Simple Summary
Pazopanib is an oral drug for metastatic pretreated uterine leiomyosarcoma that received approval in 2012, but poor data have been reported on its activity in real life since the disease is very rare. Uterine leiomyosarcoma has a poor objective response rate to other agents. We assessed the effectiveness and safety of pazopanib in everyday clinical practice, showing its activity and tolerability in patients.
Abstract
Background: Uterine leiomyosarcoma (uLMS) is characterized by aggressive behavior associated with a high risk of relapse and mortality. Several therapeutic agents have been employed in the treatment of metastatic disease, with a poor objective response rate. Pazopanib, approved in 2012, is a multi-targeted, orally active small molecule that exerts its effects by inhibiting several tyrosine kinases. To date, poor research on real-life data has been conducted. We aimed to assess the effectiveness and safety of the drug in everyday clinical practice. Methods: We present results of multicenter retrospective data on 38 patients with heavily pretreated metastatic uLMS who underwent oral pazopanib during their therapeutic journey. Results: At a median follow-up of 8.6 months, the disease control rate was 55.2%, with 17% partial responses and 15 patients (39.5%) with stable disease. At a median follow-up of 8.6 months, median progression-free survival was 4 months, and median overall survival was 19.8 months. The most common grade 3 adverse events (AEs) drug-related were hepatic toxicities, diarrhea, hypertension, nausea, and vomiting (all of them with an incidence of 5% considering the whole study cohort). No grade 4 AEs occurred. Conclusions: Pazopanib in everyday clinical practice is safe and shows a good disease control rate with prolonged survival.
1. Introduction
Uterine sarcomas represent a heterogeneous group of tumors, comprising 3–7% of all uterine malignant neoplasms and 1% of gynecological malignancies [1,2]. These tumors originate from either the myometrium or the connective tissue of the uterus. Among them, uterine leiomyosarcoma (uLMS) is the most prevalent histological type, accounting for 60% of all uterine sarcomas [3]. Uterine sarcomas have significantly different behaviors and different responses to drugs with respect to endometrial carcinomas. The more widely used staging system for uterine sarcomas is from the International Federation of Gynecology and Obstetrics (FIGO) [4]. In particular, regarding clinical behavior, patients with uterine sarcomas have more aggressive courses and worse prognoses than patients with endometrial carcinomas. Of note, their more aggressive clinical behavior does not depend on the stage at diagnosis and is often associated with a high risk of relapse and mortality, indicating an unmet medical need [2]. Surgery is the mainstay of treatment in early-stage disease; however, recurrence rates are high. For patients with localized disease confined to the uterus, the risk of relapse stands at approximately 50–70%, highlighting that novel approaches must be implemented [5].
Several therapeutic agents have been employed in the treatment of metastatic uterine sarcoma as both monotherapy and in combined modality, including docetaxel plus gemcitabine, single-agent gemcitabine, doxorubicin, pegylated liposomal doxorubicin, doxorubicin-based regimens, ifosfamide, and trabectedin [6,7,8,9,10]. Unfortunately, these treatments have demonstrated a poor objective response rate (from 9.9% to 36.0%) [6,7,8,9,10]. In addition, a small cohort of uLMS does respond to hormones and sometimes exhibit very durable responses, as in the case of aromatase inhibitors, which has shown a relatively prolonged median survival with an encouraging toxicity profile [11,12].
Pazopanib, an oral multi-targeted drug, comprises small molecules that work actively by inhibiting several tyrosine kinases, as vascular endothelial growth factor receptors (VEGFR-1, -2, -3), platelet-derived growth factor receptors (PDGFR-α and -β), fibroblast growth factor receptors (FGFR-1 and -3), and cytokine receptor (cKIT), which represent its main targets [13].
The randomized controlled phase III PALETTE trial demonstrated the efficacy of pazopanib as a single agent in patients with metastatic soft-tissue sarcomas with previous progression on standard treatment [14]. The study compared two arms: pazopanib versus placebo. In the pazopanib arm, progression-free survival (PFS) was significantly higher than the one in the placebo group, with no difference in terms of overall survival (OS) [14]. Thanks to the result of the PALATTE trial, the Food and Drug Administration (FDA) approved pazopanib in 2012, and the European Medicines Agency (EMA) approved it in 2013. Because of the rarity of the disease and the lack of literature reporting data related to everyday clinical practice [15,16,17], with our retrospective study, we aim to assess the real-life outcome of pazopanib-treated patients with pretreated uLMS in two tertiary specialized oncologic centers in Italy.
2. Materials and Methods
A retrospective study on all consecutive uLMS patients who have undergone oral pazopanib between September 2013 and March 2023 was conducted in two high-specialized oncologic centers. Adult uLMS patients aged ≥18 years (with no upper limit of age) having Eastern Cooperative Oncology Group (ECOG) performance status (PS) scores equal to or less than 2, receiving at least one previous line of chemotherapy (CT) for metastatic disease, and having histologically proven uterine high-grade leiomyosarcoma were included. The primary study objective was the effectiveness of pazopanib measured as both response rate and survival outcome. Secondary objectives were the safety and tolerability of pazopanib assessed by capturing all drug-related adverse events (AEs) occurring during treatment.
A further focus was made on the patient cohort that presented an objective response.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of IRCCS Istituto Nazionale dei Tumori di Milano (approval id INT 261-23). As for the retrospective design of the study, we received authorization to also analyze data of patients who were deceased or lost to follow-up at the time of data collection. To minimize bias, a shared data dictionary for each variable was provided to all the investigators.
Main clinical characteristics were captured from the patients’ charts. All the information was already registered in patients’ charts per clinical practice. At baseline (i.e., just prior to the first course of pazopanib), we captured the following information: detailed anamnesis, physical examination, basal imaging, echocardiography, age, ECOG PS score, and lab tests, including complete blood counts and serum chemistry panel. For study purposes, the following variables were filled in the study database and subsequently analyzed: histological subtypes, tumor grades, FIGO stage at diagnosis, sites of metastasis, and previous line of treatment/approach for uLMS (primary cytoreductive surgery, use of CT (adjuvant), radiotherapy (RT), previous lines of CT given for metastatic disease, previous surgeries).
Treatment response was evaluated as per clinical practice according to the RECIST, version 1.0. The safety and tolerability of pazopanib were assessed through the evaluation of AEs and serious AEs with the National Cancer Institute Common Terminology Criteria of AEs (v5.0).
Continuous covariates were summarized as median and range; categorical variables were summarized as absolute and percentage frequencies.
Comparisons of groups based on categorical variables were performed using Fisher’s exact test or a chi-squared test when appropriate.
The survival functions were computed by means of the Kaplan–Meier method, with a 95% confidence interval (95%CI).
All tests were two-sided, and a p-value of less than 0.05 was considered statistically significant. Statistical analyses were performed with PASS v11 software (NCSS, LLC., Kaysville, UT, USA).
3. Results
3.1. Patients’ Characteristics
From September 2013 to 2023, 38 women with metastatic uLMS (all high-grade disease) received oral pazopanib in monotherapy. Most patients (95%) had a good ECOG PS score, and the median age was 54.2 years (range of 38–72 years). The most frequent site of metastases was the lung (74%), followed by the pelvis (23.7%) and peritoneum (21.1%), and the median number of previous CT treatments was three (range of two to five) (Table 1). Twenty-six percent of patients had undergone previous RTs.

Table 1.
Patients’ characteristics at baseline.
3.2. Treatment, Effectiveness and Outcomes
The median treatment duration was 4.7 months (range of 1.2–21.9 months). At a median follow-up of 8.6 months, the disease control rate was 55.2%, with 17% (n = 6) partial responses (PRs) and 15 patients with (39.5%) stable disease (SD). We also recorded four complicated events that could also be from tumor shrinkage due to response: one pneumothorax, one intestinal perforation, one intestinal occlusion, and one hemoptysis due to pulmonary cavitation. At a median follow-up of 8.6 months, the median PFS was 4 months (95%CI 2.9–6.9), and the median OS was 19.8 months (95%CI of 10.3–25.4) (Figure 1 and Figure 2, respectively).
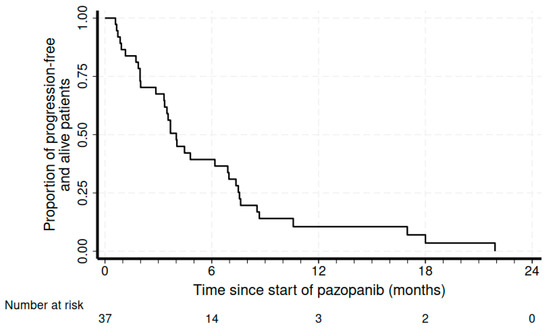
Figure 1.
Kaplan–Meier curve for progression-free survival.
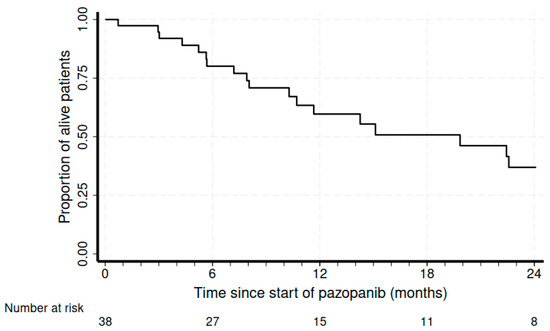
Figure 2.
Kaplan–Meier curves for overall survival.
A total of 82% of patients started pazopanib at 800 mg/die; 42.1% of patients required a dose reduction due to drug-related AEs—4 patients were reduced to 600 mg/die, and 11 patients were reduced to 400 mg/die.
Treatment was continued until disease progression, unacceptable toxic effects, or refusal by the patient. Most patients discontinued pazopanib because of disease progression; two patients (5.2%) definitively interrupted the treatment because of drug-related toxicities (one for anasarca and one for hepatic toxicity), and one patient (2.6%) was still on treatment after 10 months at the time of writing this paper.
Sixty-three patients underwent at least one further line of CT after pazopanib with a median PFS of 3 months—the most frequent CTs were pegylated liposomal doxorubicin (N = 6) and dacarbazine (N = 4).
3.3. Safety
A total of 44 drug-related AEs were registered. Seventeen (44.7%) patients had at least one AE of grade 1–2 (mild); 36.8% had one event of grade 3, and 10.5% had two AEs of grade 3 (Table 2). The most common grade 3 drug-related AEs were hepatic toxicities, diarrhea, hypertension, nausea, and vomiting (all of them with an incidence of 5% considering the whole study cohort). No grade 4 AEs or serious AEs occurred.

Table 2.
Pazopanib-related toxicities.
3.4. Patients with Objective Response
Considering the six patients with an objective response to pazopanib, the median age was 52 years (range of 44–69 years) with an ECOG PS score of 0–1. The median number of previous CT treatments was three (range of two to five). No statistically significant difference was found between this subgroup and the cohort of patients who showed progression of uLMS at the first disease assessment after treatment started (Table 3). Fifty percent of them experienced a grade 3 AE (diarrhea, cardiac toxicity with reduced ejection fraction reduction, ocular and skin toxicity), requiring a dose reduction and leading to temporary dose interruption. However, two of them had complicated events that could also be from tumor shrinkage due to response to pazopanib with gastrointestinal perforation and pneumothorax. The median PFS was 6.2 months (95%CI of 4.0 not reached), and the median OS was 10.3 months (95%CI of 7.2 not reached).

Table 3.
Characteristics of patients with objective response versus patients without response with pazopanib.
4. Discussion
Currently, the effective treatment for uLMS is an early resection (mainly complete), and CT is the principal choice in case of unresectable advanced or relapsed cases. To date, clinical trials have shown that no CT regimen (either as a single agent or combination strategy) has superiority over doxorubicin monotherapy as the first-line chemotherapy in these latter cases, mainly in terms of OS. As a second-line treatment and depending on FDA/EMA approval in different countries, pazopanib, trabectedin, and eribulin are used, but their efficacy is not still completely satisfactory, highlighting the unmet medical need and the urgency for the development of novel active agents.
Since FDA and EMA approval, little research has been conducted on the effectiveness and tolerability of oral pazopanib in the uLMS real-life setting, mainly because of the rarity of the disease and the lack of specialized centers.
Our real-life experience with pazopanib includes 38 heavily pretreated patients (median number of previous chemotherapies of three) with poorer conditions than in clinical studies, which have strictly defined inclusion and exclusion criteria. However, our retrospective results confirm the activity of pazopanib in this real-world population—fifty-five percent of patients had a disease control rate, with a median PFS in the overall population of 4 months and an OS of 19.8 months.
The limitations of our research are the retrospective nature of the study, its non-randomized design, and the absence of a control group. The strengths of the research were the homogeneity of the population and the sample size compared to the literature published in a real-life setting.
However, our results confirm those achieved in the phase III PALETTE trial (median PFS of 4.6 months and median OS of 12.5 months) with selected patients and subsequent analyses [14,18,19,20]. To note, our median OS was slightly higher than that of the PALETTE trial. On the other hand, in the PALETTE study, all soft-tissue sarcomas (metastatic non-adipocytic soft-tissue sarcoma) were included, not just those of uterine origin, like in our research. This can justify a slightly different performance in OS [14,18,19,20].
To date, only three retrospective studies have been conducted in a real-world setting [15,16,17] (Table 4). The largest study refers to the pazopanib compassionate program in which 40 uLMS patients were analyzed. Besides that analysis, our study is the largest one conducted in a real-life setting on oral pazopanib used in advanced stage/recurrent uLMS. The disease control rate ranges from 46% to 75%, with a mean PFS of 4 months (range of 2.8–5.8) and a mean OS of 13.8 (range of 6.5–20.0) (Table 4).

Table 4.
Pazopanib real-life experience studies (all retrospective).
We also recorded four complicated responses; thus, considering this possible issue in the context of a rare pathology, it is always recommended to refer patients to high-level specialized centers, as in our case.
Regarding safety, grade 3 toxicities were rare (no grade 4 AEs occurred). Each grade 3 toxicity had an incidence of less than 5%, considering the whole study sample: the drug resulted overall as safe for the patients and easily manageable for clinicians. To date, the safety profile in this study is in line with that reported in the drug characteristics of the product (e.g., hepatic toxicity), with no suspected adverse reactions to report. Patients in PR presented a high rate of grade 3 AEs (50%) with two complicated events that could also be from tumor shrinkage due to responses (intestinal perforation and pneumothorax). Nevertheless, the grade and type of AEs were consistent with the known safety profile of pazopanib [14,15,16,17,18,19,20]. In addition, our rate of discontinuation rate due to AEs was 5.2%, lower than that reported in the literature, i.e., about 13% [21,22].
Although new drugs have been studied/explored in recent years for the treatment of advanced and recurrent uLMS, we currently do not have new molecules available to offer patients except the ones within a clinical trial context or under compassionate/off-label use. Anti-angiogenetic approaches have been explored in patients with uLMS; however, both sunitinib and bevacizumab failed to show sufficient activity in this setting [23,24,25]. Other target therapies, namely nivolumab and pembrolizumab, showed promising results, but to date, they have not yet been approved by EMA for uLMS [26,27].
Discovering and implementing new drug therapies or sequential treatment approaches for unresectable advanced or relapsed uLMS are nowadays an urgent clinical challenge. Although the therapeutic efficacy of several antitumor agents, molecular-targeted drugs, and hormonal therapy have been investigated, there is no strong evidence of a satisfying tumor-reducing or control effect for uLMS patients with a previous history of CT, especially in heavily pretreated cases. Clinical trials incorporating biomarker-based patient selection as inclusion criteria for uLMS patients have not yet been conceived, and we claimed for future studies based on pharmacogenomics. This can help this particular setting with patients characterized by poor prognoses and conditioned by the rarity of the disease.
The research reports no substantial differences from a clinical point of view between patients who had an objective response and those who did not. Thus, to better understand any prognostic factors for response, further studies should be conceived from a molecular point of view, as biomarkers predictive of both response and resistance to pazopanib have still not been identified [28,29]. In fact, prospective studies on pazopanib and subsequent subgroup analyses on different types of soft-tissue sarcomas have failed to pick up (baseline) clinical or pathological features that can improve the use of this drug in terms of both response and survival [28,29].
5. Conclusions
Pazopanib in everyday clinical practice showed a good disease control rate with related prolonged survival outcomes. In addition, toxicities were mild and easily manageable, indicating pazopanib as a useful therapeutic tool for heavily pretreated uLMS patients and eligible for further treatment. Physicians should consider this agent in the therapeutic pathway of advanced/recurrent uLMS.
Author Contributions
Conceptualization, M.M. and M.D.; methodology, M.M., R.S. and G.P.; formal analysis, M.D. and L.P.; investigation, M.B., C.S., R.S. and A.F.; resources, all; data curation, all; writing—original draft preparation, M.M., M.B., M.D. and M.P.; writing—review and editing, M.M., M.B., M.P., L.P., R.S., C.F., G.P., M.L., C.C., C.S., A.F., F.R., N.C. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of IRCCS Istituto Nazionale dei Tumori di Milano.
Informed Consent Statement
Patient consent was waived because of the retrospective nature of the study.
Data Availability Statement
Data for quality control can be made available upon request to the corresponding author.
Acknowledgments
Editorial support was provided by PINCH s.r.l.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferrandina, G.; Aristei, C.; Biondetti, P.R.; Cananzi, F.C.M.; Casali, P.; Ciccarone, F.; Colombo, N.; Comandone, A.; Corvo’, R.; De Iaco, P.; et al. Italian consensus conference on management of uterine sarcomas on behalf of S.I.G.O. (Societa’ italiana di Ginecologia E Ostetricia). Eur. J. Cancer 2020, 139, 149–168. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Prat, J. Uterine sarcomas: A review. Gynecol. Oncol. 2010, 116, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Aynardi, J.T.; Chu, C.S. Uterine leiomyosarcoma: A review of the literature and update on management options. Gynecol. Oncol. 2018, 151, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Prat, J. FIGO staging for uterine sarcomas. Int. J. Gynaecol. Obstet. 2009, 104, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Giuntoli, R.L., 2nd; Metzinger, D.S.; DiMarco, C.S.; Cha, S.S.; Sloan, J.A.; Keeney, G.L.; Gostout, B.S. Retrospective review of 208 patients with leiomyosarcoma of the uterus: Prognostic indicators, surgical management, and adjuvant therapy. Gynecol. Oncol. 2003, 89, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Desar, I.M.E.; Ottevanger, P.B.; Benson, C.; van der Graaf, W.T.A. Systemic treatment in adult uterine sarcomas. Crit. Rev. Oncol. Hematol. 2018, 122, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef]
- Sutton, G.; Blessing, J.; Hanjani, P.; Kramer, P. Phase II evaluation of liposomal doxorubicin (Doxil) in recurrent or advanced leiomyosarcoma of the uterus: A Gynecologic Oncology Group study. Gynecol. Oncol. 2005, 96, 749–752. [Google Scholar] [CrossRef]
- Sutton, G.; Blessing, J.A.; Malfetano, J.H. Ifosfamide and doxorubicin in the treatment of advanced leiomyosarcomas of the uterus: A Gynecologic Oncology Group study. Gynecol. Oncol. 1996, 62, 226–229. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef]
- Asano, H.; Isoe, T.; Ito, Y.M.; Nishimoto, N.; Watanabe, Y.; Yokoshiki, S.; Watari, H. Status of the Current Treatment Options and Potential Future Targets in Uterine Leiomyosarcoma: A Review. Cancers 2022, 14, 1180. [Google Scholar] [CrossRef] [PubMed]
- Maccaroni, E.; Lunerti, V.; Agostinelli, V.; Giampieri, R.; Zepponi, L.; Pagliacci, A.; Berardi, R. New Insights into Hormonal Therapies in Uterine Sarcomas. Cancers 2022, 14, 921. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Kakutani, S.; Sato, Y.; Hanashi, A.; Kinoshita, Y.; Ishikawa, A. Drug review: Pazopanib. Jpn. J. Clin. Oncol. 2018, 48, 503–513. [Google Scholar] [CrossRef]
- Van der Graaf, W.T.; Blay, J.Y.; Chawla, S.P.; Kim, D.W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Sunar, V.; Korkmaz, V.; Akin, S.; Can Guven, D.; Arik, Z.; Ates, O.; Yilmaz, M.; Mutlu Meydanli, M.; Oksuzoglu, B. Efficacy of Pazopanib in patients with metastatic uterine sarcoma: A multi-institutional study. J. Buon 2019, 24, 2327–2332. [Google Scholar] [PubMed]
- Kim, H.J.; Kim, Y.; Lee, S.J.; Lee, J.; Park, S.H. Pazopanib monotherapy in the treatment of pretreated, metastatic uterine sarcoma: A single-center retrospective study. J. Gynecol. Oncol. 2018, 29, e3. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Judson, I.R.; Benson, C.; Merimsky, O.; Grignani, G.; Katz, D.; Freivogel, K.W.; Stein, D.; Jobanputra, M.; Mungul, A.; et al. Treatment patterns and clinical outcomes with pazopanib in patients with advanced soft tissue sarcomas in a compassionate use setting: Results of the SPIRE study. Acta Oncol. 2017, 56, 1769–1775. [Google Scholar] [CrossRef]
- Cesne, A.L.; Bauer, S.; Demetri, G.D.; Han, G.; Dezzani, L.; Ahmad, Q.; Blay, J.Y.; Judson, I.; Schöffski, P.; Aglietta, M.; et al. Safety and efficacy of Pazopanib in advanced soft tissue sarcoma: PALETTE (EORTC 62072) subgroup analyses. BMC Cancer 2019, 19, 794. [Google Scholar] [CrossRef]
- Benson, C.; Ray-Coquard, I.; Sleijfer, S.; Litière, S.; Blay, J.Y.; Le Cesne, A.; Papai, Z.; Judson, I.; Schöffski, P.; Chawla, S.; et al. Outcome of uterine sarcoma patients treated with pazopanib: A retrospective analysis based on two European Organisation for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group (STBSG) clinical trials 62043 and 62072. Gynecol. Oncol. 2016, 142, 89–94. [Google Scholar] [CrossRef]
- Nakano, K.; Motoi, N.; Inagaki, L.; Tomomatsu, J.; Gokita, T.; Ae, K.; Tanizawa, T.; Shimoji, T.; Matsumoto, S.; Takahashi, S. Differences in the responses to pazopanib and the prognoses of soft tissue sarcomas by their histological eligibility for the PALETTE study. Jpn. J. Clin. Oncol. 2015, 45, 449–455. [Google Scholar] [CrossRef]
- Schöffski, P. Pazopanib in the treatment of soft tissue sarcoma. Expert. Rev. Anticancer Ther. 2012, 12, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Wilky, B.A.; Meyer, C.F.; Trent, J.C. Pazopanib in sarcomas: Expanding the PALETTE. Curr. Opin. Oncol. 2013, 25, 373–378. [Google Scholar] [CrossRef]
- George, S.; Merriam, P.; Maki, R.G.; Van den Abbeele, A.D.; Yap, J.T.; Akhurst, T.; Harmon, D.C.; Bhuchar, G.; O’Mara, M.M.; D’Adamo, D.R.; et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J. Clin. Oncol. 2009, 27, 3154–3160. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Sill, M.W.; Scribner, D.R., Jr.; Brown, J.; Debernardo, R.L.; Hartenbach, E.M.; McCourt, C.K.; Bosscher, J.R.; Gehrig, P.A. Sunitinib malate in the treatment of recurrent or persistent uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecol. Oncol. 2009, 115, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Miller, A.; O’Malley, D.M.; Mannel, R.S.; Behbakht, K.; Bakkum-Gamez, J.N.; Michael, H. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: An NRG Oncology/Gynecologic Oncology Group study. J. Clin. Oncol. 2015, 33, 1180–1185. [Google Scholar] [CrossRef]
- D’Angelo, S.P. A multicenter phase II study of nivolumab +/− ipilimumab for patients with metastatic sarcomas (alliance A091401). J. Clin. Oncol. 2017, 35, Abst 11007. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Lee, A.T.J.; Jones, R.L.; Huang, P.H. Pazopanib in advanced soft tissue sarcomas. Signal. Transduct. Target. Ther. 2019, 4, 16. [Google Scholar] [CrossRef]
- Cope, B.M.; Traweek, R.S.; Lazcano, R.; Keung, E.Z.; Lazar, A.J.; Roland, C.L.; Nassif, E.F. Targeting the Molecular and Immunologic Features of Leiomyosarcoma. Cancers 2023, 15, 2099. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).