Breaking Barriers in Neuro-Oncology: A Scoping Literature Review on Invasive and Non-Invasive Techniques for Blood–Brain Barrier Disruption
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Study Selection and Data Extraction
3. Results
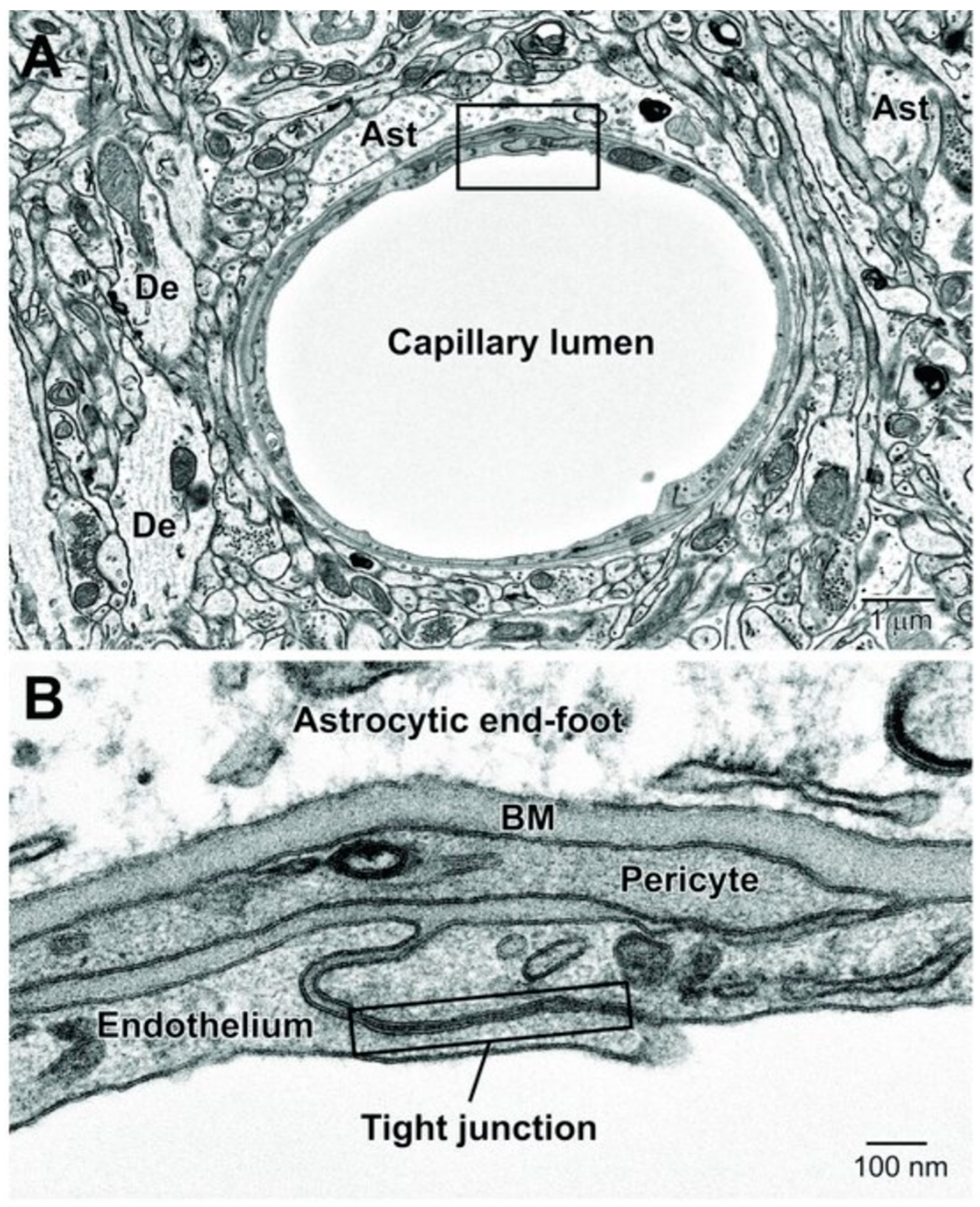
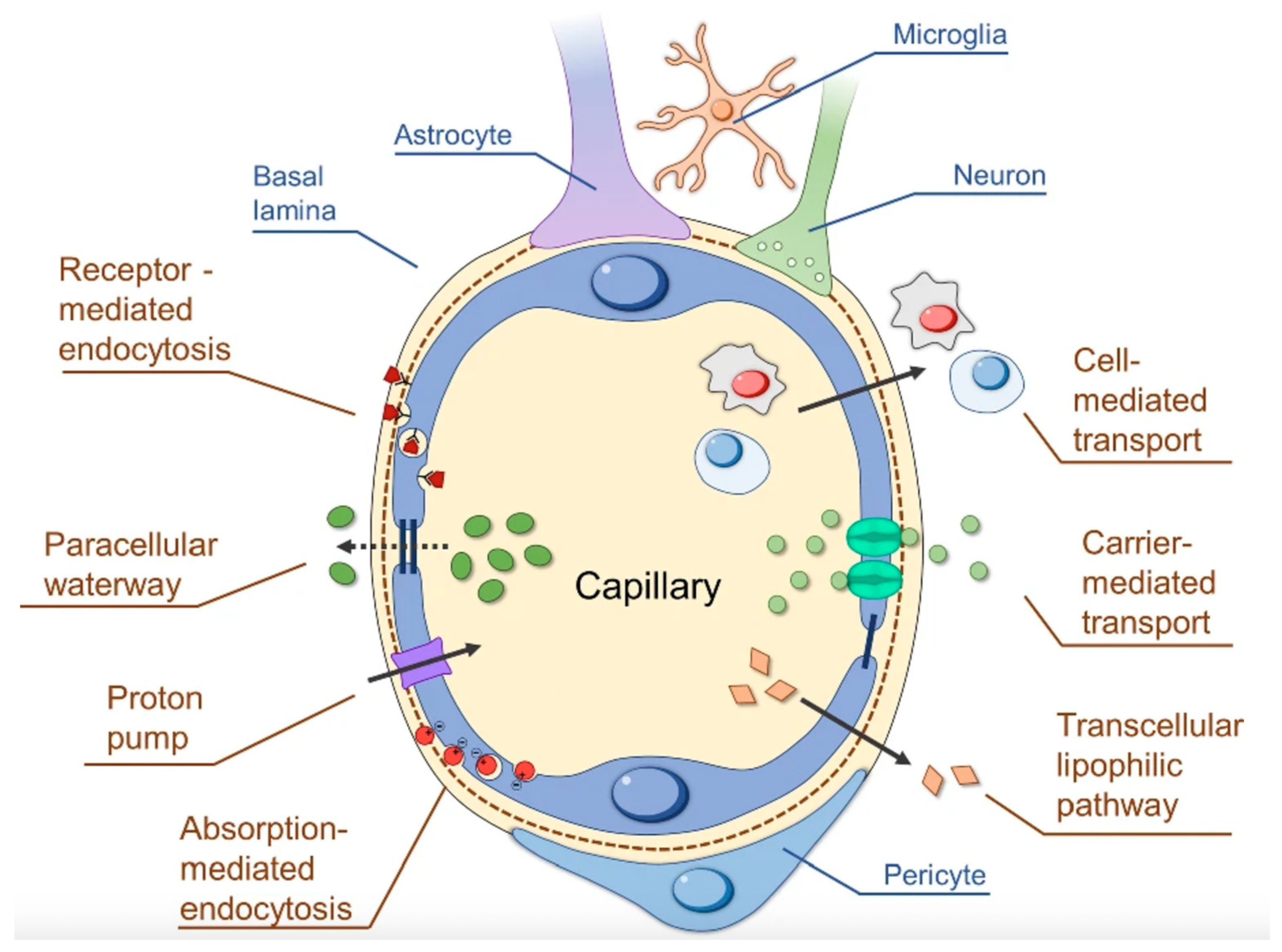
3.1. The BBB’s Structure
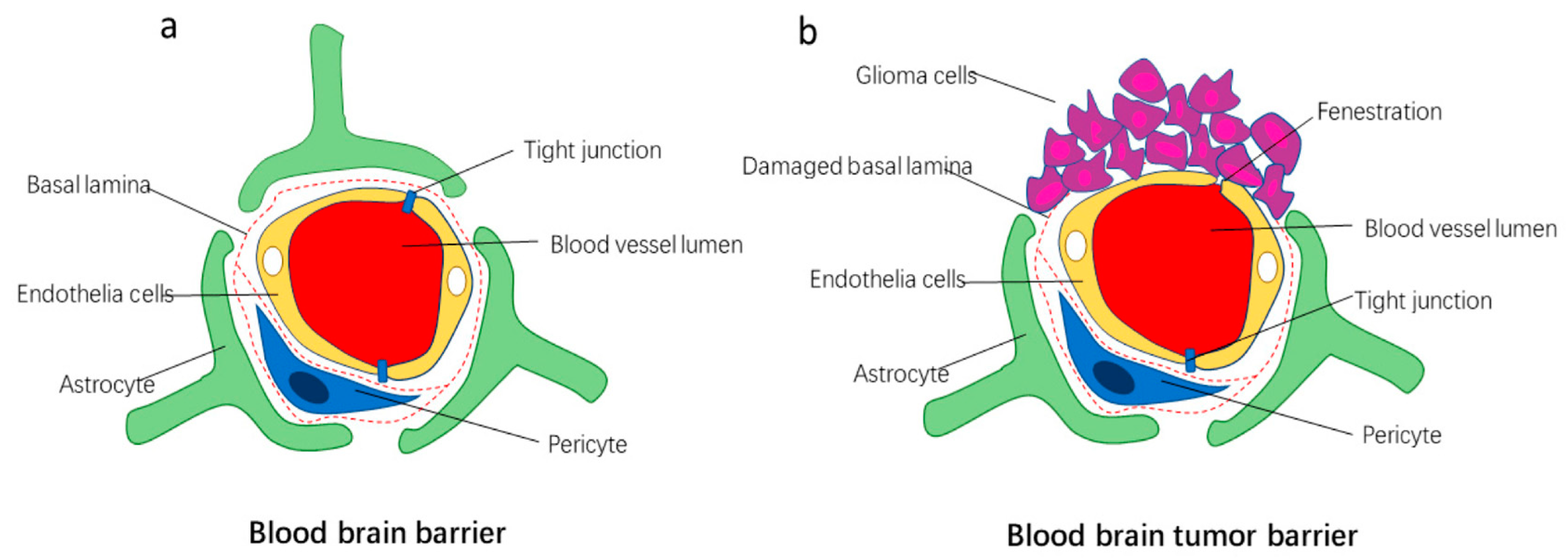
3.2. The BTB
3.3. BTB in Metastatic Cancer
3.4. BTB in Gliomas
3.5. Disruption of the BBB/BTB
3.6. Osmotic BBB/BTB Disruption
3.7. Optimizing Osmotic BBBD with Modern Imaging
3.8. Intrathecal/Intraventricular Administration
3.9. LITT
3.10. CED
4. Non-Invasive Methods of BBBD
4.1. Ultrasound-Mediated BBB Opening
High-Intensity Focused Ultrasound
4.2. Low-Intensity Pulsed Ultrasound
4.3. TTFields
5. Future Directions
6. Conclusions
Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Pardridge, W.M. Molecular biology of the blood-brain barrier. Mol. Biotechnol. 2005, 30, 57–70. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9 (Suppl. S1), S3. [Google Scholar] [CrossRef]
- Abbott, N.J. Dynamics of CNS barriers: Evolution, differentiation, and modulation. Cell Mol. Neurobiol. 2005, 25, 5–23. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef]
- Villaseñor, R.; Kuennecke, B.; Ozmen, L.; Ammann, M.; Kugler, C.; Grüninger, F.; Loetscher, H.; Freskgård, P.O.; Collin, L. Region-specific permeability of the blood-brain barrier upon pericyte loss. J. Cereb. Blood Flow. Metab. 2017, 37, 3683–3694. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Jaspan, J.B. Regional variation in transport of pancreatic polypeptide across the blood-brain barrier of mice. Pharmacol. Biochem. Behav. 1995, 51, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Moinuddin, A.; Morley, J.E.; Banks, W.A. Regional variations in the transport of interleukin-1alpha across the blood-brain barrier in ICR and aging SAMP8 mice. Neuroimmunomodulation 2000, 8, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kastin, A.J. Differential permeability of the blood-brain barrier to two pancreatic peptides: Insulin and amylin. Peptides 1998, 19, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Coomber, B.L.; Stewart, P.A. Morphometric analysis of CNS microvascular endothelium. Microvasc. Res. 1985, 30, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Ufnal, M.; Skrzypecki, J. Blood borne hormones in a cross-talk between peripheral and brain mechanisms regulating blood pressure, the role of circumventricular organs. Neuropeptides 2014, 48, 65–73. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug targeting to the brain. Pharm. Res. 2007, 24, 1733–1744. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow. Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Agarwala, S.S.; Kirkwood, J.M. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist 2000, 5, 144–151. [Google Scholar] [CrossRef]
- Steeg, P.S. The blood-tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 696–714. [Google Scholar] [CrossRef]
- Abbott, N.J. Evidence for bulk flow of brain interstitial fluid: Significance for physiology and pathology. Neurochem. Int. 2004, 45, 545–552. [Google Scholar] [CrossRef]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef]
- Marzolini, C.; Decosterd, L.A.; Shen, F.; Gander, M.; Leyvraz, S.; Bauer, J.; Buclin, T.; Biollaz, J.; Lejeune, F. Pharmacokinetics of temozolomide in association with fotemustine in malignant melanoma and malignant glioma patients: Comparison of oral, intravenous, and hepatic intra-arterial administration. Cancer Chemother. Pharmacol. 1998, 42, 433–440, Erratum in Cancer Chemother. Pharmacol. 1999, 43, 439–440. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer. 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Neurological diseases in relation to the blood-brain barrier. J. Cereb. Blood Flow. Metab. 2012, 32, 1139–1151. [Google Scholar] [CrossRef]
- Upton, D.H.; Ung, C.; George, S.M.; Tsoli, M.; Kavallaris, M.; Ziegler, D.S. Challenges and opportunities to penetrate the blood-brain barrier for brain cancer therapy. Theranostics 2022, 12, 4734–4752. [Google Scholar] [CrossRef]
- Wilhelm, I.; Nyúl-Tóth, Á.; Suciu, M.; Hermenean, A.; Krizbai, I.A. Heterogeneity of the blood-brain barrier. Tissue Barriers 2016, 4, e1143544. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Solár, P.; Zamani, A.; Lakatosová, K.; Joukal, M. The blood-brain barrier and the neurovascular unit in subarachnoid hemorrhage: Molecular events and potential treatments. Fluids Barriers CNS 2022, 19, 29. [Google Scholar] [CrossRef]
- Bamforth, S.D.; Kniesel, U.; Wolburg, H.; Engelhardt, B.; Risau, W. A dominant mutant of occludin disrupts tight junction structure and function. J. Cell Sci. 1999, 112 Pt 12, 1879–1988. [Google Scholar] [CrossRef] [PubMed]
- Mitusova, K.; Peltek, O.O.; Karpov, T.E.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Overcoming the blood-brain barrier for the therapy of malignant brain tumor: Current status and prospects of drug delivery approaches. J. Nanobiotechnol. 2022, 20, 412. [Google Scholar] [CrossRef] [PubMed]
- Aurrand-Lions, M.; Johnson-Leger, C.; Wong, C.; Du Pasquier, L.; Imhof, B.A. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood 2001, 98, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2018, 4, 78–82. [Google Scholar] [CrossRef]
- Price, B.R.; Johnson, L.A.; Norris, C.M. Reactive astrocytes: The nexus of pathological and clinical hallmarks of Alzheimer’s disease. Ageing Res. Rev. 2021, 68, 101335. [Google Scholar] [CrossRef]
- Nahirney, P.C.; Tremblay, M.E. Brain Ultrastructure: Putting the Pieces Together. Front. Cell Dev. Biol. 2021, 9, 629503. [Google Scholar] [CrossRef]
- Reese, T.S.; Karnovsky, M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Brightman, M.W.; Reese, T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969, 40, 648–677. [Google Scholar] [CrossRef]
- O’Brown, N.M.; Megason, S.G.; Gu, C. Suppression of transcytosis regulates zebrafish blood-brain barrier function. eLife 2019, 8, e47326. [Google Scholar] [CrossRef]
- Chow, B.W.; Gu, C. Gradual Suppression of Transcytosis Governs Functional Blood-Retinal Barrier Formation. Neuron 2017, 93, 1325–1333.e3. [Google Scholar] [CrossRef]
- Zaragozá, R. Transport of Amino Acids Across the Blood-Brain Barrier. Front. Physiol. 2020, 11, 973. [Google Scholar] [CrossRef]
- Hu, C.; Tao, L.; Cao, X.; Chen, L. The solute carrier transporters and the brain: Physiological and pharmacological implications. Asian J. Pharm. Sci. 2020, 15, 131–144. [Google Scholar] [CrossRef]
- Qosa, H.; Miller, D.S.; Pasinelli, P.; Trotti, D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015, 1628 Pt B, 298–316. [Google Scholar] [CrossRef]
- McConnell, H.L.; Mishra, A. Cells of the Blood-Brain Barrier: An Overview of the Neurovascular Unit in Health and Disease. Methods Mol. Biol. 2022, 2492, 3–24. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primers. 2019, 5, 5. [Google Scholar] [CrossRef]
- Qiu, Z.; Yu, Z.; Xu, T.; Wang, L.; Meng, N.; Jin, H.; Xu, B. Novel Nano-Drug Delivery System for Brain Tumor Treatment. Cells 2022, 11, 3761. [Google Scholar] [CrossRef]
- Gerstner, E.R.; Fine, R.L. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: Establishing a treatment paradigm. J. Clin. Oncol. 2007, 25, 2306–2312. [Google Scholar] [CrossRef]
- Fares, J.; Kanojia, D.; Rashidi, A.; Ulasov, I.; Lesniak, M.S. Genes that Mediate Metastasis across the Blood-Brain Barrier. Trends Cancer 2020, 6, 660–676. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S.; Woodrow, C.J.; Davies, D.C.; Costa-Martins, P.; Moss, R.F.; Krishna, S.; Bell, B.A. Occludin expression in microvessels of neoplastic and non-neoplastic human brain. Neuropathol. Appl. Neurobiol. 2001, 27, 384–395. [Google Scholar] [CrossRef]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef]
- Samala, R.; Thorsheim, H.R.; Goda, S.; Taskar, K.; Gril, B.; Steeg, P.S.; Smith, Q.R. Vinorelbine Delivery and Efficacy in the MDA-MB-231BR Preclinical Model of Brain Metastases of Breast Cancer. Pharm. Res. 2016, 33, 2904–2919. [Google Scholar] [CrossRef]
- Gril, B.; Wei, D.; Zimmer, A.S.; Robinson, C.; Khan, I.; Difilippantonio, S.; Overstreet, M.G.; Steeg, P.S. HER2 antibody-drug conjugate controls growth of breast cancer brain metastases in hematogenous xenograft models, with heterogeneous blood-tumor barrier penetration unlinked to a passive marker. Neuro Oncol. 2020, 22, 1625–1636. [Google Scholar] [CrossRef]
- Askoxylakis, V.; Ferraro, G.B.; Kodack, D.P.; Badeaux, M.; Shankaraiah, R.C.; Seano, G.; Kloepper, J.; Vardam, T.; Martin, J.D.; Naxerova, K.; et al. Preclinical Efficacy of Ado-trastuzumab Emtansine in the Brain Microenvironment. J. Natl. Cancer Inst. 2015, 108, djv313. [Google Scholar] [CrossRef]
- Terrell-Hall, T.B.; Nounou, M.I.; El-Amrawy, F.; Griffith, J.I.G.; Lockman, P.R. Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget 2017, 8, 83734–83744. [Google Scholar] [CrossRef]
- Taskar, K.S.; Rudraraju, V.; Mittapalli, R.K.; Samala, R.; Thorsheim, H.R.; Lockman, J.; Gril, B.; Hua, E.; Palmieri, D.; Polli, J.W.; et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm. Res. 2012, 29, 770–781. [Google Scholar] [CrossRef]
- Nounou, M.I.; Adkins, C.E.; Rubinchik, E.; Terrell-Hall, T.B.; Afroz, M.; Vitalis, T.; Gabathuler, R.; Tian, M.M.; Lockman, P.R. Anti-cancer Antibody Trastuzumab-Melanotransferrin Conjugate (BT2111) for the Treatment of Metastatic HER2+ Breast Cancer Tumors in the Brain: An In-Vivo Study. Pharm. Res. 2016, 33, 2930–2942. [Google Scholar] [CrossRef]
- Lin, N.U.; Carey, L.A.; Liu, M.C.; Younger, J.; Come, S.E.; Ewend, M.; Harris, G.J.; Bullitt, E.; Van den Abbeele, A.D.; Henson, J.W.; et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2008, 26, 1993–1999. [Google Scholar] [CrossRef]
- Morikawa, A.; Peereboom, D.M.; Thorsheim, H.R.; Samala, R.; Balyan, R.; Murphy, C.G.; Lockman, P.R.; Simmons, A.; Weil, R.J.; Tabar, V.; et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: A prospective study. Neuro Oncol. 2015, 17, 289–295, Erratum in Neuro Oncol. 2015, 17, 1423. [Google Scholar] [CrossRef]
- Pestalozzi, B.C.; Brignoli, S. Trastuzumab in CSF. J. Clin. Oncol. 2000, 18, 2349–2351. [Google Scholar] [CrossRef]
- Zeng, Y.D.; Liao, H.; Qin, T.; Zhang, L.; Wei, W.D.; Liang, J.Z.; Xu, F.; Dinglin, X.X.; Ma, S.X.; Chen, L.K. Blood-brain barrier permeability of gefitinib in patients with brain metastases from non-small-cell lung cancer before and during whole brain radiation therapy. Oncotarget 2015, 6, 8366–8376. [Google Scholar] [CrossRef]
- Groblewska, M.; Mroczko, B. Pro- and Antiangiogenic Factors in Gliomas: Implications for Novel Therapeutic Possibilities. Int. J. Mol. Sci. 2021, 22, 6126. [Google Scholar] [CrossRef]
- Das, S.; Marsden, P.A. Angiogenesis in glioblastoma. N. Engl. J. Med. 2013, 369, 1561–1563. [Google Scholar] [CrossRef]
- Verheul, H.M.; Pinedo, H.M. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors. Clin. Breast Cancer 2000, 1 (Suppl. S1), S80–S84. [Google Scholar] [CrossRef]
- Wei, X.; Chen, X.; Ying, M.; Lu, W. Brain tumor-targeted drug delivery strategies. Acta Pharm. Sin. B 2014, 4, 193–201. [Google Scholar] [CrossRef]
- Wen, L.; Tan, Y.; Dai, S.; Zhu, Y.; Meng, T.; Yang, X.; Liu, Y.; Liu, X.; Yuan, H.; Hu, F. VEGF-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing BBB for glioblastoma-targeting therapy. Drug Deliv. 2017, 24, 1843–1855. [Google Scholar] [CrossRef]
- Liebner, S.; Fischmann, A.; Rascher, G.; Duffner, F.; Grote, E.H.; Kalbacher, H.; Wolburg, H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000, 100, 323–331. [Google Scholar] [CrossRef]
- Saadoun, S.; Papadopoulos, M.C.; Davies, D.C.; Krishna, S.; Bell, B.A. Aquaporin-4 expression is increased in oedematous human brain tumours. J. Neurol. Neurosurg. Psychiatry 2002, 72, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Dhermain, F.G.; Hau, P.; Lanfermann, H.; Jacobs, A.H.; van den Bent, M.J. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010, 9, 906–920. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018, 20, 184–191. [Google Scholar] [CrossRef] [PubMed]
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Silbergeld, D.L.; Chicoine, M.R. Isolation and characterization of human malignant glioma cells from histologically normal brain. J. Neurosurg. 1997, 86, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Fortin, D. Drug Delivery Technology to the CNS in the Treatment of Brain Tumors: The Sherbrooke Experience. Pharmaceutics 2019, 11, 248. [Google Scholar] [CrossRef] [PubMed]
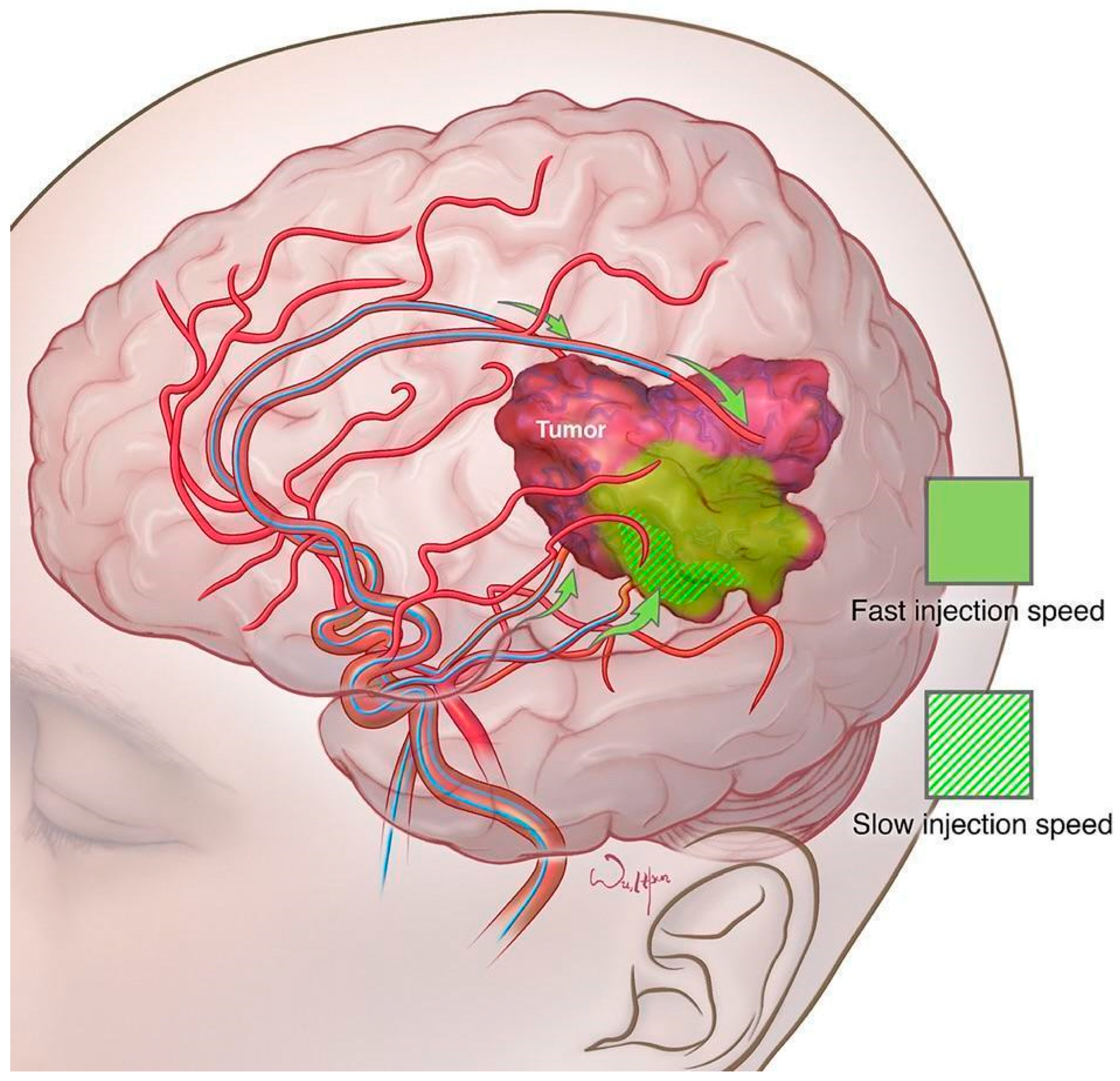
- Pinkiewicz, M.; Pinkiewicz, M.; Walecki, J.; Zawadzki, M. A systematic review on intra-arterial cerebral infusions of chemotherapeutics in the treatment of glioblastoma multiforme: The state-of-the-art. Front. Oncol. 2022, 12, 950167. [Google Scholar] [CrossRef]
- Rapoport, S.I.; Hori, M.; Klatzo, I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am. J. Physiol. 1972, 223, 323–331. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Frenkel, E.P.; Diehl, J.T.; Maravilla, K.R.; Vu, L.H.; Clark, W.K.; Rapoport, S.I.; Barnett, P.A.; Hill, S.A.; Lewis, S.E.; et al. Osmotic blood-brain barrier disruption: A new means of increasing chemotherapeutic agent delivery. Am. Neurol. Assoc. 1979, 104, 256–260. [Google Scholar]
- Williams, P.C.; Henner, W.D.; Roman-Goldstein, S.; Dahlborg, S.A.; Brummett, R.E.; Tableman, M.; Dana, B.W.; Neuwelt, E.A. Toxicity and efficacy of carboplatin and etoposide in conjunction with disruption of the blood-brain tumor barrier in the treatment of intracranial neoplasms. Neurosurgery 1995, 37, 17–27; discussion 27–28. [Google Scholar] [CrossRef]
- Miyagami, M.; Tsubokawa, T.; Tazoe, M.; Kagawa, Y. Intra-arterial ACNU chemotherapy employing 20% mannitol osmotic bloodbrain barrier disruption for malignant brain tumors. Neurol. Med. Chir. 1990, 30, 582–590. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Diehl, J.T.; Long, H.; Vu, L.H.; Hill, S.A.; Michael, A.J.; Frenkel, E.P. Monitoring of methotrexate delivery in patients with malignant brain tumors after osmotic blood-brain barrier disruption. Ann. Intern. Med. 1981, 94, 449–454. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Goldman, D.L.; Dahlborg, S.A.; Crossen, J.; Ramsey, F.; Roman-Goldstein, S.; Braziel, R.; Dana, B. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: Prolonged survival and preservation of cognitive function. J. Clin. Oncol. 1991, 9, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Han, L. Modulation of the Blood-Brain Barrier for Drug Delivery to Brain. Pharmaceutics 2021, 13, 2024. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A.; Howieson, J.; Frenkel, E.P.; Specht, H.D.; Weigel, R.; Buchan, C.G.; Hill, S.A. Therapeutic efficacy of multiagent chemotherapy with drug delivery enhancement by blood-brain barrier modification in glioblastoma. Neurosurgery 1986, 19, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.A.; Doolittle, N.D.; Daman, M.; Bruns, P.K.; Muldoon, L.; Fortin, D.; Neuwelt, E.A. Osmotic blood-brain barrier disruption chemotherapy for diffuse pontine gliomas. J. Neurooncol. 2006, 77, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.S.; Ali, R.; Feroze, A.H.; Li, G.; Lawton, M.T.; Choudhri, O. Endovascular therapies for malignant gliomas: Challenges and the future. J. Clin. Neurosci. 2016, 26, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, J.K.; Riina, H.; Shin, B.J.; Christos, P.; Kesavabhotla, K.; Hofstetter, C.P.; Tsiouris, A.J.; Boockvar, J.A. Intra-arterial delivery of bevacizumab after blood-brain barrier disruption for the treatment of recurrent glioblastoma: Progression-free survival and overall survival. World Neurosurg. 2012, 77, 130–134. [Google Scholar] [CrossRef]
- Brown, R.C.; Egleton, R.D.; Davis, T.P. Mannitol opening of the blood-brain barrier: Regional variation in the permeability of sucrose, but not 86Rb+ or albumin. Brain Res. 2004, 1014, 221–227. [Google Scholar] [CrossRef]
- Kroll, R.A.; Pagel, M.A.; Muldoon, L.L.; Roman-Goldstein, S.; Fiamengo, S.A.; Neuwelt, E.A. Improving drug delivery to intracerebral tumor and surrounding brain in a rodent model: A comparison of osmotic versus bradykinin modification of the blood-brain and/or blood-tumor barriers. Neurosurgery 1998, 43, 879–886; discussion 886–889. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Dahlborg, S.A. Chemotherapy administered in conjunction with osmotic blood-brain barrier modification in patients with brain metastases. J. Neurooncol. 1987, 4, 195–207. [Google Scholar] [CrossRef]
- Pitz, M.W.; Desai, A.; Grossman, S.A.; Blakeley, J.O. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J. Neurooncol. 2011, 104, 629–638. [Google Scholar] [CrossRef]
- Bellavance, M.A.; Blanchette, M.; Fortin, D. Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 2008, 10, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kawase, T.; Harada, S.; Takayama, H.; Suga, S. Effect of hyperosmotic solutions on human brain tumour vasculature. Acta Neurochir (Wien). Acta Neurochir. 1998, 140, 1135–1142; discussion 1141–1142. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Filippi, C.G.; Wong, T.; Ray, A.; Fralin, S.; Tsiouris, A.J.; Praminick, B.; Demopoulos, A.; McCrea, H.J.; Bodhinayake, I.; et al. Superselective intraarterial cerebral infusion of cetuximab after osmotic blood/brain barrier disruption for recurrent malignant glioma: Phase I study. J. Neurooncol. 2016, 128, 405–415, Erratum in J. Neurooncol. 2016, 128, 417. [Google Scholar] [CrossRef] [PubMed]
- Tyler, J.L.; Yamamoto, Y.L.; Diksic, M.; Théron, J.; Villemure, J.G.; Worthington, C.; Evans, A.C.; Feindel, W. Pharmacokinetics of superselective intra-arterial and intravenous [11C]BCNU evaluated by PET. J. Nucl. Med. 1986, 27, 775–780. [Google Scholar] [PubMed]
- Kroll, R.A.; Neuwelt, E.A. Outwitting the blood-brain barrier for therapeutic purposes: Osmotic opening and other means. Neurosurgery 1998, 42, 1083–1099; discussion 1099–1100. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, D.J.; Doolittle, N.D.; Gahramanov, S.; Hedrick, N.A.; Delashaw, J.B.; Neuwelt, E.A. Intra-arterial chemotherapy with osmotic blood-brain barrier disruption for aggressive oligodendroglial tumors: Results of a phase I study. Neurosurgery 2010, 66, 48–58; discussion 58. [Google Scholar]
- D’Amico, R.S.; Khatri, D.; Reichman, N.; Patel, N.V.; Wong, T.; Fralin, S.R.; Li, M.; Ellis, J.A.; Ortiz, R.; Langer, D.J.; et al. Super selective intra-arterial cerebral infusion of modern chemotherapeutics after blood-brain barrier disruption: Where are we now, and where we are going. J. Neurooncol. 2020, 147, 261–278, Erratum in J. Neurooncol. 2020, 147, 279. [Google Scholar] [CrossRef]
- Burkhardt, J.K.; Riina, H.A.; Shin, B.J.; Moliterno, J.A.; Hofstetter, C.P.; Boockvar, J.A. Intra-arterial chemotherapy for malignant gliomas: A critical analysis. Interv. Neuroradiol. 2011, 17, 286–295, Erratum in Interv. Neuroradiol. 2011, 17, 506. [Google Scholar] [CrossRef]
- Srinivasan, V.M.; Lang, F.F.; Chen, S.R.; Chen, M.M.; Gumin, J.; Johnson, J.; Burkhardt, J.K.; Kan, P. Advances in endovascular neuro-oncology: Endovascular selective intra-arterial (ESIA) infusion of targeted biologic therapy for brain tumors. J. Neurointerv. Surg. 2020, 12, 197–203. [Google Scholar] [CrossRef]
- Gobin, Y.P.; Cloughesy, T.F.; Chow, K.L.; Duckwiler, G.R.; Sayre, J.W.; Milanese, K.; Viñuela, F. Intraarterial chemotherapy for brain tumors by using a spatial dose fractionation algorithm and pulsatile delivery. Radiology 2001, 218, 724–732. [Google Scholar] [CrossRef]
- Uluc, K.; Ambady, P.; McIntyre, M.K.; Tabb, J.P.; Kersch, C.N.; Nerison, C.S.; Huddleston, A.; Liu, J.J.; Dogan, A.; Priest, R.A.; et al. Safety of intra-arterial chemotherapy with or without osmotic blood-brain barrier disruption for the treatment of patients with brain tumors. Neurooncol. Adv. 2022, 4, vdac104. [Google Scholar] [CrossRef] [PubMed]
- Boockvar, J.A.; Tsiouris, A.J.; Hofstetter, C.P.; Kovanlikaya, I.; Fralin, S.; Kesavabhotla, K.; Seedial, S.M.; Pannullo, S.C.; Schwartz, T.H.; Stieg, P.; et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clinical article. J. Neurosurg. 2011, 114, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.V.; Wong, T.; Fralin, S.R.; Li, M.; McKeown, A.; Gruber, D.; D’Amico, R.S.; Patsalides, A.; Tsiouris, A.; Stefanov, D.G.; et al. Repeated superselective intraarterial bevacizumab after blood brain barrier disruption for newly diagnosed glioblastoma: A phase I/II clinical trial. J. Neurooncol. 2021, 155, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Filippi, C.G.; Burkhardt, J.K.; Fralin, S.; Ray, A.; Wong, T.; Ortiz, R.; Langer, D.J.; Boockvar, J.A. Durability of single dose intra-arterial bevacizumab after blood/brain barrier disruption for recurrent glioblastoma. J. Exp. Ther. Oncol. 2016, 11, 261–267. [Google Scholar] [PubMed]
- Siegal, T.; Rubinstein, R.; Bokstein, F.; Schwartz, A.; Lossos, A.; Shalom, E.; Chisin, R.; Gomori, J.M. In vivo assessment of the window of barrier opening after osmotic blood-brain barrier disruption in humans. J. Neurosurg. 2000, 92, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Zünkeler, B.; Carson, R.E.; Olson, J.; Blasberg, R.G.; Devroom, H.; Lutz, R.J.; Saris, S.C.; Wright, D.C.; Kammerer, W.; Patronas, N.J.; et al. Quantification and pharmacokinetics of blood-brain barrier disruption in humans. J. Neurosurg. 1996, 85, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Ergin, A.; Wang, M.; Reif, R.; Zhang, J.; Bruce, J.N.; Bigio, I.J. Inconsistent blood brain barrier disruption by intraarterial mannitol in rabbits: Implications for chemotherapy. J. Neurooncol. 2011, 104, 11–19. [Google Scholar] [CrossRef]
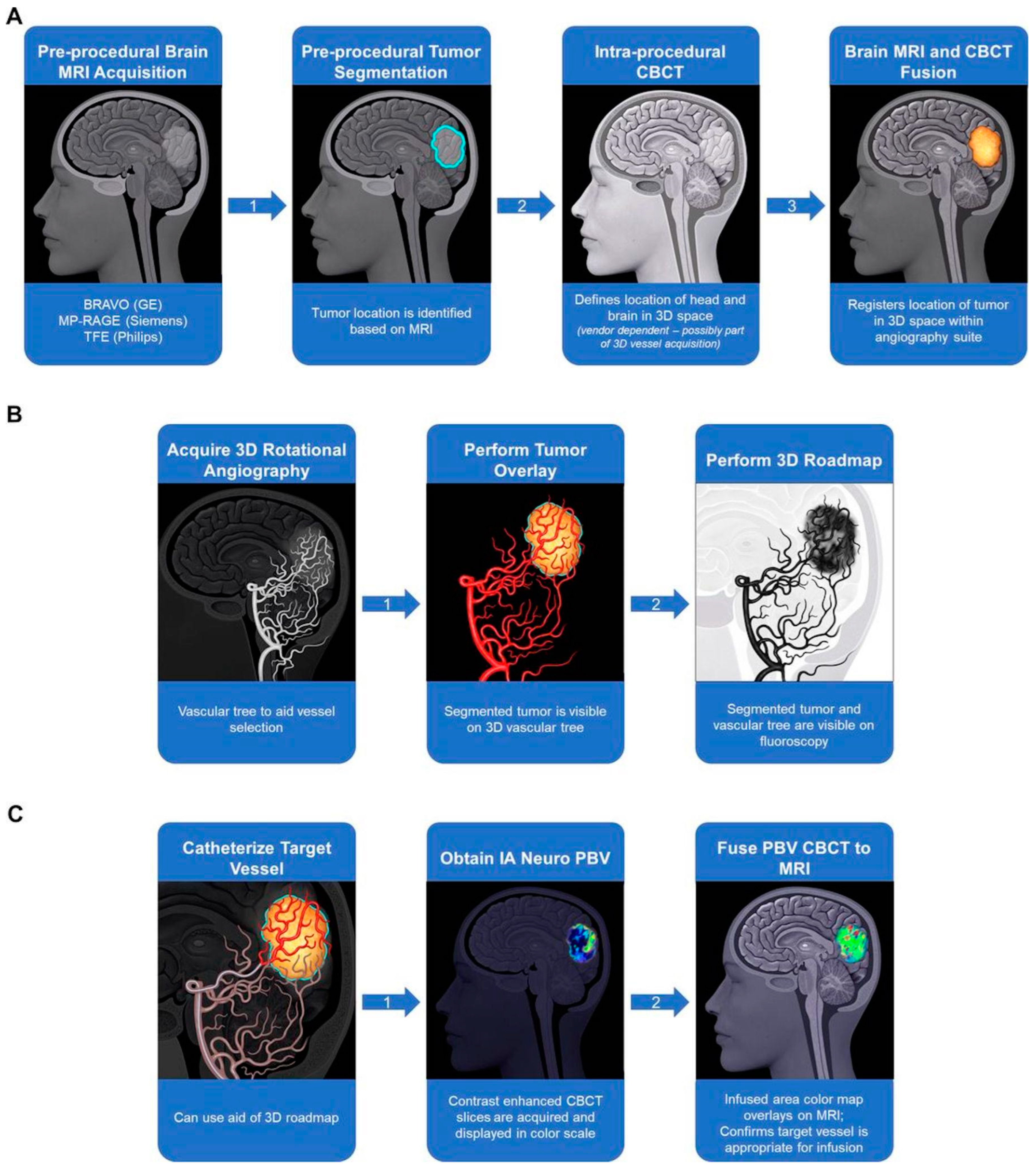
- Zawadzki, M.; Walecki, J.; Kostkiewicz, B.; Kostyra, K.; Pearl, M.S.; Solaiyappan, M.; Walczak, P.; Janowski, M. Real-time MRI guidance for intra-arterial drug delivery in a patient with a brain tumor: Technical note. BMJ Case Rep. 2019, 12, e014469. [Google Scholar] [CrossRef]
- Chu, C.; Jablonska, A.; Gao, Y.; Lan, X.; Lesniak, W.G.; Liang, Y.; Liu, G.; Li, S.; Magnus, T.; Pearl, M.; et al. Hyperosmolar blood-brain barrier opening using intra-arterial injection of hyperosmotic mannitol in mice under real-time MRI guidance. Nat. Protoc. 2022, 17, 76–94. [Google Scholar] [CrossRef]
- Kiviniemi, V.; Korhonen, V.; Kortelainen, J.; Rytky, S.; Keinänen, T.; Tuovinen, T.; Isokangas, M.; Sonkajärvi, E.; Siniluoto, T.; Nikkinen, J.; et al. Real-time monitoring of human blood-brain barrier disruption. PLoS ONE 2017, 12, e0174072. [Google Scholar] [CrossRef]
- Chen, S.R.; Chen, M.M.; Ene, C.; Lang, F.F.; Kan, P. Perfusion-guided endovascular super-selective intra-arterial infusion for treatment of malignant brain tumors. J. Neurointerv. Surg. 2022, 14, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Beauchesne, P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol. 2010, 11, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Kadoch, C.; Li, J.; Wong, V.S.; Chen, L.; Cha, S.; Munster, P.; Lowell, C.A.; Shuman, M.A.; Rubenstein, J.L. Complement activation and intraventricular rituximab distribution in recurrent central nervous system lymphoma. Clin. Cancer Res. 2014, 20, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Oberkampf, F.; Gutierrez, M.; Trabelsi Grati, O.; Le Rhun, É.; Trédan, O.; Turbiez, I.; Kadi, A.; Dubot, C.; Taillibert, S.; Vacher, S.; et al. Phase II study of intrathecal administration of trastuzumab in patients with HER2-positive breast cancer with leptomeningeal metastasis. Neuro Oncol. 2023, 25, 365–374. [Google Scholar] [CrossRef]
- Kumthekar, P.U.; Avram, M.J.; Lassman, A.B.; Lin, N.U.; Lee, E.; Grimm, S.A.; Schwartz, M.; Bell Burdett, K.L.; Lukas, R.V.; Dixit, K.; et al. A phase I/II study of intrathecal trastuzumab in human epidermal growth factor receptor 2-positive (HER2-positive) cancer with leptomeningeal metastases: Safety, efficacy, and cerebrospinal fluid pharmacokinetics. Neuro Oncol. 2023, 25, 557–565. [Google Scholar] [CrossRef]
- Kimelberg, H.K.; Kung, D.; Watson, R.E.; Reiss, F.L.; Biddlecome, S.M.; Bourke, R.S. Direct administration of methotrexate into the central nervous system of primates. Part 1: Distribution and degradation of methotrexate in nervous and systemic tissue after intraventricular injection. J. Neurosurg. 1978, 48, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Blasberg, R.G.; Patlak, C.; Fenstermacher, J.D. Intrathecal chemotherapy: Brain tissue profiles after ventriculocisternal perfusion. J. Pharmacol. Exp. Ther. 1975, 195, 73–83. [Google Scholar] [PubMed]
- Rodriguez, A.; Zugbi, S.; Requejo, F.; Deu, A.; Sampor, C.; Sgroi, M.; Bosaleh, A.; Fandiño, A.; Schaiquevich, P.; Chantada, G. Combined high-dose intra-arterial and intrathecal chemotherapy for the treatment of a case of extraocular retinoblastoma. Pediatr. Blood Cancer 2018, 65, e27385. [Google Scholar] [CrossRef]
- Urakawa, M.; Yamaguchi, K.; Tsuchida, E.; Kashiwagi, S.; Ito, H.; Matsuda, T. Blood-brain barrier disturbance following localized hyperthermia in rats. Int. J. Hyperth. 1995, 11, 709–718. [Google Scholar] [CrossRef]
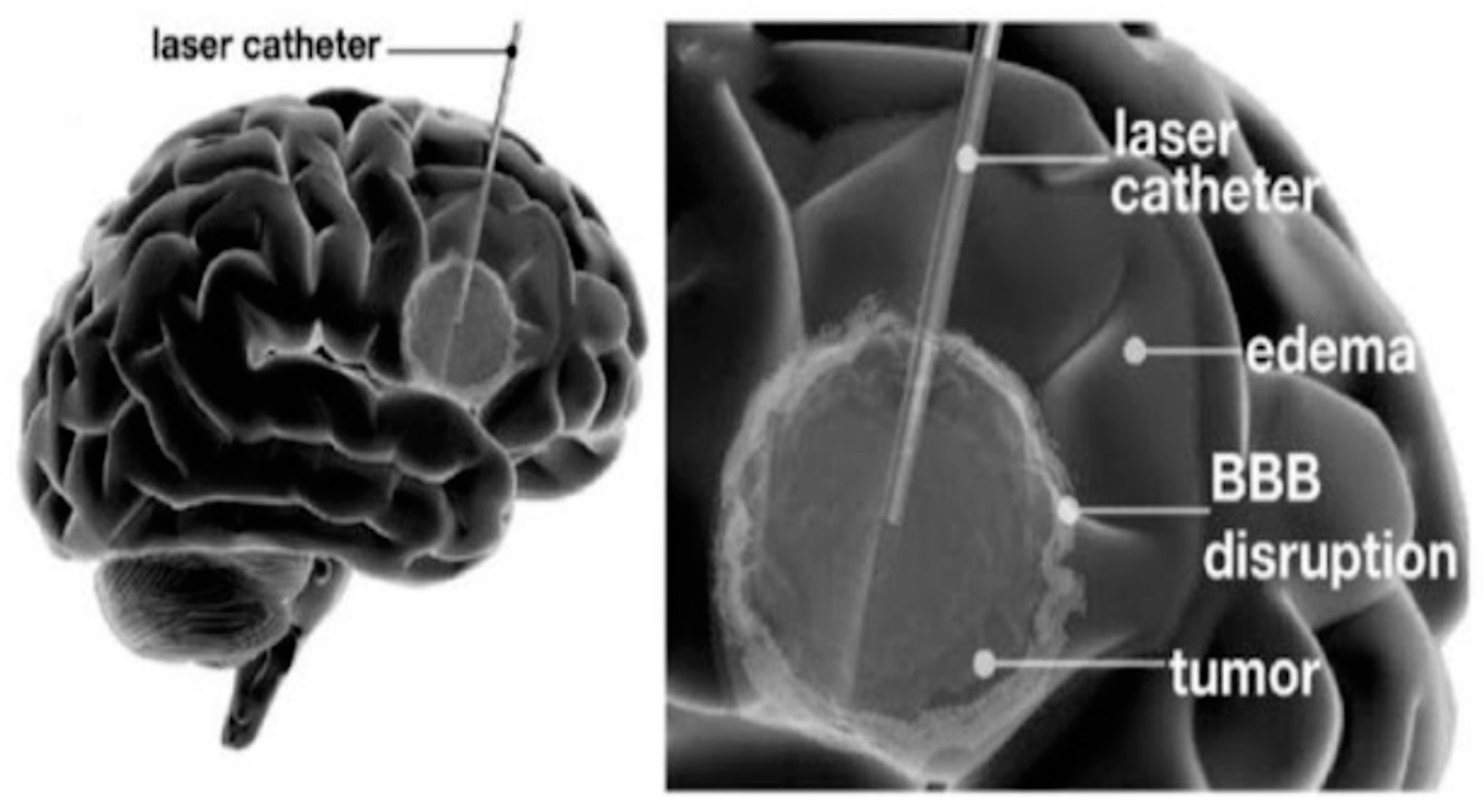
- Leuthardt, E.C.; Duan, C.; Kim, M.J.; Campian, J.L.; Kim, A.H.; Miller-Thomas, M.M.; Shimony, J.S.; Tran, D.D. Hyperthermic Laser Ablation of Recurrent Glioblastoma Leads to Temporary Disruption of the Peritumoral Blood Brain Barrier. PLoS ONE 2016, 11, e0148613. [Google Scholar] [CrossRef]
- Patel, B.; Yang, P.H.; Kim, A.H. The effect of thermal therapy on the blood-brain barrier and blood-tumor barrier. Int. J. Hyperth. 2020, 37, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Skandalakis, G.P.; Rivera, D.R.; Rizea, C.D.; Bouras, A.; Jesu Raj, J.G.; Bozec, D.; Hadjipanayis, C.G. Hyperthermia treatment advances for brain tumors. Int. J. Hyperth. 2020, 37, 3–19. [Google Scholar] [CrossRef]
- Bartlett, S.; Nagaraja, T.N.; Griffith, B.; Farmer, K.G.; Van Harn, M.; Haider, S.; Hunt, R.J.; Cabral, G.; Knight, R.A.; Valadie, O.G.; et al. Persistent Peri-Ablation Blood-Brain Barrier Opening After Laser Interstitial Thermal Therapy for Brain Tumors. Cureus 2023, 15, e37397. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Paturu, M.R.; Patel, B.; Cain, M.D.; Mahlokozera, T.; Yang, A.B.; Lin, T.H.; Leuthardt, E.C.; Yano, H.; Song, S.K.; et al. Therapeutic enhancement of blood-brain and blood-tumor barriers permeability by laser interstitial thermal therapy. Neurooncol. Adv. 2020, 2, vdaa071. [Google Scholar] [CrossRef]
- Butt, O.H.; Zhou, A.Y.; Huang, J.; Leidig, W.A.; Silberstein, A.E.; Chheda, M.G.; Johanns, T.M.; Ansstas, G.; Liu, J.; Talcott, G.; et al. A phase II study of laser interstitial thermal therapy combined with doxorubicin in patients with recurrent glioblastoma. Neurooncol. Adv. 2021, 3, vdab164, Erratum in Neurooncol. Adv. 2022, 4, vdac042. [Google Scholar] [CrossRef]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef] [PubMed]
- Sperring, C.P.; Argenziano, M.G.; Savage, W.M.; Teasley, D.E.; Upadhyayula, P.S.; Winans, N.J.; Canoll, P.; Bruce, J.N. Convection-enhanced delivery of immunomodulatory therapy for high-grade glioma. Neurooncol. Adv. 2023, 5, vdad044. [Google Scholar] [CrossRef]
- Bidros, D.S.; Liu, J.K.; Vogelbaum, M.A. Future of convection-enhanced delivery in the treatment of brain tumors. Future Oncol. 2010, 6, 117–125. [Google Scholar] [CrossRef]
- D’Amico, R.S.; Aghi, M.K.; Vogelbaum, M.A.; Bruce, J.N. Convection-enhanced drug delivery for glioblastoma: A review. J. Neurooncol. 2021, 151, 415–427. [Google Scholar] [CrossRef]
- Laske, D.W.; Youle, R.J.; Oldfield, E.H. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat. Med. 1997, 3, 1362–1368. [Google Scholar] [CrossRef]
- Raghavan, R.; Brady, M.L.; Sampson, J.H. Delivering therapy to target: Improving the odds for successful drug development. Ther. Deliv. 2016, 7, 457–481. [Google Scholar] [CrossRef]
- Rand, R.W.; Kreitman, R.J.; Patronas, N.; Varricchio, F.; Pastan, I.; Puri, R.K. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin. Cancer Res. 2000, 6, 2157–2165. [Google Scholar] [PubMed]
- Weber, F.W.; Floeth, F.; Asher, A.; Bucholz, R.; Berger, M.; Prados, M.; Chang, S.; Bruce, J.; Hall, W.; Rainov, N.G.; et al. Local convection enhanced delivery of IL4-Pseudomonas exotoxin (NBI-3001) for treatment of patients with recurrent malignant glioma. Acta Neurochir. Suppl. 2003, 88, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Lidar, Z.; Mardor, Y.; Jonas, T.; Pfeffer, R.; Faibel, M.; Nass, D.; Hadani, M.; Ram, Z. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: A phase I/II clinical study. J. Neurosurg. 2004, 100, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.J.; Shapiro, W.R.; Laske, D.W.; Jensen, R.L.; Asher, A.L.; Wessels, B.W.; Carpenter, S.P.; Shan, J.S. Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: Initial experience in 51 patients. Neurosurgery 2005, 56, 1243–1252; discussion 1252–1253. [Google Scholar] [CrossRef]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs. Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010, 12, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Akabani, G.; Archer, G.E.; Bigner, D.D.; Berger, M.S.; Friedman, A.H.; Friedman, H.S.; Herndon, J.E., 2nd; Kunwar, S.; Marcus, S.; et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J. Neurooncol. 2003, 65, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bogdahn, U.; Hau, P.; Stockhammer, G.; Venkataramana, N.K.; Mahapatra, A.K.; Suri, A.; Balasubramaniam, A.; Nair, S.; Oliushine, V.; Parfenov, V.; et al. Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: Results of a randomized and controlled phase IIb study. Neuro Oncol. 2011, 13, 132–142. [Google Scholar] [CrossRef]
- Bruce, J.N.; Fine, R.L.; Canoll, P.; Yun, J.; Kennedy, B.C.; Rosenfeld, S.S.; Sands, S.A.; Surapaneni, K.; Lai, R.; Yanes, C.L.; et al. Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery 2011, 69, 1272–1279; discussion 1279–1280. [Google Scholar] [CrossRef]
- Voges, J.; Reszka, R.; Gossmann, A.; Dittmar, C.; Richter, R.; Garlip, G.; Kracht, L.; Coenen, H.H.; Sturm, V.; Wienhard, K.; et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann. Neurol. 2003, 54, 479–487. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brewer, C.; Barnett, G.H.; Mohammadi, A.M.; Peereboom, D.M.; Ahluwalia, M.S.; Gao, S. First-in-human evaluation of the Cleveland Multiport Catheter for convection-enhanced delivery of topotecan in recurrent high-grade glioma: Results of pilot trial 1. J. Neurosurg. 2018, 1–10, ahead of print. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E., 2nd; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef]
- Thombre, R.; Mess, G.; Kempski Leadingham, K.M.; Kapoor, S.; Hersh, A.; Acord, M.; Kaovasia, T.; Theodore, N.; Tyler, B.; Manbachi, A. Towards standardization of the parameters for opening the blood-brain barrier with focused ultrasound to treat glioblastoma multiforme: A systematic review of the devices, animal models, and therapeutic compounds used in rodent tumor models. Front. Oncol. 2023, 12, 1072780. [Google Scholar] [CrossRef]
- Chen, K.T.; Wei, K.C.; Liu, H.L. Theranostic Strategy of Focused Ultrasound Induced Blood-Brain Barrier Opening for CNS Disease Treatment. Front. Pharmacol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Gorick, C.M.; Breza, V.R.; Nowak, K.M.; Cheng, V.W.T.; Fisher, D.G.; Debski, A.C.; Hoch, M.R.; Demir, Z.E.F.; Tran, N.M.; Schwartz, M.R.; et al. Applications of focused ultrasound-mediated blood-brain barrier opening. Adv. Drug Deliv. Rev. 2022, 191, 114583. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Raymond, S.; Weissleder, R.; Jolesz, F.A.; Sheikov, N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: A method for molecular imaging and targeted drug delivery. J. Neurosurg. 2006, 105, 445–454. [Google Scholar] [CrossRef]
- Chen, H.; Konofagou, E.E. The size of blood-brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J. Cereb. Blood Flow. Metab. 2014, 34, 1197–1204. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Pan, M.; Fiaz, M.; Hao, Y.; Yan, Y.; Sun, L.; Yan, F. Ultrasound-mediated blood-brain barrier opening: An effective drug delivery system for theranostics of brain diseases. Adv. Drug Deliv. Rev. 2022, 190, 114539. [Google Scholar] [CrossRef]
- Morse, S.V.; Pouliopoulos, A.N.; Chan, T.G.; Copping, M.J.; Lin, J.; Long, N.J.; Choi, J.J. Rapid Short-pulse Ultrasound Delivers Drugs Uniformly across the Murine Blood-Brain Barrier with Negligible Disruption. Radiology 2019, 291, 459–466. [Google Scholar] [CrossRef]
- Lim Kee Chang, W.; Chan, T.G.; Raguseo, F.; Mishra, A.; Chattenton, D.; de Rosales, R.T.M.; Long, N.J.; Morse, S.V. Rapid short-pulses of focused ultrasound and microbubbles deliver a range of agent sizes to the brain. Sci. Rep. 2023, 13, 6963. [Google Scholar] [CrossRef]
- Curley, C.T.; Mead, B.P.; Negron, K.; Kim, N.; Garrison, W.J.; Miller, G.W.; Kingsmore, K.M.; Thim, E.A.; Song, J.; Munson, J.M.; et al. Augmentation of brain tumor interstitial flow via focused ultrasound promotes brain-penetrating nanoparticle dispersion and transfection. Sci. Adv. 2020, 6, eaay1344. [Google Scholar] [CrossRef]
- Quadri, S.A.; Waqas, M.; Khan, I.; Khan, M.A.; Suriya, S.S.; Farooqui, M.; Fiani, B. High-intensity focused ultrasound: Past, present, and future in neurosurgery. Neurosurg. Focus 2018, 44, E16. [Google Scholar] [CrossRef]
- Hynynen, K.; Freund, W.R.; Cline, H.E.; Chung, A.H.; Watkins, R.D.; Vetro, J.P.; Jolesz, F.A. A clinical, noninvasive, MR imaging-monitored ultrasound surgery method. Radiographics 1996, 16, 185–195. [Google Scholar] [CrossRef]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef]
- Meng, Y.; Pople, C.B.; Suppiah, S.; Llinas, M.; Huang, Y.; Sahgal, A.; Perry, J.; Keith, J.; Davidson, B.; Hamani, C.; et al. MR-guided focused ultrasound liquid biopsy enriches circulating biomarkers in patients with brain tumors. Neuro Oncol. 2021, 23, 1789–1797. [Google Scholar] [CrossRef]
- Kuniyil Ajith Singh, M.; Steenbergen, W. Photoacoustic-guided focused ultrasound (PAFUSion) for identifying reflection artifacts in photoacoustic imaging. Photoacoustics 2015, 3, 123–131. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, C.; Zeng, J.; Sun, Q.; Wang, G.; Wang, Y.; Wu, Y.; Dou, S.; Gao, M.; Li, Z. Ambient Aqueous Synthesis of Ultrasmall PEGylated Cu2-x Se Nanoparticles as a Multifunctional Theranostic Agent for Multimodal Imaging Guided Photothermal Therapy of Cancer. Adv. Mater. 2016, 28, 8927–8936. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Gould, A.; Amidei, C.; Ward, R.; Schmidt, K.A.; Zhang, D.Y.; Gomez, C.; Bebawy, J.F.; Liu, B.P.; Bouchoux, G.; et al. Repeated blood-brain barrier opening with an implantable ultrasound device for delivery of albumin-bound paclitaxel in patients with recurrent glioblastoma: A phase 1 trial. Lancet Oncol. 2023, 24, 509–522. [Google Scholar] [CrossRef]
- Xin, Z.; Lin, G.; Lei, H.; Lue, T.F.; Guo, Y. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. Transl. Androl. Urol. 2016, 5, 255–266. [Google Scholar] [CrossRef]
- Beccaria, K.; Sabbagh, A.; de Groot, J.; Canney, M.; Carpentier, A.; Heimberger, A.B. Blood-brain barrier opening with low intensity pulsed ultrasound for immune modulation and immune therapeutic delivery to CNS tumors. J. Neurooncol. 2021, 151, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Patel, C.B.; Pohling, C.; Young, C.; Song, J.; Flores, T.A.; Zeng, Y.; Joubert, L.-M.; Arami, H.; Natarajan, A.; et al. Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov. 2018, 4, 113. [Google Scholar] [CrossRef] [PubMed]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Salvador, E.; Kessler, A.F.; Domröse, D.; Hörmann, J.; Schaeffer, C.; Giniunaite, A.; Burek, M.; Tempel-Brami, C.; Voloshin, T.; Volodin, A.; et al. Tumor Treating Fields (TTFields) Reversibly Permeabilize the Blood-Brain Barrier In Vitro and In Vivo. Biomolecules 2022, 12, 1348. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs. Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316, Erratum in JAMA 2018, 319, 1824. [Google Scholar] [CrossRef]
| Methods of Overcoming/Disrupting BBB in Drug Delivery to the CNS | ||||
|---|---|---|---|---|
| Method | Mechanism of BBBD | Duration of BBB Disruption | Advantages | Disadvantages |
| Invasive methods of overcoming/disrupting BBB | ||||
| OBBBD | Infusion of an osmotic agent, e.g., mannitol leads to dehydration of endothelial cells and subsequent disruption of the tight junctions between endothelial cells of the BBB. | The maximum effect in humans lasts up to 40 min and returns to baseline levels only after 6 to 8 h [105] ^. |
|
|
| Intrathecal/intraventricular administration | NA | NA |
|
|
| LITT | Disruption of endothelial tight junctions and increases endothelial cell transcytosis. | ‡ the peak permeability of the BBB occurs one to two weeks after LITT and returns to baseline by eight weeks postoperatively. |
|
|
| CED | A stereotactically placed catheter is connected to an infusion pump that generates the pressure gradient to facilitate the controlled and direct infusion of therapeutic agents into the extracellular space of the brain. | NA |
|
|
| Non-invasive methods of overcoming/disrupting BBB | ||||
| FUS-MB | FUS-triggered oscillation of microbubbles opens tight junctions in the BBB. Up-regulation of vesicles and carrier proteins or modulation of mechanosensitive ion channels. | 20 h * [156]. |
|
|
| LIPU-MB | Microbubbles oscillate upon stimulation by ultrasound, producing mechanical stress on the endothelial wall that disrupts the BBB. | most BBB integrity is restored within 1 h after LIPU-MB [160]. |
| |
| TTFields | Disruption of the localization of tight-junction proteins such as claudin 5 and ZO-1. | The BBB permeability normalizes within 24 h of ceasing TTFields treatment [164]. |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinkiewicz, M.; Pinkiewicz, M.; Walecki, J.; Zaczyński, A.; Zawadzki, M. Breaking Barriers in Neuro-Oncology: A Scoping Literature Review on Invasive and Non-Invasive Techniques for Blood–Brain Barrier Disruption. Cancers 2024, 16, 236. https://doi.org/10.3390/cancers16010236
Pinkiewicz M, Pinkiewicz M, Walecki J, Zaczyński A, Zawadzki M. Breaking Barriers in Neuro-Oncology: A Scoping Literature Review on Invasive and Non-Invasive Techniques for Blood–Brain Barrier Disruption. Cancers. 2024; 16(1):236. https://doi.org/10.3390/cancers16010236
Chicago/Turabian StylePinkiewicz, Miłosz, Mateusz Pinkiewicz, Jerzy Walecki, Artur Zaczyński, and Michał Zawadzki. 2024. "Breaking Barriers in Neuro-Oncology: A Scoping Literature Review on Invasive and Non-Invasive Techniques for Blood–Brain Barrier Disruption" Cancers 16, no. 1: 236. https://doi.org/10.3390/cancers16010236
APA StylePinkiewicz, M., Pinkiewicz, M., Walecki, J., Zaczyński, A., & Zawadzki, M. (2024). Breaking Barriers in Neuro-Oncology: A Scoping Literature Review on Invasive and Non-Invasive Techniques for Blood–Brain Barrier Disruption. Cancers, 16(1), 236. https://doi.org/10.3390/cancers16010236







