Evaluation of Three Imaging Methods to Quantify Key Events in Pelvic Bone Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
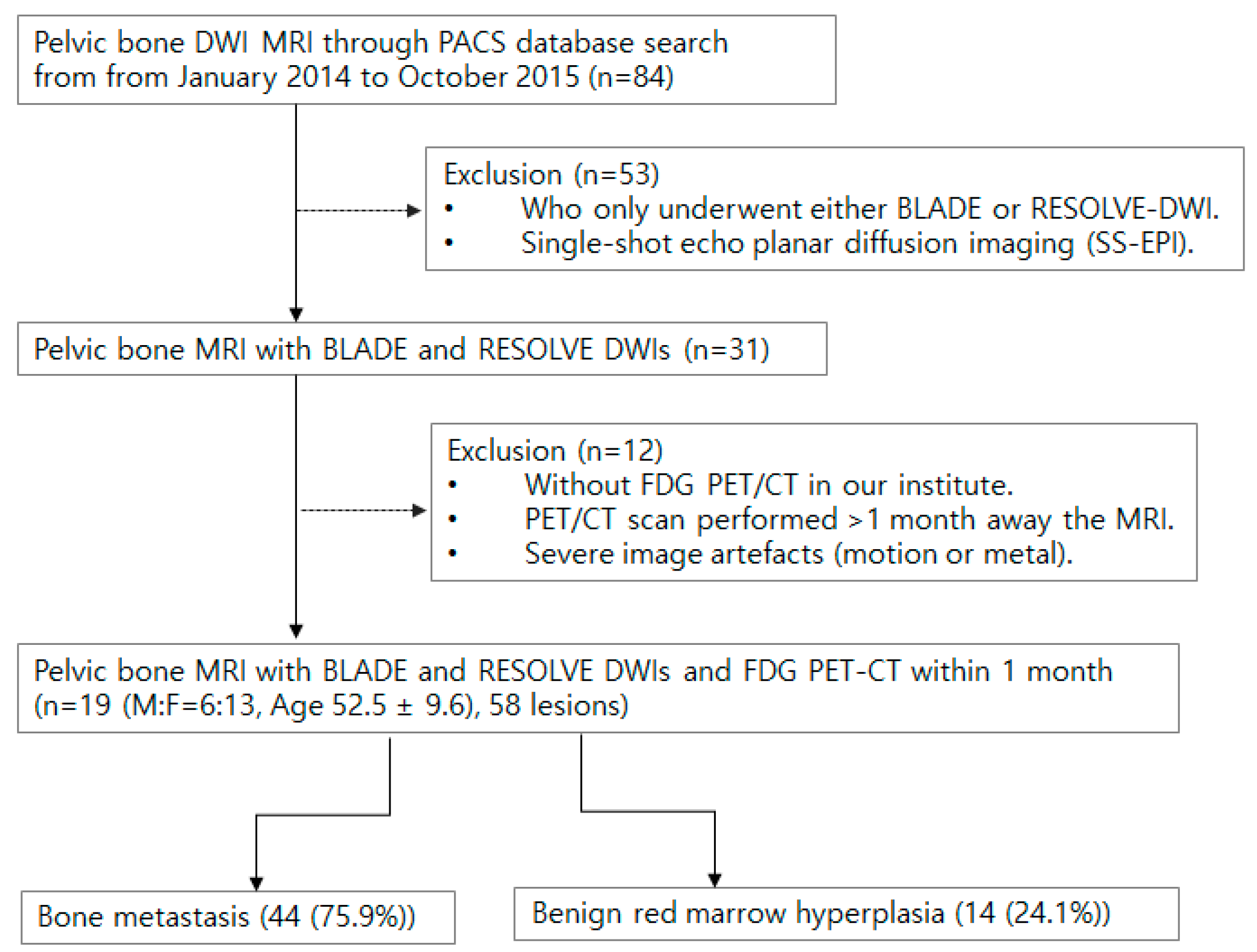
2.1. Patient Selection
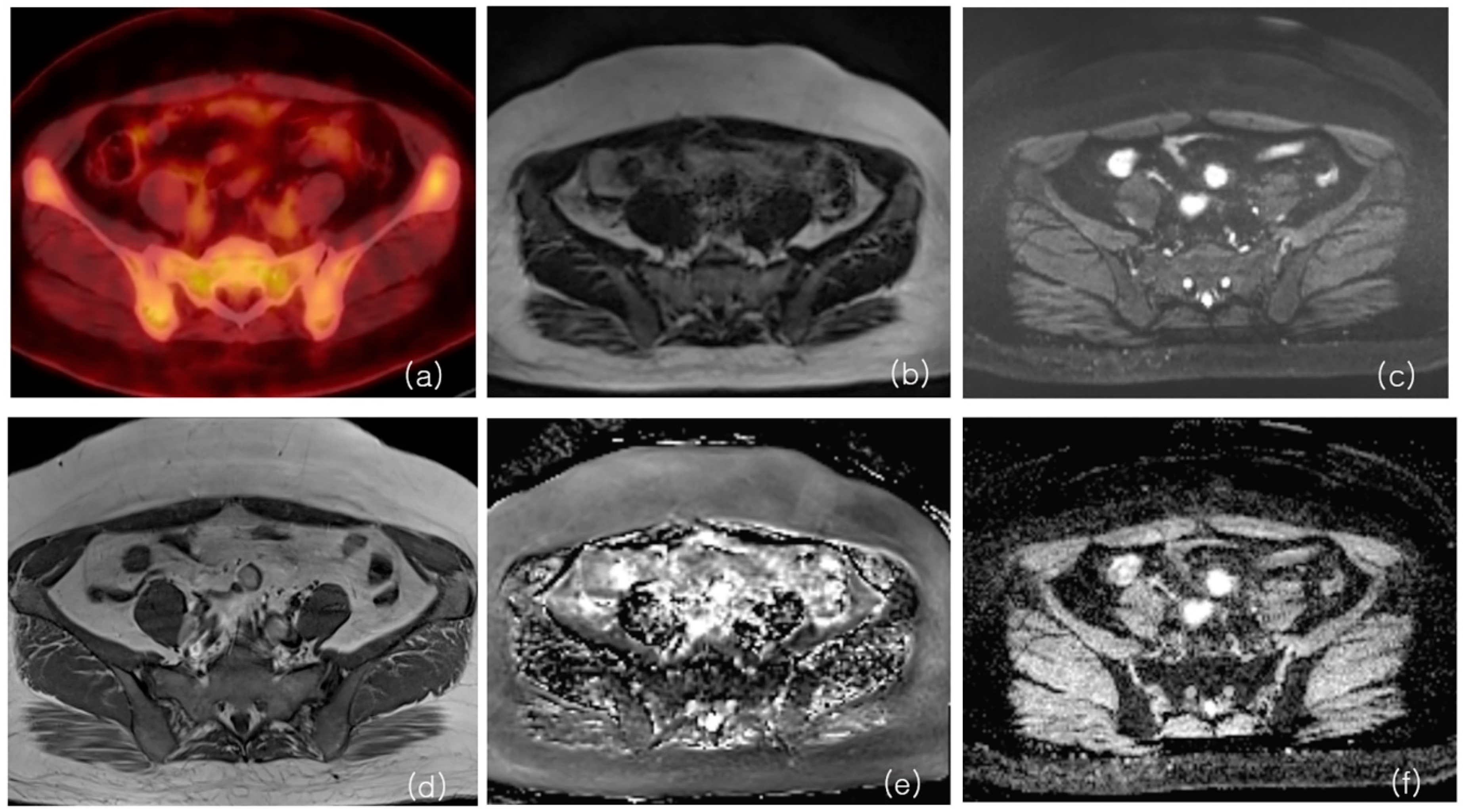
2.2. Magnetic Resonance Imaging
2.3. F-18 FDG PET/CT
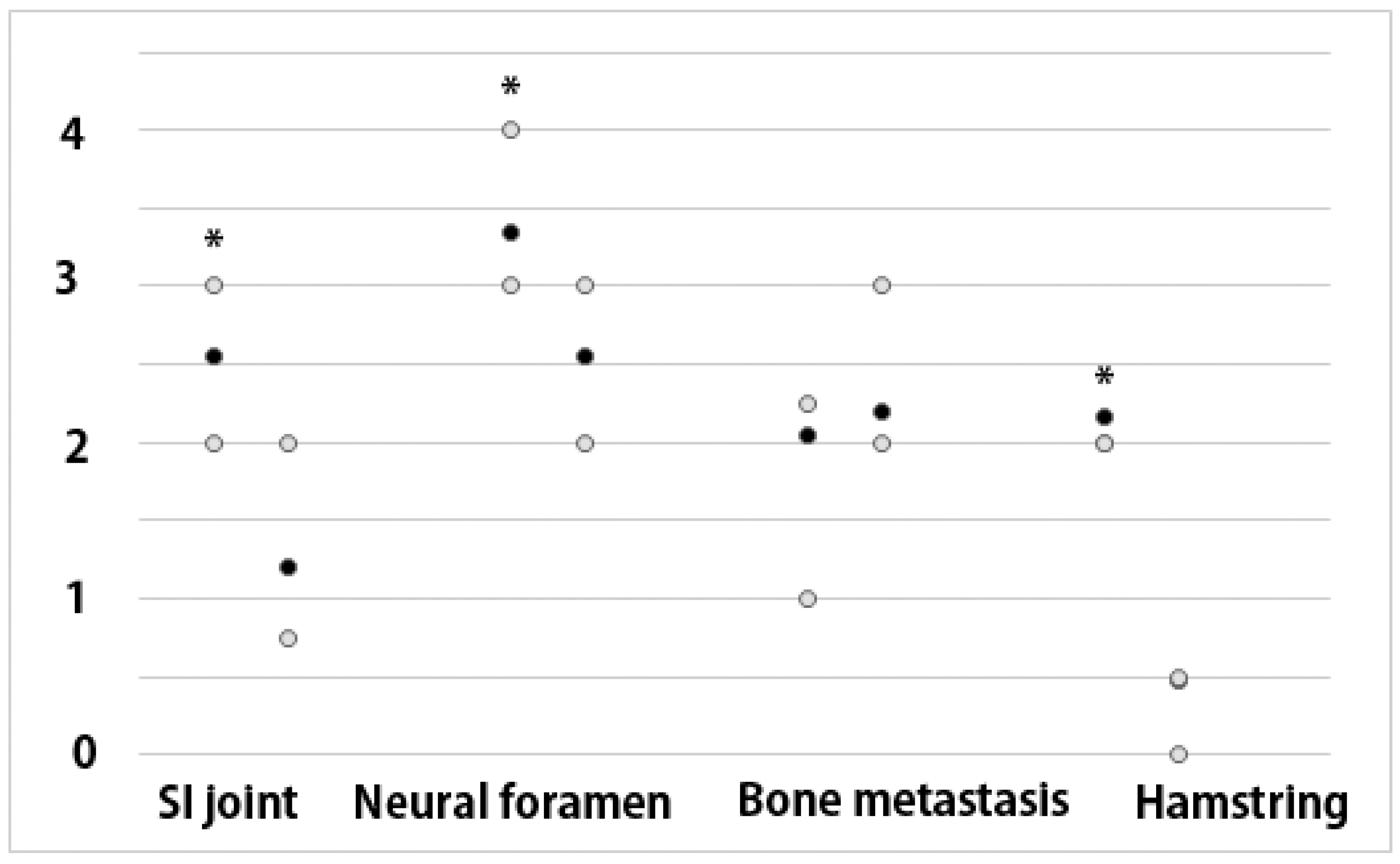
2.4. Quantification
2.5. Statistical Analysis
Ethics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ko, C.C.; Yeh, L.R.; Kuo, Y.T.; Chen, J.H. Imaging biomarkers for evaluating tumor response: RECIST and beyond. Biomark. Res. 2021, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Costelloe, C.M.; Chuang, H.H.; Madewell, J.E.; Ueno, N.T. Cancer Response Criteria and Bone Metastases: RECIST 1.1, MDA and PERCIST. J. Cancer 2010, 1, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, A.; Cao, X.; Liao, C.; Schifitto, G.; Zhong, J. Diffusion Encoding Methods in MRI: Perspectives and Challenges. Investig. Magn. Reson. Imaging 2022, 26, 208–219. [Google Scholar] [CrossRef]
- Sun, W.; Li, M.; Gu, Y.; Sun, Z.; Qiu, Z.; Zhou, Y. Diagnostic value of whole-body DWI with background body suppression plus calculation of apparent diffusion coefficient at 3 T versus 18F-FDG PET/CT for detection of bone metastases. Am. J. Roentgenol. 2020, 214, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, Z.; Sha, Y.; Wang, S.; Ye, X.; Pan, Y.; Wang, S. Readout-segmented echo-planar imaging in the evaluation of sinonasal lesions: A comprehensive comparison of image quality in single-shot echo-planar imaging. Magn. Reson. Imaging 2016, 34, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Morita, S.; Goto, Y.; Tadenuma, H.; Nishina, Y.; Yoneyama, M.; Tanaka, I.; Sakai, S. Artifact-robust diffusion-weighted whole-body imaging with background suppression at 3 T using improved turbo spin-echo diffusion-weighted imaging. Br. J. Radiol. 2019, 92, 20180489. [Google Scholar] [CrossRef]
- Yeom, K.W.; Holdsworth, S.J.; Van, A.T.; Iv, M.; Skare, S.; Lober, R.M.; Bammer, R. Comparison of readout-segmented echo-planar imaging (EPI) and single-shot EPI in clinical application of diffusion-weighted imaging of the pediatric brain. Am. J. Roentgenol. 2013, 200, W437–W443. [Google Scholar] [CrossRef]
- Saifuddin, A.; Tyler, P.; Rajakulasingam, R. Imaging of bone marrow pitfalls with emphasis on MRI. Br. J. Radiol. 2023, 96, 20220063. [Google Scholar] [CrossRef]
- Bordalo-Rodrigues, M.; Galant, C.; Lonneux, M.; Clause, D.; Vande Berg, B.C. Focal nodular hyperplasia of the hematopoietic marrow simulating vertebral metastasis on FDG positron emission tomography. Am. J. Roentgenol. 2003, 180, 669–671. [Google Scholar] [CrossRef]
- Hong, S.; Youk, T.; Lee, S.J.; Kim, K.M.; Vajdic, C.M. Bone metastasis and skeletal-related events in patients with solid cancer: A Korean nationwide health insurance database study. PLoS ONE 2020, 15, e0234927. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Liao, T.; Rao, Z.; Gong, W.; Ou, L.; Chen, Y.; Zhang, C. Comparison of the Relative Diagnostic Performance of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F]FDG PET/CT for the Detection of Bone Metastasis in Patients With Different Cancers. Front. Oncol. 2021, 11, 737827. [Google Scholar] [CrossRef] [PubMed]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T. Multidisciplinary Approach for Bone Metastasis: A Review. Cancers 2018, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Liu, T.; Wang, X.M.; Xu, Y.; Deng, S.M. Diagnosis of bone metastases: A meta-analysis comparing (1)(8)FDG PET, CT, MRI and bone scintigraphy. Eur. Radiol. 2011, 21, 2604–2617. [Google Scholar] [CrossRef] [PubMed]
- Peer, S.; Gopinath, R.; Saini, J.; Kumar, P.; Srinivas, D.; Nagaraj, C. Evaluation of the Diagnostic Performance of F18-Fluorodeoxyglucose-Positron Emission Tomography, Dynamic Susceptibility Contrast Perfusion, and Apparent Diffusion Coefficient in Differentiation between Recurrence of a High-grade Glioma and Radiation Necrosis. Indian J. Nucl. Med. 2023, 38, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; McAllister, A.S.; Jin, N.; Lubeley, L.J.; Selvaraj, B.; Smith, M.; Krishnamurthy, R.; Zhou, K. Comparison of 2D BLADE Turbo Gradient- and Spin-Echo and 2D Spin-Echo Echo-Planar Diffusion-Weighted Brain MRI at 3 T: Preliminary Experience in Children. Acad. Radiol. 2019, 26, 1597–1604. [Google Scholar] [CrossRef]
- Lin, M.; Sha, Y.; Sheng, Y.; Chen, W. Accuracy of 2D BLADE Turbo Gradient- and Spin-Echo Diffusion Weighted Imaging for the Diagnosis of Primary Middle Ear Cholesteatoma. Otol. Neurotol. 2022, 43, e651–e657. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Cai, J.; Chang, H.C. Motion-resolved four-dimensional abdominal diffusion-weighted imaging using PROPELLER EPI (4D-DW-PROPELLER-EPI). Magn. Reson. Med. 2023, 90, 2454–2471. [Google Scholar] [CrossRef]
- Perez-Lopez, R.; Mateo, J.; Mossop, H.; Blackledge, M.D.; Collins, D.J.; Rata, M.; Morgan, V.A.; Macdonald, A.; Sandhu, S.; Lorente, D.; et al. Diffusion-weighted Imaging as a Treatment Response Biomarker for Evaluating Bone Metastases in Prostate Cancer: A Pilot Study. Radiology 2017, 283, 168–177. [Google Scholar] [CrossRef]
- Chinnappan, S.; Chandra, P.; Kumar, J.S.; Chandran, G.; Nath, S. SUVmax/ADC Ratio as a Molecular Imaging Biomarker for Diagnosis of Biopsy-Naive Primary Prostate Cancer. Indian J. Nucl. Med. 2021, 36, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Polat, A.V.; Tosun, F.C.; Selcuk, M.B. Does the SUVmax of FDG-PET/CT Correlate with the ADC Values of DWI in Musculoskeletal Malignancies? J. Belg. Soc. Radiol. 2021, 105, 11. [Google Scholar] [CrossRef] [PubMed]
- Rakheja, R.; Chandarana, H.; DeMello, L.; Jackson, K.; Geppert, C.; Faul, D.; Glielmi, C.; Friedman, K.P. Correlation between standardized uptake value and apparent diffusion coefficient of neoplastic lesions evaluated with whole-body simultaneous hybrid PET/MRI. Am. J. Roentgenol. 2013, 201, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Yamano, T.; Fukushima, K.; Miyoshi, Y.; Hirota, S.; Kawanaka, Y.; Miya, M.; Doi, H.; Yamakado, K.; Hirota, S. Correlation of the SUVmax of FDG-PET and ADC values of diffusion-weighted MR imaging with pathologic prognostic factors in breast carcinoma. Eur. J. Radiol. 2016, 85, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Tyng, C.J.; Guimarães, M.D.; Bitencourt, A.G.V.; dos Santos, L.C.M.; Barbosa, P.N.V.P.; Zurstrassen, C.E.; Pereira, E.N.; Gross, J.L.; Chojniak, R. Correlation of the ADC values assessed by diffusion-weighted MRI and 18F–FDG PET/CT SUV in patients with lung cancer. Appl. Cancer Res. 2018, 38, 9. [Google Scholar] [CrossRef]
- Wahl, R.L.; Henry, C.A.; Ethier, S.P. Serum glucose: Effects on tumor and normal tissue accumulation of 2-[F-18]-fluoro-2-deoxy-D-glucose in rodents with mammary carcinoma. Radiology 1992, 183, 643–647. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J. How We Read Oncologic FDG PET/CT. Cancer Imaging 2016, 16, 35. [Google Scholar] [CrossRef]
- Azad, G.K.; Taylor, B.P.; Green, A.; Sandri, I.; Swampillai, A.; Harries, M.; Kristeleit, H.; Mansi, J.; Goh, V.; Cook, G.J.R. Prediction of therapy response in bone-predominant metastatic breast cancer: Comparison of [(18)F] fluorodeoxyglucose and [(18)F]-fluoride PET/CT with whole-body MRI with diffusion-weighted imaging. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 821–830. [Google Scholar] [CrossRef]
- Hamaoka, T.; Madewell, J.E.; Podoloff, D.A.; Hortobagyi, G.N.; Ueno, N.T. Bone imaging in metastatic breast cancer. J. Clin. Oncol. 2004, 22, 2942–2953. [Google Scholar] [CrossRef]
- Rajakulasingam, R.; Saifuddin, A. Focal nodular marrow hyperplasia: Imaging features of 53 cases. Br. J. Radiol. 2020, 93, 20200206. [Google Scholar] [CrossRef]
- Park, S.; Do Huh, J. Differentiation of bone metastases from benign red marrow depositions of the spine: The role of fat-suppressed T2-weighted imaging compared to fat fraction map. Eur. Radiol. 2022, 32, 6730–6738. [Google Scholar] [CrossRef] [PubMed]


| Modality | Metrics | Metastasis | Red Marrow |
|---|---|---|---|
| BLADE | ADCMin | 600.9 ± 57.9 (p = 0.01) * | 136 ± 61.7 |
| ADCMax | 1460.4 ± 107.8 (p = 0.002) * | 413.4 ± 74.4 | |
| ADCAvr | 972.8 ± 71.6 (p = 0.002) * | 260.2 ± 71.9 | |
| RESOLVE | ADCMin | 610.1 ± 67.3 (p = 0.005) * | 22.0 ± 22.0 |
| ADCMax | 1250.4 ± 82.2 (p = 0.005) * | 523.8 ± 81.8 | |
| ADCAvr | 883.9 ± 8.2 (p = 0.002) * | 166.2 ± 34.4 | |
| F-18 FDG PET | SUVpeak | 4.9 ± 0.5 (p = 0.03) * | 1.6 ± 0.1 |
| SUVmean | 3.3 ± 0.2 (p = 0.006) * | 1.4 ± 0.2 | |
| SUVmax | 5.4 ± 0.5 (p = 0.018) * | 1.9 ± 0.2 |
| R2 | SUVpeak | C.I., p Values | SUVmean | C.I., p Values | SUVmax | C.I., p Values | |
|---|---|---|---|---|---|---|---|
| BLADE | ADCMin | 0.1 | (−0.21~0.39, p = 0.510) | 0.04 | (−0.271~0.339, p = 0.810) | 0.07 | (−0.239~0.368, p = 0.650) |
| ADCMax | 0.21 | (−0.10~0.48, p = 0.170) | 0.14 | (−0.177~0.423, p = 0.380) | 0.14 | (−0.175~0.425, p = 0.370) | |
| ADCAvr | 0.23 | (−0.08~0.50, p = 0.130) | 0.16 | (−0.155~0.441, p = 0.310) | 0.16 | (−0.156~0.440, p = 0.310) | |
| RESOLVE | ADCMin | 0.11 | (−0.20~0.40, p = 0.470) | 0.07 | (−0.239~0.369, p = 0.640) | 0.1 | (−0.216~0.390, p = 0.540) |
| ADCMax | 0.31 * | (0.01~0.57, p = 0.040) | 0.27 | (−0.038~0.532, p = 0.070) | 0.24 | (−0.074~0.505, p = 0.120) | |
| ADCAvr | 0.23 | (−0.08~04, p = 0.130) | 0.19 | (−0.124~0.466, p = 0.220) | 0.2 | (−0.117~0.472, p = 0.200) |
| BLADE DWI | RESOLVE DWI | |
|---|---|---|
| SNR | 712.6 ± 236.5 * | 216.4 ± 16.6 |
| Imaging Time | 6 min 3 s ± 25 s * | 3 min 47 s ± 16 s |
| Variables | Area | Cut-Off Value | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| BLADE | ADC Min | 0.891 | 355.0 | 77.3 | 100 |
| ADC Max | 0.982 | 686.5 | 90.9 | 100 | |
| ADC Average | 0.950 | 531.0 | 818 | 100 | |
| RESOLVE | ADC Min | 0.918 | 112.50 | 81.8 | 100 |
| ADC Max | 0.982 | 737.0 | 90.9 | 100 | |
| ADC Average | 0.995 | 273.0 | 97.7 | 100 | |
| FDG-PET | SUV peak | 0.877 | 2.06 | 79.5 | 100 |
| SUV mean | 0.889 | 1.44 | 88.6 | 80 | |
| SUV max | 0.895 | 2.59 | 77.3 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Ahn, T.R.; Hwang, K.H.; Lee, S.-W. Evaluation of Three Imaging Methods to Quantify Key Events in Pelvic Bone Metastasis. Cancers 2024, 16, 214. https://doi.org/10.3390/cancers16010214
Lee H, Ahn TR, Hwang KH, Lee S-W. Evaluation of Three Imaging Methods to Quantify Key Events in Pelvic Bone Metastasis. Cancers. 2024; 16(1):214. https://doi.org/10.3390/cancers16010214
Chicago/Turabian StyleLee, Haejun, Tae Ran Ahn, Kyung Hoon Hwang, and Sheen-Woo Lee. 2024. "Evaluation of Three Imaging Methods to Quantify Key Events in Pelvic Bone Metastasis" Cancers 16, no. 1: 214. https://doi.org/10.3390/cancers16010214
APA StyleLee, H., Ahn, T. R., Hwang, K. H., & Lee, S.-W. (2024). Evaluation of Three Imaging Methods to Quantify Key Events in Pelvic Bone Metastasis. Cancers, 16(1), 214. https://doi.org/10.3390/cancers16010214






