Patient-Reported Sexual Function, Bladder Function and Quality of Life for Patients with Low Rectal Cancers with or without a Permanent Ostomy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Distribution and Data Collection
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APR | abdominoperineal resection |
| CRT | chemoradiation |
| FACTG7 | Functional Assessment of Cancer Therapy—General 7 Question Survey |
| FLUTS | Female Lower Urinary Tract Symptom Score |
| FSFI | Female Sexual Function Index |
| IIEF | International Index of Erectile Function |
| MLUTS | Male Lower Urinary Tract Symptom Score |
| NOM | non-operative management |
| PROM | patient-reported outcome measure |
| QOL | quality of life |
| SSS | sphincter-sparing surgery |
| TNT | total neoadjuvant therapy |
References
- Hawkins, A.T.; Albutt, K.; Wise, P.E.; Alavi, K.; Sudan, R.; Kaiser, A.M.; Bordeianou, L.; Continuing Education Committee of the SSAT. Abdominoperineal Resection for Rectal Cancer in the Twenty-First Century: Indications, Techniques, and Outcomes. J. Gastrointest. Surg. 2018, 22, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Bordeianou, L.; Maguire, L.H.; Alavi, K.; Sudan, R.; Wise, P.E.; Kaiser, A.M. Sphincter-Sparing Surgery in Patients with Low-Lying Rectal Cancer: Techniques, Oncologic Outcomes, and Functional Results. J. Gastrointest. Surg. 2014, 18, 1358–1372. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.-R.; Villanueva, M.-T.; Witzigmann, H.; et al. Preoperative versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial after a Median Follow-up of 11 Years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Henriquez, N.; Galante, D.J.; Monson, J.R.T. Selection and Outcomes in Abdominoperineal Resection. Front. Oncol. 2020, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Mulita, F.; Lotfollahzadeh, S. Intestinal Stoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, É.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant Chemotherapy with FOLFIRINOX and Preoperative Chemoradiotherapy for Patients with Locally Advanced Rectal Cancer (UNICANCER-PRODIGE 23): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-Course Radiotherapy Followed by Chemotherapy before Total Mesorectal Excision (TME) versus Preoperative Chemoradiotherapy, TME, and Optional Adjuvant Chemotherapy in Locally Advanced Rectal Cancer (RAPIDO): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef] [PubMed]
- McMullen, C.K.; Bulkley, J.E.; Altschuler, A.; Wendel, C.S.; Grant, M.; Hornbrook, M.C.; Sun, V.; Krouse, R.S. Greatest Challenges of Rectal Cancer Survivors: Results of a Population-Based Survey. Dis. Colon Rectum 2016, 59, 1019–1027. [Google Scholar] [CrossRef]
- Wiatrek, R.L.; Thomas, J.S.; Papaconstantinou, H.T. Perineal Wound Complications after Abdominoperineal Resection. Clin. Colon Rectal Surg. 2008, 21, 76–85. [Google Scholar] [CrossRef]
- Du, P.; Wang, S.-Y.; Zheng, P.-F.; Mao, J.; Hu, H.; Cheng, Z.-B. Comparison of Overall Survival and Quality of Life between Patients Undergoing Anal Reconstruction and Patients Undergoing Traditional Lower Abdominal Stoma after Radical Resection. Clin. Transl. Oncol. 2019, 21, 1390–1397. [Google Scholar] [CrossRef]
- Engel, J.; Kerr, J.; Schlesinger-Raab, A.; Eckel, R.; Sauer, H.; Hölzel, D. Quality of Life in Rectal Cancer Patients: A Four-Year Prospective Study. Ann. Surg. 2003, 238, 203–213. [Google Scholar] [CrossRef]
- Grumann, M.M.; Noack, E.M.; Hoffmann, I.A.; Schlag, P.M. Comparison of Quality of Life in Patients Undergoing Abdominoperineal Extirpation or Anterior Resection for Rectal Cancer. Ann. Surg. 2001, 233, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Feddern, M.-L.; Emmertsen, K.J.; Laurberg, S. Quality of Life with or without Sphincter Preservation for Rectal Cancer. Color. Dis. 2019, 21, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Konanz, J.; Herrle, F.; Weiss, C.; Post, S.; Kienle, P. Quality of Life of Patients after Low Anterior, Intersphincteric, and Abdominoperineal Resection for Rectal Cancer--a Matched-Pair Analysis. Int. J. Color. Dis. 2013, 28, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Bong, J.W.; Lim, S.-B.; Lee, J.L.; Kim, C.W.; Yoon, Y.S.; Park, I.J.; Yu, C.S.; Kim, J.C. Comparison of Anthropometric Parameters after Ultralow Anterior Resection and Abdominoperineal Resection in Very Low-Lying Rectal Cancers. Gastroenterol. Res. Pract. 2018, 2018, 9274618. [Google Scholar] [CrossRef] [PubMed]
- Wani, R.A.; Bhat, I.-U.-A.; Parray, F.Q.; Chowdri, N.A. Quality of Life After “Total Mesorectal Excision (TME)” for Rectal Carcinoma: A Study from a Tertiary Care Hospital in Northern India. Indian J. Surg. Oncol. 2017, 8, 499–505. [Google Scholar] [CrossRef]
- Näsvall, P.; Dahlstrand, U.; Löwenmark, T.; Rutegård, J.; Gunnarsson, U.; Strigård, K. Quality of Life in Patients with a Permanent Stoma after Rectal Cancer Surgery. Qual. Life Res. 2017, 26, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Herrinton, L.J.; Altschuler, A.; McMullen, C.K.; Bulkley, J.E.; Hornbrook, M.C.; Sun, V.; Wendel, C.S.; Grant, M.; Baldwin, C.M.; Demark-Wahnefried, W.; et al. Conversations for Providers Caring for Patients with Rectal Cancer: Comparison of Long-Term Patient-Centered Outcomes for Patients with Low Rectal Cancer Facing Ostomy or Sphincter-Sparing Surgery. CA Cancer J. Clin. 2016, 66, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Verheij, F.S.; Omer, D.M.; Williams, H.; Lin, S.T.; Qin, L.-X.; Buckley, J.T.; Thompson, H.M.; Yuval, J.B.; Kim, J.K.; Dunne, R.F.; et al. Long-Term Results of Organ Preservation in Patients with Rectal Adenocarcinoma Treated with Total Neoadjuvant Therapy: The Randomized Phase II OPRA Trial. J. Clin. Oncol. 2023, JCO2301208. [Google Scholar] [CrossRef]
- Rooney, M.K.; De, B.; Corrigan, K.; Smith, G.L.; Taniguchi, C.; Minsky, B.D.; Ludmir, E.B.; Koay, E.J.; Das, P.; Koong, A.C.; et al. Patient-Reported Bowel Function and Bowel-Related Quality of Life After Pelvic Radiation for Rectal Adenocarcinoma: The Impact of Radiation Fractionation and Surgical Resection. Clin. Color. Cancer 2023, 22, 211–221. [Google Scholar] [CrossRef]
- Basch, E.; Dueck, A.C.; Mitchell, S.A.; Mamon, H.; Weiser, M.; Saltz, L.; Gollub, M.; Rogak, L.; Ginos, B.; Mazza, G.L.; et al. Patient-Reported Outcomes During and After Treatment for Locally Advanced Rectal Cancer in the PROSPECT Trial (Alliance N1048). J. Clin. Oncol. 2023, 41, 3724–3734. [Google Scholar] [CrossRef]
- Yanez, B.; Pearman, T.; Lis, C.G.; Beaumont, J.L.; Cella, D. The FACT-G7: A Rapid Version of the Functional Assessment of Cancer Therapy-General (FACT-G) for Monitoring Symptoms and Concerns in Oncology Practice and Research. Ann. Oncol. 2013, 24, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Peters, T.J.; Abrams, P.; Brookes, S.T.; de aa Rosette, J.J.; Schäfer, W. Scoring the Short Form ICSmaleSF Questionnaire. International Continence Society. J. Urol. 2000, 164, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Brookes, S.T.; Donovan, J.L.; Wright, M.; Jackson, S.; Abrams, P. A Scored Form of the Bristol Female Lower Urinary Tract Symptoms Questionnaire: Data from a Randomized Controlled Trial of Surgery for Women with Stress Incontinence. Am. J. Obs. Gynecol. 2004, 191, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Cappelleri, J.C.; Smith, M.D.; Lipsky, J.; Peña, B.M. Development and Evaluation of an Abridged, 5-Item Version of the International Index of Erectile Function (IIEF-5) as a Diagnostic Tool for Erectile Dysfunction. Int. J. Impot. Res. 1999, 11, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Wiegel, M.; Meston, C.; Rosen, R. The Female Sexual Function Index (FSFI): Cross-Validation and Development of Clinical Cutoff Scores. J. Sex Marital. Ther. 2005, 31, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Krouse, R.; Grant, M.; Ferrell, B.; Dean, G.; Nelson, R.; Chu, D. Quality of Life Outcomes in 599 Cancer and Non-Cancer Patients with Colostomies. J. Surg. Res. 2007, 138, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)--a Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inf. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Thaysen, H.V.; Jess, P.; Laurberg, S. Health-related Quality of Life after Surgery for Primary Advanced Rectal Cancer and Recurrent Rectal Cancer: A Review. Color. Dis. 2012, 14, 797–803. [Google Scholar] [CrossRef]
- Schrag, D.; Shi, Q.; Weiser, M.R.; Gollub, M.J.; Saltz, L.B.; Musher, B.L.; Goldberg, J.; Al Baghdadi, T.; Goodman, K.A.; McWilliams, R.R.; et al. Preoperative Treatment of Locally Advanced Rectal Cancer. N. Engl. J. Med. 2023, 389, 322–334. [Google Scholar] [CrossRef]
- Du, X.; Mao, L.; Leng, Y.; Chen, F. Validation of the FACT-G7 in Patients with Hematologic Malignancies. Front. Oncol. 2023, 13, 1183632. [Google Scholar] [CrossRef]
- Mah, K.; Swami, N.; Le, L.W.; Chow, R.; Hannon, B.L.; Rodin, G.; Zimmermann, C. Validation of the 7-item Functional Assessment of Cancer Therapy-General (FACT-G7) as a Short Measure of Quality of Life in Patients with Advanced Cancer. Cancer 2020, 126, 3750–3757. [Google Scholar] [CrossRef]
- Pearman, T.; Yanez, B.; Peipert, J.; Wortman, K.; Beaumont, J.; Cella, D. Ambulatory Cancer and US General Population Reference Values and Cutoff Scores for the Functional Assessment of Cancer Therapy. Cancer 2014, 120, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, S.; Maalouf, M.F.; Penta, R.; Joshua, T.G.; Liberman, A.S.; Fiore, J.F.; Feldman, L.S.; Lee, L. The Impact of Restorative Proctectomy versus Permanent Colostomy on Health-Related Quality of Life after Rectal Cancer Surgery Using the Patient-Generated Index. Surgery 2023, 174, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Vonk-Klaassen, S.M.; de Vocht, H.M.; den Ouden, M.E.M.; Eddes, E.H.; Schuurmans, M.J. Ostomy-Related Problems and Their Impact on Quality of Life of Colorectal Cancer Ostomates: A Systematic Review. Qual. Life Res. 2016, 25, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Maguire, B.; Clancy, C.; Connelly, T.M.; Mehigan, B.J.; McCormick, P.; Altomare, D.F.; Gosselink, M.P.; Larkin, J.O. Quality of Life Meta-Analysis Following Coloanal Anastomosis versus Abdominoperineal Resection for Low Rectal Cancer. Color. Dis. 2022, 24, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Fucini, C.; Gattai, R.; Urena, C.; Bandettini, L.; Elbetti, C. Quality of Life among Five-Year Survivors after Treatment for Very Low Rectal Cancer with or without a Permanent Abdominal Stoma. Ann. Surg. Oncol. 2008, 15, 1099–1106. [Google Scholar] [CrossRef]
- Orsini, R.G.; Thong, M.S.Y.; van de Poll-Franse, L.V.; Slooter, G.D.; Nieuwenhuijzen, G.a.P.; Rutten, H.J.T.; de Hingh, I.H.J.T. Quality of Life of Older Rectal Cancer Patients Is Not Impaired by a Permanent Stoma. Eur. J. Surg. Oncol. 2013, 39, 164–170. [Google Scholar] [CrossRef]
- Allal, A.S.; Bieri, S.; Pelloni, A.; Spataro, V.; Anchisi, S.; Ambrosetti, P.; Sprangers, M.A.; Kurtz, J.M.; Gertsch, P. Sphincter-Sparing Surgery after Preoperative Radiotherapy for Low Rectal Cancers: Feasibility, Oncologic Results and Quality of Life Outcomes. Br. J. Cancer 2000, 82, 1131–1137. [Google Scholar] [CrossRef]
- Tschann, P.; Weigl, M.; Brock, T.; Frick, J.; Sturm, O.; Presl, J.; Jäger, T.; Weitzendorfer, M.; Schredl, P.; Clemens, P.; et al. Identification of Risk Factors for Sexual Dysfunction after Multimodal Therapy of Locally Advanced Rectal Cancer and Their Impact on Quality of Life: A Single-Center Trial. Cancers 2022, 14, 5796. [Google Scholar] [CrossRef]
- Li, K.; He, X.; Tong, S.; Zheng, Y. Risk Factors for Sexual Dysfunction after Rectal Cancer Surgery in 948 Consecutive Patients: A Prospective Cohort Study. Eur. J. Surg. Oncol. 2021, 47, 2087–2092. [Google Scholar] [CrossRef]
- Guzelsoy, M.; Erkan, A.; Ozturk, M.; Zengin, S.; Coban, S.; Turkoglu, A.R.; Koc, A. Comparison of Three Questionnaire Forms Used in the Diagnosis of Lower Urinary Tract Symptoms: A Prospective Study. Prostate Int. 2022, 10, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.; Bock, D.; Asplund, D.; Ohlsson, B.; Rosenberg, J.; Angenete, E. Urinary Dysfunction in Patients with Rectal Cancer: A Prospective Cohort Study. Color. Dis. 2020, 22, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hrabe, J.E.; Kapadia, M.R. Guiding Patients Through a “Watch-and-Wait” Approach for Rectal Cancer-Understanding the Functional Outcomes. JAMA Surg. 2023, 158, e230165. [Google Scholar] [CrossRef] [PubMed]
- Loria, A.; Tejani, M.A.; Temple, L.K.; Justiniano, C.F.; Melucci, A.D.; Becerra, A.Z.; Monson, J.R.T.; Aquina, C.T.; Fleming, F.J. Practice Patterns for Organ Preservation in US Patients With Rectal Cancer, 2006–2020. JAMA Oncol. 2023. [Google Scholar] [CrossRef]
- Custers, P.A.; van der Sande, M.E.; Grotenhuis, B.A.; Peters, F.P.; van Kuijk, S.M.J.; Beets, G.L.; Breukink, S.O.; Dutch Watch-and-Wait Consortium. Long-Term Quality of Life and Functional Outcome of Patients with Rectal Cancer Following a Watch-and-Wait Approach. JAMA Surg. 2023, 158, e230146. [Google Scholar] [CrossRef]
- Mulita, F.; Tepetes, K.; Verras, G.-I.; Liolis, E.; Tchabashvili, L.; Kaplanis, C.; Perdikaris, I.; Velissaris, D.; Maroulis, I. Perineal Colostomy: Advantages and Disadvantages. Gastroenterol. Rev. 2022, 17, 89–95. [Google Scholar] [CrossRef]
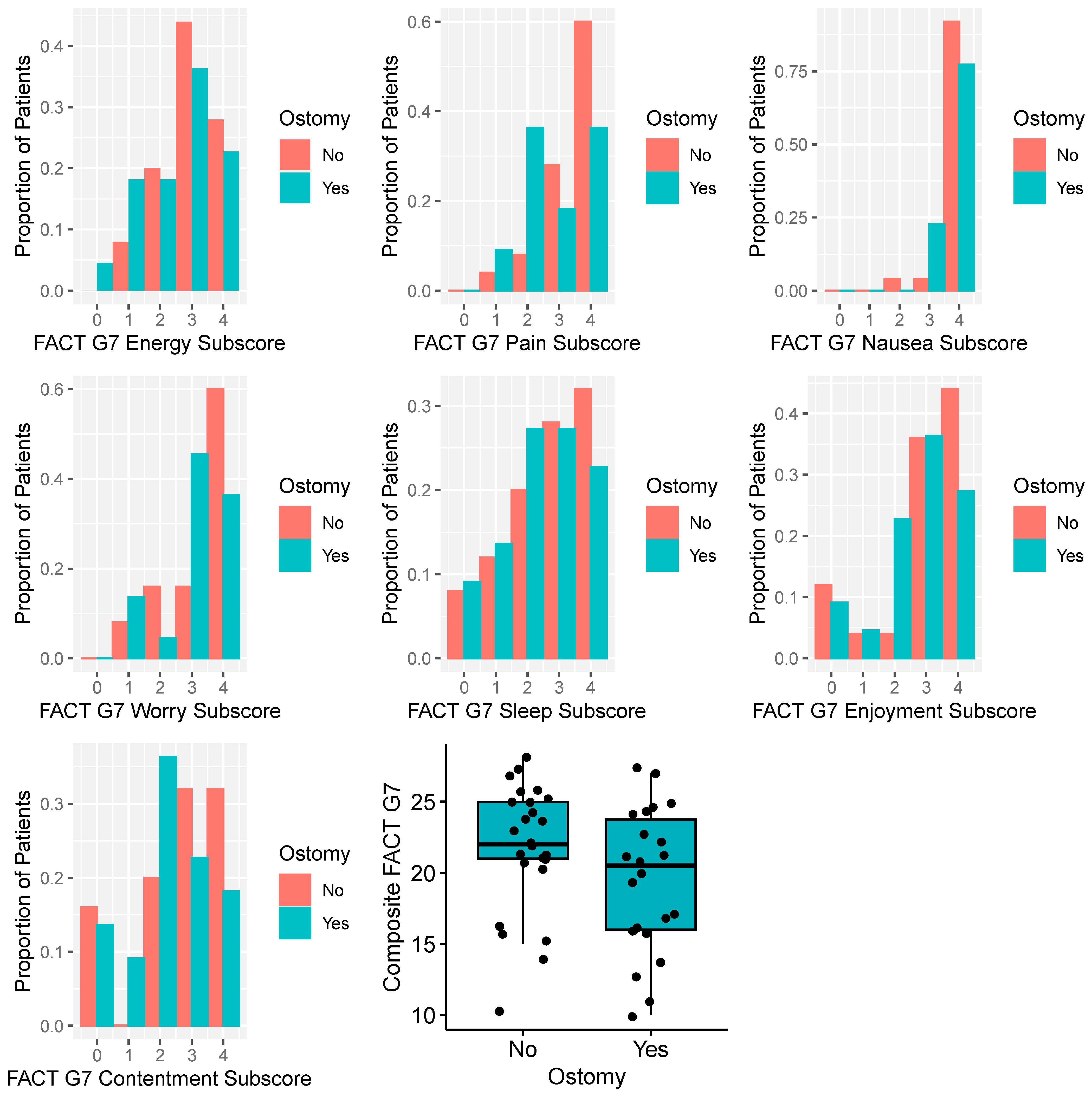

| Score | Presence of a Permanent Ostomy | |||
|---|---|---|---|---|
| No | Yes | Overall | p-Value | |
| FACTG7 | n = 25 | n = 22 | n = 47 | |
| Mean (SD) | 21.8 (4.54) | 19.5 (4.99) | 20.7 (4.84) | 0.12 |
| Median [Q1, Q3] | 22 [20.5, 25] | 20.5 [16, 24] | 21 [16, 25] | |
| IIEF | n = 17 | n = 12 | n = 29 | |
| Mean (SD) | 15.5 (6.37) | 11.9 (7.33) | 14 (6.89) | 0.18 |
| Median [Q1, Q3] | 16 [9, 22] | 8.5 [6.5, 19.5] | 14 [7.5, 21] | |
| FSFI | n = 4 | n = 6 | n = 10 | |
| Mean (SD) | 18.3 (9.19) | 15.1 (5.78) | 16.4 (7.02) | 0.83 |
| Median [Q1, Q3] | 18.6 [10.4, 26.15] | 14.2 [10.5, 16.6] | 14.2 [10.5, 25.4] | |
| MLUTS | n = 18 | n = 12 | n = 30 | |
| Mean (SD) | 6.50 (4.66) | 14.3 (13.7) | 9.60 (9.92) | 0.06 |
| Median [Q1, Q3] | 5.50 [3, 8] | 11.0 [4.5, 17] | 6.50 [3, 13] | |
| FLUTS | n = 6 | n = 9 | n = 15 | |
| Mean (SD) | 5.33 (3.27) | 15.8 (7.92) | 11.6 (8.23) | 0.01 |
| Median [Q1, Q3] | 5.50 [3, 7] | 17 [8.5, 22] | 10 [4, 18] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rooney, M.K.; Pasli, M.; Chang, G.J.; Das, P.; Koay, E.J.; Koong, A.C.; Ludmir, E.B.; Minsky, B.D.; Noticewala, S.S.; Peacock, O.; et al. Patient-Reported Sexual Function, Bladder Function and Quality of Life for Patients with Low Rectal Cancers with or without a Permanent Ostomy. Cancers 2024, 16, 153. https://doi.org/10.3390/cancers16010153
Rooney MK, Pasli M, Chang GJ, Das P, Koay EJ, Koong AC, Ludmir EB, Minsky BD, Noticewala SS, Peacock O, et al. Patient-Reported Sexual Function, Bladder Function and Quality of Life for Patients with Low Rectal Cancers with or without a Permanent Ostomy. Cancers. 2024; 16(1):153. https://doi.org/10.3390/cancers16010153
Chicago/Turabian StyleRooney, Michael K., Melisa Pasli, George J. Chang, Prajnan Das, Eugene J. Koay, Albert C. Koong, Ethan B. Ludmir, Bruce D. Minsky, Sonal S. Noticewala, Oliver Peacock, and et al. 2024. "Patient-Reported Sexual Function, Bladder Function and Quality of Life for Patients with Low Rectal Cancers with or without a Permanent Ostomy" Cancers 16, no. 1: 153. https://doi.org/10.3390/cancers16010153
APA StyleRooney, M. K., Pasli, M., Chang, G. J., Das, P., Koay, E. J., Koong, A. C., Ludmir, E. B., Minsky, B. D., Noticewala, S. S., Peacock, O., Smith, G. L., & Holliday, E. B. (2024). Patient-Reported Sexual Function, Bladder Function and Quality of Life for Patients with Low Rectal Cancers with or without a Permanent Ostomy. Cancers, 16(1), 153. https://doi.org/10.3390/cancers16010153






