Simple Summary
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer with high mortality. Most patients present with an advanced stage of the disease, highlighting the urgent need for early detection. Recent studies of individuals at high risk of PDAC showed benefits from participating in clinical management and surveillance programs. PDAC clinical management and surveillance programs are suggested for individuals with a germline pathogenic variant in a cancer predisposition gene or a strong family history. In the present study, we performed a systematic literature review to investigate the mutational portrait of the main genes (ATM, BRCA1, BRCA2, CDKN2A, EPCAM, MLH1, MSH2, MSH6, PALB2, PMS2, STK11, TP53) involved in PDAC susceptibility. Our findings may support the development of tailored management and follow-up strategies in PDAC patients with specific germline genetic variants.
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most fatal malignancies worldwide. While population-wide screening recommendations for PDAC in asymptomatic individuals are not achievable due to its relatively low incidence, pancreatic cancer surveillance programs are recommended for patients with germline causative variants in PDAC susceptibility genes or a strong family history. In this study, we sought to determine the prevalence and significance of germline alterations in major genes (ATM, BRCA1, BRCA2, CDKN2A, EPCAM, MLH1, MSH2, MSH6, PALB2, PMS2, STK11, TP53) involved in PDAC susceptibility. We performed a systematic review of PubMed publications reporting germline variants identified in these genes in PDAC patients. Overall, the retrieved articles included 1493 PDAC patients. A high proportion of these patients (n = 1225/1493, 82%) were found to harbor alterations in genes (ATM, BRCA1, BRCA2, PALB2) involved in the homologous recombination repair (HRR) pathway. Specifically, the remaining PDAC patients were reported to carry alterations in genes playing a role in other cancer pathways (CDKN2A, STK11, TP53; n = 181/1493, 12.1%) or in the mismatch repair (MMR) pathway (MLH1, MSH2, MSH6, PMS2; n = 87/1493, 5.8%). Our findings highlight the importance of germline genetic characterization in PDAC patients for better personalized targeted therapies, clinical management, and surveillance.
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most fatal malignancies worldwide, with a 5-year overall survival of about 5–10% and a 10-year overall survival of less than 1% [,]. Recent epidemiological data indicate that the incidence of PDAC is rising. It currently ranks 12th among the most common cancers worldwide and is the seventh-highest cause of cancer death in both sexes [,]. Most patients present with surgically unresectable disease or distant metastases, highlighting the urgent need for early detection []. The most common metastatic sites for PDAC are the liver and peritoneum. Less frequently, lung, brain, and bone metastases are also detected in PDAC patients []. Focusing on early diagnosis of metastatic PDAC, with a particular emphasis on unusual symptoms such as bone pain, which might be related to skeletal metastases, is a key priority to extend survival and improve the quality of life of PDAC patients [].
Currently, population-wide screening recommendations for PDAC in asymptomatic individuals are not achievable due to the relatively low incidence of this cancer []. Conversely, pancreatic surveillance programs are recommended for patients with germline causative variants in genes involved in PDAC susceptibility or a strong family history []. Nowadays, it is estimated that approximately 10% of individuals with PDAC have alterations in genes associated with known hereditary cancer syndromes that are also associated with an increased risk of developing this disease []. More specifically, patients with pathogenic or likely pathogenic variants (PVs/LPVs) in the BRCA1 and BRCA2 genes, which are associated with hereditary breast and ovarian cancer, have a 5–10% increased risk of developing PDAC []. Likewise, a higher risk of developing PDAC has been associated with germline genetic alterations in the MLH1, MSH2, MSH6, and EPCAM genes, which are involved in Lynch syndrome (LS) [], and in the STK11 gene, which is responsible for Peutz–Jeghers syndrome []. Additionally, germline PVs/LPVs in other genes such as PALB2, ATM, CDKN2A, and TP53, which are involved in other hereditary cancer syndromes, also confer an increased risk of PDAC [,]. The identification of PVs/LPVs in one of these PDAC susceptibility genes has relevant implications for therapeutic strategies. For example, patients harboring genetic alterations in BRCA1 or BRCA2 may benefit from targeted therapies []. Considering the clinical impact of this evidence, the National Comprehensive Cancer Network (NCCN) currently recommends genetic testing for all individuals with a PDAC diagnosis []. In addition, the American Society of Clinical Oncology released a provisional clinical opinion according to which all individuals with newly diagnosed PDAC should undergo risk assessment for hereditary cancer syndromes known to be associated with an increased risk of PDAC, and germline genetic testing should be offered to patients with a personal history of pancreatic cancer, even if they have no remarkable family history []. In the present study, we performed a systematic literature review to investigate the mutational portrait of the main genes involved in PDAC susceptibility.
2. Materials and Methods
2.1. Search Strategy and Study Selection
This systematic review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta–Analyses) guidelines (Table S1) [] and was not registered in PROSPERO (international prospective register of systematic reviews). It was performed in PubMed by searching for studies reporting PDAC cases carrying germline variants in the main genes associated with PDAC susceptibility. The timeframe of the search was from February 1976 to September 2023. The search comprised the keywords (((pancreatic cancer) OR (pancreatic adenocarcinoma) OR (pancreatic ductal adenocarcinoma)) AND ((germline variant) OR (germline mutation) OR (pathogenic germline variant)) NOT (neuroendocrine)). Only studies including PDAC patients harboring a germline genetic variant in one of the following PDAC susceptibility genes were selected: ATM, BRCA1, BRCA2, CDKN2A, EPCAM, MLH1, MSH2, MSH6, PALB2, PMS2, STK11, and TP53. The population of interest was composed of individuals with any type or stage of PDAC. Studies reporting patients with pancreatic neuroendocrine tumors or PDAC patients not harboring disease-associated germline variants, or PDAC patients with only somatic alterations in PDAC susceptibility genes were excluded. Studies not including relevant information (genetic sequence variant nomenclature) were also excluded. Moreover, patients described in different studies were included only once.
2.2. Data Extraction
Ten reviewers independently assessed the title, abstract, main text, and supplementary material of the identified articles to determine study inclusion or exclusion. Relevant information regarding PDAC patients with disease-causative genetic sequence variants was extracted from the full text of the included articles. Any disagreement was resolved by a consensus meeting among the reviewers. The extracted study details included information about the article (author(s), year of publication, title, DOI), genetic sequence variant nomenclature (gene, gene transcript, DNA change, protein change, type of variant), and the number of patients carrying each genetic variant.
3. Results
3.1. Literature Search and Study Selection
The initial search of the literature using the keywords chosen based on the inclusion criteria retrieved 912 records. After screening these 912 records, no article was excluded. The full-text articles were studied and assessed for retrieval, and 66 studies were not retrieved and, therefore, excluded. The remaining 846 studies were assessed for eligibility. Among these, 527 studies were excluded for different reasons (articles not in English, PDAC patients without PVs/LPVs in the selected genes, patients carrying only somatic alterations in PDAC susceptibility genes), and 150 studies were excluded because the evaluated patients had endocrine pancreatic cancer. As a result, 169 published studies fulfilled the inclusion criteria [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. The selected 169 published studies included different types of study designs, mostly consisting of case studies and cohort studies (Table S2). The language of all articles was English. Figure 1 shows the flow diagram for study selection.
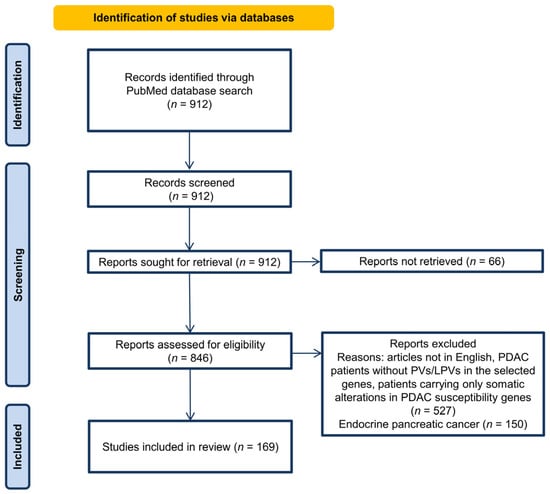
Figure 1.
PRISMA flow-chart diagram showing the article selection process.
3.2. Gene Alteration Frequency in PDAC Patients
Overall, the identified articles included 1493 PDAC patients who were found to carry PVs/LPVs in one of the selected genes. Genetic testing was performed in PDAC individuals with or without a family history of different cancer types (i.e., pancreatic, breast, colorectal, or melanoma). Full details of all the genetic causative or likely causative variants reported are shown in Table S3 [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. Overall, most of these PDAC patients (n = 1225, 82%) were found to harbor PVs/LPVs in genes (ATM, BRCA1, BRCA2, PALB2) involved in the DNA homologous recombination repair (HRR) pathway. Among this subset of patients, 561 (45.8%) carried PVs/LPVs in BRCA2, 310 (25.3%) in ATM, 239 (19.5%) in BRCA1, and 115 (9.4%) in PALB2. A lower proportion of patients (n = 181, 12.1%) were reported to harbor PVs/LPVs in genes (CDKN2A, STK11, TP53) involved in other cancer-related pathways. Of these 181 patients, 146 (80.7%) carried PVs/LPVs in CDKN2A, 24 (13.2%) in TP53, and 11 (6.1%) in STK11. The remaining 87 (5.8%) patients were found to harbor PVs/LPVs in genes (MLH1, MSH2, MSH6, and PMS2) involved in the DNA mismatch repair (MMR) pathway. Of these 87 patients, 26 (29.9%) carried PVs/LPVs in MSH6, 23 (26.4%) in MLH1, 20 (23%) in PMS2, and 18 (20.7%) in MSH2 (Figure 2).
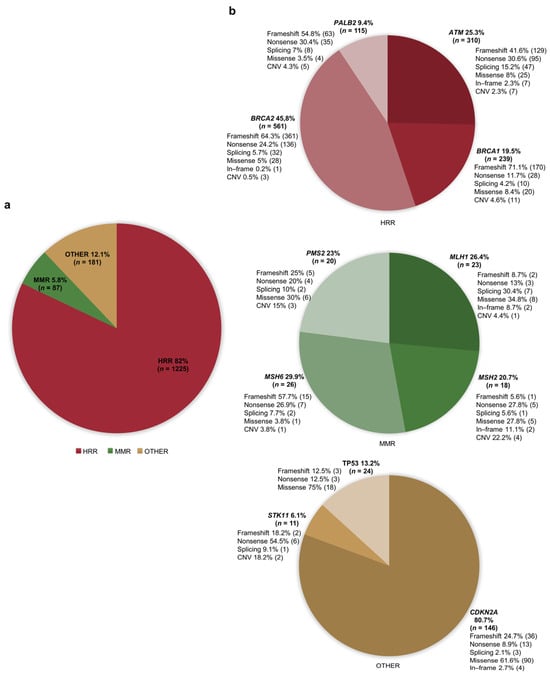
Figure 2.
Percentage distribution of patients with pancreatic ductal adenocarcinoma (PDAC) carrying germline pathogenic/likely pathogenic variants (PVs/LPVs). (a) Distribution of patients based on functional gene groups (HRR, MMR, and others). (b) Distribution of patients with PVs/LPVs in MMR, HRR, and other genes based on the specific gene. Abbreviations: CNV: copy number variation; HRR: homologous recombination repair; MMR: mismatch repair.
3.3. Types of Variants Identified in HRR Genes (ATM, BRCA1, BRCA2, PALB2)
As indicated above, a total of 1225 out of 1493 (82%) PDAC patients carried PVs/LPVs in genes involved in the HRR pathway (Figure 2 and Table S3) [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
Among the 561 patients with BRCA2 germline PVs/LPVs (n = 308), 258 truncating variants (171 frameshift and 87 nonsense) were identified in 497 (88.5%) patients, 26 splicing alterations were identified in 32 (5.7%) patients, 20 missense variants were identified in 28 (5%) patients, and a unique in-frame deletion variant was identified in a single (0.2%) patient. Moreover, three (0.5%) patients were found to harbor different large deletions involving the BRCA2 gene (Figure 3a and Table S3).
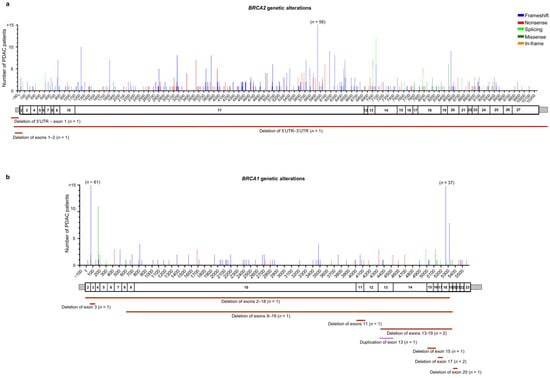
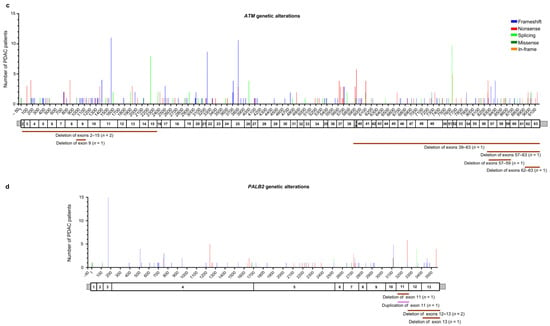
Figure 3.
Distribution of the alterations identified in HRR genes in the pancreatic ductal adenocarcinoma (PDAC) patients analyzed in this study: (a) BRCA2 (NM_000059.4), (b) BRCA1 (NM_7294.4), (c) ATM (NM_000051.4), and (d) PALB2 (NM_024675.4). For each gene, the upper panel shows the identified genetic alterations, with colored vertical bars representing the type of pathogenic variants (PVs) and likely pathogenic variants (LPVs). Color codes are as follows: blue bars = frameshift variants, red bars = nonsense variants, light green bars = splicing variants, dark green bars = missense variants, orange bars = in–frame variants. The height of the bar represents the number of PDAC patients harboring a genetic variant at the specified position. The x-axis represents the coding sequence of each gene. For each gene, the lower panel shows a schematic representation of its exons (rectangles). Copy number variants (CNVs) are represented by color codes: brown lines = large deletions, violet lines = large duplications.
Among the 239 patients with BRCA1 germline PVs/LPVs (n = 95), 70 truncating variants (50 frameshift and 20 nonsense) were identified in 198 patients (82.8%), nine splicing variants were identified in 10 (4.2%) patients, and seven missense variants were identified in 20 patients (8.4%). Moreover, 11 (4.6%) patients were found to carry nine different copy number variations (CNVs) involving the BRCA1 gene (Figure 3b and Table S3).
Among the 310 patients with ATM germline PVs/LPVs (n = 198), 137 truncating variants (81 frameshift and 56 nonsense) were identified in 224 (72.2%) patients, 31 splicing variants were identified in 47 (15.2%) patients, 21 missense variants were identified in 25 patients (8.1%), and three in–frame variants were identified in seven (2.3%) patients. Moreover, six CNVs (large deletions) involving the ATM gene were found in seven (2.3%) patients (Figure 3c and Table S3).
Among the 115 patients with PALB2 germline PVs/LPVs (n = 70), 57 truncating variants (38 frameshift and 19 nonsense) were identified in 98 (85.2%) patients, five splicing variants were identified in eight (7%) patients, and four missense variants were identified in four (3.5%) patients. Additionally, four CNVs were identified in five (4.3%) patients (Figure 3d and Table S3).
3.4. Types of Variants Identified in MMR Genes (EPCAM, MLH1, MSH2, MSH6, and PMS2)
As indicated above, a total of 87 out of 1493 (5.8%) PDAC patients carried PVs/LPVs in genes involved in the MMR (Figure 2 and Table S3). No individual was found to harbor germline causative genetic alterations involving the EPCAM gene.
Among the 23 patients with MLH1 germline PVs/LPVs (n = 19), five truncating variants (three nonsense and two frameshift) were identified in five patients (21.7%), four splicing variants were identified in seven patients (30.4%), and seven missense variants were identified in eight patients (34.8%). Additionally, two in-frame deletions were identified in two patients (8.7%). As regards CNVs, a unique CNV (large duplication involving MLH1 exons 9 and 12) was found in one patient (4.4%) (Figure 4a and Table S3).
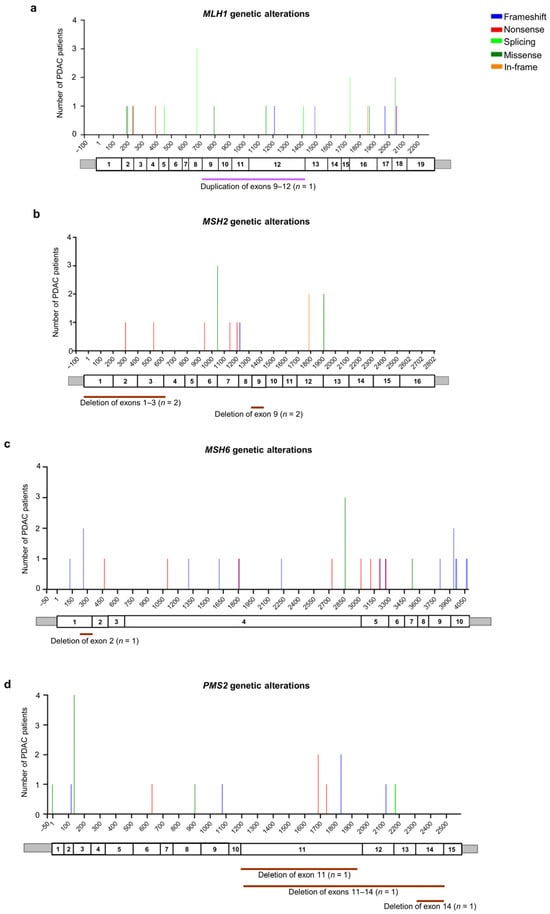
Figure 4.
Distribution of the alterations identified in MMR genes in the pancreatic ductal adenocarcinoma (PDAC) patients analyzed in this study: (a) MLH1 (NM_000249.4), (b) MSH2 (NM_000251.3), (c) MSH6 (NM_000179.3), and (d) PMS2 (NM_000535.7). For each gene, the upper panel shows the identified genetic alterations, with colored vertical bars representing the type of pathogenic variants (PVs) and likely pathogenic variants (LPVs). Color codes are as follows: blue bars = frameshift variants, red bars = nonsense variants, light green bars = splicing variants, dark green bars = missense variants, orange bars = in-frame variants. The height of the bar represents the number of PDAC patients harboring a genetic variant at the specified position. The x-axis represents the coding sequence of each gene. For each gene, the lower panel shows a schematic representation of its exons (rectangles). Copy number variants (CNVs) are represented by color codes: brown lines = large deletions, violet lines = large duplications.
Among the 18 patients with MSH2 germline PVs/LPVs (n = 12), six truncating variants (five nonsense and one frameshift) were identified in six patients (33.4%), and a single splicing variant was identified in one patient (5.6%). Moreover, two missense variants were identified in five patients (27.8%). In addition, two patients (11.1%) were found to carry one in-frame deletion and four patients (22.2%) were found to carry two different CNVs (large MSH2 deletions) (Figure 4b and Table S3).
Among the 26 patients with MSH6 germline PVs/LPVs (n = 25), 21 truncating variants (14 frameshift and seven nonsense) were identified in 22 patients (84.6%), two splicing variants were identified in two patients (7.7%), and a unique missense variant was identified in one patient (3.8%). Moreover, one patient (3.8%) was found to harbor a deletion involving MSH6 exon 2 (Figure 4c and Table S3).
Among the 20 patients with PMS2 germline PVs/LPVs (n = 16), eight truncating variants (five frameshift and three nonsense) were identified in nine patients (45%), two splicing variants were identified in two patients (10%), and three missense variants were identified in six patients (30%). Additionally, three CNVs (large deletions) involving the PMS2 gene were found in three patients (15%) (Figure 4d and Table S3).
3.5. Types of Variants Identified in Other Cancer–Related Genes (CDKN2A, STK11, TP53)
As indicated above, a total of 181 out of 1493 (12.1%) PDAC patients carried PVs/LPVs in genes (CDKN2A, STK11, TP53) involved in other cancer-related pathways (Figure 2 and Table S3).
Our analysis identified 146 patients with CDKN2A germline PVs/LPVs (n = 41). More specifically, 18 truncating variants (13 frameshift and five nonsense) were identified in 49 patients (33.6%), a single splicing variant was identified in three patients (2.1%), 19 missense variants were found in 90 patients (61.6%), and three in-frame deletions/duplications were identified in four patients (2.7%) (Figure 5a and Table S3).
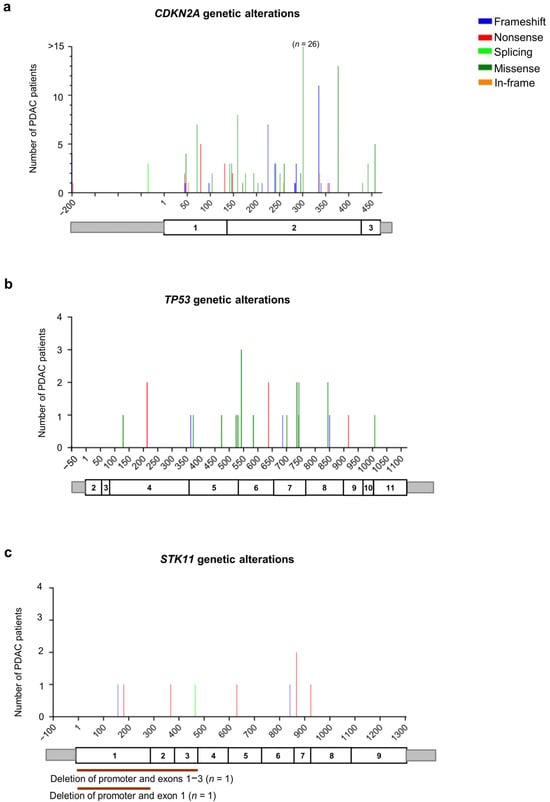
Figure 5.
Distribution of the alterations identified in genes involved in other cancer-related pathways in the pancreatic ductal adenocarcinoma (PDAC) patients analyzed in this study: (a) CDKN2A (NM_000077.5), (b) TP53 (NM_000546.6), and (c) STK11 (NM_000455.5). For each gene, the upper panel shows the identified genetic alterations, with colored vertical bars representing the type of pathogenic variants (PVs) and likely pathogenic variants (LPVs). Color codes are as follows: blue bars = frameshift variants, red bars = nonsense variants, light green bars = splicing variants, dark green bars = missense variants, orange bars = in-frame variants. The height of the bar represents the number of PDAC patients harboring a genetic variant at the specified position. The x-axis represents the coding sequence of each gene. For each gene, the lower panel shows a schematic representation of its exons (rectangles). Copy number variants (CNVs) are represented by color codes: brown lines = large deletions, violet lines = large duplications.
Among the 24 patients with TP53 germline PVs/LPVs (n = 20), we detected five truncating variants (three frameshift and two nonsense) in six patients (25%), and 15 missense variants in 18 patients (75%) (Figure 5b and Table S3).
Among the 11 patients with germline PVs/LPVs (n = 10) in the STK11 gene, we detected seven truncating variants (two frameshift and five nonsense) in eight patients (72.4%), and a unique splicing variant in a single patient (9.1%). Moreover, two deletions involving the promoter and part of the coding region (exon 1 and exons 1–3) of the STK11 gene were found in two patients (18.2%) (Figure 5d and Table S3).
4. Discussion
In this study, we sought to determine the distribution and type of germline pathogenic variants in the main genes associated with PDAC susceptibility. The identification of germline PVs/LPVs in susceptibility genes may enable specific surveillance programs to be provided to individuals at high risk of developing PDAC []. Specific PDAC surveillance programs have been proven to support the identification of premalignant lesions or pancreatic cancer at an early stage in high–risk individuals with or without a family history of PDAC [].
Based on the PDAC patients with germline PVs/LPVs identified through this systematic literature review, PVs/LPVs were most frequently reported in the ATM, BRCA1, BRCA2, and PALB2 genes. All these genes participate in the HRR pathway, which repairs DNA double–strand breaks (DSBs) [,]. BRCA1 and BRCA2 play a pivotal role in DNA damage response via the HRR pathway []. BRCA1/2–deficient cells lack HRR activity and accumulate DSBs, which results in genomic instability and increased cancer risk []. Germline genetic alterations affecting BRCA1/2 are primarily responsible for breast and ovarian cancer. In addition, PVs/LPVs involving these genes are associated with an increased risk of colon and prostate cancer []. Moreover, BRCA1/2 germline alterations have been reported in about 5–10% of patients with familial PDAC and about 3% of patients with apparently sporadic PDAC [,]. In the current study, we determined the frequency and distribution of HRR gene alterations in the PDAC patients reported in the articles included in our literature review.
Overall, a higher rate of PDAC patients (561/1225, 45.8%) was found to harbor PVs/LPVs in the BRCA2 gene as compared to PDAC patients harboring PVs/LPVs in other HRR genes (BRCA1, ATM, and PALB2). The vast majority of these patients (497/561, 88.5%) carried truncating variants, among which six alterations (c.3170_3174del, p.Lys1057fs; c.3847_3848del, p.Val1283Lysfs; c.5946del, p.Ser1982Argfs*22; c.6174del, p.Phe2058Leufs*12; c.6275_6276del, p.Leu2092Profs*7, c.8537_8538del, p.Glu2846Glyfs*22) recurred frequently.
In particular, the BRCA2 c.5946del (p.Ser1982Argfs*) recurring variant is reported to be a founder mutation in the Ashkenazi Jewish population [] and was identified in 56 PDAC patients. Other BRCA2 variant types (i.e., missense, in–frame deletion, CNV, and splicing) were identified with a low frequency in PDAC patients.
As regards PDAC patients (n = 239) harboring BRCA1 germline alterations, most of them (198/239, 82.8%) had truncating variants. Of these, three were commonly occurring founder mutations (c.68_69delAG, p.Glu23fs; c.5266dup, p.Gln1756Pfs*74; c.5319dup, p.Asn1774Glnfs*56) [,,]. Other BRCA1 variant types (i.e., missense, and splicing) were identified with a lower frequency in PDAC patients (12.6%) as compared to truncating variants.
The initial phase of the HRR pathway is orchestrated by the kinases ATM and ATR, which are responsible for the phosphorylation of several proteins involved in downstream steps of the repair cascade []. Biallelic germline variants in the ATM gene cause ataxia–telangiectasia, a disorder characterized by neuronal degeneration, immune deficiency, and increased cancer risk []. On the other hand, monoallelic ATM germline variants have been shown to be associated with an increased risk of developing malignancies, including breast, pancreatic, prostate, stomach, ovarian, colorectal, and melanoma []. Importantly, monoallelic ATM germline alterations have also been reported in familial and sporadic cases of PDAC []. In addition, biallelic ATM inactivation has been found with a higher frequency in PDAC specimens from familial pancreatic cancer patients compared to sporadic cases []. In one study, the relative risk of pancreatic cancer was estimated to be 2.41 in patients carrying a monoallelic ATM germline variant []. In our literature review analysis, 198 distinct germline mutations in the ATM gene were detected in 310 (25.3%) PDAC patients. Within this subset, a high proportion of PVs/LPVs (137, 69.2%) were truncating variants. Four of these truncating variants (c.1564_1565del, p.Glu522fs; c.3245_3247delinsTGAT, p.His1082Leufs*14; c.3802del, p.Val1268fs; c.5932G>T, p.Glu1978*) recurred in more than five PDAC patients. Interestingly, among the several ATM splicing variants (n = 31, 15.6%) identified in 47 PDAC patients in our literature review, one (c.7630-2A>C) recurred in 10 patients.
The PALB2 gene plays a crucial role in the HRR pathway via modulation of BRCA2 and RAD51 recruitment to DSBs. Germline PALB2 pathogenic variants have been associated with an increased risk of breast, ovarian, and pancreatic cancer [,]. Our literature review analysis showed that a lower rate of PDAC patients (115/1225, 9.4%) was found to harbor PVs/LPVs in the PALB2 gene as compared to PDAC patients harboring PVs/LPVs in other HRR genes (BRCA1, BRCA2, and ATM). In this subgroup, most patients (98/115, 85.2%) carried truncating variants. Four of these PALB2 truncating variants (c.172_175del, p.Gln60Argfst*7; c.1240C>T, p.Arg414*; c.3116del, p.Asn1039fs; c.3256C>T, p.Arg1086*) recurred in five or more PDAC unrelated patients. Interestingly, other PALB2 variant types (i.e., missense and splicing) were identified with a lower frequency in PDAC patients (n = 12, 10.5%) as compared to truncating variants. The remaining four (5.7%) genetic alterations were large PALB2 deletions/duplications and were identified in five (4.3%) patients. Of note, all of them involved the C–terminal region of the gene.
Clinical oncology research is increasingly focusing on the identification of germline and somatic alterations in HRR genes as a way to guide targeted therapy in different types of cancers, including PDAC. Recent retrospective [,] and prospective studies [] examining the use of the DSB–inducing drug cisplatin in PDAC patients with altered BRCA1/2 have shown clinical benefit. Moreover, poly(ADP–ribose) polymerase (PARP) inhibitors have shown promise as a treatment for tumors with BRCA mutations. In particular, PARP inhibitors were found to increase progression–free survival when used as a maintenance therapy for patients with pancreatic cancer who responded to first–line platinum–based therapy [].
Recent studies have shown that germline alterations in MMR genes (MLH1, MSH2, MSH6, PMS2, and EPCAM) are primarily responsible for LS, a common hereditary colorectal cancer syndrome [,]. LS confers an increased risk of multiple types of malignancies, including colorectal, endometrial, ovarian, stomach, small intestine, hepatobiliary system, urinary tract, and brain cancer [,]. The loss of DNA MMR activity determines the high microsatellite instability (MSI–H) phenotype observed in tumor samples of these patients [,]. Moreover, patients with a molecular diagnosis of LS also have an estimated 9– to 11–fold increased risk of pancreatic cancer [,].
To obtain further insight into the involvement of MMR gene alterations in PDAC, we determined their frequency and distribution in the PDAC patients reported in the articles included in our literature review. Collectively, germline mutations in the MLH1, MSH2, PMS2, and MSH6 genes were detected in 5.8% of PDAC patients, with comparable frequencies for each of these genes. No germline alteration was identified in the EPCAM gene.
As regards the MLH1 genetic alterations identified in PDAC patients, they were evenly distributed throughout the coding region, without obvious hotspots. Among these PVs/LPVs, a similar proportion of missense and truncating variants was observed in PDAC patients. Interestingly, three patients harbored the splicing variant c.677+3A>G, which has been reported to cause skipping of MLH1 exon 8 []. Furthermore, two PDAC patients carried the splicing variant c.1731G>A (p.Ser577=), which is located next to the exon 15 splicing donor site and has been functionally shown to cause skipping of MLH1 exon 15 []. Additionally, two patients were found to carry the missense variant c.2041G>A (p.Ala681Thr), which has been reported as pathogenic despite inconclusive data on protein expression and normal MMR activity resulting from experimental evaluation of different studies [].
Our analysis also showed that 18 PDAC patients (20.7%) had PVs/LPVs in the MSH2 gene. Intriguingly, these PVs/LPVs (missense, splicing, and truncating variants) were found to cluster in a hotspot ranging from nucleotide 900 to nucleotide 1300 of the MSH2 coding region. The MSH2 missense variant c.1046C>T (p.Pro349Leu) was found in three patients [].
Moreover, we found that 26 PDAC patients carried distinct germline PVs/LPVs in the MSH6 gene. Of these, truncating variants were identified with a higher frequency (22/26, 84.6%) as compared to other alteration types (missense and splicing), which were found in four patients (15.4%).
Concerning PMS2, a total of 20 PDAC patients were identified as harboring genetic alterations in this gene. Interestingly, a higher rate of PDAC patients (9/20, 45%) had PMS2 truncating variants as compared to PDAC patients (6/20, 30%) with PMS2 missense variants.
MSI-H pancreatic cancers are defective in DNA MMR and can occur in inherited disorders such as LS. Patients with this type of pancreatic cancer are less responsive to fluorouracil and gemcitabine and more responsive to FOLFIRINOX [,]. Furthermore, pembrolizumab, an inhibitor of the immune checkpoint protein PD–1, has been approved for several MMR-deficient tumor types []. Indeed, a review of immune–based treatment approaches for patients with MSI–H pancreatic cancer suggests that immune checkpoint inhibition therapy is effective and has the potential to provide a good response in this subgroup of patients [].
Of note, a subset of pancreatic cancers arises in the background of Peutz–Jeghers syndrome (PJS), which is caused by germline alterations in the STK11 gene. STK11 encodes for a tumor suppressor serine/threonine protein kinase that controls the activity of AMPK family members []. Individuals with PJS have gastrointestinal polyps and an increased risk of CRC as well as extracolonic malignancies, including stomach, lung, breast, ovarian, cervical, testes, and pancreatic cancer []. Based on literature data, the cumulative risk of developing PDAC at the age of 70 years in these patients ranges from 11 to 36%, which represents a 132–fold increase compared to the general population [,]. Our literature review analysis showed that 10 germline variants in the STK11 gene were detected in 11 (6.1%) PDAC patients. Most of these alterations (n = 7, 70%) were truncating variants, which were interspersed throughout the region encoding for STK11 kinase catalytic domain (aa 49–309). The remaining alterations (n = 3, 30%) were splicing variants and large deletions involving the promoter and the first exons (exon 1 and/or 3) of the STK11 gene. Importantly, somatic alterations in the STK11 gene have emerged as potential therapy targets in patients with non–small cell lung cancer (NSCLC), an approach that may also lead to novel therapeutic opportunities in PDAC []. Germline alterations in the CDKN2A (p16INK4a/p14ARF) gene are responsible for familial melanoma–pancreatic cancer syndrome. In addition, CDKN2A is a susceptibility gene for head and neck squamous cell carcinoma (HNSCC), lung cancer, esophageal cancer, neural system cancer, breast carcinoma, and sarcomas []. CDKN2A is a tumor suppressor gene playing a central role in the regulation of the cell cycle and induction of apoptosis. Patients with CDKN2A germline alterations have a 20– to 47–fold increased risk of developing PDAC [,]. Our literature review analysis showed that 41 germline variants in the CDKN2A gene were detected in 146 PDAC patients. Twenty–seven of these alterations were found to be recurrent, i.e., occurring in more than one individual, while the remaining fourteen were identified in one patient each. Several of these CDKN2A PVs/PLVs were previously identified as founder mutations in different populations: c.301G>T, p.Gly101Trp (detected in 26 PDAC patients) in southeastern Europe [], c.377T>A, p.Val126Asp (detected in 13 PDAC patients) in North America [], c.335_337dup, p.Arg112dup (detected in 11 PDAC patients) in Sweden [], and c.225_243del, p.Ala76Cysfs*64 (detected in 7 PDAC patients) in the Netherlands []. Moreover, another CDKN2A founder mutation (c.159G>C; p.Met53Ile), mostly occurring in the Scottish population [], was detected in eight patients. While limited achievements have been reported in targeted monotherapy in pancreatic cancer harboring CDKN2A loss-of-function genomic alterations [], a recent pilot study demonstrated efficacy in a small group of PDAC patients with CDKN2A somatic alterations treated with a combination of genomically matched targeted agents [].
Germline mutations in the TP53 gene are responsible for Li–Fraumeni syndrome, which is characterized by autosomal dominant inheritance and early onset of a wide range of tumor types, including soft tissue sarcomas and osteosarcomas, breast cancer, brain tumors, leukemia, and adrenocortical carcinoma. Moreover, this syndrome is characterized by a high risk of pancreatic cancer []. Our literature review analysis showed that a few germline variants in the TP53 gene were detected in 24 (13.2%) PDAC patients, most of which were missense mutations (75%) interspersed throughout the coding region of the gene. Moreover, three missense variants (c.542G>A, p.Arg181His; c.736A>G, p.Met246Val; c.844C>T, p.Arg282Trp) were identified in each of two unrelated individuals. Of note, TP53 mutations are critical drivers that influence the carcinogenesis and prognosis of PDAC patients []. Recently, a phase I trial demonstrated the therapeutic efficacy of gene therapy based on SGT–53, an antitumor agent comprising a cationic liposome encapsulating a plasmid encoding wild–type p53, in the treatment of different solid tumors []. Interestingly, a phase II clinical trial of SGT–53 plus gemcitabine and nab–paclitaxel (NCT02340117) is being conducted for advanced PDAC. Overall, the mutational status of the TP53 gene may prove useful to guide therapeutic strategies in PDAC patients [].
In the era of precision medicine, the integration of germline and somatic genetic profiling is gaining a central role in medical oncology. Indeed, germline and somatic genetic testing may help personalize therapeutic decisions for targeted therapies according to each patient’s mutational status []. Moreover, germline characterization of genetic variants underlying cancer susceptibility may enable the identification of individuals at risk of developing a particular cancer and guide precision prevention strategies []. Each individual’s overall risk of cancer depends at least in part on the type of genetic variant and the phenotypic effect of the altered genes. As regards PDAC susceptibility genes, BRCA1 and BRCA2 are considered high penetrance genes in breast and ovarian cancer, and MLH1 and MSH2 are considered high penetrance genes in LS CRC []. On the other hand, ATM and PALB2 are considered moderate penetrance genes in breast and ovarian cancer [], and MSH6, PMS2, and EPCAM are considered moderate penetrance genes in LS CRC []. Additionally, CDKN2A, STK11, and TP53 are considered highly penetrant genes in melanoma, juvenile polyposis syndrome (JPS), and Li–Fraumeni syndrome, respectively. In this study, a high proportion of PDAC patients were found to harbor truncating variants, which are expected to have a more deleterious effect on gene function, in moderate penetrance genes (ATM, PALB2, MSH6, PMS2). Conversely, alterations that are likely to have a lesser impact on gene function, such as missense variants, were predominantly detected in the subgroup of high penetrance genes (CDKN2A, TP53) in these patients. Instead, missense and truncating variants involving the MLH1 and MSH2 genes were identified in a similar proportion of PDAC patients. As regards the STK11 gene, which has high penetrance for the development of mucocutaneous pigmentation and gastrointestinal hamartomatous polyposis in JPS, a higher proportion of PDAC patients were found to carry truncating variants as compared to missense variants. Moreover, truncating variants were predominantly detected in the high penetrant genes BRCA1 and BRCA2 in these patients.
Altogether, these findings help decipher the genetic landscape of relevant cancer susceptibility genes in PDAC patients. As such, they may support the development of tailored management and follow–up strategies in PDAC patients with specific germline genetic variants and a personal and/or familial history of cancer.
However, this systematic review study has various limitations.
First, we only included studies that focused on PDAC cancer patients who underwent genetic testing for hereditary syndromes known to be associated with an increased risk of PDAC, which limits a more comprehensive understanding of the role of other emerging genes on genetic predisposition to PDAC. Second, the present systematic literature review includes studies published from 1976 to 2023. The inclusion of outdated scientific articles may have influenced the accuracy or relevance of the results due to older methodologies associated with clinical screening and genetic testing. Third, this systematic review study excludes scientific articles not providing a detailed genetic annotation of genetic variants, which limits a broader integration of clinical information of other PDAC patients. Moreover, the present study includes PDAC patients described in scientific articles, which may not reflect the broader global diversity of all patients with PDAC, thus weakening the generalizability of the findings. A further limitation of the present study is represented by the inclusion of PDAC patients with a known genetic predisposition to PDAC. Notably, PDAC is a multifactorial disease influenced by a combination of genetic, environmental, and lifestyle factors. The integration of these aspects into future research could provide a more holistic understanding of PDAC risk and development.
This systematic review study aimed to decipher the genetic landscape of patients with any type or stage of PDAC. In this regard, the lack of standardized clinical data at the individual patient level in the selected articles limits the comprehension of specific associations between genetic findings and disease characteristics.
5. Conclusions
In this systematic literature review study, we explored the genetic landscape of PDAC in patients with germline genetic variants in major PDAC susceptibility genes. The molecular characterization of these patients highlights the importance of personalized medicine to provide tailored genetic counseling, management, and surveillance to families with PDAC and hereditary cancer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16010056/s1; Table S1: PRISMA checklist; Table S2: Data characteristics of the included studies; Table S3: Genetic causative variants in patients with pancreatic ductal adenocarcinoma (PDAC).
Author Contributions
Conceptualization, A.P. and C.S.; validation, A.P., G.F., C.F. and V.D.; formal A.P., G.F., C.F., M.L.S., P.S., K.D.M., E.D.N., M.L., V.G. and V.D.; investigation, A.P., G.F., C.F., M.L.S., P.S., K.D.M., E.D.N., M.L., V.G. and V.D.; resources, V.G. and C.S.; writing, A.P., G.F. and V.D.; writing—review and editing, A.P., G.F., V.D. and C.S.; visualization, A.P., G.F. and V.D.; supervision, C.S. and V.G.; project administration, V.G. and C.S.; funding acquisition, C.S., V.D., C.F., M.L.S., P.S. and V.G. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results has received funding from Italian Association for Cancer Research: IG-23794 2020–2024 to Cristiano Simone; Italian Association for Cancer Research: Airc Fellowship for Italy ID 26678-2021 to Martina Lepore Signorile; Italian Ministry of Health: Ricerca Corrente 2021–2023 to Cristiano Simone; Italian Ministry of Health: Ricerca Corrente 2022-2024 to Vittoria Disciglio; Italian Ministry of Health: Ricerca Corrente 2022-2024 to Candida Fasano; Italian Ministry of Health: Ricerca Corrente 2023-2025 to Valentina Grossi; Italian Ministry of Health: Starting Grant SG-2019-12371540 to Paola Sanese.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
We thank Francesco Paolo Jori for his helpful discussion during the preparation of the manuscript and for editorial assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Pancreatic Cancer Collaborators. The Global, Regional, and National Burden of Pancreatic Cancer and Its Attributable Risk Factors in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947. [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, S.; Wu, B.; Wang, Z. Clinicopathological Features, Prognostic Factors and Survival in Patients with Pancreatic Cancer Bone Metastasis. Front. Oncol. 2022, 12, 759403. [Google Scholar] [CrossRef]
- Argentiero, A.; Calabrese, A.; Solimando, A.G.; Notaristefano, A.; Panarelli, M.M.G.; Brunetti, O. Bone Metastasis as Primary Presentation of Pancreatic Ductal Adenocarcinoma: A Case Report and Literature Review. Clin. Case Rep. 2019, 7, 1972–1976. [Google Scholar] [CrossRef]
- Klatte, D.C.F.; Boekestijn, B.; Onnekink, A.M.; Dekker, F.W.; van der Geest, L.G.; Wasser, M.N.J.M.; Feshtali, S.; Mieog, J.S.D.; Luelmo, S.A.C.; Morreau, H.; et al. Surveillance for Pancreatic Cancer in High-Risk Individuals Leads to Improved Outcomes: A Propensity Score-Matched Analysis. Gastroenterology 2023, 164, 1223–1231.e4. [Google Scholar] [CrossRef]
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic Cancer: Advances and Challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef]
- Pilarski, R. The Role of BRCA Testing in Hereditary Pancreatic and Prostate Cancer Families. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 79–86. [Google Scholar] [CrossRef]
- Chaffee, K.G.; Oberg, A.L.; McWilliams, R.R.; Majithia, N.; Allen, B.A.; Kidd, J.; Singh, N.; Hartman, A.-R.; Wenstrup, R.J.; Petersen, G.M. Prevalence of Germline Mutations in Cancer Genes among Pancreatic Cancer Patients with Positive Family History. Genet. Med. 2018, 20, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Klatte, D.C.F.; Wallace, M.B.; Löhr, M.; Bruno, M.J.; van Leerdam, M.E. Hereditary Pancreatic Cancer. Best Pract. Res. Clin. Gastroenterol. 2022, 58–59, 101783. [Google Scholar] [CrossRef] [PubMed]
- Klatte, D.C.F.; Boekestijn, B.; Wasser, M.N.J.M.; Feshtali Shahbazi, S.; Ibrahim, I.S.; Mieog, J.S.D.; Luelmo, S.A.C.; Morreau, H.; Potjer, T.P.; Inderson, A.; et al. Pancreatic Cancer Surveillance in Carriers of a Germline CDKN2A Pathogenic Variant: Yield and Outcomes of a 20-Year Prospective Follow-Up. J. Clin. Oncol. 2022, 40, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L.; Yoo, H.K.; Hettle, R.; Cui, K.Y.; Joo, S.; Locker, G.Y.; Golan, T. Patient-Centered Outcomes in the POLO Study of Active Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. Cancer 2023, 129, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Crowley, F.; Gandhi, S.; Rudshteyn, M.; Sehmbhi, M.; Cohen, D.J. Adherence to NCCN Genetic Testing Guidelines in Pancreatic Cancer and Impact on Treatment. Oncology 2023, 28, 486–493. [Google Scholar] [CrossRef]
- Stoffel, E.M.; McKernin, S.E.; Brand, R.; Canto, M.; Goggins, M.; Moravek, C.; Nagarajan, A.; Petersen, G.M.; Simeone, D.M.; Yurgelun, M.; et al. Evaluating Susceptibility to Pancreatic Cancer: ASCO Provisional Clinical Opinion. J. Clin. Oncol. 2019, 37, 153–164. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Golan, T.; O’Kane, G.M.; Denroche, R.E.; Raitses-Gurevich, M.; Grant, R.C.; Holter, S.; Wang, Y.; Zhang, A.; Jang, G.H.; Stossel, C.; et al. Genomic Features and Classification of Homologous Recombination Deficient Pancreatic Ductal Adenocarcinoma. Gastroenterology 2021, 160, 2119–2132.e9. [Google Scholar] [CrossRef]
- Shindo, K.; Yu, J.; Suenaga, M.; Fesharakizadeh, S.; Cho, C.; Macgregor-Das, A.; Siddiqui, A.; Witmer, P.D.; Tamura, K.; Song, T.J.; et al. Deleterious Germline Mutations in Patients with Apparently Sporadic Pancreatic Adenocarcinoma. JCO 2017, 35, 3382–3390. [Google Scholar] [CrossRef]
- Puccini, A.; Ponzano, M.; Dalmasso, B.; Vanni, I.; Gandini, A.; Puglisi, S.; Borea, R.; Cremante, M.; Bruno, W.; Andreotti, V.; et al. Clinical Significance of Germline Pathogenic Variants among 51 Cancer Predisposition Genes in an Unselected Cohort of Italian Pancreatic Cancer Patients. Cancers 2022, 14, 4447. [Google Scholar] [CrossRef]
- Lowery, M.A.; Wong, W.; Jordan, E.J.; Lee, J.W.; Kemel, Y.; Vijai, J.; Mandelker, D.; Zehir, A.; Capanu, M.; Salo-Mullen, E.; et al. Prospective Evaluation of Germline Alterations in Patients with Exocrine Pancreatic Neoplasms. JNCI J. Natl. Cancer Inst. 2018, 110, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; LaDuca, H.; Shimelis, H.; Polley, E.C.; Lilyquist, J.; Hart, S.N.; Na, J.; Thomas, A.; Lee, K.Y.; Davis, B.T.; et al. Multigene Hereditary Cancer Panels Reveal High-Risk Pancreatic Cancer Susceptibility Genes. JCO Precis. Oncol. 2018, 2, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Astiazaran-Symonds, E.; Kim, J.; Haley, J.S.; Kim, S.Y.; Rao, H.S.; Genetics Center, R.; Carey, D.J.; Stewart, D.R.; Goldstein, A.M. A Genome-First Approach to Estimate Prevalence of Germline Pathogenic Variants and Risk of Pancreatic Cancer in Select Cancer Susceptibility Genes. Cancers 2022, 14, 3257. [Google Scholar] [CrossRef] [PubMed]
- Antwi, S.O.; Fagan, S.E.; Chaffee, K.G.; Bamlet, W.R.; Hu, C.; Polley, E.C.; Hart, S.N.; Shimelis, H.; Lilyquist, J.; Gnanaolivu, R.D.; et al. Risk of Different Cancers Among First-Degree Relatives of Pancreatic Cancer Patients: Influence of Probands’ Susceptibility Gene Mutation Status. JNCI J. Natl. Cancer Inst. 2019, 111, 264–271. [Google Scholar] [CrossRef]
- Xiong, A.; Ma, N.; Wei, G.; Li, C.; Li, K.; Wang, B. Genomic Alterations in Tumor Tissue and ctDNA from Chinese Pancreatic Cancer Patients. Am. J. Cancer Res. 2021, 11, 4551–4567. [Google Scholar]
- Chittenden, A.; Haraldsdottir, S.; Ukaegbu, C.; Underhill-Blazey, M.; Gaonkar, S.; Uno, H.; Brais, L.K.; Perez, K.; Wolpin, B.M.; Syngal, S.; et al. Implementing Systematic Genetic Counseling and Multigene Germline Testing for Individuals with Pancreatic Cancer. JCO Oncol. Pract. 2021, 17, e236–e247. [Google Scholar] [CrossRef]
- Cremin, C.; Lee, M.K.; Hong, Q.; Hoeschen, C.; Mackenzie, A.; Dixon, K.; McCullum, M.; Nuk, J.; Kalloger, S.; Karasinska, J.; et al. Burden of Hereditary Cancer Susceptibility in Unselected Patients with Pancreatic Ductal Adenocarcinoma Referred for Germline Screening. Cancer Med. 2020, 9, 4004–4013. [Google Scholar] [CrossRef]
- Dal Buono, A.; Poliani, L.; Greco, L.; Bianchi, P.; Barile, M.; Giatti, V.; Bonifacio, C.; Carrara, S.; Malesci, A.; Laghi, L. Prevalence of Germline Mutations in Cancer Predisposition Genes in Patients with Pancreatic Cancer or Suspected Related Hereditary Syndromes: Historical Prospective Analysis. Cancers 2023, 15, 1852. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hart, S.N.; Polley, E.C.; Gnanaolivu, R.; Shimelis, H.; Lee, K.Y.; Lilyquist, J.; Na, J.; Moore, R.; Antwi, S.O.; et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA 2018, 319, 2401. [Google Scholar] [CrossRef]
- Mizukami, K.; Iwasaki, Y.; Kawakami, E.; Hirata, M.; Kamatani, Y.; Matsuda, K.; Endo, M.; Sugano, K.; Yoshida, T.; Murakami, Y.; et al. Genetic Characterization of Pancreatic Cancer Patients and Prediction of Carrier Status of Germline Pathogenic Variants in Cancer-Predisposing Genes. eBioMedicine 2020, 60, 103033. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.G.; Stevens, M.A.; Hernández, A.T.; Chandra, S.; Hubbard, J.M.; Kemppainen, J.L.; Majumder, S.; Petersen, G.M. Pancreatic Cancer Risk to Siblings of Probands in Bilineal Cancer Settings. Genet. Med. 2022, 24, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wei, J.; Lu, Z.; Huang, S.; Gao, H.; Chen, J.; Guo, F.; Tu, M.; Xiao, B.; Xi, C.; et al. Prevalence of Germline Sequence Variations Among Patients with Pancreatic Cancer in China. JAMA Netw. Open 2022, 5, e2148721. [Google Scholar] [CrossRef] [PubMed]
- Slavin, T.P.; Neuhausen, S.L.; Nehoray, B.; Niell-Swiller, M.; Solomon, I.; Rybak, C.; Blazer, K.; Adamson, A.; Yang, K.; Sand, S.; et al. The Spectrum of Genetic Variants in Hereditary Pancreatic Cancer Includes Fanconi Anemia Genes. Fam. Cancer 2018, 17, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Takai, E.; Yachida, S.; Shimizu, K.; Furuse, J.; Kubo, E.; Ohmoto, A.; Suzuki, M.; Hruban, R.H.; Okusaka, T.; Morizane, C.; et al. Germline Mutations in Japanese Familial Pancreatic Cancer Patients. Oncotarget 2016, 7, 74227–74235. [Google Scholar] [CrossRef] [PubMed]
- Takai, E.; Nakamura, H.; Chiku, S.; Kubo, E.; Ohmoto, A.; Totoki, Y.; Shibata, T.; Higuchi, R.; Yamamoto, M.; Furuse, J.; et al. Whole-Exome Sequencing Reveals New Potential Susceptibility Genes for Japanese Familial Pancreatic Cancer. Ann. Surg. 2022, 275, e652–e658. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Wong, C.; Cuggia, A.; Borgida, A.; Holter, S.; Hall, A.; Connor, A.A.; Bascuñana, C.; Asselah, J.; Bouganim, N.; et al. Reflex Testing for Germline BRCA1, BRCA2, PALB2, and ATM Mutations in Pancreatic Cancer: Mutation Prevalence and Clinical Outcomes from Two Canadian Research Registries. JCO Precis. Oncol. 2018, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, D.; Jiang, Z.; Skaro, M.; Weiss, M.J.; Wolfgang, C.L.; Makary, M.A.; He, J.; Cameron, J.L.; Zheng, L.; Klimstra, D.S.; et al. Histomorphology of Pancreatic Cancer in Patients with Inherited ATM Serine/Threonine Kinase Pathogenic Variants. Mod. Pathol. 2019, 32, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.B.; Zhao, L.; Wang, X.; Ghelman, Y.; Overman, M.J.; Javle, M.M.; Shroff, R.T.; Varadhachary, G.R.; Wolff, R.A.; McAllister, F.; et al. Germline DNA Sequencing Reveals Novel Mutations Predictive of Overall Survival in a Cohort of Patients with Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 1385–1394. [Google Scholar] [CrossRef]
- Walker, E.J.; Carnevale, J.; Pedley, C.; Blanco, A.; Chan, S.; Collisson, E.A.; Tempero, M.A.; Ko, A.H. Referral Frequency, Attrition Rate, and Outcomes of Germline Testing in Patients with Pancreatic Adenocarcinoma. Fam. Cancer 2019, 18, 241–251. [Google Scholar] [CrossRef]
- Zimmermann, M.T.; Mathison, A.J.; Stodola, T.; Evans, D.B.; Abrudan, J.L.; Demos, W.; Tschannen, M.; Aldakkak, M.; Geurts, J.; Lomberk, G.; et al. Interpreting Sequence Variation in PDAC-Predisposing Genes Using a Multi-Tier Annotation Approach Performed at the Gene, Patient, and Cohort Level. Front. Oncol. 2021, 11, 606820. [Google Scholar] [CrossRef]
- Ryu, K.H.; Park, S.; Chun, J.W.; Cho, E.; Choi, J.; Lee, D.-E.; Shim, H.; Kim, Y.-H.; Han, S.-S.; Park, S.-J.; et al. Prevalence and Risk Factors of Germline Pathogenic Variants in Pancreatic Ductal Adenocarcinoma. Cancer Res. Treat. 2023, 55, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Shui, L.; Li, X.; Peng, Y.; Tian, J.; Li, S.; He, D.; Li, A.; Tian, B.; Li, M.; Gao, H.; et al. The Germline/Somatic DNA Damage Repair Gene Mutations Modulate the Therapeutic Response in Chinese Patients with Advanced Pancreatic Ductal Adenocarcinoma. J. Transl. Med. 2021, 19, 301. [Google Scholar] [CrossRef] [PubMed]
- Dudley, B.; Karloski, E.; Monzon, F.A.; Singhi, A.D.; Lincoln, S.E.; Bahary, N.; Brand, R.E. Germline Mutation Prevalence in Individuals with Pancreatic Cancer and a History of Previous Malignancy. Cancer 2018, 124, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Huang, F.; Chen, X.; Zhang, L.; Shen, M.; Pan, B.; Wang, B.; Guo, W. Germline Mutations in Homologous Recombination Repair Genes among Chinese Pancreatic Ductal Adenocarcinoma Patients Detected Using Next-Generation Sequencing. Molec. Genet. Gen. Med. 2023, 11, e2170. [Google Scholar] [CrossRef]
- Schwartz, M.; Korenbaum, C.; Benfoda, M.; Mary, M.; Colas, C.; Coulet, F.; Parrin, M.; Jonveaux, P.; Ingster, O.; Granier, S.; et al. Familial Pancreatic Adenocarcinoma: A Retrospective Analysis of Germline Genetic Testing in a French Multicentre Cohort. Clin. Genet. 2019, 96, 579–584. [Google Scholar] [CrossRef]
- Walker, E.J.; Goldberg, D.; Gordon, K.M.; Pedley, C.; Carnevale, J.; Cinar, P.; Collisson, E.A.; Tempero, M.A.; Ko, A.H.; Blanco, A.M.; et al. Implementation of an Embedded In-Clinic Genetic Testing Station to Optimize Germline Testing for Patients with Pancreatic Adenocarcinoma. Oncology 2021, 26, e1982–e1991. [Google Scholar] [CrossRef]
- Brand, R.; Borazanci, E.; Speare, V.; Dudley, B.; Karloski, E.; Peters, M.L.B.; Stobie, L.; Bahary, N.; Zeh, H.; Zureikat, A.; et al. Prospective Study of Germline Genetic Testing in Incident Cases of Pancreatic Adenocarcinoma. Cancer 2018, 124, 3520–3527. [Google Scholar] [CrossRef]
- Grant, R.C.; Al-Sukhni, W.; Borgida, A.E.; Holter, S.; Kanji, Z.S.; McPherson, T.; Whelan, E.; Serra, S.; Trinh, Q.M.; Peltekova, V.; et al. Exome Sequencing Identifies Nonsegregating Nonsense ATM and PALB2variants in Familial Pancreatic Cancer. Hum. Genom. 2013, 7, 11. [Google Scholar] [CrossRef]
- Grant, R.C.; Selander, I.; Connor, A.A.; Selvarajah, S.; Borgida, A.; Briollais, L.; Petersen, G.M.; Lerner-Ellis, J.; Holter, S.; Gallinger, S. Prevalence of Germline Mutations in Cancer Predisposition Genes in Patients with Pancreatic Cancer. Gastroenterology 2015, 148, 556–564. [Google Scholar] [CrossRef]
- Roberts, N.J.; Norris, A.L.; Petersen, G.M.; Bondy, M.L.; Brand, R.; Gallinger, S.; Kurtz, R.C.; Olson, S.H.; Rustgi, A.K.; Schwartz, A.G.; et al. Whole Genome Sequencing Defines the Genetic Heterogeneity of Familial Pancreatic Cancer. Cancer Discov. 2016, 6, 166–175. [Google Scholar] [CrossRef]
- Zhan, Q.; Wen, C.; Zhao, Y.; Fang, L.; Jin, Y.; Zhang, Z.; Zou, S.; Li, F.; Yang, Y.; Wu, L.; et al. Identification of Copy Number Variation-Driven Molecular Subtypes Informative for Prognosis and Treatment in Pancreatic Adenocarcinoma of a Chinese Cohort. eBioMedicine 2021, 74, 103716. [Google Scholar] [CrossRef] [PubMed]
- Tavano, F.; Gioffreda, D.; Fontana, A.; Palmieri, O.; Gentile, A.; Latiano, T.; Latiano, A.; Latiano, T.P.; Scaramuzzi, M.; Maiello, E.; et al. Evaluation of Inherited Germline Mutations in Cancer Susceptibility Genes among Pancreatic Cancer Patients: A Single-Center Study. Mol. Med. 2023, 29, 14. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.J.; Nowak, J.A.; Camarda, N.D.; Moffitt, R.A.; Ghazani, A.A.; Hazar-Rethinam, M.; Raghavan, S.; Kim, J.; Brais, L.K.; Ragon, D.; et al. Real-Time Genomic Characterization of Advanced Pancreatic Cancer to Enable Precision Medicine. Cancer Discov. 2018, 8, 1096–1111. [Google Scholar] [CrossRef] [PubMed]
- Yurgelun, M.B.; Chittenden, A.B.; Morales-Oyarvide, V.; Rubinson, D.A.; Dunne, R.F.; Kozak, M.M.; Qian, Z.R.; Welch, M.W.; Brais, L.K.; Da Silva, A.; et al. Germline Cancer Susceptibility Gene Variants, Somatic Second Hits, and Survival Outcomes in Patients with Resected Pancreatic Cancer. Genet. Med. 2019, 21, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Borazanci, E.; Korn, R.; Liang, W.S.; Guarnieri, C.; Haag, S.; Snyder, C.; Hendrickson, K.; Caldwell, L.; Von Hoff, D.; Jameson, G. An Analysis of Patients with DNA Repair Pathway Mutations Treated with a PARP Inhibitor. Oncology 2020, 25, e60–e67. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Lowery, M.A.; Berger, M.F.; Kemel, Y.; Taylor, B.; Zehir, A.; Srinivasan, P.; Bandlamudi, C.; Chou, J.; Capanu, M.; et al. Ampullary Cancer: Evaluation of Somatic and Germline Genetic Alterations and Association with Clinical Outcomes. Cancer 2019, 125, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.A.; Denroche, R.E.; Jang, G.H.; Timms, L.; Kalimuthu, S.N.; Selander, I.; McPherson, T.; Wilson, G.W.; Chan-Seng-Yue, M.A.; Borozan, I.; et al. Association of Distinct Mutational Signatures with Correlates of Increased Immune Activity in Pancreatic Ductal Adenocarcinoma. JAMA Oncol. 2017, 3, 774. [Google Scholar] [CrossRef] [PubMed]
- Lovecek, M.; Janatova, M.; Skalicky, P.; Zemanek, T.; Havlik, R.; Ehrmann, J.; Strouhal, O.; Zemankova, P.; Lhotova, K.; Borecka, M.; et al. Genetic Analysis of Subsequent Second Primary Malignant Neoplasms in Long-Term Pancreatic Cancer Survivors Suggests New Potential Hereditary Genetic Alterations. CMAR 2019, 11, 599–609. [Google Scholar] [CrossRef]
- Yang, X.R.; Rotunno, M.; Xiao, Y.; Ingvar, C.; Helgadottir, H.; Pastorino, L.; Van Doorn, R.; Bennett, H.; Graham, C.; Sampson, J.N.; et al. Multiple Rare Variants in High-Risk Pancreatic Cancer-Related Genes May Increase Risk for Pancreatic Cancer in a Subset of Patients with and without Germline CDKN2A Mutations. Hum. Genet. 2016, 135, 1241–1249. [Google Scholar] [CrossRef]
- He, Y.; Huang, W.; Tang, Y.; Li, Y.; Peng, X.; Li, J.; Wu, J.; You, N.; Li, L.; Liu, C.; et al. Clinical and Genetic Characteristics in Pancreatic Cancer from Chinese Patients Revealed by Whole Exome Sequencing. Front. Oncol. 2023, 13, 1167144. [Google Scholar] [CrossRef]
- Slater, E.P.; Wilke, L.M.; Böhm, L.B.; Strauch, K.; Lutz, M.; Gercke, N.; Matthäi, E.; Hemminki, K.; Försti, A.; Schlesner, M.; et al. Combinations of Low-Frequency Genetic Variants Might Predispose to Familial Pancreatic Cancer. JPM 2021, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Uson, P.L.S.; Samadder, N.J.; Riegert-Johnson, D.; Boardman, L.; Borad, M.J.; Ahn, D.; Sonbol, M.B.; Faigel, D.O.; Fukami, N.; Pannala, R.; et al. Clinical Impact of Pathogenic Germline Variants in Pancreatic Cancer: Results from a Multicenter, Prospective, Universal Genetic Testing Study. Clin. Transl. Gastroenterol. 2021, 12, e00414. [Google Scholar] [CrossRef] [PubMed]
- Llach, J.; Moreno, L.; Sánchez, A.; Herrera-Pariente, C.; Ocaña, T.; Cuatrecasas, M.; Rivero-Sánchez, L.; Moreira, R.; Díaz, M.; Jung, G.; et al. Genetic Counseling for Hereditary Gastric and Pancreatic Cancer in High-Risk Gastrointestinal Cancer Clinics: An Effective Strategy. Cancers 2020, 12, 2386. [Google Scholar] [CrossRef] [PubMed]
- Johns, A.L.; McKay, S.H.; Humphris, J.L.; Pinese, M.; Chantrill, L.A.; Mead, R.S.; Tucker, K.; Andrews, L.; Goodwin, A.; Leonard, C.; et al. Lost in Translation: Returning Germline Genetic Results in Genome-Scale Cancer Research. Genome Med. 2017, 9, 41. [Google Scholar] [CrossRef]
- Mandelker, D.; Marra, A.; Zheng-Lin, B.; Selenica, P.; Blanco-Heredia, J.; Zhu, Y.; Gazzo, A.; Wong, D.; Yelskaya, Z.; Rai, V.; et al. Genomic Profiling Reveals Germline Predisposition and Homologous Recombination Deficiency in Pancreatic Acinar Cell Carcinoma. JCO 2023, 41, JCO2300561. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.-K.; Lin, R.-T.; Yeh, C.-C.; Yang, C.-Y.; Wu, C.-J.; Chen, P.-L.; Lin, J.-T. Diagnostic Rate of Germline Pathogenic Variants in Pancreatic Ductal Adenocarcinoma Patients Using Whole Genome Sequencing. Front. Genet. 2023, 14, 1172365. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.J.; Jiao, Y.; Yu, J.; Kopelovich, L.; Petersen, G.M.; Bondy, M.L.; Gallinger, S.; Schwartz, A.G.; Syngal, S.; Cote, M.L.; et al. ATM Mutations in Patients with Hereditary Pancreatic Cancer. Cancer Discov. 2012, 2, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Ding, D.; Lin, C.; Cunningham, D.; Wright, M.; Javed, A.A.; Azad, N.; Lee, V.; Donehower, R.; De Jesus-Acosta, A.; et al. RAD51B Harbors Germline Mutations Associated with Pancreatic Ductal Adenocarcinoma. JCO Precis. Oncol. 2022, 6, e2100404. [Google Scholar] [CrossRef]
- Martino, C.; Pandya, D.; Lee, R.; Levy, G.; Lo, T.; Lobo, S.; Frank, R.C. ATM-Mutated Pancreatic Cancer: Clinical and Molecular Response to Gemcitabine/Nab-Paclitaxel After Genome-Based Therapy Resistance. Pancreas 2020, 49, 143–147. [Google Scholar] [CrossRef]
- Mandelker, D.; Zhang, L.; Kemel, Y.; Stadler, Z.K.; Joseph, V.; Zehir, A.; Pradhan, N.; Arnold, A.; Walsh, M.F.; Li, Y.; et al. Mutation Detection in Patients with Advanced Cancer by Universal Sequencing of Cancer-Related Genes in Tumor and Normal DNA vs Guideline-Based Germline Testing. JAMA 2017, 318, 825. [Google Scholar] [CrossRef]
- Golan, T.; Kindler, H.L.; Park, J.O.; Reni, M.; Macarulla, T.; Hammel, P.; Van Cutsem, E.; Arnold, D.; Hochhauser, D.; McGuinness, D.; et al. Geographic and Ethnic Heterogeneity of Germline BRCA1 or BRCA2 Mutation Prevalence Among Patients with Metastatic Pancreatic Cancer Screened for Entry Into the POLO Trial. JCO 2020, 38, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Zheng-Lin, B.; Rainone, M.; Varghese, A.M.; Yu, K.H.; Park, W.; Berger, M.; Mehine, M.; Chou, J.; Capanu, M.; Mandelker, D.; et al. Methylation Analyses Reveal Promoter Hypermethylation as a Rare Cause of “Second Hit” in Germline BRCA1-Associated Pancreatic Ductal Adenocarcinoma. Mol. Diagn. Ther. 2022, 26, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Ozer, M.; Ranganathan, M.; Lecomte, N.; Schvartzman, J.M.; Walch, H.S.; Chatila, W.K.; Hong, J.; Carlo, M.I.; Walsh, M.F.; Sheehan, M.; et al. Concurrent Germline BRCA1/2 and Mismatch Repair Mutations in Young-Onset Pancreatic and Colorectal Cancer: The Importance of Comprehensive Germline and Somatic Characterization to Inform Therapeutic Options. JCO Precis. Oncol. 2022, 6, e2100560. [Google Scholar] [CrossRef] [PubMed]
- Zhen, D.B.; Rabe, K.G.; Gallinger, S.; Syngal, S.; Schwartz, A.G.; Goggins, M.G.; Hruban, R.H.; Cote, M.L.; McWilliams, R.R.; Roberts, N.J.; et al. BRCA1, BRCA2, PALB2, and CDKN2A Mutations in Familial Pancreatic Cancer: A PACGENE Study. Genet. Med. 2015, 17, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; Kelsen, D.P.; Stadler, Z.K.; Yu, K.H.; Janjigian, Y.Y.; Ludwig, E.; D’Adamo, D.R.; Salo-Mullen, E.; Robson, M.E.; Allen, P.J.; et al. An Emerging Entity: Pancreatic Adenocarcinoma Associated with a Known BRCA Mutation: Clinical Descriptors, Treatment Implications, and Future Directions. Oncology 2011, 16, 1397–1402. [Google Scholar] [CrossRef]
- Golan, T.; Stossel, C.; Atias, D.; Buzhor, E.; Halperin, S.; Cohen, K.; Raitses-Gurevich, M.; Glick, Y.; Raskin, S.; Yehuda, D.; et al. Recapitulating the Clinical Scenario of BRCA-associated Pancreatic Cancer in Pre-Clinical Models. Intl. J. Cancer 2018, 143, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.L.; Frado, L.E.; Hwang, C.; Kumar, S.; Khanna, L.G.; Levinson, E.J.; Chabot, J.A.; Chung, W.K.; Frucht, H. BRCA1 and BRCA2 Germline Mutations Are Frequently Demonstrated in Both High-Risk Pancreatic Cancer Screening and Pancreatic Cancer Cohorts. Cancer 2014, 120, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, C.R.; Levine, D.A.; Tang, L.H.; Allen, P.J.; Jarnagin, W.; Brennan, M.F.; Offit, K.; Robson, M.E. BRCA Germline Mutations in Jewish Patients with Pancreatic Adenocarcinoma. JCO 2009, 27, 433–438. [Google Scholar] [CrossRef]
- Salo-Mullen, E.E.; O’Reilly, E.M.; Kelsen, D.P.; Ashraf, A.M.; Lowery, M.A.; Yu, K.H.; Reidy, D.L.; Epstein, A.S.; Lincoln, A.; Saldia, A.; et al. Identification of Germline Genetic Mutations in Patients with Pancreatic Cancer. Cancer 2015, 121, 4382–4388. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Lee, J.W.; Lowery, M.A.; Capanu, M.; Stadler, Z.K.; Moore, M.J.; Dhani, N.; Kindler, H.L.; Estrella, H.; Maynard, H.; et al. Phase 1 Trial Evaluating Cisplatin, Gemcitabine, and Veliparib in 2 Patient Cohorts: Germline BRCA Mutation Carriers and Wild-type BRCA Pancreatic Ductal Adenocarcinoma. Cancer 2018, 124, 1374–1382. [Google Scholar] [CrossRef]
- Golan, T.; Sella, T.; O’Reilly, E.M.; Katz, M.H.; Epelbaum, R.; Kelsen, D.P.; Borgida, A.; Maynard, H.; Kindler, H.; Friedmen, E.; et al. Overall survival and clinical characteristics of BRCA mutation carriers with stage I/II pancreatic cancer. Br. J. Cancer 2017, 116, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Dagan, E. Predominant Ashkenazi BRCA1/2 Mutations in Families with Pancreatic Cancer. Genet. Test. 2008, 12, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.L.; Shakya, R.; Lipsyc, M.D.; Mitchel, E.B.; Kumar, S.; Hwang, C.; Deng, L.; Devoe, C.; Chabot, J.A.; Szabolcs, M.; et al. High Prevalence of BRCA1 and BRCA2 Germline Mutations with Loss of Heterozygosity in a Series of Resected Pancreatic Adenocarcinoma and Other Neoplastic Lesions. Clin. Cancer Res. 2013, 19, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Fam, H. Delineating the Effects BRCA1 and BRCA2 Loss of Heterozygosity in Pancreatic Cancer Progression. Clin. Genet. 2014, 85, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Kanji, Z.S.; Epelbaum, R.; Devaud, N.; Dagan, E.; Holter, S.; Aderka, D.; Paluch-Shimon, S.; Kaufman, B.; Gershoni-Baruch, R.; et al. Overall Survival and Clinical Characteristics of Pancreatic Cancer in BRCA Mutation Carriers. Br. J. Cancer 2014, 111, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Lener, M.R.; Scott, R.J.; Kluźniak, W.; Baszuk, P.; Cybulski, C.; Wiechowska-Kozłowska, A.; Huzarski, T.; Byrski, T.; Kładny, J.; Pietrzak, S.; et al. Do Founder Mutations Characteristic of Some Cancer Sites Also Predispose to Pancreatic Cancer?: Do Founder Mutations Predispose to Pancreatic Cancer? Int. J. Cancer 2016, 139, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Holter, S.; Borgida, A.; Dodd, A.; Grant, R.; Semotiuk, K.; Hedley, D.; Dhani, N.; Narod, S.; Akbari, M.; Moore, M.; et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients with Pancreatic Adenocarcinoma. JCO 2015, 33, 3124–3129. [Google Scholar] [CrossRef] [PubMed]
- Alimirzaie, S.; Mohamadkhani, A.; Masoudi, S.; Sellars, E.; Boffetta, P.; Malekzadeh, R.; Akbari, M.R.; Pourshams, A. Mutations in Known and Novel Cancer Susceptibility Genes in Young Patients with Pancreatic Cancer. Arch. Iran Med. 2018, 21, 228–233. [Google Scholar]
- Ghiorzo, P.; Pensotti, V.; Fornarini, G.; Sciallero, S.; Battistuzzi, L.; Belli, F.; Bonelli, L.; Borgonovo, G.; Bruno, W.; Gozza, A.; et al. Contribution of Germline Mutations in the BRCA and PALB2 Genes to Pancreatic Cancer in Italy. Fam. Cancer 2012, 11, 41–47. [Google Scholar] [CrossRef]
- Park, J.H.; Jo, J.H.; Jang, S.I.; Chung, M.J.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y.; Lee, H.S.; Cho, J.H. BRCA 1/2 Germline Mutation Predicts the Treatment Response of FOLFIRINOX with Pancreatic Ductal Adenocarcinoma in Korean Patients. Cancers 2022, 14, 236. [Google Scholar] [CrossRef]
- Al-Sukhni, W.; Rothenmund, H.; Eppel Borgida, A.; Zogopoulos, G.; O’Shea, A.-M.; Pollett, A.; Gallinger, S. Germline BRCA1 Mutations Predispose to Pancreatic Adenocarcinoma. Hum. Genet. 2008, 124, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Saha, G.; Singh, R.; Mandal, A.; Das, S.; Chattopadhyay, E.; Panja, P.; Roy, P.; DeSarkar, N.; Gulati, S.; Ghatak, S.; et al. A Novel Hotspot and Rare Somatic Mutation p.A138V, at TP53 Is Associated with Poor Survival of Pancreatic Ductal and Periampullary Adenocarcinoma Patients. Mol. Med. 2020, 26, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Golesworthy, B.; Cuggia, A.; Domecq, C.; Chaudhury, P.; Barkun, J.; Metrakos, P.; Asselah, J.; Bouganim, N.; Gao, Z.-H.; et al. Oncology Clinic-Based Germline Genetic Testing for Exocrine Pancreatic Cancer Enables Timely Return of Results and Unveils Low Uptake of Cascade Testing. J. Med. Genet. 2022, 59, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Albanese, L.; Signoriello, G.; Napoli, C.; Molinari, A.M. Pancreatic Cancer with Mutation in BRCA1/2, MLH1, and APC Genes: Phenotype Correlation and Detection of a Novel Germline BRCA2 Mutation. Genes 2022, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Lundy, J.; McKay, O.; Croagh, D.; Ganju, V. Exceptional Response to Olaparib and Pembrolizumab for Pancreatic Adenocarcinoma with Germline BRCA1 Mutation and High Tumor Mutation Burden: Case Report and Literature Review. JCO Precis. Oncol. 2022, 6, e2100437. [Google Scholar] [CrossRef]
- Lal, G.; Liu, G.; Schmocker, B.; Kaurah, P.; Ozcelik, H.; Narod, S.A.; Redston, M.; Gallinger, S. Inherited Predisposition to Pancreatic Adenocarcinoma: Role of Family History and Germ-Line P16, BRCA1, and BRCA2 Mutations. Cancer Res. 2000, 60, 409–416. [Google Scholar] [PubMed]
- Tibiletti, M.G.; Carnevali, I.; Pensotti, V.; Chiaravalli, A.M.; Facchi, S.; Volorio, S.; Mariette, F.; Mariani, P.; Fortuzzi, S.; Pierotti, M.A.; et al. OncoPan®: An NGS-Based Screening Methodology to Identify Molecular Markers for Therapy and Risk Assessment in Pancreatic Ductal Adenocarcinoma. Biomedicines 2022, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Lertwilaiwittaya, P.; Roothumnong, E.; Nakthong, P.; Dungort, P.; Meesamarnpong, C.; Tansa-Nga, W.; Pongsuktavorn, K.; Wiboonthanasarn, S.; Tititumjariya, W.; Thongnoppakhun, W.; et al. Thai Patients Who Fulfilled NCCN Criteria for Breast/Ovarian Cancer Genetic Assessment Demonstrated High Prevalence of Germline Mutations in Cancer Susceptibility Genes: Implication to Asian Population Testing. Breast Cancer Res. Treat. 2021, 188, 237–248. [Google Scholar] [CrossRef]
- Pinto, P.; Peixoto, A.; Santos, C.; Rocha, P.; Pinto, C.; Pinheiro, M.; Leça, L.; Martins, A.T.; Ferreira, V.; Bartosch, C.; et al. Analysis of Founder Mutations in Rare Tumors Associated with Hereditary Breast/Ovarian Cancer Reveals a Novel Association of BRCA2 Mutations with Ampulla of Vater Carcinomas. PLoS ONE 2016, 11, e0161438. [Google Scholar] [CrossRef]
- Palacio, S.; McMurry, H.S.; Ali, R.; Donenberg, T.; Silva-Smith, R.; Wideroff, G.; Sussman, D.A.; Rocha Lima, C.M.S.; Hosein, P.J. DNA Damage Repair Deficiency as a Predictive Biomarker for FOLFIRINOX Efficacy in Metastatic Pancreatic Cancer. J. Gastrointest. Oncol. 2019, 10, 1133–1139. [Google Scholar] [CrossRef]
- Terashima, T.; Morizane, C.; Ushiama, M.; Shiba, S.; Takahashi, H.; Ikeda, M.; Mizuno, N.; Tsuji, K.; Yasui, K.; Azemoto, N.; et al. Germline Variants in Cancer-Predisposing Genes in Pancreatic Cancer Patients with a Family History of Cancer. Jpn. J. Clin. Oncol. 2022, 52, hyac110. [Google Scholar] [CrossRef] [PubMed]
- Lecuelle, J.; Aarnink, A.; Tharin, Z.; Truntzer, C.; Ghiringhelli, F. Using Exome Sequencing to Improve Prediction of FOLFIRINOX First Efficacy for Pancreatic Adenocarcinoma. Cancers 2021, 13, 1851. [Google Scholar] [CrossRef]
- Golan, T.; Barenboim, A.; Lahat, G.; Nachmany, I.; Goykhman, Y.; Shacham-Shmueli, E.; Halpern, N.; Brazowski, E.; Geva, R.; Wolf, I.; et al. Increased Rate of Complete Pathologic Response After Neoadjuvant FOLFIRINOX for BRCA Mutation Carriers with Borderline Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2020, 27, 3963–3970. [Google Scholar] [CrossRef] [PubMed]
- Goehringer, C.; Sutter, C.; Kloor, M.; Gebert, J.; Slater, E.P.; Keller, M.; Treiber, I.; Ganschow, P.; Kadmon, M.; Moog, U. Double Germline Mutations in APC and BRCA2 in an Individual with a Pancreatic Tumor. Fam. Cancer 2017, 16, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.J.; Lowery, M.A.; Basturk, O.; Allen, P.J.; Yu, K.H.; Tabar, V.; Beal, K.; Reidy, D.L.; Yamada, Y.; Janjigian, Y.; et al. Brain Metastases in Pancreatic Ductal Adenocarcinoma: Assessment of Molecular Genotype–Phenotype Features—An Entity With an Increasing Incidence? Clin. Color. Cancer 2018, 17, e315–e321. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Cassidy, L.D.; Pisupati, V.; Jonasson, J.G.; Bjarnason, H.; Eyfjord, J.E.; Karreth, F.A.; Lim, M.; Barber, L.M.; Clatworthy, S.A.; et al. Germline Brca2 Heterozygosity Promotes KrasG12D -Driven Carcinogenesis in a Murine Model of Familial Pancreatic Cancer. Cancer Cell 2010, 18, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Sonnenblick, A.; Kadouri, L.; Appelbaum, L.; Peretz, T.; Sagi, M.; Goldberg, Y.; Hubert, A. Complete Remission, in BRCA2 Mutation Carrier with Metastatic Pancreatic Adenocarcinoma, Treated with Cisplatin Based Therapy. Cancer Biol. Ther. 2011, 12, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mou, Y.; Hou, S.; Cao, D.; Li, A. Response of Germline BRCA2-Mutated Advanced Pancreatic Acinar Cell Carcinoma to Olaparib: A Case Report. Medicine 2018, 97, e13113. [Google Scholar] [CrossRef]
- Chapman, J.S.; Asthana, S.; Cade, L.; Chang, M.T.; Wang, Z.; Zaloudek, C.J.; Ueda, S.; Collisson, E.A.; Taylor, B.S. Clinical Sequencing Contributes to a BRCA -Associated Cancer Rediagnosis That Guides an Effective Therapeutic Course. J. Natl. Compr. Canc. Netw. 2015, 13, 835–845. [Google Scholar] [CrossRef]
- Hata, T.; Mizuma, M.; Motoi, F.; Ishida, M.; Ohtsuka, H.; Nakagawa, K.; Morikawa, T.; Furukawa, T.; Unno, M. Germline DNA Damage Repair Gene Mutations in Pancreatic Cancer Patients with Personal/Family Histories of Pancreas/Breast/Ovarian/Prostate Cancer in a Japanese Population. Ann. Gastroent. Surg. 2021, 5, 853–864. [Google Scholar] [CrossRef]
- Dreikhausen, L.; Schulte, N.; Belle, S.; Weidner, P.; Moersdorf, J.; Reissfelder, C.; Ebert, M.P.; Zhan, T. Pancreatic Acinar Cell Carcinoma with Germline BRCA2 Mutation and Severe Pancreatic Panniculitis: A Case Report. Visc. Med. 2021, 37, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.B.; Groot, V.P.; Gemenetzis, G.; Wei, J.; Cameron, J.L.; Weiss, M.J.; Goggins, M.; Wolfgang, C.L.; Yu, J.; He, J. BRCA1/BRCA2 Germline Mutation Carriers and Sporadic Pancreatic Ductal Adenocarcinoma. J. Am. Coll. Surg. 2018, 226, 630–637e1. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.A.; Greenhalf, B.; Ellis, I.; Sina-Frey, M.; Rieder, H.; Korte, B.; Gerdes, B.; Kress, R.; Ziegler, A.; Raeburn, J.A.; et al. BRCA2 Germline Mutations in Familial Pancreatic Carcinoma. JNCI J. Natl. Cancer Inst. 2003, 95, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Sakamoto, H.; Takeuchi, S.; Ameri, M.; Kuboki, Y.; Yamamoto, T.; Hatori, T.; Yamamoto, M.; Sugiyama, M.; Ohike, N.; et al. Whole Exome Sequencing Reveals Recurrent Mutations in BRCA2 and FAT Genes in Acinar Cell Carcinomas of the Pancreas. Sci. Rep. 2015, 5, 8829. [Google Scholar] [CrossRef] [PubMed]
- Murali, K.; Dwarte, T.M.; Nikfarjam, M.; Tucker, K.M.; Vaughan, R.B.; Efthymiou, M.; Collins, A.; Spigelman, A.D.; Salmon, L.; Johns, A.L.; et al. Significant Detection of New Germline Pathogenic Variants in Australian Pancreatic Cancer Screening Program Participants. Hered. Cancer Clin. Pract. 2021, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Liu, S.; Huang, D.; Han, X.; Wu, X.; Shao, Y.W.; Hu, Y. Acquired Multiple Secondary BRCA2 Mutations upon PARPi Resistance in a Metastatic Pancreatic Cancer Patient Harboring a BRCA2 Germline Mutation. Am. J. Transl. Res. 2020, 12, 612–617. [Google Scholar]
- Castro, M.; Vierkoetter, K.; Prager, D.; Montgomery, S.; Sedgwick, K. Synchronous Onset of Breast and Pancreatic Cancers: Results of Germline and Somatic Genetic Analysis. Case Rep. Oncol. 2016, 9, 387–394. [Google Scholar] [CrossRef]
- Wang, H.; Mao, C.; Li, N.; Sun, L.; Zheng, Y.; Xu, N. A Case Report of a Dramatic Response to Olaparib in a Patient with Metastatic Pancreatic Cancer Harboring a Germline BRCA2 Mutation. Medicine 2019, 98, e17443. [Google Scholar] [CrossRef]
- Ozçelik, H.; Schmocker, B.; Di Nicola, N.; Shi, X.H.; Langer, B.; Moore, M.; Taylor, B.R.; Narod, S.A.; Darlington, G.; Andrulis, I.L.; et al. Germline BRCA2 6174delT Mutations in Ashkenazi Jewish Pancreatic Cancer Patients. Nat. Genet. 1997, 16, 17–18. [Google Scholar] [CrossRef]
- Figer, A.; Irmin, L.; Geva, R.; Flex, D.; Sulkes, J.; Sulkes, A.; Friedman, E. The Rate of the 6174delT Founder Jewish Mutation in BRCA2 in Patients with Non-Colonic Gastrointestinal Tract Tumours in Israel. Br. J. Cancer 2001, 84, 478–481. [Google Scholar] [CrossRef]
- Murphy, K.M.; Brune, K.A.; Griffin, C.; Sollenberger, J.E.; Petersen, G.M.; Bansal, R.; Hruban, R.H.; Kern, S.E. Evaluation of Candidate Genes MAP2K4, MADH4, ACVR1B, and BRCA2 in Familial Pancreatic Cancer: Deleterious BRCA2 Mutations in 17%. Cancer Res. 2002, 62, 3789–3793. [Google Scholar] [PubMed]
- Takeuchi, S.; Doi, M.; Ikari, N.; Yamamoto, M.; Furukawa, T. Mutations in BRCA1, BRCA2, and PALB2, and a Panel of 50 Cancer-Associated Genes in Pancreatic Ductal Adenocarcinoma. Sci. Rep. 2018, 8, 8105. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Biankin, A.V.; Bailey, P.; Chang, D.K.; Laheru, D.; Wolfgang, C.L.; Brody, J.R. BRCA2 Secondary Mutation-Mediated Resistance to Platinum and PARP Inhibitor-Based Therapy in Pancreatic Cancer. Br. J. Cancer 2017, 116, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- White, K.; Held, K.R.; Weber, B.H.F. A BRCA2 Germ-Line Mutation in Familial Pancreatic Carcinoma. Int. J. Cancer 2001, 91, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.B.; Carus, A.; Sunde, L.; Hamilton-Dutoit, S.; Ladekarl, M. BRCA-Associated Pancreatico-Biliary Neoplasms: Four Cases Illustrating the Emerging Clinical Impact of Genotyping. Acta Oncol. 2016, 55, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yoo, C.; Kim, K.; Park, K.-J.; Chang, H.-M.; Kim, T.W.; Lee, J.-L.; Lee, W.; Lee, S.S.; Park, D.H.; et al. Germline BRCA Mutations in Asian Patients with Pancreatic Adenocarcinoma: A Prospective Study Evaluating Risk Category for Genetic Testing. Investig. New Drugs 2018, 36, 163–169. [Google Scholar] [CrossRef]
- Schultheis, A.M.; Nguyen, G.P.; Ortmann, M.; Kruis, W.; Büttner, R.; Schildhaus, H.-U.; Markiefka, B. Squamous Cell Carcinoma of the Pancreas in a Patient with Germline BRCA2 Mutation-Response to Neoadjuvant Radiochemotherapy. Case Rep. Oncol. Med. 2014, 2014, 860532. [Google Scholar] [CrossRef]
- Kryklyva, V.; Haj Mohammad, N.; Morsink, F.H.M.; Ligtenberg, M.J.L.; Offerhaus, G.J.A.; Nagtegaal, I.D.; De Leng, W.W.J.; Brosens, L.A.A. Pancreatic Acinar Cell Carcinoma Is Associated with BRCA2 Germline Mutations: A Case Report and Literature Review. Cancer Biol. Ther. 2019, 20, 949–955. [Google Scholar] [CrossRef]
- Shimmura, H.; Kuramochi, H.; Jibiki, N.; Katagiri, S.; Nishino, T.; Araida, T. Dramatic Response of FOLFIRINOX Regimen in a Collision Pancreatic Adenocarcinoma Patient with a Germline BRCA2 Mutation: A Case Report. Jpn. J. Clin. Oncol. 2019, 49, 1049–1054. [Google Scholar] [CrossRef]
- Bruno, W.; Andreotti, V.; Bisio, A.; Pastorino, L.; Fornarini, G.; Sciallero, S.; Bianchi-Scarrà, G.; Inga, A.; Ghiorzo, P. Functional Analysis of a CDKN2A 5’UTR Germline Variant Associated with Pancreatic Cancer Development. PLoS ONE 2017, 12, e0189123. [Google Scholar] [CrossRef]
- Ghiorzo, P.; Fornarini, G.; Sciallero, S.; Battistuzzi, L.; Belli, F.; Bernard, L.; Bonelli, L.; Borgonovo, G.; Bruno, W.; De Cian, F.; et al. CDKN2A Is the Main Susceptibility Gene in Italian Pancreatic Cancer Families. J. Med. Genet. 2012, 49, 164–170. [Google Scholar] [CrossRef]
- Maker, A.V.; Warth, J.A.; Zinner, M.J. Novel Presentation of a Familial Pancreatic Cancer Syndrome. J. Gastrointest. Surg. 2009, 13, 1151–1154. [Google Scholar] [CrossRef]
- Bottillo, I.; Valiante, M.; Menale, L.; Paiardini, A.; Papi, L.; Janson, G.; Sestini, R.; Iorio, A.; De Simone, P.; Frascione, P.; et al. A Novel CDKN2A In-Frame Deletion Associated with Pancreatic Cancer-Melanoma Syndrome. Dermatol. Online J. 2020, 15, 26. [Google Scholar] [CrossRef]
- Pissa, M.; Helkkula, T.; Appelqvist, F.; Silander, G.; Borg, Å.; Pettersson, J.; Lapins, J.; Nielsen, K.; Höiom, V.; Helgadottir, H. CDKN2A Genetic Testing in Melanoma-Prone Families in Sweden in the Years 2015–2020: Implications for Novel National Recommendations. Acta Oncol. 2021, 60, 888–896. [Google Scholar] [CrossRef]
- Ghiorzo, P.; Gargiulo, S.; Nasti, S.; Pastorino, L.; Battistuzzi, L.; Bruno, W.; Bonelli, L.; Taveggia, P.; Pugliese, V.; Borgonovo, G.; et al. Predicting the Risk of Pancreatic Cancer: On CDKN2A Mutations in the Melanoma-Pancreatic Cancer Syndrome in Italy. JCO 2007, 25, 5336–5337. [Google Scholar] [CrossRef]
- Moore, P.S.; Zamboni, G.; Falconi, M.; Bassi, C.; Scarpa, A. A Novel Germline Mutation, P48T, in theCDKN2A/P16 Gene in a Patient with Pancreatic Carcinoma. Hum. Mutat. 2000, 16, 447–448. [Google Scholar] [CrossRef]
- Dębniak, T.; Van De Wetering, T.; Scott, R.; Nagay, L.; Cybulski, C.; Górski, B.; Jakubowska, A.; Gronwald, J.; Masojć, B.; Huzarski, T.; et al. Low Prevalence of CDKN2A/ARF Mutations among Early-Onset Cancers of Breast, Pancreas and Malignant Melanoma in Poland. Eur. J. Cancer Prev. 2008, 17, 389–391. [Google Scholar] [CrossRef]
- Lal, G.; Liu, L.; Hogg, D.; Lassam, N.J.; Redston, M.S.; Gallinger, S. Patients with Both Pancreatic Adenocarcinoma and Melanoma May Harbor Germline CDKN2A Mutations. Genes Chromosomes Cancer 2000, 27, 358–361. [Google Scholar] [CrossRef]
- Bartsch, D.K.; Sina-Frey, M.; Lang, S.; Wild, A.; Gerdes, B.; Barth, P.; Kress, R.; Grützmann, R.; Colombo-Benkmann, M.; Ziegler, A.; et al. CDKN2A Germline Mutations in Familial Pancreatic Cancer. Ann. Surg. 2002, 236, 730–737. [Google Scholar] [CrossRef]
- Lee, C.L.; Holter, S.; Borgida, A.; Dodd, A.; Ramotar, S.; Grant, R.; Wasson, K.; Elimova, E.; Jang, R.W.; Moore, M.; et al. Germline BRCA2 Variants in Advanced Pancreatic Acinar Cell Carcinoma: A Case Report and Review of Literature. World J. Gastroenterol. 2022, 28, 6421–6432. [Google Scholar] [CrossRef]
- Levin, T.; Mæhle, L. Uptake of Genetic Counseling, Genetic Testing and Surveillance in Hereditary Malignant Melanoma (CDKN2A) in Norway. Fam. Cancer 2017, 16, 257–265. [Google Scholar] [CrossRef]
- Soufir, N.; Lacapere, J.J.; Bertrand, G.; Matichard, E.; Meziani, R.; Mirebeau, D.; Descamps, V.; Gérard, B.; Archimbaud, A.; Ollivaud, L.; et al. Germline Mutations of the INK4a-ARF Gene in Patients with Suspected Genetic Predisposition to Melanoma. Br. J. Cancer 2004, 90, 503–509. [Google Scholar] [CrossRef][Green Version]
- Juan, F.M.D.; Escribano, M.R.; Lapiedra, C.M.; Alcantara, F.M.D.; Soto, M.C.; Fons, A.C.; Machado, I. Pancreatic Adenosquamous Carcinoma and Intraductal Papillary Mucinous Neoplasm in a CDKN2A Germline Mutation Carrier. WJGO 2017, 9, 390. [Google Scholar] [CrossRef]
- Gerdes, B.; Bartsch, D.K.; Ramaswamy, A.; Kersting, M.; Wild, A.; Schuermann, M.; Frey, M.; Rothmund, M. Multiple Primary Tumors as an Indicator for p16INK4a Germline Mutations in Pancreatic Cancer Patients? Pancreas 2000, 21, 369–375. [Google Scholar] [CrossRef]
- Potjer, T.P.; Kranenburg, H.E.; Bergman, W.; De Vos Tot Nederveen Cappel, W.H.; Van Monsjou, H.S.; Barge-Schaapveld, D.Q.C.M.; Vasen, H.F.A. Prospective Risk of Cancer and the Influence of Tobacco Use in Carriers of the P16-Leiden Germline Variant. Eur. J. Hum. Genet. 2015, 23, 711–714. [Google Scholar] [CrossRef][Green Version]
- Koorstra, J.-B.M.; Maitra, A.; Morsink, F.H.M.; Drillenburg, P.; Ten Kate, F.J.W.; Hruban, R.H.; Offerhaus, J.A. Undifferentiated Carcinoma with Osteoclastic Giant Cells (UCOCGC) of the Pancreas Associated With the Familial Atypical Multiple Mole Melanoma Syndrome (FAMMM). Am. J. Surg. Pathol. 2008, 32, 1905–1909. [Google Scholar] [CrossRef]
- Yang, X.R.; Jessop, L.; Myers, T.; Amundadottir, L.; Pfeiffer, R.M.; Wheeler, W.; Pike, K.M.; Yuenger, J.; Burdett, L.; Yeager, M.; et al. Lack of Germline PALB2 Mutations in Melanoma-Prone Families with CDKN2A Mutations and Pancreatic Cancer. Fam. Cancer 2011, 10, 545–548. [Google Scholar] [CrossRef]
- Goldstein, A.M. Prospective Risk of Cancer in CDKN2A Germline Mutation Carriers. J. Med. Genet. 2004, 41, 421–424. [Google Scholar] [CrossRef]
- McWilliams, R.R.; Wieben, E.D.; Rabe, K.G.; Pedersen, K.S.; Wu, Y.; Sicotte, H.; Petersen, G.M. Prevalence of CDKN2A Mutations in Pancreatic Cancer Patients: Implications for Genetic Counseling. Eur. J. Hum. Genet. 2011, 19, 472–478. [Google Scholar] [CrossRef]
- Ghiorzo, P.; Pastorino, L.; Bonelli, L.; Cusano, R.; Nicora, A.; Zupo, S.; Queirolo, P.; Sertoli, M.; Pugliese, V.; Bianchi-Scarrà, G. INK4/ARF Germline Alterations in Pancreatic Cancer Patients. Ann. Oncol. 2004, 15, 70–78. [Google Scholar] [CrossRef]
- Borg, A.; Sandberg, T.; Nilsson, K.; Johannsson, O.; Klinker, M.; Masback, A.; Westerdahl, J.; Olsson, H.; Ingvar, C. High Frequency of Multiple Melanomas and Breast and Pancreas Carcinomas in CDKN2A Mutation-Positive Melanoma Families. JNCI J. Natl. Cancer Inst. 2000, 92, 1260–1266. [Google Scholar] [CrossRef]
- Llach, J.; Aguilera, P.; Sánchez, A.; Ginès, A.; Fernández-Esparrach, G.; Soy, G.; Sendino, O.; Vaquero, E.; Carballal, S.; Ausania, F.; et al. Pancreatic Cancer Surveillance in Carriers of a Germline Pathogenic Variant in CDKN2A. Cancers 2023, 15, 1690. [Google Scholar] [CrossRef]
- Parker, J.F. Pancreatic Carcinoma Surveillance in Patients with Familial Melanoma. Arch. Dermatol. 2003, 139, 1019. [Google Scholar] [CrossRef]
- Puig, S.; Potrony, M.; Cuellar, F.; Puig-Butille, J.A.; Carrera, C.; Aguilera, P.; Nagore, E.; Garcia-Casado, Z.; Requena, C.; Kumar, R.; et al. Characterization of Individuals at High Risk of Developing Melanoma in Latin America: Bases for Genetic Counseling in Melanoma. Genet. Med. 2016, 18, 727–736. [Google Scholar] [CrossRef]
- Tanyi, M.; Olasz, J.; Lukács, G.; Tanyi, J.L.; Tóth, L.; Antal-Szalmás, P.; Ress, Z.; Bubán, T.; András, C.; Damjanovich, L. A New Mutation in Muir-Torre Syndrome Associated with Familiar Transmission of Different Gastrointestinal Adenocarcinomas. Eur. J. Surg. Oncol. EJSO 2009, 35, 1128–1130. [Google Scholar] [CrossRef]
- Latham, A.; Srinivasan, P.; Kemel, Y.; Shia, J.; Bandlamudi, C.; Mandelker, D.; Middha, S.; Hechtman, J.; Zehir, A.; Dubard-Gault, M.; et al. Microsatellite Instability Is Associated with the Presence of Lynch Syndrome Pan-Cancer. JCO 2019, 37, 286–295. [Google Scholar] [CrossRef]
- Grandval, P.; Barouk-Simonet, E.; Bronner, M.; Buisine, M.-P.; Moretta, J.; Tinat, J.; Olschwang, S. Is the Controversy on Breast Cancer as Part of the Lynch-Related Tumor Spectrum Still Open? Fam. Cancer 2012, 11, 681–683. [Google Scholar] [CrossRef]
- Malgerud, L.; Lindberg, J.; Wirta, V.; Gustafsson-Liljefors, M.; Karimi, M.; Moro, C.F.; Stecker, K.; Picker, A.; Huelsewig, C.; Stein, M.; et al. Bioinformatory-assisted Analysis of Next-generation Sequencing Data for Precision Medicine in Pancreatic Cancer. Mol. Oncol. 2017, 11, 1413–1429. [Google Scholar] [CrossRef]
- Banville, N.; Geraghty, R.; Fox, E.; Leahy, D.; Green, A.; Keegan, D.; Geoghegan, J.; Odonoghue, D.; Hyland, J.; Sheahan, K. Medullary Carcinoma of the Pancreas in a Man with Hereditary Nonpolyposis Colorectal Cancer Due to a Mutation of the MSH2 Mismatch Repair Gene. Hum. Pathol. 2006, 37, 1498–1502. [Google Scholar] [CrossRef]
- Gargiulo, S.; Torrini, M.; Ollila, S.; Nasti, S.; Pastorino, L.; Cusano, R.; Bonelli, L.; Battistuzzi, L.; Mastracci, L.; Bruno, W.; et al. Germline MLH1 and MSH2 Mutations in Italian Pancreatic Cancer Patients with Suspected Lynch Syndrome. Fam. Cancer 2009, 8, 547–553. [Google Scholar] [CrossRef]
- Peng, S.-H.; Chen, B.-B.; Kuo, T.-C.; Lee, J.-C.; Yang, S.-H. Maintenance Therapy of Low-Dose Nivolumab, S-1, and Leucovorin in Metastatic Pancreatic Adenocarcinoma with a Germline Mutation of MSH6: A Case Report. Front. Immunol. 2022, 13, 1077840. [Google Scholar] [CrossRef] [PubMed]
- Ohmoto, A.; Yachida, S.; Kubo, E.; Takai, E.; Suzuki, M.; Shimada, K.; Okusaka, T.; Morizane, C. Clinicopathologic Features and Germline Sequence Variants in Young Patients (≤40 Years Old) with Pancreatic Ductal Adenocarcinoma. Pancreas 2016, 45, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Sadaqat, R.; Naeemi, H.; Masood, I.; Hassan, U.; Ijaz, B.; Hanif, F.; Syed, A.A.; Yusuf, M.A.; Rashid, M.U. Contribution of Germline PALB2 Variants to an Unselected and Prospectively Registered Pancreatic Cancer Patient Cohort in Pakistan. HPB 2022, 24, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Lener, M.R.; Kashyap, A.; Kluźniak, W.; Cybulski, C.; Soluch, A.; Pietrzak, S.; Huzarski, T.; Gronwald, J.; Lubiński, J. The Prevalence of Founder Mutations among Individuals from Families with Familial Pancreatic Cancer Syndrome. Cancer Res. Treat. 2017, 49, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Borecka, M.; Zemankova, P.; Vocka, M.; Soucek, P.; Soukupova, J.; Kleiblova, P.; Sevcik, J.; Kleibl, Z.; Janatova, M. Mutation Analysis of the PALB2 Gene in Unselected Pancreatic Cancer Patients in the Czech Republic. Cancer Genet. 2016, 209, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Leslie, G.; Doroszuk, A.; Schneider, S.; Allen, J.; Decker, B.; Dunning, A.M.; Redman, J.; Scarth, J.; Plaskocinska, I.; et al. Cancer Risks Associated with Germline PALB2 Pathogenic Variants: An International Study of 524 Families. JCO 2020, 38, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Slater, E.; Langer, P.; Niemczyk, E.; Strauch, K.; Butler, J.; Habbe, N.; Neoptolemos, J.; Greenhalf, W.; Bartsch, D. PALB2 Mutations in European Familial Pancreatic Cancer Families. Clin. Genet. 2010, 78, 490–494. [Google Scholar] [CrossRef]
- Peterlongo, P.; Catucci, I.; Pasquini, G.; Verderio, P.; Peissel, B.; Barile, M.; Varesco, L.; Riboni, M.; Fortuzzi, S.; Manoukian, S.; et al. Palb2 Germline Mutations in Familial Breast Cancer Cases with Personal and Family History of Pancreatic Cancer. Breast Cancer Res. Treat. 2011, 126, 825–828. [Google Scholar] [CrossRef]
- Abe, K.; Ueki, A.; Urakawa, Y.; Kitago, M.; Yoshihama, T.; Nanki, Y.; Kitagawa, Y.; Aoki, D.; Kosaki, K.; Hirasawa, A. Familial Pancreatic Cancer with PALB2 and NBN Pathogenic Variants: A Case Report. Hered. Cancer Clin. Pract. 2021, 19, 5. [Google Scholar] [CrossRef]
- Boeck, S.; Mehraein, Y.; Ormanns, S.; Kruger, S.; Westphalen, C.B.; Haas, M.; Jung, A.; Kirchner, T.; Heinemann, V. Mismatch-Repair-Deficient Metastatic Pancreatic Ductal Adenocarcinoma with a Germline PALB2 Mutation: Unusual Genetics, Unusual Clinical Course. Ann. Oncol. 2017, 28, 438–439. [Google Scholar] [CrossRef]
- Jones, S.; Hruban, R.H.; Kamiyama, M.; Borges, M.; Zhang, X.; Parsons, D.W.; Lin, J.C.-H.; Palmisano, E.; Brune, K.; Jaffee, E.M.; et al. Exomic Sequencing Identifies PALB2 as a Pancreatic Cancer Susceptibility Gene. Science 2009, 324, 217. [Google Scholar] [CrossRef] [PubMed]
- Bernstein Molho, R.; Zalmanoviz, S.; Laitman, Y.; Friedman, E. De Novo Pathogenic Germline Variant in PALB2 in a Patient with Pancreatic Cancer. Fam. Cancer 2020, 19, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Hofstatter, E.W.; Domchek, S.M.; Miron, A.; Garber, J.; Wang, M.; Componeschi, K.; Boghossian, L.; Miron, P.L.; Nathanson, K.L.; Tung, N. PALB2 Mutations in Familial Breast and Pancreatic Cancer. Fam. Cancer 2011, 10, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Tischkowitz, M.D.; Sabbaghian, N.; Hamel, N.; Borgida, A.; Rosner, C.; Taherian, N.; Srivastava, A.; Holter, S.; Rothenmund, H.; Ghadirian, P.; et al. Analysis of the Gene Coding for the BRCA2-Interacting Protein PALB2 in Familial and Sporadic Pancreatic Cancer. Gastroenterology 2009, 137, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cuggia, A.; Pacis, A.; Boileau, J.-C.; Marcus, V.A.; Gao, Z.-H.; Chong, G.; Foulkes, W.D.; Zogopoulos, G. Pancreatic Cancer Progression in a Patient with Lynch Syndrome Receiving Immunotherapy: A Cautionary Tale. J. Natl. Compr. Cancer Netw. 2021, 19, 883–887. [Google Scholar] [CrossRef]
- Sato, N.; Rosty, C.; Jansen, M.; Fukushima, N.; Ueki, T.; Yeo, C.J.; Cameron, J.L.; Iacobuzio-Donahue, C.A.; Hruban, R.H.; Goggins, M. STK11/LKB1 Peutz-Jeghers Gene Inactivation in Intraductal Papillary-Mucinous Neoplasms of the Pancreas. Am. J. Pathol. 2001, 159, 2017–2022. [Google Scholar] [CrossRef]
- Su, G.H.; Hruban, R.H.; Bansal, R.K.; Bova, G.S.; Tang, D.J.; Shekher, M.C.; Westerman, A.M.; Entius, M.M.; Goggins, M.; Yeo, C.J.; et al. Germline and Somatic Mutations of the STK11/LKB1 Peutz-Jeghers Gene in Pancreatic and Biliary Cancers. Am. J. Pathol. 1999, 154, 1835–1840. [Google Scholar] [CrossRef]
- Resta, N.; Pierannunzio, D.; Lenato, G.M.; Stella, A.; Capocaccia, R.; Bagnulo, R.; Lastella, P.; Susca, F.C.; Bozzao, C.; Loconte, D.C.; et al. Cancer Risk Associated with STK11/LKB1 Germline Mutations in Peutz–Jeghers Syndrome Patients: Results of an Italian Multicenter Study. Dig. Liver Dis. 2013, 45, 606–611. [Google Scholar] [CrossRef]
- Wang, Y.; Cuggia, A.; Chen, Y.-I.; Parent, J.; Stanek, A.; Denroche, R.E.; Zhang, A.; Grant, R.C.; Domecq, C.; Golesworthy, B.; et al. Is Biannual Surveillance for Pancreatic Cancer Sufficient in Individuals with Genetic Syndromes or Familial Pancreatic Cancer? J. Natl. Compr. Cancer Netw. 2022, 20, 663–673.e12. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Yu, C.; Zhu, J.; Liu, C.; Li, X.; Ren, L.; Li, T. Intraductal Oncocytic Papillary Neoplasm Arising in Peutz-Jeghers Syndrome Bile Duct: A Unique Case Report. Diagn. Pathol. 2022, 17, 96. [Google Scholar] [CrossRef]
- Ruijs, M.W.G.; Verhoef, S.; Rookus, M.A.; Pruntel, R.; Van Der Hout, A.H.; Hogervorst, F.B.L.; Kluijt, I.; Sijmons, R.H.; Aalfs, C.M.; Wagner, A.; et al. TP53 Germline Mutation Testing in 180 Families Suspected of Li-Fraumeni Syndrome: Mutation Detection Rate and Relative Frequency of Cancers in Different Familial Phenotypes. J. Med. Genet. 2010, 47, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Terado, Y.; Tokiwa, S.; Nishio, K.; Saito, K.; Isotani, S.; Kamiyama, Y.; Muto, S.; Imamura, T.; Horie, S. Novel Germ Line Mutation P53-P177R in Adult Adrenocortical Carcinoma Producing Neuron-Specific Enolase as a Possible Marker. Jpn. J. Clin. Oncol. 2010, 40, 815–818. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, R.R.; Wieben, E.D.; Chaffee, K.G.; Antwi, S.O.; Raskin, L.; Olopade, O.I.; Li, D.; Highsmith, W.E.; Colon-Otero, G.; Khanna, L.G.; et al. CDKN2A Germline Rare Coding Variants and Risk of Pancreatic Cancer in Minority Populations. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Helgadottir, H.; Höiom, V.; Jönsson, G.; Tuominen, R.; Ingvar, C.; Borg, Å.; Olsson, H.; Hansson, J. High Risk of Tobacco-Related Cancers in CDKN2A Mutation-Positive Melanoma Families. J. Med. Genet. 2014, 51, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Paiella, S.; Capurso, G.; Cavestro, G.M.; Butturini, G.; Pezzilli, R.; Salvia, R.; Signoretti, M.; Crippa, S.; Carrara, S.; Frigerio, I.; et al. Results of First-Round of Surveillance in Individuals at High-Risk of Pancreatic Cancer from the AISP (Italian Association for the Study of the Pancreas) Registry. Am. J. Gastroenterol. 2019, 114, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Paiella, S.; Capurso, G.; Carrara, S.; Secchettin, E.; Casciani, F.; Frigerio, I.; Zerbi, A.; Archibugi, L.; Bonifacio, C.; Malleo, G.; et al. Outcomes of a 3-Year Prospective Surveillance in Individuals at High-Risk for Pancreatic Cancer. Am. J. Gastroenterol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, L.; Piombino, C.; Toss, A. Germline Mutations in Other Homologous Recombination Repair-Related Genes Than BRCA1/2: Predictive or Prognostic Factors? J. Pers. Med. 2021, 11, 245. [Google Scholar] [CrossRef]
- Venkitaraman, A.R. Cancer Susceptibility and the Functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182. [Google Scholar] [CrossRef]
- Walsh, C.S. Two Decades beyond BRCA1/2: Homologous Recombination, Hereditary Cancer Risk and a Target for Ovarian Cancer Therapy. Gynecol. Oncol. 2015, 137, 343–350. [Google Scholar] [CrossRef]
- Angeli, D.; Salvi, S.; Tedaldi, G. Genetic Predisposition to Breast and Ovarian Cancers: How Many and Which Genes to Test? IJMS 2020, 21, 1128. [Google Scholar] [CrossRef]
- Alvarez, C.; Tapia, T.; Perez-Moreno, E.; Gajardo-Meneses, P.; Ruiz, C.; Rios, M.; Missarelli, C.; Silva, M.; Cruz, A.; Matamala, L.; et al. BRCA1 and BRCA2 Founder Mutations Account for 78% of Germline Carriers among Hereditary Breast Cancer Families in Chile. Oncotarget 2017, 8, 74233–74243. [Google Scholar] [CrossRef] [PubMed]
- ElBiad, O.; Laraqui, A.; El Boukhrissi, F.; Mounjid, C.; Lamsisi, M.; Bajjou, T.; Elannaz, H.; Lahlou, A.I.; Kouach, J.; Benchekroune, K.; et al. Prevalence of Specific and Recurrent/Founder Pathogenic Variants in BRCA Genes in Breast and Ovarian Cancer in North Africa. BMC Cancer 2022, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Hamel, N.; Feng, B.-J.; Foretova, L.; Stoppa-Lyonnet, D.; Narod, S.A.; Imyanitov, E.; Sinilnikova, O.; Tihomirova, L.; Lubinski, J.; Gronwald, J.; et al. On the Origin and Diffusion of BRCA1 c.5266dupC (5382insC) in European Populations. Eur. J. Hum. Genet. 2011, 19, 300–306. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, P.J. ATM and Ataxia Telangiectasia. EMBO Rep. 2004, 5, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Borja, N.A.; Silva-Smith, R.; Huang, M.; Parekh, D.J.; Sussman, D.; Tekin, M. Atypical ATMs: Broadening the Phenotypic Spectrum of ATM-Associated Hereditary Cancer. Front. Oncol. 2023, 13, 1068110. [Google Scholar] [CrossRef]
- Kim, H.; Saka, B.; Knight, S.; Borges, M.; Childs, E.; Klein, A.; Wolfgang, C.; Herman, J.; Adsay, V.N.; Hruban, R.H.; et al. Having Pancreatic Cancer with Tumoral Loss of ATM and Normal TP53 Protein Expression Is Associated with a Poorer Prognosis. Clin. Cancer Res. 2014, 20, 1865–1872. [Google Scholar] [CrossRef]
- Thompson, D.; Duedal, S.; Kirner, J.; McGuffog, L.; Last, J.; Reiman, A.; Byrd, P.; Taylor, M.; Easton, D.F. Cancer Risks and Mortality in Heterozygous ATM Mutation Carriers. J. Natl. Cancer Inst. 2005, 97, 813–822. [Google Scholar] [CrossRef]
- Tischkowitz, M.; Balmaña, J.; Foulkes, W.D.; James, P.; Ngeow, J.; Schmutzler, R.; Voian, N.; Wick, M.J.; Stewart, D.R.; Pal, T.; et al. Management of Individuals with Germline Variants in PALB2: A Clinical Practice Resource of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 1416–1423. [Google Scholar] [CrossRef]
- Wattenberg, M.M.; Asch, D.; Yu, S.; O’Dwyer, P.J.; Domchek, S.M.; Nathanson, K.L.; Rosen, M.A.; Beatty, G.L.; Siegelman, E.S.; Reiss, K.A. Platinum Response Characteristics of Patients with Pancreatic Ductal Adenocarcinoma and a Germline BRCA1, BRCA2 or PALB2 Mutation. Br. J. Cancer 2020, 122, 333–339. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Lee, J.W.; Zalupski, M.; Capanu, M.; Park, J.; Golan, T.; Tahover, E.; Lowery, M.A.; Chou, J.F.; Sahai, V.; et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin with or without Veliparib in Patients with Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J. Clin. Oncol. 2020, 38, 1378–1388. [Google Scholar] [CrossRef]
- Pantaleo, A.; Forte, G.; Cariola, F.; Valentini, A.M.; Fasano, C.; Sanese, P.; Grossi, V.; Buonadonna, A.L.; De Marco, K.; Lepore Signorile, M.; et al. Tumor Testing and Genetic Analysis to Identify Lynch Syndrome Patients in an Italian Colorectal Cancer Cohort. Cancers 2023, 15, 5061. [Google Scholar] [CrossRef]
- Lepore Signorile, M.; Disciglio, V.; Di Carlo, G.; Pisani, A.; Simone, C.; Ingravallo, G. From Genetics to Histomolecular Characterization: An Insight into Colorectal Carcinogenesis in Lynch Syndrome. Int. J. Mol. Sci. 2021, 22, 6767. [Google Scholar] [CrossRef] [PubMed]
- Kastrinos, F.; Mukherjee, B.; Tayob, N.; Wang, F.; Sparr, J.; Raymond, V.M.; Bandipalliam, P.; Stoffel, E.M.; Gruber, S.B.; Syngal, S. Risk of Pancreatic Cancer in Families with Lynch Syndrome. JAMA 2009, 302, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Win, A.K.; Young, J.P.; Lindor, N.M.; Tucker, K.M.; Ahnen, D.J.; Young, G.P.; Buchanan, D.D.; Clendenning, M.; Giles, G.G.; Winship, I.; et al. Colorectal and Other Cancer Risks for Carriers and Noncarriers from Families with a DNA Mismatch Repair Gene Mutation: A Prospective Cohort Study. J. Clin. Oncol. 2012, 30, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Betz, B.; Theiss, S.; Aktas, M.; Konermann, C.; Goecke, T.O.; Möslein, G.; Schaal, H.; Royer-Pokora, B. Comparative in Silico Analyses and Experimental Validation of Novel Splice Site and Missense Mutations in the Genes MLH1 and MSH2. J. Cancer Res. Clin. Oncol. 2010, 136, 123–134. [Google Scholar] [CrossRef]
- Tournier, I.; Vezain, M.; Martins, A.; Charbonnier, F.; Baert-Desurmont, S.; Olschwang, S.; Wang, Q.; Buisine, M.P.; Soret, J.; Tazi, J.; et al. A Large Fraction of Unclassified Variants of the Mismatch Repair Genes MLH1 and MSH2 Is Associated with Splicing Defects. Hum. Mutat. 2008, 29, 1412–1424. [Google Scholar] [CrossRef]
- Tricarico, R.; Kasela, M.; Mareni, C.; Thompson, B.A.; Drouet, A.; Staderini, L.; Gorelli, G.; Crucianelli, F.; Ingrosso, V.; Kantelinen, J.; et al. Assessment of the InSiGHT Interpretation Criteria for the Clinical Classification of 24 MLH1 and MSH2 Gene Variants. Hum. Mutat. 2017, 38, 64–77. [Google Scholar] [CrossRef]
- Lindor, N.M.; Petersen, G.M.; Spurdle, A.B.; Thompson, B.; Goldgar, D.E.; Thibodeau, S.N. Pancreatic Cancer and a Novel MSH2 Germline Alteration. Pancreas 2011, 40, 1138–1140. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Katz, M.H.G.; Wang, H.; Cuddy, A.; You, Y.N. Clinical and Genetic Implications of DNA Mismatch Repair Deficiency in Patients with Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2017, 152, 1086–1088. [Google Scholar] [CrossRef]
- Riazy, M.; Kalloger, S.E.; Sheffield, B.S.; Peixoto, R.D.; Li-Chang, H.H.; Scudamore, C.H.; Renouf, D.J.; Schaeffer, D.F. Mismatch repair status may predict response to adjuvant chemotherapy in resectable pancreatic ductal adenocarcinoma. Mod. Pathol. 2015, 28, 1383–1389. [Google Scholar] [CrossRef]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden-High Solid Tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, M.; Lampis, A.; Mirchev, M.B.; Okuducu, A.F.; Ratti, M.; Valeri, N.; Hahne, J.C. Immune-Based Therapies and the Role of Microsatellite Instability in Pancreatic Cancer. Genes 2020, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Cariola, F.; De Marco, K.; Manghisi, A.; Guglielmi, F.A.; Armentano, R.; Lippolis, G.; Giorgio, P.; Simone, C.; Disciglio, V. A Novel STK11 Gene Mutation (c.388dupG, p.Glu130Glyfs∗33) in a Peutz-Jeghers Family and Evidence of Higher Gastric Cancer Susceptibility Associated with Alterations in STK11 Region Aa 107–170. Genes Dis. 2022, 9, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Welsh, S.B.; Hamilton, S.R.; Offerhaus, G.J.; Gittelsohn, A.M.; Booker, S.V.; Krush, A.J.; Yardley, J.H.; Luk, G.D. Increased Risk of Cancer in the Peutz-Jeghers Syndrome. N. Engl. J. Med. 1987, 316, 1511–1514. [Google Scholar] [CrossRef] [PubMed]
- Korsse, S.E.; Harinck, F.; van Lier, M.G.F.; Biermann, K.; Offerhaus, G.J.A.; Krak, N.; Looman, C.W.N.; van Veelen, W.; Kuipers, E.J.; Wagner, A.; et al. Pancreatic Cancer Risk in Peutz-Jeghers Syndrome Patients: A Large Cohort Study and Implications for Surveillance. J. Med. Genet. 2013, 50, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Laderian, B.; Mundi, P.; Fojo, T.; Bates, S.E. Emerging Therapeutic Implications of STK11 Mutation: Case Series. Oncologist 2020, 25, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Chiang, J.; Ngeow, J. CDKN2A Germline Alterations and the Relevance of Genotype-Phenotype Associations in Cancer Predisposition. Hered. Cancer Clin. Pract. 2021, 19, 21. [Google Scholar] [CrossRef]
- De Snoo, F.A.; Bishop, D.T.; Bergman, W.; van Leeuwen, I.; van der Drift, C.; van Nieuwpoort, F.A.; Out-Luiting, C.J.; Vasen, H.F.; ter Huurne, J.A.; Frants, R.R.; et al. Increased risk of cancer other than melanoma in CDKN2A founder mutation (p16-Leiden)-positive melanoma families. Clin. Cancer Res. 2008, 14, 7151–7157. [Google Scholar] [CrossRef]
- Vasen, H.F.; Gruis, N.A.; Frants, R.R.; van Der Velden, P.A.; Hille, E.T.; Bergman, W. Risk of Developing Pancreatic Cancer in Families with Familial Atypical Multiple Mole Melanoma Associated with a Specific 19 Deletion of P16 (P16-Leiden). Int. J. Cancer 2000, 87, 809–811. [Google Scholar] [CrossRef]
- Auroy, S.; Avril, M.F.; Chompret, A.; Pham, D.; Goldstein, A.M.; Bianchi-Scarrà, G.; Frebourg, T.; Joly, P.; Spatz, A.; Rubino, C.; et al. Sporadic Multiple Primary Melanoma Cases: CDKN2A Germline Mutations with a Founder Effect. Genes Chromosomes Cancer 2001, 32, 195–202. [Google Scholar] [CrossRef]
- Cremin, C.; Howard, S.; Le, L.; Karsan, A.; Schaeffer, D.F.; Renouf, D.; Schrader, K.A. CDKN2A Founder Mutation in Pancreatic Ductal Adenocarcinoma Patients without Cutaneous Features of Familial Atypical Multiple Mole Melanoma (FAMMM) Syndrome. Hered. Cancer Clin. Pract. 2018, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Borg, A.; Johannsson, U.; Johannsson, O.; Häkansson, S.; Westerdahl, J.; Mäsbäck, A.; Olsson, H.; Ingvar, C. Novel Germline P16 Mutation in Familial Malignant Melanoma in Southern Sweden. Cancer Res. 1996, 56, 2497–2500. [Google Scholar] [PubMed]
- Gruis, N.A.; van der Velden, P.A.; Sandkuijl, L.A.; Prins, D.E.; Weaver-Feldhaus, J.; Kamb, A.; Bergman, W.; Frants, R.R. Homozygotes for CDKN2 (P16) Germline Mutation in Dutch Familial Melanoma Kindreds. Nat. Genet. 1995, 10, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Hayward, N.; Goldgar, D.; Tsao, H.; Hogg, D.; Palmer, J.; Stark, M.; Tobias, E.S.; MacKie, R. The M53I Mutation in CDKN2A Is a Founder Mutation That Predominates in Melanoma Patients with Scottish Ancestry. Genes Chromosomes Cancer 2007, 46, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Al Baghdadi, T.; Halabi, S.; Garrett-Mayer, E.; Mangat, P.K.; Ahn, E.R.; Sahai, V.; Alvarez, R.H.; Kim, E.S.; Yost, K.J.; Rygiel, A.L.; et al. Palbociclib in Patients with Pancreatic and Biliary Cancer with CDKN2A Alterations: Results From the Targeted Agent and Profiling Utilization Registry Study. JCO Precis. Oncol. 2019, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shaya, J.; Kato, S.; Adashek, J.J.; Patel, H.; Fanta, P.T.; Botta, G.P.; Sicklick, J.K.; Kurzrock, R. Personalized Matched Targeted Therapy in Advanced Pancreatic Cancer: A Pilot Cohort Analysis. NPJ Genom. Med. 2023, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Maddalena, M.; Mallel, G.; Nataraj, N.B.; Shreberk-Shaked, M.; Hassin, O.; Mukherjee, S.; Arandkar, S.; Rotkopf, R.; Kapsack, A.; Lambiase, G.; et al. TP53 Missense Mutations in PDAC Are Associated with Enhanced Fibrosis and an Immunosuppressive Microenvironment. Proc. Natl. Acad. Sci. USA 2021, 118, e2025631118. [Google Scholar] [CrossRef]
- Senzer, N.; Nemunaitis, J.; Nemunaitis, D.; Bedell, C.; Edelman, G.; Barve, M.; Nunan, R.; Pirollo, K.F.; Rait, A.; Chang, E.H. Phase I Study of a Systemically Delivered P53 Nanoparticle in Advanced Solid Tumors. Mol. Ther. 2013, 21, 1096–1103. [Google Scholar] [CrossRef]
- Gao, J.; Chen, X.; Li, X.; Miao, F.; Fang, W.; Li, B.; Qian, X.; Lin, X. Differentiating TP53 Mutation Status in Pancreatic Ductal Adenocarcinoma Using Multiparametric MRI-Derived Radiomics. Front. Oncol. 2021, 11, 632130. [Google Scholar] [CrossRef]
- Yap, T.A.; Stadler, Z.K.; Stout, L.A.; Schneider, B.P. Aligning Germline Cancer Predisposition with Tumor-Based Next-Generation Sequencing for Modern Oncology Diagnosis, Interception, and Therapeutic Development. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390738. [Google Scholar] [CrossRef]
- Taeubner, J.; Wieczorek, D.; Yasin, L.; Brozou, T.; Borkhardt, A.; Kuhlen, M. Penetrance and Expressivity in Inherited Cancer Predisposing Syndromes. Trends Cancer 2018, 4, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Turchiano, A.; Piglionica, M.; Martino, S.; Bagnulo, R.; Garganese, A.; De Luisi, A.; Chirulli, S.; Iacoviello, M.; Stasi, M.; Tabaku, O.; et al. Impact of High-to-Moderate Penetrance Genes on Genetic Testing: Looking over Breast Cancer. Genes 2023, 14, 1530. [Google Scholar] [CrossRef] [PubMed]
- Nolano, A.; Medugno, A.; Trombetti, S.; Liccardo, R.; De Rosa, M.; Izzo, P.; Duraturo, F. Hereditary Colorectal Cancer: State of the Art in Lynch Syndrome. Cancers 2022, 15, 75. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).