70 | 13 (32.5%) |
| ≥70 | 27 (67.5%) |
| Extent of resection | |
| Biopsy | 30 (75.0) |
| Partial resection | 1 (2.5) |
| Subtotal resection | 6 (15.0) |
| Gross total resection | 3 (7.5) |
| Pathology | |
| Glioblastoma, IDH-wildtype | 31 (77.5) |
| Diffuse midline glioma H3K27M-mutant | 3 (7.5) |
| Astrocytoma, IDH-wildtype | 4 (10.0) |
| Astrocytoma, IDH-mutant | 2 (5.0) |
| MGMT promoter methylation | 11 (27.5) |
| Temozolomide-based CCRT | 28 (70.0) |
| Adjuvant temozolomide | 25 (62.5) |
| Overall Survival | ||||||
|---|---|---|---|---|---|---|
| Variables | Event a | Median (Months) | HR | 95% CI | p-Value | |
| Age, years | ≤80 | 19/28 | 12.2 | Reference | - | 0.799 |
| >80 | 8/12 | 14.1 | 0.89 | 0.38–2.11 | ||
| Sex | Female | 8/15 | 15.1 | Reference | - | 0.158 |
| Male | 19/25 | 9.1 | 1.83 | 0.79–4.24 | ||
| KPS | <70 | 9/13 | 9.5 | Reference | - | 0.689 |
| ≥70 | 18/27 | 12.2 | 1.19 | 0.51–2.78 | ||
| Extent of resection | GTR | 1/3 | 8.7 | Reference | - | |
| STR/PR | 6/7 | 9.5 | 1.15 | 0.13–10.2 | 0.903 | |
| bx/no bx | 20/30 | 12.7 | 1.23 | 0.16–9.47 | 0.842 | |
| MGMT promoter | Methylated | 6/11 | 13.6 | Reference | - | 0.873 |
| Others | 21/29 | 12.1 | 1.08 | 0.43–2.69 | ||
| CCRT | No | 8/12 | 13.6 | Reference | - | 0.368 |
| Yes | 19/28 | 12.2 | 1.50 | 0.62–3.61 | ||
| Adjuvant TMZ | No | 8/15 | 12.1 | Reference | - | 0.840 |
| Yes | 19/25 | 12.7 | 0.92 | 0.40–2.12 | ||
| BED, Gy | <72 | 20/29 | 12.2 | Reference | - | 0.871 |
| ≥72 | 7/11 | 15.5 | 0.93 | 0.39–2.24 | ||
| GTV, cc | Continuous | 43.8 b | 12.2 | 1.00 | 1.00–1.01 | 0.114 |
| Progression-Free Survival | ||||||
| Variables | Event a | Median (Months) | HR | 95% CI | p-Value | |
| Age, years | ≤80 | 25/28 | 4.5 | Reference | - | 0.613 |
| >80 | 11/12 | 5.8 | 0.83 | 0.39–1.73 | ||
| Sex | Female | 15/15 | 4.1 | Reference | - | 0.899 |
| Male | 21/25 | 5.0 | 1.05 | 0.53–2.07 | ||
| KPS | <70 | 11/13 | 6.7 | Reference | - | 0.127 |
| ≥70 | 25/27 | 4.5 | 1.82 | 0.84–3.94 | ||
| Extent of resection | GTR | 2/3 | 7.9 | Reference | - | |
| STR/PR | 7/7 | 5.1 | 1.03 | 0.20–5.24 | 0.971 | |
| bx/no bx | 27/30 | 4.2 | 1.33 | 0.31–5.66 | 0.702 | |
| MGMT promoter | Methylated | 8/11 | 5.0 | Reference | - | 0.935 |
| Others | 28/29 | 4.2 | 1.03 | 0.46–2.30 | ||
| CCRT | No | 10/12 | 4.2 | Reference | - | 0.247 |
| Yes | 26/28 | 4.5 | 1.61 | 0.72–3.59 | ||
| Adjuvant TMZ | No | 13/15 | 3.1 | Reference | - | 0.103 |
| Yes | 23/25 | 6.7 | 0.55 | 0.27–1.13 | ||
| BED, Gy | <72 | 27/29 | 4.5 | Reference | - | 0.825 |
| ≥72 | 9/11 | 4.6 | 0.92 | 0.43–1.97 | ||
| GTV, cc | Continuous | 43.8 b | 4.5 | 1.00 | 1.00–1.01 | 0.579 |
| Characteristics | Total N = 30 | Salvage Tx N = 12 | BSC N = 18 | p-Value |
|---|---|---|---|---|
| Initial treatment | ||||
| Age, years (median, [IQR]) | 74 [67–81] | 78 [66–81] | 72 [67–81] | 0.496 |
| Sex | 0.501 | |||
| Female | 14 (46.7) | 7 (58.3) | 7 (38.9) | |
| Male | 16 (53.3) | 5 (41.7) | 11 (61.1) | |
| KPS | 1.000 | |||
| <70 | 9 (30.0) | 4 (33.3) | 5 (27.8) | |
| ≥70 | 21 (70.0) | 8 (66.7) | 13 (72.2) | |
| Extent of resection | 0.199 | |||
| Biopsy/no surgery | 22 (73.3) | 8 (66.7) | 14 (77.8) | |
| Partial/subtotal resection | 6 (20.0) | 2 (16.7) | 4 (22.2) | |
| Gross total resection | 2 (6.7) | 2 (16.7) | 0 (0.0) | |
| Pathology | 0.273 | |||
| Glioblastoma, IDH-wild-type | 22 (73.3) | 7 (58.3) | 15 (83.3) | |
| Others | 8 (26.7) | 5 (41.7) | 3 (16.7) | |
| MGMT promoter methylation | 6 (20.0) | 2 (16.7) | 4 (22.2) | 1.000 |
| Temozolomide-based CCRT | 21 (70.0) | 7 (58.3) | 14 (77.8) | 0.464 |
| Adjuvant temozolomide | 19 (63.3) | 6 (50.0) | 13 (72.2) | 0.395 |
| RT modality | 1.000 | |||
| 3D CRT | 3 (10.0) | 1 (8.3) | 2 (11.1) | |
| IMRT | 27 (90.0) | 11 (91.7) | 16 (88.9) | |
| Total dose, Gy (median, [IQR]) | 56 [56–59] | 56 [56–58] | 56 [56–59] | 0.631 |
| BED, Gy (median, [IQR]) | 71.7 [71.7–76.4] | 71.7 [71.7–74.9] | 71.7 [71.7–76.4] | 0.631 |
| GTV, cc (median, [IQR]) | 46.5 [22.9–61.9] | 46.5 [21.8–60.5] | 44.8 [23.0–64.4] | 0.692 |
| CTV, cc (median, [IQR]) | 176.5 [121.9–289.8] | 153.1 [104.6–207.0] | 230.3 [143.0–318.6] | 0.079 |
| At disease progression | ||||
| Recurrence-free interval | 4.0 [2.8–7.9] | 7.2 [3.0–8.8] | 3.8 [2.8–5.1] | 0.189 |
| Age, years (median, [IQR]) | 74 [67–81] | 78 [67–82] | 72 [67–81] | 0.432 |
| KPS | 0.048 | |||
| <70 | 20 (66.7%) | 5 (41.7%) | 15 (83.3%) | |
| ≥70 | 10 (33.3%) | 7 (58.3%) | 3 (16.7%) | |
| Site of disease progression | 0.478 | |||
| Local | 26 (86.6%) | 11 (91.7%) | 15 (83.3%) | |
| Noncontiguous intracranial failure | 2 (6.7%) | 1 (8.3%) | 1 (5.6%) | |
| Leptomeningeal seeding | 2 (6.7%) | 0 (0.0%) | 2 (11.1%) |
| Variables | Event a | Median (Months) | HR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Age, years | ≤80 | 14/20 | 7.5 | Reference | - | 0.159 |
| >80 | 7/10 | 4.4 | 2.02 | 0.76–5.36 | ||
| Sex | Female | 7/14 | 7.0 | Reference | - | 0.536 |
| Male | 14/16 | 7.5 | 1.35 | 0.53–3.44 | ||
| KPS at | <70 | 15/20 | 7.5 | Reference | - | 0.141 |
| progression | ≥70 | 6/10 | 8.3 | 0.46 | 0.16–1.30 | |
| Initial EOR | GTR | 1/2 | 0.8 | Reference | - | |
| STR/PR | 5/6 | 3.4 | 0.87 | 0.10–7.83 | 0.903 | |
| Bx/no Bx | 15/22 | 7.5 | 0.62 | 0.08–4.88 | 0.648 | |
| MGMT | Methylated | 4/6 | 8.9 | Reference | - | 0.432 |
| promoter | Others | 17/24 | 7.0. | 1.56 | 0.52–4.71 | |
| Management | BSC | 16/18 | 4.6 | Reference | - | 0.032 |
| at progression | Salvage Tx | 5/12 | 9.6 | 0.29 | 0.10–0.90 | |
| RFI, month | Continuous | 4.0 b | 7.0 | 0.99 | 0.91–1.08 | 0.891 |
| Study | Inclusion Criteria | No. | CCRT (%) | Surgery (%) | RT Regimen | BED (EQD2) (Gy) | Median OS |
|---|---|---|---|---|---|---|---|
| Roa [11] | ≥60 y and KPS ≥ 50 | 48 | 0 | 65 | 40 Gy/15 fx | 50.7 (42.2) | 5.6 m |
| Malmstrom [8] | 60–70 y and ECOG PS 0–2 | 58 | 0 | 78 | 34 Gy/10 fx | 45.6 (38.0) | 8.8 m |
| >70 y and ECOG PS 0–2 | 40 | 0 | 67 | 34 Gy/10 fx | 45.6 (38.0) | 7.0 m | |
| Wick [19] | >65 y and KPS ≥ 60 | 178 | 0 | 61 | 60 Gy/30 fx | 72.0 (60.0) | 9.4 m |
| Minniti [9] | ≥70 y and KPS ≥ 60 | 71 | 100 | 87 | 40 Gy/15 fx | 50.7 (42.2) | 12.4 m |
| Perry [10] | >65 y and ECOG PS 0–2 | 281 | 100 | 68 | 40 Gy/15 fx | 50.7 (42.2) | 9.3 m |
| 281 | 0 | 68 | 40 Gy/15 fx | 50.7 (42.2) | 7.6 m | ||
| Wee [20] | >65 y | 196 | 100 | 88 | 60 Gy/30 fx | 72.0 (60.6) | 17.6 m |
| 64 | 100 | 67 | 45 Gy/15 fx | 58.5 (48.8) | 13.2 m | ||
| Current study | >70 y or KPS ≤ 70 | 28 | 100 | 29 | 56 Gy/20 fx | 71.7 (59.7) | 12.2 m |
| 12 | 0 | 17 | 56 Gy/20 fx | 71.7 (59.7) | 13.6 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Shin, H.; Lim, D.H.; Nam, D.-H.; Lee, J.-I.; Seol, H.J.; Kong, D.-S.; Choi, J.W.; Chong, K.; Lee, W.J. Treatment Outcomes after Dose-Escalated Moderately Hypofractionated Radiotherapy for Frail Patients with High-Grade Glioma. Cancers 2024, 16, 64. https://doi.org/10.3390/cancers16010064
Kim N, Shin H, Lim DH, Nam D-H, Lee J-I, Seol HJ, Kong D-S, Choi JW, Chong K, Lee WJ. Treatment Outcomes after Dose-Escalated Moderately Hypofractionated Radiotherapy for Frail Patients with High-Grade Glioma. Cancers. 2024; 16(1):64. https://doi.org/10.3390/cancers16010064
Chicago/Turabian StyleKim, Nalee, Hyunju Shin, Do Hoon Lim, Do-Hyun Nam, Jung-Il Lee, Ho Jun Seol, Doo-Sik Kong, Jung Won Choi, Kyuha Chong, and Won Jae Lee. 2024. "Treatment Outcomes after Dose-Escalated Moderately Hypofractionated Radiotherapy for Frail Patients with High-Grade Glioma" Cancers 16, no. 1: 64. https://doi.org/10.3390/cancers16010064
APA StyleKim, N., Shin, H., Lim, D. H., Nam, D.-H., Lee, J.-I., Seol, H. J., Kong, D.-S., Choi, J. W., Chong, K., & Lee, W. J. (2024). Treatment Outcomes after Dose-Escalated Moderately Hypofractionated Radiotherapy for Frail Patients with High-Grade Glioma. Cancers, 16(1), 64. https://doi.org/10.3390/cancers16010064






