Immune Checkpoint Profiling in Humanized Breast Cancer Mice Revealed Cell-Specific LAG-3/PD-1/TIM-3 Co-Expression and Elevated PD-1/TIM-3 Secretion
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Breast Cancer Cell Lines
2.2. Isolation of Human CD34+ Hematopoietic Stem Cells from Umbilical Cord Blood
2.3. Generation of Humanized Tumor Mice
2.4. Ethics Statements
2.5. Flow Cytometry
2.6. BioLegend’s LEGENDplex™ Bead-Based Immunoassay
2.7. ELISA
2.8. Database Analysis
2.9. Statistical Analyses
3. Results
3.1. Colonization of Human Immune Cells upon HSC Transplantation
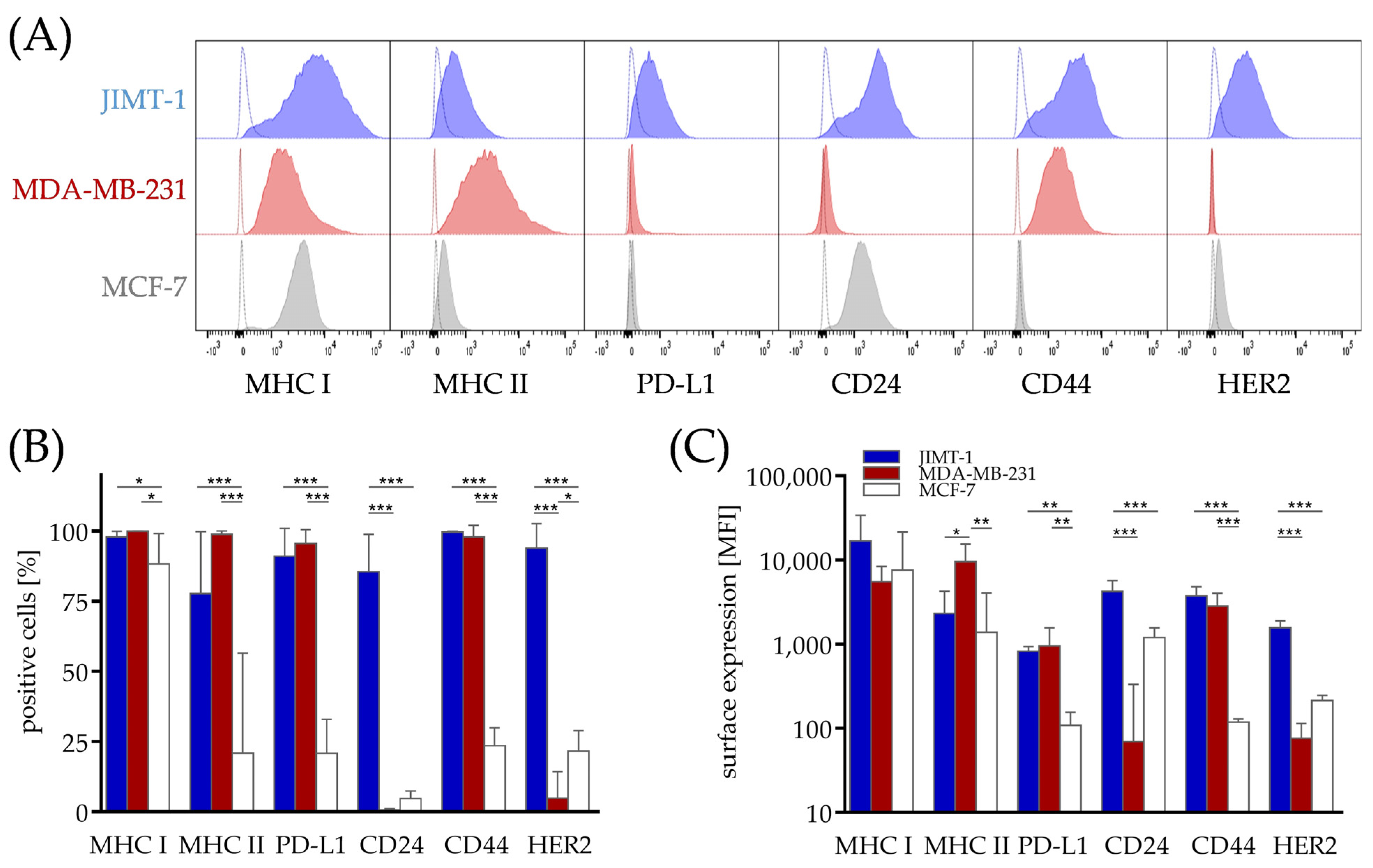
3.2. Differences in Phenotype of Breast Cancer Cell Lines from Different Subtypes, Which Might Contribute to Immunogenicity
3.3. Checkpoint Therapy-Related Soluble Proteins Are Elevated in the Serum of Humanized Mice Transplanted with Breast Cancer
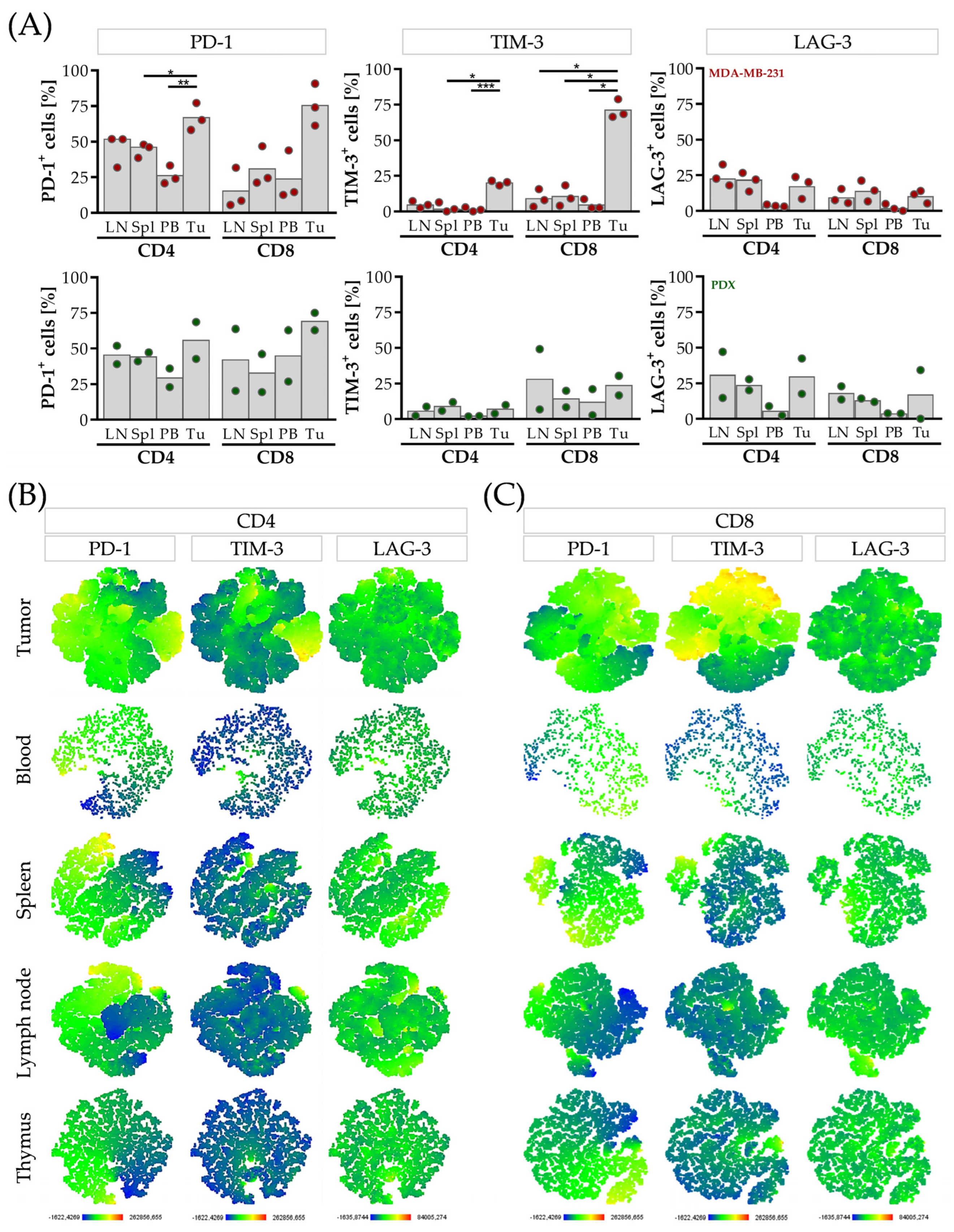
3.4. CD4 T Cells Are Characterized by Elevated LAG-3, CD8 T Cells and by Elevated TIM-3 Expression in the Tumor Microenvironment of HTMs
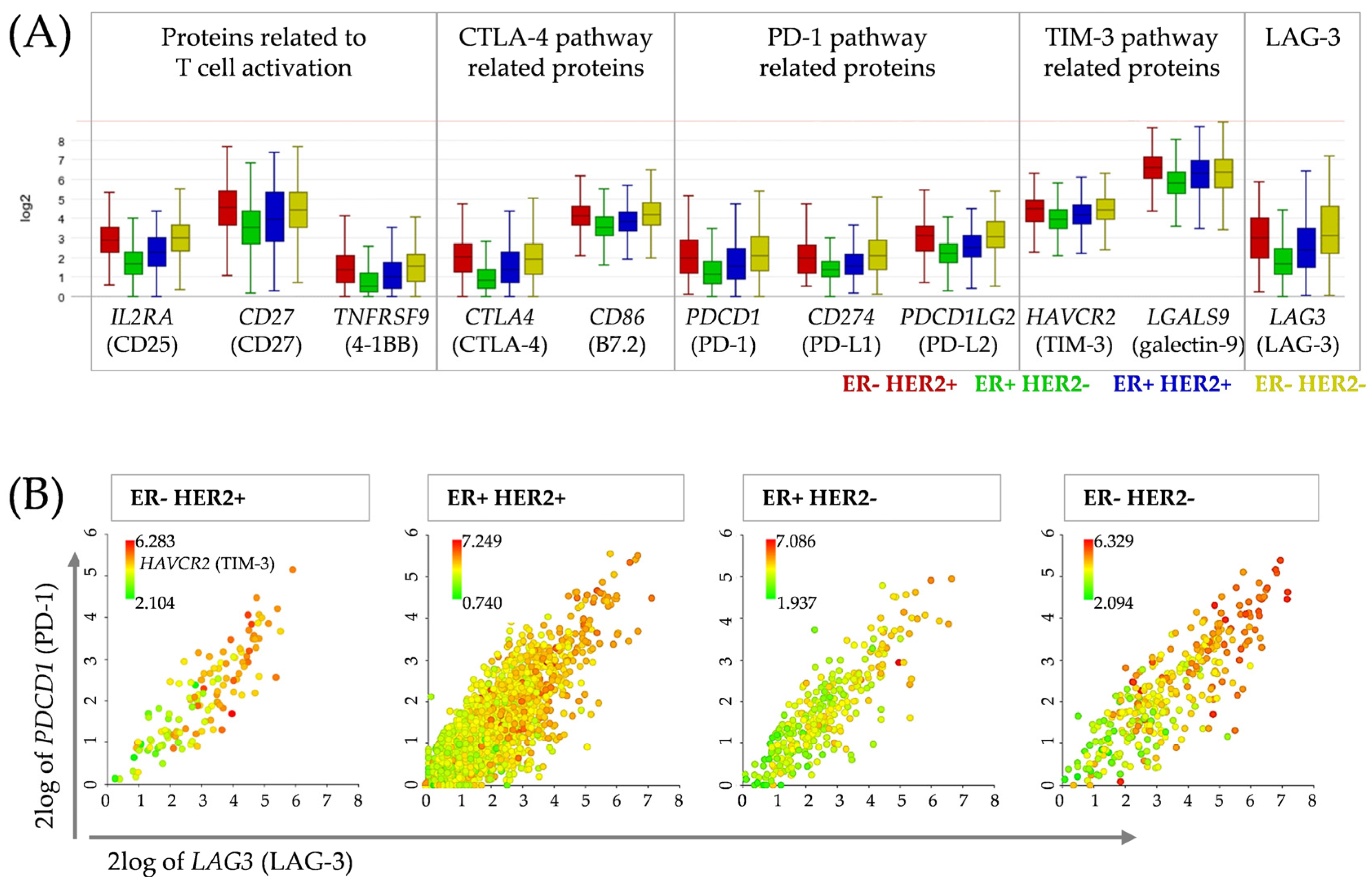
3.5. Correlation Analysis between Candidates for Concomitant Checkpoint Blockade Confirms PD-1, TIM-3 and LAG-3 as Targets in Breast Cancer Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balar, A.V.; Weber, J.S. PD-1 and PD-L1 antibodies in cancer: Current status and future directions. Cancer Immunol. Immunother. 2017, 66, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Graydon, C.G.; Mohideen, S.; Fowke, K.R. LAG3’s Enigmatic Mechanism of Action. Front. Immunol. 2021, 11, 615317. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; González-Rodríguez, A.P.; Payer, Á.R.; González-García, E.; López-Soto, A.; Gonzalez, S. LAG-3 Blockade with Relatlimab (BMS-986016) Restores Anti-Leukemic Responses in Chronic Lymphocytic Leukemia. Cancers 2021, 13, 2112. [Google Scholar] [CrossRef]
- Liang, B.; Workman, C.; Lee, J.; Chew, C.; Dale, B.M.; Colonna, L.; Flores, M.; Li, N.; Schweighoffer, E.; Greenberg, S.; et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J. Immunol. 2008, 180, 5916–5926. [Google Scholar] [CrossRef]
- Gagliani, N.; Magnani, C.F.; Huber, S.; Gianolini, M.E.; Pala, M.; Licona-Limon, P.; Guo, B.; Herbert, D.’B.R.; Bulfone, A.; Trentini, F.; et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 2013, 19, 739–746. [Google Scholar] [CrossRef]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef]
- Woo, S.-R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, S.; Fan, L.; Zhang, B.; Xu, S. TIM-3: An update on immunotherapy. Int. Immunopharmacol. 2021, 99, 107933. [Google Scholar] [CrossRef]
- Curtin, J.F.; Liu, N.; Candolfi, M.; Xiong, W.; Assi, H.; Yagiz, K.; Edwards, M.R.; Michelsen, K.S.; Kroeger, K.M.; Liu, C.; et al. HMGB1 Mediates Endogenous TLR2 Activation and Brain Tumor Regression. PLoS Med. 2009, 6, e1000010. [Google Scholar] [CrossRef] [PubMed]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T.; Herbst, R.; Coyle, A.J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Schlichtner, S.; Yasinska, I.M.; Lall, G.S.; Berger, S.M.; Ruggiero, S.; Cholewa, D.; Aliu, N.; Gibbs, B.F.; Fasler-Kan, E.; Sumbayev, V.V. T lymphocytes induce human cancer cells derived from solid malignant tumors to secrete galectin-9 which facilitates immunosuppression in cooperation with other immune checkpoint proteins. J. Immunother. Cancer 2023, 11, e005714. [Google Scholar] [CrossRef]
- Shayan, G.; Srivastava, R.; Li, J.; Schmitt, N.; Kane, L.P.; Ferris, R.L. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology 2017, 6, e1261779. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Wege, A.K.; Ernst, W.; Eckl, J.; Frankenberger, B.; Vollmann-Zwerenz, A.; Männel, D.N.; Ortmann, O.; Kroemer, A.; Brockhoff, G. Humanized tumor mice—A new model to study and manipulate the immune response in advanced cancer therapy. Int. J. Cancer 2011, 129, 2194–2206. [Google Scholar] [CrossRef]
- Wege, A.K.; Kirchhammer, N.; Kazandjian, L.V.; Prassl, S.; Brandt, M.; Piendl, G.; Ortmann, O.; Fischer, S.; Brockhoff, G. A novel rabbit derived anti-HER2 antibody with pronounced therapeutic effectiveness on HER2-positive breast cancer cells in vitro and in humanized tumor mice (HTM). J. Transl. Med. 2020, 18, 316. [Google Scholar] [CrossRef]
- Wege, A.K.; Rom-Jurek, E.-M.; Jank, P.; Denkert, C.; Ugocsai, P.; Solbach, C.; Blohmer, J.-U.; Sinn, B.; van Mackelenbergh, M.; Möbus, V.; et al. mdm2 gene amplification is associated with luminal breast cancer progression in humanized PDX mice and a worse outcome of estrogen receptor positive disease. Int. J. Cancer 2022, 150, 1357–1372. [Google Scholar] [CrossRef]
- Wege, A.K.; Weber, F.; Kroemer, A.; Ortmann, O.; Nimmerjahn, F.; Brockhoff, G. IL-15 enhances the anti-tumor activity of trastuzumab against breast cancer cells but causes fatal side effects in humanized tumor mice (HTM). Oncotarget 2017, 8, 2731–2744. [Google Scholar] [CrossRef]
- Bruss, C.; Kellner, K.; Ortmann, O.; Seitz, S.; Brockhoff, G.; Hutchinson, J.A.; Wege, A.K. Advanced Immune Cell Profiling by Multiparameter Flow Cytometry in Humanized Patient-Derived Tumor Mice. Cancers 2022, 14, 2214. [Google Scholar] [CrossRef]
- Dalal, H.; Dahlgren, M.; Gladchuk, S.; Brueffer, C.; Gruvberger-Saal, S.K.; Saal, L.H. Clinical associations of ESR2 (estrogen receptor beta) expression across thousands of primary breast tumors. Sci. Rep. 2022, 12, 4696. [Google Scholar] [CrossRef] [PubMed]
- Rom-Jurek, E.-M.; Kirchhammer, N.; Ugocsai, P.; Ortmann, O.; Wege, A.K.; Brockhoff, G. Regulation of Programmed Death Ligand 1 (PD-L1) Expression in Breast Cancer Cell Lines In Vitro and in Immunodeficient and Humanized Tumor Mice. Int. J. Mol. Sci. 2018, 19, 563. [Google Scholar] [CrossRef]
- Wang, M.; Yao, L.-C.; Cheng, M.; Cai, D.; Martinek, J.; Pan, C.-X.; Shi, W.; Ma, A.-H.; De Vere White Ralph, W.; Airhart, S.; et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018, 32, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Rosato, R.R.; Dávila-González, D.; Choi, D.S.; Qian, W.; Chen, W.; Kozielski, A.J.; Wong, H.; Dave, B.; Chang, J.C. Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Res. 2018, 20, 108. [Google Scholar] [CrossRef] [PubMed]
- Capasso, A.; Lang, J.; Pitts, T.M.; Jordan, K.R.; Lieu, C.H.; Davis, S.L.; Diamond, J.R.; Kopetz, S.; Barbee, J.; Peterson, J.; et al. Characterization of immune responses to anti-PD-1 mono and combination immunotherapy in hematopoietic humanized mice implanted with tumor xenografts. J. Immunother. Cancer 2019, 7, 37. [Google Scholar] [CrossRef]
- Choi, B.; Lee, J.S.; Kim, S.J.; Hong, D.; Park, J.B.; Lee, K.-Y. Anti-tumor effects of anti-PD-1 antibody, pembrolizumab, in humanized NSG PDX mice xenografted with dedifferentiated liposarcoma. Cancer Lett. 2020, 478, 56–69. [Google Scholar] [CrossRef]
- Rios-Doria, J.; Stevens, C.; Maddage, C.; Lasky, K.; Koblish, H.K. Characterization of human cancer xenografts in humanized mice. J. Immunother. Cancer 2020, 8, e000416. [Google Scholar] [CrossRef]
- Liu, W.N.; Fong, S.Y.; Tan, W.W.S.; Tan, S.Y.; Liu, M.; Cheng, J.Y.; Lim, S.; Suteja, L.; Huang, E.K.; Chan, J.K.Y.; et al. Establishment and Characterization of Humanized Mouse NPC-PDX Model for Testing Immunotherapy. Cancers 2020, 12, 1025. [Google Scholar] [CrossRef]
- Yin, L.; Wang, X.-J.; Chen, D.-X.; Liu, X.-N.; Wang, X.-J. Humanized mouse model: A review on preclinical applications for cancer immunotherapy. Am. J. Cancer Res. 2020, 10, 4568–4584. [Google Scholar]
- Tian, J.; Chen, X.; Fu, S.; Zhang, R.; Pan, L.; Cao, Y.; Wu, X.; Xiao, H.; Lin, H.-J.; Lo, H.-W.; et al. Bazedoxifene is a novel IL-6/GP130 inhibitor for treating triple-negative breast cancer. Breast Cancer Res. Treat. 2019, 175, 553–566. [Google Scholar] [CrossRef]
- Conroy, M.J.; Lysaght, J. CX3CL1 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1231, 1–12. [Google Scholar] [CrossRef]
- Xiong, X.; Liao, X.; Qiu, S.; Xu, H.; Zhang, S.; Wang, S.; Ai, J.; Yang, L. CXCL8 in Tumor Biology and Its Implications for Clinical Translation. Front. Mol. Biosci. 2022, 9, 723846. [Google Scholar] [CrossRef] [PubMed]
- Burugu, S.; Gao, D.; Leung, S.; Chia, S.K.; Nielsen, T.O. LAG-3+ tumor infiltrating lymphocytes in breast cancer: Clinical correlates and association with PD-1/PD-L1+ tumors. Ann. Oncol. 2017, 28, 2977–2984. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Kashiwagi, S.; Takada, K.; Ishihara, S.; Goto, W.; Morisaki, T.; Shibutani, M.; Tanaka, H.; Hirakawa, K.; Ohira, M. Clinical Significance of Expression of Immunoadjuvant Molecules (LAG-3, TIM-3, OX-40) in Neoadjuvant Chemotherapy for Breast Cancer. Anticancer Res. 2022, 42, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Miglietta, F.; Guarneri, V. Immune Infiltrates in Breast Cancer: Recent Updates and Clinical Implications. Cells 2021, 10, 223. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Forbes, K.; Vignali, K.M.; Heale, B.S.; Saftig, P.; Hartmann, D.; Black, R.A.; Rossi, J.J.; Blobel, C.P.; et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007, 26, 494–504. [Google Scholar] [CrossRef]
- Frigola, X.; Inman, B.A.; Lohse, C.M.; Krco, C.J.; Cheville, J.C.; Thompson, R.H.; Leibovich, B.; Blute, M.L.; Dong, H.; Kwon, E.D. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin. Cancer Res. 2011, 17, 1915–1923. [Google Scholar] [CrossRef]
- Bailly, C.; Thuru, X.; Quesnel, B. Soluble Programmed Death Ligand-1 (sPD-L1): A Pool of Circulating Proteins Implicated in Health and Diseases. Cancers 2021, 13, 3034. [Google Scholar] [CrossRef]
- Burnell, S.E.A.; Capitani, L.; MacLachlan, B.J.; Mason, G.H.; Gallimore, A.M.; Godkin, A. Seven mysteries of LAG-3: A multi-faceted immune receptor of increasing complexity. Immunother. Adv. 2022, 2, ltab025. [Google Scholar] [CrossRef]
- Buisson, S.; Triebel, F. LAG-3 (CD223) reduces macrophage and dendritic cell differentiation from monocyte precursors. Immunology 2005, 114, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, B.L.; Steel, H.C.; Hlatshwayo, N.; Theron, A.J.; Meyer, P.W.A.; Nayler, S.; Benn, C.-A.; Smit, T.; Kwofie, L.L.I.; Heyman, L.; et al. Systemic Immune Dysregulation in Early Breast Cancer Is Associated With Decreased Plasma Levels of Both Soluble Co-Inhibitory and Co-Stimulatory Immune Checkpoint Molecules. Front Immunol 2022, 13, 823842. [Google Scholar] [CrossRef] [PubMed]
- Triebel, F.; Hacene, K.; Pichon, M.-F. A soluble lymphocyte activation gene-3 (sLAG-3) protein as a prognostic factor in human breast cancer expressing estrogen or progesterone receptors. Cancer Lett. 2006, 235, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, T.; Xuan, Q.; Zhao, H.; Qin, L.; Zhang, Q. Lymphocyte-Activation Gene-3 Expression and Prognostic Value in Neoadjuvant-Treated Triple-Negative Breast Cancer. J. Breast Cancer 2018, 21, 124–133. [Google Scholar] [CrossRef]
- Botticelli, A.; Zizzari, I.G.; Scagnoli, S.; Pomati, G.; Strigari, L.; Cirillo, A.; Cerbelli, B.; Di Filippo, A.; Napoletano, C.; Scirocchi, F.; et al. The Role of Soluble LAG3 and Soluble Immune Checkpoints Profile in Advanced Head and Neck Cancer: A Pilot Study. J. Pers. Med. 2021, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Qi, F.; Rao, Q.; Sun, J.; Du, X.; Qi, Z.; Yang, B.; Xia, J. Serum LAG-3 Predicts Outcome and Treatment Response in Hepatocellular Carcinoma Patients With Transarterial Chemoembolization. Front. Immunol. 2021, 12, 754961. [Google Scholar] [CrossRef]
- Machiraju, D.; Wiecken, M.; Lang, N.; Hülsmeyer, I.; Roth, J.; Schank, T.E.; Eurich, R.; Halama, N.; Enk, A.; Hassel, J.C. Soluble immune checkpoints and T-cell subsets in blood as biomarkers for resistance to immunotherapy in melanoma patients. Oncoimmunology 2021, 10, 1926762. [Google Scholar] [CrossRef]
- Li, N.; Jilisihan, B.; Wang, W.; Tang, Y.; Keyoumu, S. Soluble LAG3 acts as a potential prognostic marker of gastric cancer and its positive correlation with CD8+T cell frequency and secretion of IL-12 and INF-γ in peripheral blood. Cancer Biomark. 2018, 23, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qi, Y.; Zhai, J.; Kong, X.; Wang, X.; Wang, Z.; Fang, Y.; Wang, J. Molecular and Clinical Characterization of LAG3 in Breast Cancer Through 2994 Samples. Front. Immunol. 2021, 12, 599207. [Google Scholar] [CrossRef]
- Sidaway, P. Breast cancer: LAG3 expression indicates favourable outcomes. Nat. Rev. Clin. Oncol. 2017, 14, 712. [Google Scholar] [CrossRef]
- Bottai, G.; Raschioni, C.; Losurdo, A.; Di Tommaso, L.; Tinterri, C.; Torrisi, R.; Reis-Filho, J.S.; Roncalli, M.; Sotiriou, C.; Santoro, A.; et al. An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Res. 2016, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Morais, A.L.; Cerdá, S.; de Miguel, M. New Checkpoint Inhibitors on the Road: Targeting TIM-3 in Solid Tumors. Curr. Oncol. Rep. 2022, 24, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-X.; Huang, D.-J.; Baloche, V.; Zhang, L.; Xu, J.-X.; Li, B.-W.; Zhao, X.-R.; He, J.; Mai, H.-Q.; Chen, Q.-Y.; et al. Galectin-9 promotes a suppressive microenvironment in human cancer by enhancing STING degradation. Oncogenesis 2020, 9, 65. [Google Scholar] [CrossRef]
- Yang, R.; Sun, L.; Li, C.-F.; Wang, Y.-H.; Yao, J.; Li, H.; Yan, M.; Chang, W.-C.; Hsu, J.-M.; Cha, J.-H.; et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef]
- Yamauchi, A.; Kontani, K.; Kihara, M.; Nishi, N.; Yokomise, H.; Hirashima, M. Galectin-9, a novel prognostic factor with antimetastatic potential in breast cancer. Breast J. 2006, 12, S196-200. [Google Scholar] [CrossRef]
- Holtan, S.G.; Mansfield, A.S.; Creedon, D.J.; Nevala, W.K.; Haluska, P.; Leontovich, A.A.; Markovic, S.N. An organ system based approach to prognosis in advanced melanoma. Front. Biosci. 2012, 4, 2723–2733. [Google Scholar] [CrossRef]
- Sideras, K.; Biermann, K.; Verheij, J.; Takkenberg, B.R.; Mancham, S.; Hansen, B.E.; Schutz, H.M.; de Man, R.A.; Sprengers, D.; Buschow, S.I.; et al. PD-L1, Galectin-9 and CD8+ tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. Oncoimmunology 2017, 6, e1273309. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Ma, C.; Gao, W.; Song, B.; Xue, H.; Chen, W.; Chen, X.; Zhang, Y.; Shao, Q.; et al. Reduced Expression of Galectin-9 Contributes to a Poor Outcome in Colon Cancer by Inhibiting NK Cell Chemotaxis Partially through the Rho/ROCK1 Signaling Pathway. PLoS ONE 2016, 11, e0152599. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Fu, Q.; Wang, Z.; Fu, H.; Liu, W.; Wang, Y.; Xu, J. Galectin-9 as a prognostic and predictive biomarker in bladder urothelial carcinoma. Urol. Oncol. 2017, 35, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Grosset, A.-A.; Labrie, M.; Vladoiu, M.C.; Yousef, E.M.; Gaboury, L.; St-Pierre, Y. Galectin signatures contribute to the heterogeneity of breast cancer and provide new prognostic information and therapeutic targets. Oncotarget 2016, 7, 18183–18203. [Google Scholar] [CrossRef]
- Fu, H.; Liu, Y.; Le, X.; Liu, W.; Fu, Q.; Liu, H.; Zhang, W.; Xu, J. Galectin-9 predicts postoperative recurrence and survival of patients with clear-cell renal cell carcinoma. Tumour Biol. 2015, 36, 5791–5799. [Google Scholar] [CrossRef]
- Choi, S.I.; Seo, K.W.; Kook, M.C.; Kim, C.G.; Kim, Y.W.; Cho, S.J. Prognostic value of tumoral expression of galectin-9 in gastric cancer. Turk. J. Gastroenterol. 2017, 28, 166–170. [Google Scholar] [CrossRef]
- Ohue, Y.; Kurose, K.; Nozawa, R.; Isobe, M.; Nishio, Y.; Tanaka, T.; Doki, Y.; Hori, T.; Fukuoka, J.; OKa, M.; et al. Survival of Lung Adenocarcinoma Patients Predicted from Expression of PD-L1, Galectin-9, and XAGE1 (GAGED2a) on Tumor Cells and Tumor-Infiltrating T Cells. Cancer Immunol. Res. 2016, 4, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, Y.; Yue, F. Prognostic Value of Soluble Programmed Cell Death Ligand-1 (sPD-L1) in Various Cancers: A Meta-analysis. Target. Oncol. 2021, 16, 13–26. [Google Scholar] [CrossRef]
- Han, B.; Dong, L.; Zhou, J.; Yang, Y.; Guo, J.; Xuan, Q.; Gao, K.; Xu, Z.; Lei, W.; Wang, J.; et al. The clinical implication of soluble PD-L1 (sPD-L1) in patients with breast cancer and its biological function in regulating the function of T lymphocyte. Cancer Immunol. Immunother. 2021, 70, 2893–2909. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, X.; Sun, L.; Mao, Y. Correlation Between PD-L2 Expression and Clinical Outcome in Solid Cancer Patients: A Meta-Analysis. Front. Oncol. 2019, 9, 47. [Google Scholar] [CrossRef]
- Chervoneva, I.; Peck, A.R.; Sun, Y.; Yi, M.; Udhane, S.S.; Langenheim, J.F.; Girondo, M.A.; Jorns, J.M.; Chaudhary, L.N.; Kamaraju, S.; et al. High PD-L2 Predicts Early Recurrence of ER-Positive Breast Cancer. JCO Precis. Oncol. 2023, 7, e2100498. [Google Scholar] [CrossRef]
- Hoffmann, O.; Wormland, S.; Bittner, A.-K.; Collenburg, M.; Horn, P.A.; Kimmig, R.; Kasimir-Bauer, S.; Rebmann, V. Programmed death receptor ligand-2 (PD-L2) bearing extracellular vesicles as a new biomarker to identify early triple-negative breast cancer patients at high risk for relapse. J. Cancer Res. Clin. Oncol. 2023, 149, 1159–1174. [Google Scholar] [CrossRef]
- Buderath, P.; Schwich, E.; Jensen, C.; Horn, P.A.; Kimmig, R.; Kasimir-Bauer, S.; Rebmann, V. Soluble Programmed Death Receptor Ligands sPD-L1 and sPD-L2 as Liquid Biopsy Markers for Prognosis and Platinum Response in Epithelial Ovarian Cancer. Front. Oncol. 2019, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Chrétien, S.; Zerdes, I.; Bergh, J.; Matikas, A.; Foukakis, T. Beyond PD-1/PD-L1 Inhibition: What the Future Holds for Breast Cancer Immunotherapy. Cancers 2019, 11, 628. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernández-Rubio, L.; Bocanegra, A.; Echaide, M.; Garnica, M.; Ramos, P.; Fernández-Hinojal, G.; Vera, R.; et al. Clinical landscape of LAG-3-targeted therapy. Immunooncol. Technol. 2022, 14, 100079. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Patel, M.R.; Hamilton, E.P.; Chmielowski, B.; Ulahannan, S.V.; Kindler, H.L.; Bahadur, S.W.; Clingan, P.R.; Mallesara, G.; Weickhardt, A.J.; et al. A phase I, first-in-human, open-label, dose-escalation study of MGD013, a bispecific DART molecule binding PD-1 and LAG-3, in patients with unresectable or metastatic neoplasms. J. Clin. Oncol. 2020, 38, 3004. [Google Scholar] [CrossRef]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, D.; Forde, P.M.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.-C.; Hodi, F.S.; et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3620–3629. [Google Scholar] [CrossRef] [PubMed]

| Cytokines and Ligands for Chemokines | Ligands for Checkpoint Proteins | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | TGF-ß | IL-4 | IL-1ß | TNF | CXCL8 | MCP-1 | CXCL10 | CX3CL1 | HMGB1 | Gal-9 | sPD-L1 | sPD-L2 | ||
| JIMT-1 | 214 | (-) | (-) | (-) | (-) | 14 | (-) | (-) | (-) | 44,578 | (-) | (-) | 257 | |
| ±82 | ±12 | ±12,795 | ±106 | |||||||||||
| MDA-MB-231 | 5672 | 44 | (-) | (-) | (-) | 1194 | 120 | 71 | 6751 | 52,257 | 59 | (-) | 206 | |
| ±1237 | ±32 | ±110 | ±36 | ±13 | ±729 | ±7768 | ±36 | ±69 | ||||||
| MCF-7 | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | (-) | 32,056 | (-) | (-) | (-) | |
| ±18,210 | ||||||||||||||
| Correlation Coefficient | ||||
|---|---|---|---|---|
| ER− HER2+ | ER+ HER2+ | ER+ HER2− | ER− HER2− | |
| PDCD1 vs. LAG3 | 0.865 | 0.850 | 0.777 | 0.835 |
| PDCD1 vs. HAVCR2 | 0.495 | 0.474 | 0.433 | 0.622 |
| LAG3 vs. HAVCR2 | 0.590 | 0.549 | 0.490 | 0.635 |
| LAG3 vs. LGALS9 | 0.567 | 0.659 | 0.587 | 0.706 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruss, C.; Kellner, K.; Albert, V.; Hutchinson, J.A.; Seitz, S.; Ortmann, O.; Brockhoff, G.; Wege, A.K. Immune Checkpoint Profiling in Humanized Breast Cancer Mice Revealed Cell-Specific LAG-3/PD-1/TIM-3 Co-Expression and Elevated PD-1/TIM-3 Secretion. Cancers 2023, 15, 2615. https://doi.org/10.3390/cancers15092615
Bruss C, Kellner K, Albert V, Hutchinson JA, Seitz S, Ortmann O, Brockhoff G, Wege AK. Immune Checkpoint Profiling in Humanized Breast Cancer Mice Revealed Cell-Specific LAG-3/PD-1/TIM-3 Co-Expression and Elevated PD-1/TIM-3 Secretion. Cancers. 2023; 15(9):2615. https://doi.org/10.3390/cancers15092615
Chicago/Turabian StyleBruss, Christina, Kerstin Kellner, Veruschka Albert, James A. Hutchinson, Stephan Seitz, Olaf Ortmann, Gero Brockhoff, and Anja K. Wege. 2023. "Immune Checkpoint Profiling in Humanized Breast Cancer Mice Revealed Cell-Specific LAG-3/PD-1/TIM-3 Co-Expression and Elevated PD-1/TIM-3 Secretion" Cancers 15, no. 9: 2615. https://doi.org/10.3390/cancers15092615
APA StyleBruss, C., Kellner, K., Albert, V., Hutchinson, J. A., Seitz, S., Ortmann, O., Brockhoff, G., & Wege, A. K. (2023). Immune Checkpoint Profiling in Humanized Breast Cancer Mice Revealed Cell-Specific LAG-3/PD-1/TIM-3 Co-Expression and Elevated PD-1/TIM-3 Secretion. Cancers, 15(9), 2615. https://doi.org/10.3390/cancers15092615







