New Regional Dynamic Cancer Model across the European Union
Abstract
:Simple Summary
Abstract
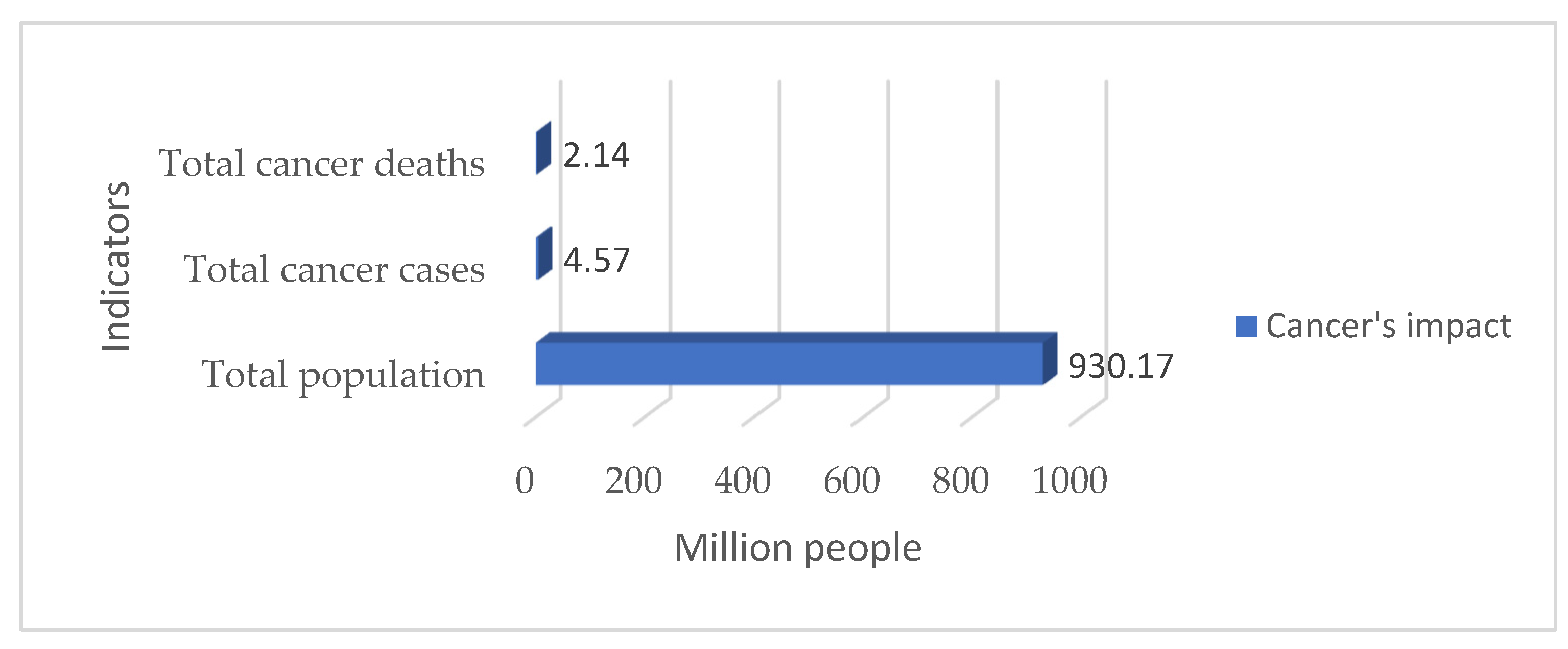
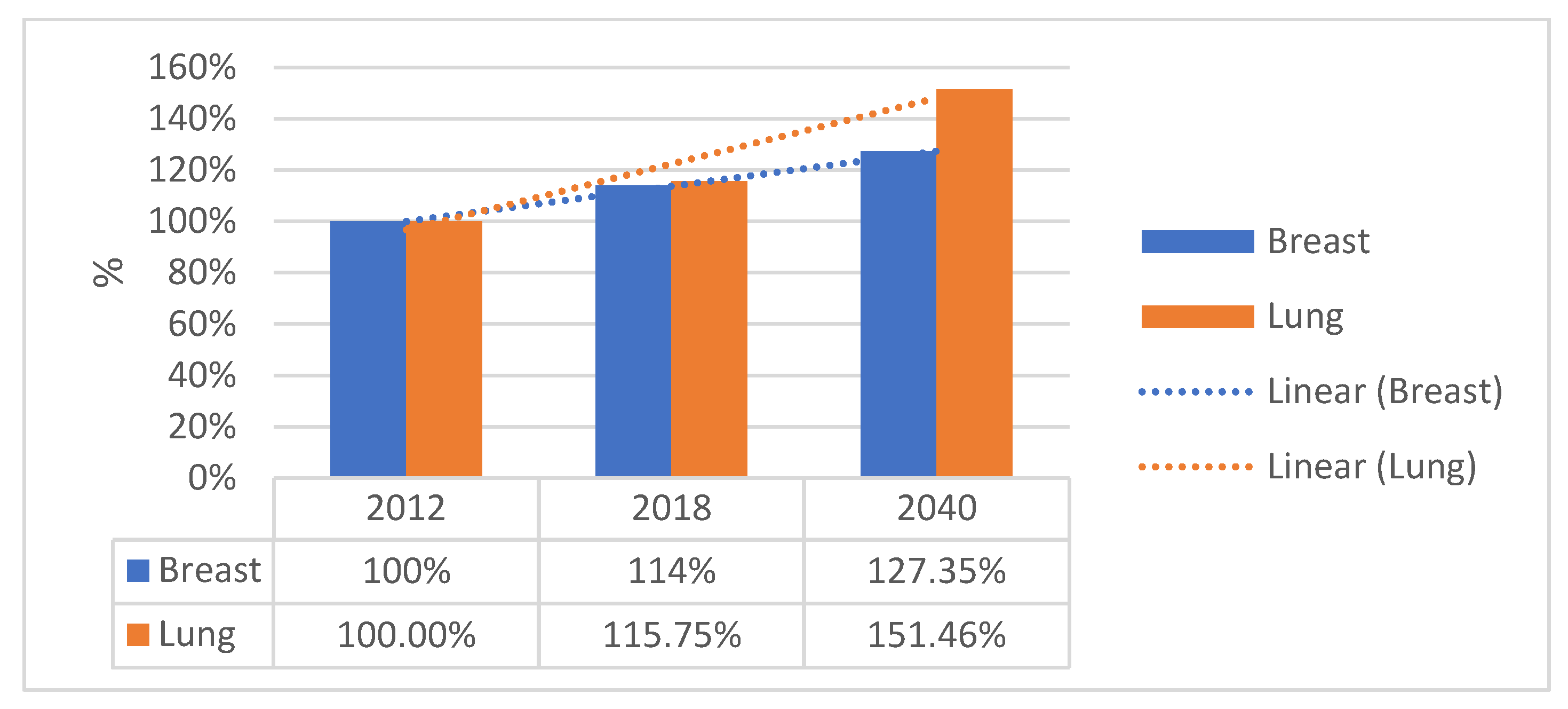
1. Introduction
2. Literature Review
3. Materials and Methods
- As far as lip, oral cavity and pharyngeal cancer was concerned, the increase in its incidence for men varied between 3.08 units per 100,000 inhabitants, while the economic wealth of men increased by one unit in 1993. In 2021, the incidence rate became 0.381 for an increase of one unit in the economic well-being of the men in the sample analysed. The maximum value was reached in 1994, when the incidence rate was 3.82 units for one unit increase in men’s economic well-being, while the minimum value was recorded in 2012, with a negative incidence rate with a correlation coefficient of −1.72 related to the dependent variable increase in men’s economic welfare.
- Analysis of the disease situation for the female population in the 25 EU member states for the lip, oral cavity, and pharyngeal cancer type showed a much wider variation in the indicator, from 4.06 incidence points in 1993 to −0.68 points in 2021. The maximum value was reached in 1996, when the incidence rate was 22.65 units for one unit increase in women’s economic well-being, while the minimum value was recorded in 1998, i.e., a negative incidence rate with a correlation coefficient of −7.17 related to the dependent variable increase in women’s economic welfare.
- As far as lip, oral cavity, and pharyngeal cancer was concerned, the increase in its mortality for men ranged from −8.77 units per 100,000 inhabitants to −8.77 units per 100,000 inhabitants, while men’s economic well-being increased by one unit in 1993 (maximum value). In 2021, the mortality rate became −1.3 for an increase of one unit in the economic well-being of men in the analysed sample (minimum value).
- Analysis of disease mortality for the female population in the 25 EU member states for the lip, oral cavity, and pharyngeal cancer type showed a much wider variation in the indicator, from −35.3 incidence points in 1993 to −6.7 points in 2021. The maximum value was reached in 1994, when the mortality rate was −51.17 units for one unit increase in women’s economic well-being, while the minimum value was recorded in 2019, i.e., negative mortality rate with a correlation coefficient of −4.55 related to the dependent variable increase in women’s economic welfare.
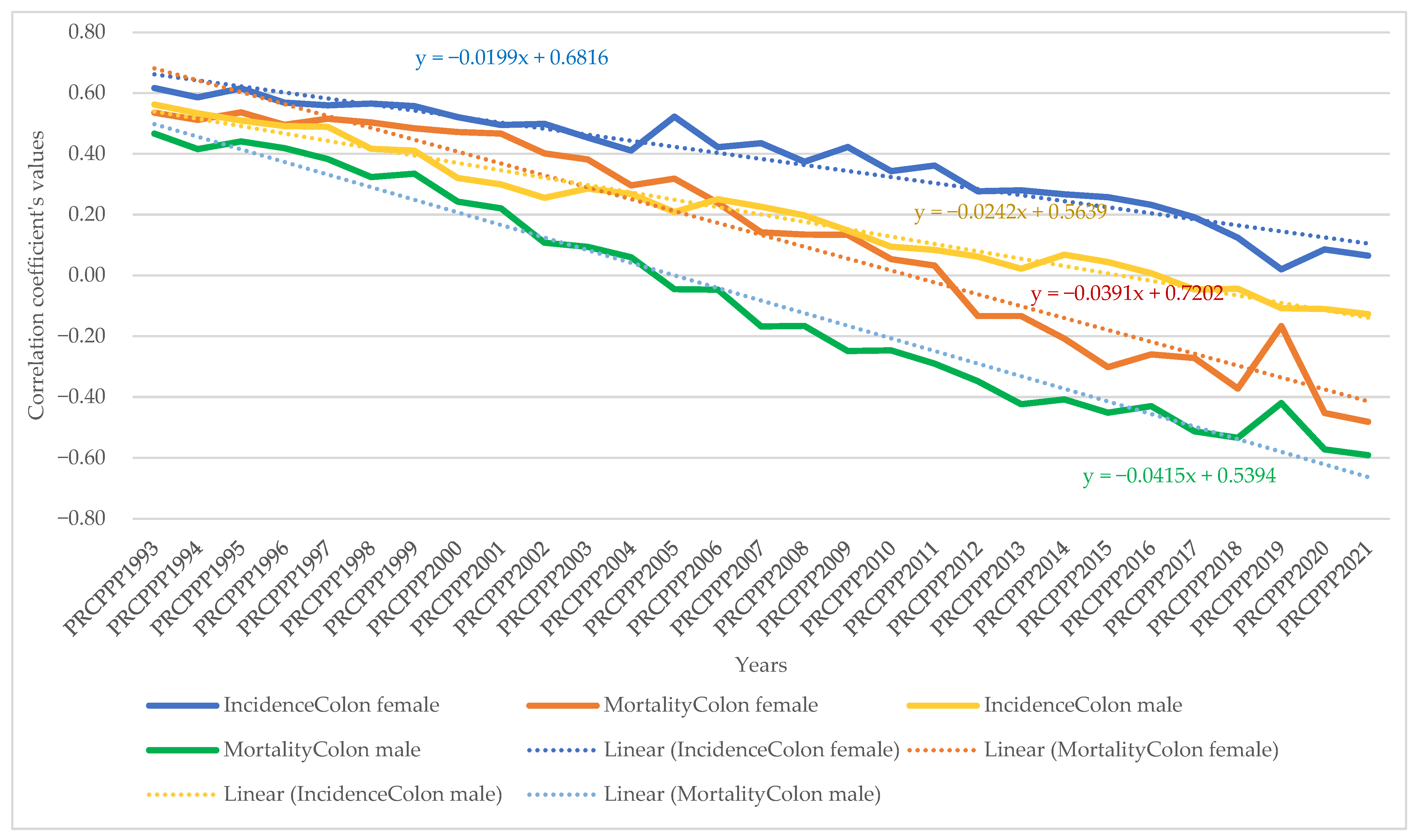
- As far as colon cancer was concerned, the increase in its incidence varied for men between 2.22 units per 100,000 inhabitants, while the economic wealth of men increased by one unit in 1993. In 2021, the incidence rate became 1.03 for an increase of one unit in the economic welfare of the men in the sample analysed. The maximum value was reached in 1996, when the incidence rate was 5.7 units for one unit increase in men’s economic well-being, while the minimum value was recorded in 2001, namely a negative incidence rate with a correlation coefficient of −1.3 related to thedependent variable increase in men’s economic welfare.
- Analysis of the disease situation for the female population in the 25 EU member states for colon cancer showed much wider variation, from 3.11 incidence points in 1993 to 1.21 points in 2021. The maximum value was reached in 1995, when the incidence rate was 4.61 units for one unit increase in women’s economic well-being, while the minimum value was recorded in 2010, i.e., a negative incidence rate with a correlation coefficient of −2.08 related to thedependent variable increase in women’s economic welfare.
- As far as colon cancer was concerned, the increase in colon cancer mortality for men ranged from −5.9 units per 100,000 inhabitants, while the economic welfare of men increased by one unit in 1993 (maximum value). In 2021, the mortality rate became −2.95 for an increase of one unit in the economic welfare of the men in the sample analysed (minimum value). The maximum value was reached in 2012 (1.66) and the minimum was reached in 1994 (−9.12).
- Analysis of disease mortality for the female population in the 25 EU member states for the colon cancer type showed a much wider variation of the indicator, from −2.77 incidence points in 1993 to −4.98 points in 2021. The maximum value was reached in 2009, when the mortality rate was 2.02 units for one unit increase in women’s economic welfare, while the minimum value was recorded in 1995, i.e., a negative mortality rate with a correlation coefficient of −7.07 related to the dependent variable increase in women’s economic welfare.
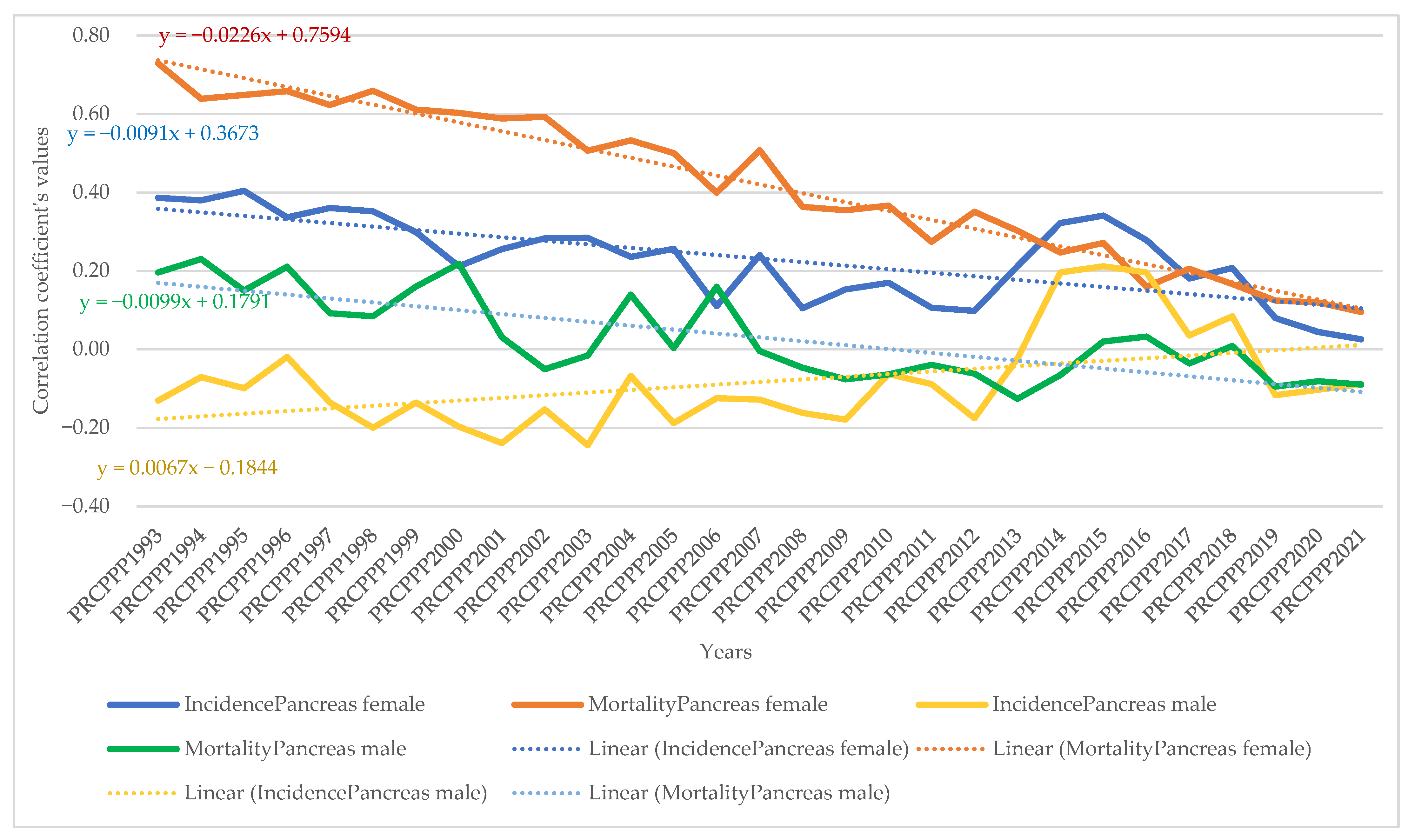
- As far as pancreatic cancer was concerned, the increase in its incidence in men ranged from −9.9 units per 100,000 inhabitants, while the economic welfare of men increased by one unit in 1993. In 2021, the incidence rate became −1.93 for an increase of one unit in the economic welfare of the men in the sample analysed. The maximum value was reached in 2014, when the incidence rate was 4.35 units for the one unit increase in men’s economic welfare, while the minimum value was recorded in 1998, with a negative incidence rate with a correlation coefficient of −13.62 related to the dependent variable increase in men’s economic welfare.
- Analysis of the disease situation for the female population in the 25 EU member states for pancreatic cancer showed a much wider variation in the indicator, from −8.99 incidence points in 1993 to −2.69 points in 2021. The maximum value was reached in 2013, when the incidence rate was 1.49 units for one unit increase in women’s economic welfare, while the minimum value was recorded in 1998, i.e., a negative incidence rate with a correlation coefficient of −11.11 related to the dependent variable increase in women’s economic welfare.
- As far as pancreatic cancer was concerned, the increase in pancreatic cancer mortality for men ranged from 21.87 units per 100,000 population, while the economic welfare of men increased by one unit in 1993. In 2021, the mortality rate became 0.06 for an increase of one unit in the economic welfare of the men in the analysed sample. The maximum value was reached in 1994 (23.79) and the minimum value was reached in 2014 (−2.39).
- Analysis of disease mortality for the female population in the 25 EU member states for pancreatic cancer showed a much wider variation in the indicator, from 16.7 incidence points in 1993 to 2.0 points in 2021. The maximum value was reached in 1999, when the mortality rate was 20.5 units for one unit increase in women’s economic welfare, while the minimum value was recorded in 2013, i.e., a negative mortality rate with a correlation coefficient of −0.78 related to the dependent variable increase in women’s economic welfare.
- As far as lung cancer was concerned, the increase in its incidence varied for men between −0.35 units per 100,000 inhabitants, while the economic welfare of men increased by one unit in 1993. In 2021, the incidence rate became 0.27 for an increase of one unit in the economic welfare of the men in the analysed sample. The maximum value was reached in 2011, when the incidence rate was 1.9 units for one unit increase in men’s economic welfare, while the minimum value was recorded in 1998, with a negative incidence rate with a correlation coefficient of −1.6 related to the dependent variable increase in men’s economic welfare.
- Analysis of the disease situation for the female population in the 25 EU member states for the lung cancer type showed a much wider variation in the indicator, from −0.9 incidence points in 1993 to 1.08 points in 2021. The maximum value was reached in 2002, when the incidence rate was 3.78 units for one unit increase in women’s economic welfare, while the minimum value was recorded in 1995, i.e., a negative incidence rate with a correlation coefficient of −4.98 related to the dependent variable increase in women’s economic welfare.
- As far as lung cancer was concerned, the increase in mortality for men ranged from 0.8 units per 100,000 inhabitants, while the economic welfare of men increased by one unit in 1993. In 2021, the mortality rate became −0.8 for an increase of one unit in the economic welfare of the men in the analysed sample. The maximum value was reached in 1998 (1.9) and the minimum value was reached in 2011 (−3.39).
- Analysis of disease mortality for the female population in the 25 EU member states for the lung cancer type showed a much wider variation in the indicator, from 0.42 incidence points in 1993 to −0.63 points in 2021. The maximum value was reached in 1996, when the mortality rate was 4.2 units for one unit increase in women’s economic welfare, while the minimum value was recorded in 2002, i.e., a negative mortality rate with a correlation coefficient of −3.5 related to the dependent variable increase in women’s economic welfare.
- As far as leukaemia was concerned, the increase in its incidence in men varied between 12.4 units per 100,000 inhabitants, while the economic welfare of men increased by one unit in 1993. In 2021, the incidence rate became −0.07 for an increase of one unit in the economic welfare of men in the analysed sample. The maximum value was reached in 2001, when the incidence rate was 12.95 units for one unit increase in men’s economic welfare, while the minimum value was recorded in 2007, with a negative incidence rate with a correlation coefficient of −2.5 related to the dependent variable increase in men’s economic welfare.
- Analysis of the disease situation for the female population in the 25 EU member states for the leukaemia cancer type showed a much wider variation in the indicator, from 6.59 incidence points in 1993 to 1.87 points in 2021. The maximum value was reached in 1997, when the incidence rate was 13.5 units for one unit increase in women’s economic welfare, while the minimum value was recorded in 2005, i.e., a negative incidence rate with a correlation coefficient of −4.07 related to the dependent variable increase in women’s economic welfare.
- As far as leukaemia was concerned, the increase in its mortality for men ranged from −10 units per 100,000 inhabitants while the economic wealth of men increased by one unit in 1993. In 2021, the mortality rate became 2.97 for an increase of one unit in the economic welfare of the men in the analysed sample. The maximum value was reached in 2018 (7.66) and the minimum value was reached in 1997 (−20.5).
- Analysis of disease mortality for the female population in the 25 EU member states for the leukaemia cancer type showed a much wider variation in the indicator, from −5.8 incidence points in 1993 to 2.33 points in 2021. The maximum value was reached in 2008, when the mortality rate was 7.47 units for one unit increase in women’s economic welfare, while the minimum value was recorded in the year 1997, i.e., a negative mortality rate with a correlation coefficient of −21.7 related to the dependent variable increase in women’s economic welfare.
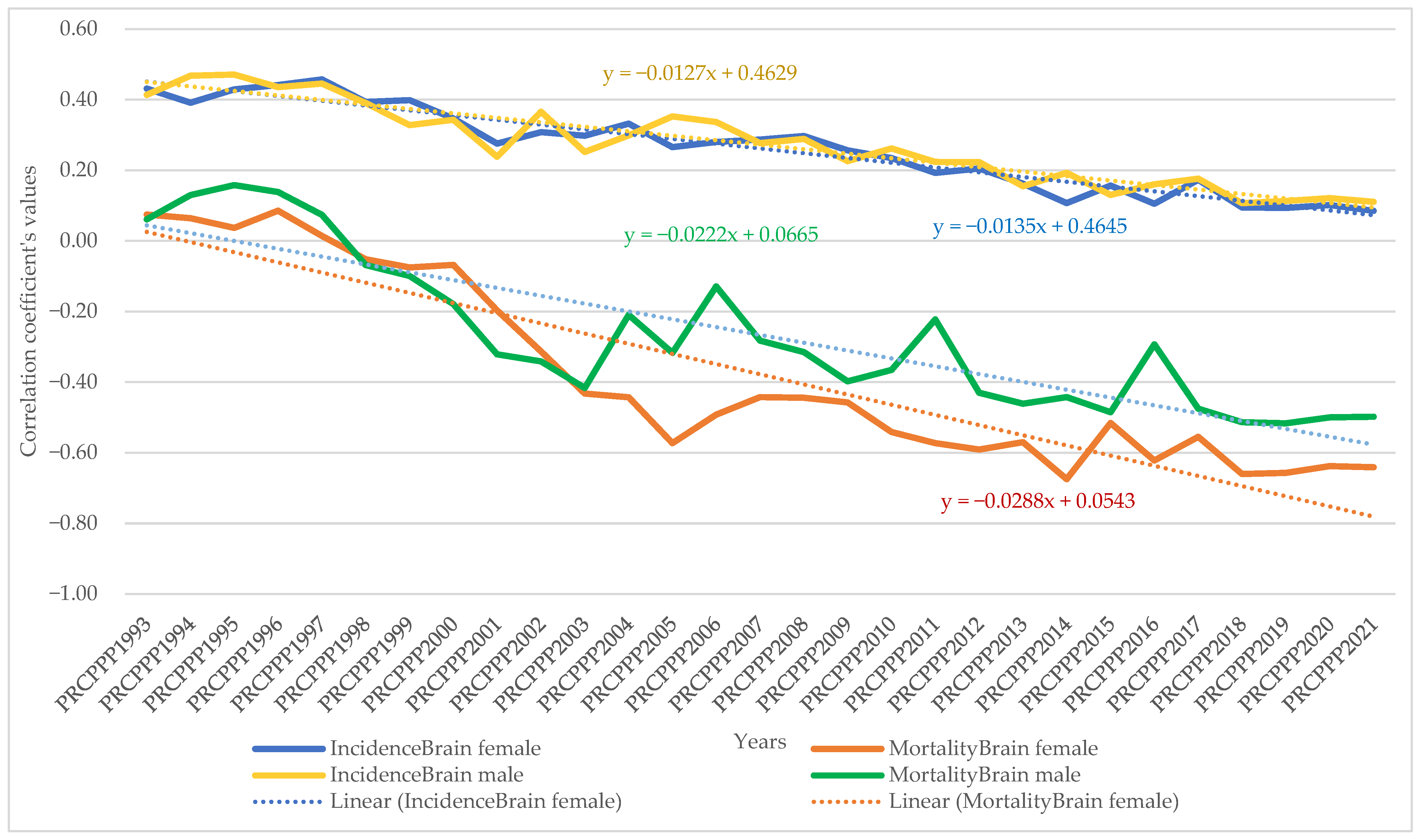
- For brain and central nervous system cancer, the increase in its incidence varied for men between 4.28 units per 100,000 inhabitants, while the economic welfare of men increased by one unit in 1993. In 2021, the incidence rate became 1.19 for an increase of one unit in the economic welfare of the men in the analysed sample. The maximum value was reached in 1997, when the incidence rate was 9.53 units for one unit increase in men’s economic welfare, while the minimum value was recorded in 2016, with a negative incidence rate with a correlation coefficient of −0.57 related to the dependent variable increase in men’s economic welfare.
- Analysis of the disease situation for the female population in the 25 EU member states for the brain and central nervous system cancer type showed a much wider variation in the indicator, from 3.96 incidence points in 1993 to 0.72 points in 2021. The maximum value was reached in 1997, when the incidence rate was 5.1 units for one unit increase in women’s economic welfare, while the minimum value was recorded in 2014, i.e., a negative incidence rate with a correlation coefficient of 0.27 related to the dependent variable increase in women’s economic welfare.
- As far as brain and central nervous system cancer was concerned, the increase in its mortality for men ranged from −15.5 units per 100,000 inhabitants, while the economic welfare of men increased by one unit in 1993. In 2021, the mortality rate became −6.25 for a one unit increase in the economic welfare of men in the analysed sample. The maximum value was reached in 2004 (1.17) and the minimum value was reached in 2001 (−19.12).
- Analysis of disease mortality for the female population in the 25 EU member states for the brain and central nervous system cancer type showed a much wider variation in the indicator, from −13.54 incidence points in 1993 to −10.13 points in 2021. The maximum value was reached in 2001, when the mortality rate was −23.87 units for one unit increase in women’s economic welfare, while the minimum value was recorded in 1997, i.e., a negative mortality rate with a correlation coefficient of −3.4 related to the dependent variable increase in women’s economic welfare.
4. Results
5. Discussion
- Additional measures to ensure early detection of early stages of lip, oral cavity, and pharyngeal, pancreatic, and brain and central nervous system cancers;
- Additional funding for lip, oral cavity, and pharyngeal cancer and lung cancer treatments to prevent mortality among women;
- Additional funding for brain and central nervous system cancer treatments to prevent mortality in men;
- Additional financial support to compensate for the costs of medicines in the treatment of various types of cancer;
- Strengthening support to provide palliative treatment for brain and central nervous system cancer and lung cancer;
- Reducing disparities in the financing of treatment costs between EU member states;
- Supporting a proactive approach to cancer by patients and their families.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Indicator | Females | Males | Total | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Std. Err of Mean | Min | Max | Std. Deviation | Mean | Median | Std. Err of Mean | Min | Max | Std. Deviation | Mean | Median | Std. Err of Mean | Min | Max | Std. Deviation | |
| IncidenceLip1993 * | 4.73 | 4.50 | 0.32 | 2.50 | 8.10 | 1.58 | 18.29 | 16.90 | 1.72 | 7.50 | 43.30 | 8.60 | 11.51 | 7.65 | 1.30 | 2.50 | 43.30 | 9.19 |
| IncidenceLip2021 * | 8.88 | 8.90 | 0.58 | 4.30 | 15.10 | 2.89 | 23.11 | 21.30 | 1.89 | 10.80 | 57.10 | 9.44 | 16.00 | 14.65 | 1.41 | 4.30 | 57.10 | 9.97 |
| MortalityLip1993 * | 1.96 | 1.90 | 0.12 | 1.00 | 4.10 | 0.61 | 9.34 | 8.30 | 1.00 | 3.10 | 24.20 | 5.00 | 5.65 | 3.15 | 0.73 | 1.00 | 24.20 | 5.13 |
| MortalityLip2021 * | 2.97 | 2.90 | 0.18 | 1.20 | 6.30 | 0.91 | 10.81 | 8.50 | 1.25 | 4.30 | 24.80 | 6.23 | 6.89 | 4.35 | 0.84 | 1.20 | 24.80 | 5.92 |
| IncidenceColon1993 * | 27.40 | 24.20 | 2.25 | 11.20 | 54.60 | 11.26 | 26.87 | 26.20 | 2.21 | 12.10 | 53.30 | 11.04 | 27.13 | 25.90 | 1.56 | 11.20 | 54.60 | 11.04 |
| IncidenceColon2021 * | 42.53 | 41.10 | 2.20 | 20.10 | 66.20 | 10.99 | 52.41 | 53.00 | 2.89 | 25.40 | 78.00 | 14.44 | 47.47 | 45.55 | 1.93 | 20.10 | 78.00 | 13.64 |
| MortalityColon1993 * | 16.82 | 16.50 | 1.32 | 7.10 | 29.90 | 6.60 | 16.57 | 16.40 | 1.15 | 7.60 | 25.60 | 5.77 | 16.70 | 16.45 | 0.87 | 7.10 | 29.90 | 6.13 |
| MortalityColon2021 * | 20.00 | 20.60 | 0.97 | 6.30 | 30.00 | 4.84 | 24.98 | 23.40 | 1.57 | 8.20 | 44.40 | 7.86 | 22.49 | 21.10 | 0.98 | 6.30 | 44.40 | 6.93 |
| IncidencePancreas1993 * | 10.35 | 9.50 | 0.63 | 5.30 | 15.30 | 3.16 | 12.07 | 12.20 | 0.51 | 8.20 | 17.20 | 2.56 | 11.21 | 11.65 | 0.42 | 5.30 | 17.20 | 2.98 |
| IncidencePancreas2021 * | 15.59 | 14.70 | 1.01 | 9.00 | 29.40 | 5.06 | 16.62 | 15.40 | 0.94 | 9.30 | 26.90 | 4.69 | 16.11 | 15.30 | 0.69 | 9.00 | 29.40 | 4.85 |
| MortalityPancreas1993 * | 10.24 | 10.20 | 0.69 | 4.30 | 18.20 | 3.47 | 12.02 | 11.90 | 0.45 | 8.80 | 17.00 | 2.26 | 11.13 | 10.90 | 0.43 | 4.30 | 18.20 | 3.03 |
| MortalityPancreas2021 * | 18.16 | 18.10 | 0.67 | 10.60 | 22.70 | 3.36 | 19.11 | 19.20 | 0.57 | 11.50 | 23.70 | 2.84 | 18.64 | 19.15 | 0.44 | 10.60 | 23.70 | 3.12 |
| IncidenceLung1993 * | 17.66 | 15.40 | 1.85 | 7.10 | 47.60 | 9.24 | 79.80 | 81.60 | 4.24 | 39.50 | 117.40 | 21.18 | 48.73 | 40.70 | 4.99 | 7.10 | 117.40 | 35.31 |
| IncidenceLung2021 * | 43.47 | 42.20 | 3.88 | 19.70 | 93.60 | 19.38 | 86.20 | 85.50 | 4.33 | 40.70 | 128.90 | 21.64 | 64.83 | 62.70 | 4.19 | 19.70 | 128.90 | 29.65 |
| MortalityLung1993 * | 16.70 | 13.60 | 1.71 | 7.20 | 46.50 | 8.55 | 75.06 | 73.90 | 3.43 | 41.30 | 115.00 | 17.17 | 45.88 | 42.55 | 4.58 | 7.20 | 115.00 | 32.39 |
| MortalityLung2021 * | 34.96 | 34.10 | 2.88 | 17.70 | 72.30 | 14.40 | 74.18 | 73.00 | 4.05 | 37.20 | 117.90 | 20.24 | 54.57 | 53.65 | 3.73 | 17.70 | 117.90 | 26.35 |
| IncidenceLeukaemia1993 * | 8.97 | 8.80 | 0.48 | 3.00 | 14.40 | 2.42 | 11.06 | 11.50 | 0.61 | 4.40 | 17.80 | 3.04 | 10.02 | 9.75 | 0.41 | 3.00 | 17.80 | 2.92 |
| IncidenceLeukaemia2021 * | 10.04 | 10.50 | 0.74 | 5.30 | 17.10 | 3.72 | 17.13 | 16.70 | 0.72 | 9.90 | 28.00 | 3.60 | 13.59 | 14.20 | 0.72 | 5.30 | 28.00 | 5.09 |
| MortalityLeukaemia1993 * | 6.25 | 6.30 | 0.29 | 3.40 | 8.70 | 1.47 | 8.02 | 8.10 | 0.31 | 5.00 | 10.80 | 1.53 | 7.14 | 7.10 | 0.25 | 3.40 | 10.80 | 1.73 |
| MortalityLeukaemia2021 * | 7.59 | 7.40 | 0.32 | 4.00 | 10.20 | 1.62 | 9.78 | 9.60 | 0.37 | 6.60 | 14.50 | 1.86 | 8.68 | 8.85 | 0.29 | 4.00 | 14.50 | 2.05 |
| IncidenceBrain1993 * | 10.29 | 6.60 | 1.21 | 3.40 | 23.00 | 6.07 | 11.40 | 9.20 | 1.25 | 4.60 | 27.80 | 6.23 | 10.84 | 9.20 | 0.86 | 3.40 | 27.80 | 6.11 |
| IncidenceBrain2021 * | 17.37 | 13.30 | 2.20 | 7.00 | 45.40 | 10.98 | 15.58 | 14.00 | 1.35 | 9.30 | 32.50 | 6.73 | 16.47 | 13.55 | 1.28 | 7.00 | 45.40 | 9.06 |
| MortalityBrain1993 * | 4.83 | 4.80 | 0.19 | 3.10 | 7.00 | 0.95 | 6.12 | 5.90 | 0.26 | 4.20 | 10.20 | 1.28 | 5.47 | 5.30 | 0.18 | 3.10 | 10.20 | 1.29 |
| MortalityBrain2021 * | 7.10 | 6.80 | 0.39 | 3.80 | 10.70 | 1.94 | 8.82 | 8.30 | 0.35 | 6.20 | 12.70 | 1.77 | 7.96 | 7.90 | 0.29 | 3.80 | 12.70 | 2.03 |
| COFOG1993 *** | 49.39 | 47.00 | 3.01 | 27.30 | 83.80 | 15.03 | 49.39 | 47.00 | 3.01 | 27.30 | 83.80 | 15.03 | 49.39 | 47.00 | 2.10 | 27.30 | 83.80 | 14.87 |
| COFOG2021 *** | 44.68 | 44.10 | 1.53 | 19.80 | 57.90 | 7.63 | 44.68 | 44.10 | 1.53 | 19.80 | 57.90 | 7.63 | 44.68 | 44.10 | 1.07 | 19.80 | 57.90 | 7.55 |
| PRCPPP1993 ** | 92.57 | 75.99 | 12.98 | 10.90 | 265.47 | 64.89 | 92.57 | 75.99 | 12.98 | 10.90 | 265.47 | 64.89 | 92.57 | 75.99 | 9.08 | 10.90 | 265.47 | 64.23 |
| PRCPPP2021 ** | 90.08 | 74.57 | 8.28 | 33.50 | 171.83 | 41.42 | 90.08 | 74.57 | 8.28 | 33.50 | 171.83 | 41.42 | 90.08 | 74.57 | 5.80 | 33.50 | 171.83 | 41.00 |
References
- World Health Organization Burden of Cancer. Available online: https://cdn.who.int/media/docs/default-source/ncds/ncd-surveillance/cancer-profiles-2020/euro-cancer-profile-2020.pdf?sfvrsn=6fbcb00e_3 (accessed on 15 January 2023).
- Cabasag, C.J.; Vignat, J.; Ferlay, J.; Arndt, V.; Lemmens, V.; Praagman, J.; Bray, F.; Soerjomataram, I. The Preventability of Cancer in Europe: A Quantitative Assessment of Avoidable Cancer Cases across 17 Cancer Sites and 38 Countries in 2020. Eur. J. Cancer 2022, 177, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Soerjomataram, I.; Bray, F. Planning for Tomorrow: Global Cancer Incidence and the Role of Prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Huang, J.; Chan, S.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno III, D.E.; Xu, W.; Zheng, Z.-J.; Elcarte, E.; Withers, M.; et al. Global Incidence, Mortality and Temporal Trends of Cancer in Children: A Joinpoint Regression Analysis. Cancer Med. 2022, 12, 1903–1911. [Google Scholar] [CrossRef]
- Dalmartello, M.; La Vecchia, C.; Bertuccio, P.; Boffetta, P.; Levi, F.; Negri, E.; Malvezzi, M. European Cancer Mortality Predictions for the Year 2022 with Focus on Ovarian Cancer. Ann. Oncol. 2022, 33, 330–339. [Google Scholar] [CrossRef]
- Global Burden of Disease 2019 Cancer Collaboration. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- Ward, Z.J.; Scott, A.M.; Hricak, H.; Atun, R. Global Costs, Health Benefits, and Economic Benefits of Scaling up Treatment and Imaging Modalities for Survival of 11 Cancers: A Simulation-Based Analysis. Lancet Oncol. 2021, 22, 341–350. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Luo, Q.; O’Connell, D.L.; Yu, X.Q.; Kahn, C.; Caruana, M.; Pesola, F.; Sasieni, P.; Grogan, P.B.; Aranda, S.; Cabasag, C.J.; et al. Cancer Incidence and Mortality in Australia from 2020 to 2044 and an Exploratory Analysis of the Potential Effect of Treatment Delays during the COVID-19 Pandemic: A Statistical Modelling Study. Lancet Public Health 2022, 7, e537–e548. [Google Scholar] [CrossRef]
- Changfa, X.; Xuesi, D.; He, L.; Maomao, C.; Dianqin, S.; Siyi, H.; Fan, Y.; Xinxin, Y.; Shaoli, Z.; Ni, L.; et al. Cancer Statistics in China and United States, 2022: Profiles, Trends, and Determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef]
- Tudor, C. A Novel Approach to Modeling and Forecasting Cancer Incidence and Mortality Rates through Web Queries and Automated Forecasting Algorithms: Evidence from Romania. Biology 2022, 11, 857. [Google Scholar] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Chenyu, L.; Na, L.; Bin, L.; Jie, C.; Ming, L.; Yuhan, Z.; Hongda, C.; Min, D.; Jing, N. Global and Regional Trends in Incidence and Mortality of Female Breast Cancer and Associated Factors at National Level in 2000 to 2019. Chin. Med. J. 2022, 135, 42–51. [Google Scholar] [CrossRef]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar]
- Huang, J.; Deng, Y.; Tin, M.S.; Lok, V.; Ngai, C.H.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.-J.; Elcarte, E.; et al. Distribution, Risk Factors, and Temporal Trends for Lung Cancer Incidence and Mortality: A Global Analysis. Chest 2022, 161, 1101–1111. [Google Scholar] [CrossRef]
- Sharma, R.; Aashima; Nanda, M.; Fronterre, C.; Sewagudde, P.; Ssentongo, A.E.; Yenney, K.; Arhin, N.D.; Oh, J.; Amponsah-Manu, F.; et al. Mapping Cancer in Africa: A Comprehensive and Comparable Characterization of 34 Cancer Types Using Estimates from GLOBOCAN 2020. Front. Public Health 2022, 10, 839835. [Google Scholar] [CrossRef]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European Cancer Burden in 2020: Incidence and Mortality Estimates for 40 Countries and 25 Major Cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef] [PubMed]
- Belot, A.; Pohar-Perme, M. Social Disparities in Cancer Survival: Methodological Considerations BT-Social Environment and Cancer in Europe: Towards an Evidence-Based Public Health Policy; Launoy, G., Zadnik, V., Coleman, M.P., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 39–54. ISBN 978-3-030-69329-9. [Google Scholar]
- Sofi-Mahmudi, A.; Masinaei, M.; Shamsoddin, E.; Tovani-Palone, M.R.; Heydari, M.-H.; Shoaee, S.; Ghasemi, E.; Azadnajafabad, S.; Roshani, S.; Rezaei, N.; et al. Global, Regional, and National Burden and Quality of Care Index (QCI) of Lip and Oral Cavity Cancer: A Systematic Analysis of the Global Burden of Disease Study 1990–2017. BMC Oral Health 2021, 21, 558. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.; Kabir, Z.; Harding, M. Lip, Oral Cavity and Pharyngeal Cancer Burden in the European Union from 1990–2019 Using the 2019 Global Burden of Disease Study. Int. J. Environ. Res. Public Health 2022, 19, 6532. [Google Scholar] [CrossRef] [PubMed]
- Damgacioglu, H.; Sonawane, K.; Zhu, Y.; Li, R.; Balasubramanian, B.A.; Lairson, D.R.; Giuliano, A.R.; Deshmukh, A.A. Oropharyngeal Cancer Incidence and Mortality Trends in All 50 States in the US, 2001–2017. JAMA Otolaryngol. Neck Surg. 2022, 148, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, F.; Zheng, Y.; Lin, Y.; Wang, H.-L. Worldwide Trend in Human Papillomavirus–Attributable Cancer Incidence Rates between 1990 and 2012 and Bayesian Projection to 2030. Cancer 2021, 127, 3172–3182. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; De Schutter, H.; Van Damme, N.; Valerianova, Z.; Atanasov, T.; Májek, O.; et al. Colorectal Cancer Incidence, Mortality, and Stage Distribution in European Countries in the Colorectal Cancer Screening Era: An International Population-Based Study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.; Lok, V.; Wang, J.; Fung, F.; Ding, H.; Zheng, Z.-J. Differences in Incidence and Mortality Trends of Colorectal Cancer Worldwide Based on Sex, Age, and Anatomic Location. Clin. Gastroenterol. Hepatol. 2021, 19, 955–966. [Google Scholar] [CrossRef]
- Akimoto, N.; Ugai, T.; Zhong, R.; Hamada, T.; Fujiyoshi, K.; Giannakis, M.; Wu, K.; Cao, Y.; Ng, K.; Ogino, S. Rising Incidence of Early-Onset Colorectal Cancer—A Call to Action. Nat. Rev. Clin. Oncol. 2021, 18, 230–243. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Ionescu, E.M.; Tieranu, C.G.; Maftei, D.; Grivei, A.; Olteanu, A.O.; Arbanas, T.; Calu, V.; Musat, S.; Mihaescu-Pintia, C.; Cucu, I.C. Colorectal Cancer Trends of 2018 in Romania—An Important Geographical Variation Between Northern and Southern Lands and High Mortality Versus European Averages. J. Gastrointest. Cancer 2021, 52, 222–228. [Google Scholar] [CrossRef]
- Henderson, R.H.; French, D.; Maughan, T.; Adams, R.; Allemani, C.; Minicozzi, P.; Coleman, M.P.; McFerran, E.; Sullivan, R.; Lawler, M. The Economic Burden of Colorectal Cancer across Europe: A Population-Based Cost-of-Illness Study. Lancet Gastroenterol. Hepatol. 2021, 6, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Carioli, G.; Malvezzi, M.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European Cancer Mortality Predictions for the Year 2021 with Focus on Pancreatic and Female Lung Cancer. Ann. Oncol. 2021, 32, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.-J.; Wong, M.C.S. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, N.; El-Serag, H.B.; Abrams, H.R.; Thrift, A.P. Burden of Pancreatic Cancer: From Epidemiology to Practice. Clin. Gastroenterol. Hepatol. 2021, 19, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, X.; He, W.; Ye, W. Burden of Pancreatic Cancer along with Attributable Risk Factors in Europe between 1990 and 2019, and Projections until 2039. Int. J. Cancer 2021, 149, 993–1001. [Google Scholar] [CrossRef]
- Aryannejad, A.; Tabary, M.; Ebrahimi, N.; Mohammadi, E.; Fattahi, N.; Roshani, S.; Masinaei, M.; Naderimagham, S.; Azadnajafabad, S.; Jamshidi, K.; et al. Global, Regional, and National Survey on the Burden and Quality of Care of Pancreatic Cancer: A Systematic Analysis for the Global Burden of Disease Study 1990–2017. Pancreatology 2021, 21, 1443–1450. [Google Scholar] [CrossRef]
- Tonini, V.; Zanni, M. Pancreatic Cancer in 2021: What You Need to Know to Win. World J. Gastroenterol. 2021, 27, 5851–5889. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, L.; He, X.; Luo, Y. Gastrointestinal Cancers in China, the USA, and Europe. Gastroenterol. Rep. 2021, 9, 91–104. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, M.J.; Yun, E.H.; Jung, K.-W. Epidemiology of Gastric Cancer in Korea: Trends in Incidence and Survival Based on Korea Central Cancer Registry Data (1999–2019). J. Gastric Cancer 2022, 22, 160–168. [Google Scholar] [CrossRef]
- Oudkerk, M.; Liu, S.; Heuvelmans, M.A.; Walter, J.E.; Field, J.K. Lung Cancer LDCT Screening and Mortality Reduction—Evidence, Pitfalls and Future Perspectives. Nat. Rev. Clin. Oncol. 2021, 18, 135–151. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Severi, G.; Andersen, Z.J.; Atkinson, R.; Bauwelinck, M.; Bellander, T.; Boutron-Ruault, M.-C.; Brandt, J.; Brunekreef, B.; Cesaroni, G.; et al. Long-Term Low-Level Ambient Air Pollution Exposure and Risk of Lung Cancer—A Pooled Analysis of 7 European Cohorts. Environ. Int. 2021, 146, 106249. [Google Scholar] [CrossRef] [PubMed]
- Hvidtfeldt, U.A.; Chen, J.; Andersen, Z.J.; Atkinson, R.; Bauwelinck, M.; Bellander, T.; Brandt, J.; Brunekreef, B.; Cesaroni, G.; Concin, H.; et al. Long-Term Exposure to Fine Particle Elemental Components and Lung Cancer Incidence in the ELAPSE Pooled Cohort. Environ. Res. 2021, 193, 110568. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.M.; Bibeau, F.; Thunnissen, E.; Botling, J.; Ryška, A.; Wolf, J.; Öhrling, K.; Burdon, P.; Malapelle, U.; Büttner, R. The Evolving Landscape of Biomarker Testing for Non-Small Cell Lung Cancer in Europe. Lung Cancer 2021, 154, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Man, J.; Chen, H.; Zhang, T.; Yin, X.; He, Q.; Lu, M. Temporal Trends of the Lung Cancer Mortality Attributable to Smoking from 1990 to 2017: A Global, Regional and National Analysis. Lung Cancer 2021, 152, 49–57. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, X.; Li, F.; Jin, J.; Wang, H. The Global Burden and Attributable Risk Factors of Chronic Lymphocytic Leukemia in 204 Countries and Territories from 1990 to 2019: Analysis Based on the Global Burden of Disease Study 2019. Biomed. Eng. Online 2022, 21, 4. [Google Scholar] [CrossRef]
- Ou, Y.; Long, Y.; Ji, L.; Zhan, Y.; Qiao, T.; Wang, X.; Chen, H.; Cheng, Y. Trends in Disease Burden of Chronic Lymphocytic Leukemia at the Global, Regional, and National Levels From 1990 to 2019, and Projections Until 2030: A Population-Based Epidemiologic Study. Front. Oncol. 2022, 12, 840616. [Google Scholar] [CrossRef]
- Huang, J.; Chan, S.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.-J.; Elcarte, E.; Withers, M.; et al. Disease Burden, Risk Factors, and Trends of Leukaemia: A Global Analysis. Front. Oncol. 2022, 12, 904292. [Google Scholar] [CrossRef]
- Kósa, F.; Nečasová, T.; Špaček, M.; Giannopoulos, K.; Hus, I.; Jurková, T.; Koriťáková, E.; Chrápavá, M.; Nováčková, M.; Katinová, I.; et al. Secondary Malignancies and Survival of FCR-Treated Patients with Chronic Lymphocytic Leukemia in Central Europe. Cancer Med. 2022, 12, 1961–1971. [Google Scholar] [CrossRef]
- Sekeroglu, B.; Tuncal, K. Prediction of Cancer Incidence Rates for the European Continent Using Machine Learning Models. Health Inform. J. 2021, 27, 1460458220983878. [Google Scholar] [CrossRef]
- Iragorri, N.; de Oliveira, C.; Fitzgerald, N.; Essue, B. The Out-of-Pocket Cost Burden of Cancer Care—A Systematic Literature Review. Curr. Oncol. 2021, 28, 1216–1248. [Google Scholar] [CrossRef]
- Fitch, M.I.; Longo, C.J.; Chan, R.J. Cancer Patients’ Perspectives on Financial Burden in a Universal Healthcare System: Analysis of Qualitative Data from Participants from 20 Provincial Cancer Centers in Canada. Patient Educ. Couns. 2021, 104, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, M.; Gucciardi, G.; Rizzo, L. Savings from Public Procurement Centralization in the Healthcare System. Eur. J. Political Econ. 2021, 66, 101963. [Google Scholar] [CrossRef]
- Fundytus, A.; Sengar, M.; Lombe, D.; Hopman, W.; Jalink, M.; Gyawali, B.; Trapani, D.; Roitberg, F.; De Vries, E.G.E.; Moja, L.; et al. Access to Cancer Medicines Deemed Essential by Oncologists in 82 Countries: An International, Cross-Sectional Survey. Lancet Oncol. 2021, 22, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Godman, B.; Hill, A.; Simoens, S.; Selke, G.; Selke Krulichová, I.; Zampirolli Dias, C.; Martin, A.P.; Oortwijn, W.; Timoney, A.; Gustafsson, L.L.; et al. Potential Approaches for the Pricing of Cancer Medicines across Europe to Enhance the Sustainability of Healthcare Systems and the Implications. Expert Rev. Pharmacoecon. Outcomes Res. 2021, 21, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Gultekin, M.; Morice, P.; Nieminen, P.; Cruickshank, M.; Poortmans, P.; Kelly, D.; Poljak, M.; Bergeron, C.; Ritchie, D.; et al. The European Response to the WHO Call to Eliminate Cervical Cancer as a Public Health Problem. Int. J. Cancer 2021, 148, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.; Mayer, D.K.; Fielding, R.; Eicher, M.; Verdonck-de Leeuw, I.M.; Johansen, C.; Soto-Perez-de-Celis, E.; Foster, C.; Chan, R.; Alfano, C.M.; et al. Management of Cancer and Health After the Clinic Visit: A Call to Action for Self-Management in Cancer Care. JNCI J. Natl. Cancer Inst. 2021, 113, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.; Balachandrakumar, V.K.; Driver, R.J.; Tataru, D.; Paley, L.; Marshall, A.; Alexander, G.; Rowe, I.A.; Palmer, D.H.; Cross, T.J.S.; et al. Regional Variations in Hepatocellular Carcinoma Incidence, Routes to Diagnosis, Treatment and Survival in England. Br. J. Cancer 2022, 126, 804–814. [Google Scholar] [CrossRef]
- Calthorpe, L.; Romero-Hernandez, F.; Miller, P.; Conroy, P.C.; Hirose, K.; Kim, A.; Kirkwood, K.; Nakakura, E.; Corvera, C.; Maker, A.V.; et al. Contemporary Trends in Malignant Peritoneal Mesothelioma: Incidence and Survival in the United States. Cancers 2023, 15, 229. [Google Scholar] [CrossRef]
- Malagón, T.; Yong, J.H.E.; Tope, P.; Miller, W.H., Jr.; Franco, E.L.; Care, M.T.F.; McGill Task Force on the Impact of COVID-19 on Cancer Control and Care. Predicted Long-Term Impact of COVID-19 Pandemic-Related Care Delays on Cancer Mortality in Canada. Int. J. Cancer 2022, 150, 1244–1254. [Google Scholar] [CrossRef]
- Mao, J.J.; Pillai, G.G.; Andrade, C.J.; Ligibel, J.A.; Basu, P.; Cohen, L.; Khan, I.A.; Mustian, K.M.; Puthiyedath, R.; Dhiman, K.S.; et al. Integrative Oncology: Addressing the Global Challenges of Cancer Prevention and Treatment. CA Cancer J. Clin. 2022, 72, 144–164. [Google Scholar] [CrossRef]
- Ribeiro, M.F.A.; Oliveira, M.C.M.; Leite, A.C.; Bruzinga, F.F.B.; Mendes, P.A.; Grossmann, S.D.M.C.; de Araújo, V.E.; Souto, G.R. Assessment of Screening Programs as a Strategy for Early Detection of Oral Cancer: A Systematic Review. Oral Oncol. 2022, 130, 105936. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.; Goel, A.K.; Oberoi, S.; Jain, S.; Singh, D.; Kapoor, R. Impact of Demographic Factors on Delayed Presentation of Oral Cancers: A Questionnaire-Based Cross-Sectional Study from a Rural Cancer Center. Cancer Res. Stat. Treat. 2022, 5, 45–51. [Google Scholar]
- Tranby, E.P.; Heaton, L.J.; Tomar, S.L.; Kelly, A.L.; Fager, G.L.; Backley, M.; Frantsve-Hawley, J. Oral Cancer Prevalence, Mortality, and Costs in Medicaid and Commercial Insurance Claims Data. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Shang, Z.-J. Oral Cancer Incidence, Mortality, and Mortality-to-Incidence Ratio Are Associated with Human Development Index in China, 1990–2019. BioMed Res. Int. 2022, 2022, 6457840. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, F.; Zhao, G.; Zhang, X.; Zhang, X.; Li, T.; Hu, C.; Zhu, W.; Li, D. Trends in the Global Burden of Oral Cancer Joint with Attributable Risk Factors: Results from the Global Burden of Disease Study 2019. Oral Oncol. 2022, 134, 106189. [Google Scholar] [CrossRef]
- Zorzi, M.; Urso, E.D.L. Impact of Colorectal Cancer Screening on Incidence, Mortality and Surgery Rates: Evidences from Programs Based on the Fecal Immunochemical Test in Italy. Dig. Liver Dis. 2023, 55, 336–341. [Google Scholar] [CrossRef]
- Gurba, A.; Taciak, P.; Sacharczuk, M.; Młynarczuk-Biały, I.; Bujalska-Zadrożny, M.; Fichna, J. Gold (III) Derivatives in Colon Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 724. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic Colorectal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Yu, K.-X.; Yuan, W.-J.; Huang, C.-H.; Xiao, L.; Xiao, R.-S.; Zeng, P.-W.; Chen, L.; Chen, Z.-H. Socioeconomic Deprivation and Survival Outcomes in Patients with Colorectal Cancer. Am. J. Cancer Res. 2022, 12, 829–838. [Google Scholar]
- Smits, L.J.H.; Vink-Börger, E.; van Lijnschoten, G.; Focke-Snieders, I.; van der Post, R.S.; Tuynman, J.B.; van Grieken, N.C.T.; Nagtegaal, I.D. Diagnostic Variability in the Histopathological Assessment of Advanced Colorectal Adenomas and Early Colorectal Cancer in a Screening Population. Histopathology 2022, 80, 790–798. [Google Scholar] [CrossRef]
- Andersson, R.; Haglund, C.; Seppänen, H.; Ansari, D. Pancreatic Cancer—The Past, the Present, and the Future. Scand. J. Gastroenterol. 2022, 57, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Ettrich, T.J.; Sturm, N.; Güthle, M.; Hüttner, F.J.; Perkhofer, L. Pancreatic Cancer: Current Multimodality Treatment Options and the Future Impact of Molecular Biological Profiling. Visc. Med. 2022, 38, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Coll-Ortega, C.; Prades, J.; Manchón-Walsh, P.; Borras, J.M. Centralisation of Surgery for Complex Cancer Diseases: A Scoping Review of the Evidence Base on Pancreatic Cancer. J. Cancer Policy 2022, 32, 100334. [Google Scholar] [CrossRef]
- Hsu, D.S.; Kumar, N.S.; Le, S.T.; Chang, A.L.; Kazantsev, G.; Spitzer, A.L.; Peng, P.D.; Chang, C.-K. Centralization of Pancreatic Cancer Treatment within an Integrated Healthcare System Improves Overall Survival. Am. J. Surg. 2022, 223, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, A.; Berlin, J.; Chari, S.; Kindler, H.; Matrisian, L.; Mayoral, A.; Mills, J.; Nissen, N.; Picozzi, V.; Zelada-Arenas, F.; et al. Management of Patients with Pancreatic Cancer Using the “Right Track” Model. Oncologist 2023, oyad080. [Google Scholar] [CrossRef]
- Emmerick, I.C.M.; Uy, K.; Guiab, K.; Powers, M.; Lou, F.; Lin, P.; Maxfield, M.; Voland, R.; Varlotto, J. Impact of the National Lung Screening Trial (NLST) Publication and Medicare Lung Cancer Screening Payment on Lung Cancer Incidence Rates: An Interrupted Time Series Analysis. J. Cancer Policy 2022, 31, 100318. [Google Scholar] [CrossRef]
- Zhou, B.; Zang, R.; Zhang, M.; Song, P.; Liu, L.; Bie, F.; Peng, Y.; Bai, G.; Gao, S. Worldwide Burden and Epidemiological Trends of Tracheal, Bronchus, and Lung Cancer: A Population-Based Study. eBioMedicine 2022, 78, 103951. [Google Scholar] [CrossRef]
- Xu, K.; Mei, R.; Liang, L.; Sun, W. Regional Convergence Analysis of Sustainable Innovation Efficiency in European Union Countries. J. Environ. Manag. 2023, 325, 116636. [Google Scholar] [CrossRef]
- Frost, N.; Unger, K.; Blum, T.G.; Misch, D.; Kurz, S.; Lüders, H.; Olive, E.; Raspe, M.; Hilbrandt, M.; Koch, M.; et al. Management, Risk Factors and Prognostic Impact of Checkpoint-Inhibitor Pneumonitis (CIP) in Lung Cancer—A Multicenter Observational Analysis. Lung Cancer 2023, 179, 107184. [Google Scholar] [CrossRef]
- Xiao, H.; Shi, Z.; Zou, Y.; Xu, K.; Yu, X.; Wen, L.; Liu, Y.; Chen, H.; Long, H.; Chen, J.; et al. One-off Low-Dose CT Screening of Positive Nodules in Lung Cancer: A Prospective Community-Based Cohort Study. Lung Cancer 2023, 177, 1–10. [Google Scholar] [CrossRef]
- Howlader, N.; Sharon, E.; Bhattacharya, M.; Ehrlich, L.A.; Richardson, N.C.; Gormley, N.J.; de Claro, R.A.; Wood, A.E.; Mariotto, A.B.; Cronin, K.A. The Impact of Improved Treatments on Survival of Adult US Leukemia Patients: 1990–2018. Cancer Epidemiol. Biomarkers Prev. 1171. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The Trends Projection Analysis. Chem. Biol. Lett. 2022, 10, 451. [Google Scholar]
- Du, M.; Chen, W.; Liu, K.; Wang, L.; Hu, Y.; Mao, Y.; Sun, X.; Luo, Y.; Shi, J.; Shao, K.; et al. The Global Burden of Leukemia and Its Attributable Factors in 204 Countries and Territories: Findings from the Global Burden of Disease 2019 Study and Projections to 2030. J. Oncol. 2022, 2022, 1612702. [Google Scholar] [CrossRef]
- Atsou, K.M.; Rachet, B.; Cornet, E.; Chretien, M.-L.; Rossi, C.; Remontet, L.; Roche, L.; Giorgi, R.; Gauthier, S.; Girard, S.; et al. Factors Influencing Access to Specialised Haematology Units during Acute Myeloblastic Leukaemia Patient Care: A Population-Based Study in France. Cancer Med. [CrossRef]
- Xiang, D.; Hu, S.; Mai, T.; Zhang, X.; Zhang, L.; Wang, S.; Jin, K.; Huang, J. Worldwide Cancer Statistics of Adults over 75 Years Old in 2019: A Systematic Analysis of the Global Burden of Disease Study 2019. BMC Public Health 2022, 22, 1979. [Google Scholar] [CrossRef]
- Yao, Y.; Ji, P.; Chen, H.; Ge, J.; Xu, Y.; Wang, P.; Xu, L.; Yan, Z. Ferroptosis-Based Drug Delivery System as a New Therapeutic Opportunity for Brain Tumors. Front. Oncol. 2023, 13, 1084289. [Google Scholar] [CrossRef] [PubMed]
- Thierheimer, M.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Ostrom, Q.T.; Barnholtz-Sloan, J.S. Mortality Trends in Primary Malignant Brain and Central Nervous System Tumors Vary by Histopathology, Age, Race, and Sex. J. Neurooncol. 2023, 162, 167–177. [Google Scholar] [CrossRef]
- Cioffi, G.; Waite, K.A.; Edelson, J.L.; Kruchko, C.; Ostrom, Q.T.; Barnholtz-Sloan, J.S. Changes in Survival over Time for Primary Brain and Other CNS Tumors in the United States, 2004–2017. J. Neurooncol. 2022, 160, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Che, W.; Liu, J.; Fu, T.; Wang, X.; Lyu, J. Recent Trends in Synchronous Brain Metastasis Incidence and Mortality in the United States: Ten-Year Multicenter Experience. Curr. Oncol. 2022, 29, 8374–8389. [Google Scholar] [CrossRef]
- Chieffo, D.P.; Lino, F.; Ferrarese, D.; Belella, D.; Della Pepa, G.M.; Doglietto, F. Brain Tumor at Diagnosis: From Cognition and Behavior to Quality of Life. Diagnostics 2023, 13, 541. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Disease Surveillance, Monitoring and Reporting. Available online: https://www.who.int/teams/noncommunicable-diseases/surveillance/data/cancer-profiles (accessed on 15 January 2023).
- World Health Organization Age-Standardized Rate (World) per 100 000, Mortality, Males and Females. Available online: https://gco.iarc.fr/overtime/en/dataviz/trends?populations=75200_10000&sexes=1_2&types=1&multiple_populations=1 (accessed on 15 January 2023).
- World Health Organization. Crude Rate per 100 000, Incidence and Mortality, Males and Females. Available online: https://gco.iarc.fr/overtime/en/dataviz/trends?populations=4000&sexes=1_2&types=0_1&multiple_populations=1&mode=cancer&group_populations=1&multiple_cancers=1&cancers=1&key=crude_rate (accessed on 16 January 2023).
- Eurostat General Government Expenditure by Function (COFOG). Available online: https://ec.europa.eu/eurostat/databrowser/view/gov_10a_exp/default/table?lang=en (accessed on 16 January 2023).
- World Bank. GDP per Capita (Current US$)—European Union. Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=EU-AT (accessed on 16 January 2023).
- Duran-Romero, A.J.; Infante-Cossio, P.; Pereyra-Rodriguez, J.-J. Trends in Mortality Rates for Oral and Oropharyngeal Cancer in Spain, 1979–2018. Oral Dis. 2022, 28, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Mapping of Global, Regional and National Incidence, Mortality and Mortality-to-Incidence Ratio of Lung Cancer in 2020 and 2050. Int. J. Clin. Oncol. 2022, 27, 665–675. [Google Scholar] [CrossRef]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and Other Central Nervous System Tumor Statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]





| Type of Cancer | Type of Indicator | Unit Measure | Symbol | |
|---|---|---|---|---|
| Lip, oral cavity, and pharyngeal | Incidence | Crude rate per 100,000, incidence, males and females | IncidenceLip | |
| Mortality | Crude rate per 100,000, incidence, males and females | MortalityLip | ||
| Colon | Incidence | Crude rate per 100,000, incidence, males and females | IncidenceColon | |
| Mortality | Crude rate per 100,000, incidence, males and females | MortalityColon | ||
| Pancreatic | Incidence | Crude rate per 100,000, incidence, males and females | IncidencePancreas | |
| Mortality | Crude rate per 100,000, incidence, males and females | MortalityPancreas | ||
| Lung | Incidence | Crude rate per 100,000, incidence, males and females | IncidenceLung | |
| Mortality | Crude rate per 100,000, incidence, males and females | MortalityLung | ||
| Leukaemia | Incidence | Crude rate per 100,000, incidence, males and females | IncidenceLeukaemia | |
| Mortality | Crude rate per 100,000, incidence, males and females | MortalityLeukaemia | ||
| Brain and central nervous system | Incidence | Crude rate per 100,000, incidence, males and females | IncidenceBrain | |
| Mortality | Crude rate per 100,000, incidence, males and females | MortalityBrain | ||
| General government expenditure by function (COFOG) | Percentage of gross domestic product (GDP) | COFOG | ||
| Purchasing power parity (PPP), price level index, and real expenditures | Nominal expenditure per inhabitant (in euro) | PRCPPP 1 | ||
| Unstandardised Coefficients | (Constant) | COFOG | Lip | Colon | Pancreatic | Lung | Leukaemia | Brain | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Gender | Incidence | Morta lity | Incidence | Morta lity | Incidence | Morta lity | Incidence | Morta lity | Incidence | Morta lity | Incidence | Morta lity | ||
| 1993 | male | −86.040 | 1.136 | 3.080 | −8.770 | 2.225 | −5.907 | −9.906 | 21.875 | −0.353 | 0.799 | 12.417 | −10.003 | 4.281 | −15.500 |
| female | 19.140 | 0.344 | 4.062 | −35.331 | 3.112 | −2.769 | −8.988 | 16.703 | −0.880 | 0.418 | 6.595 | −5.806 | 3.961 | −13.540 | |
| 1994 | male | −124.771 | 1.298 | 3.822 | −8.542 | 4.951 | −9.119 | −9.143 | 23.787 | 0.328 | 0.224 | 0.528 | −7.791 | 6.543 | −8.244 |
| female | −82.802 | 1.892 | 18.590 | −51.174 | 2.413 | −4.751 | −8.479 | 16.526 | −1.437 | 1.233 | 7.505 | −1.428 | 3.110 | −9.641 | |
| 1995 | male | −81.949 | 1.985 | 3.202 | −5.972 | 4.783 | −9.010 | −10.265 | 17.292 | 0.556 | −0.592 | 1.839 | 0.461 | 5.239 | −11.036 |
| female | −99.790 | 1.558 | 16.638 | −33.630 | 4.614 | −7.073 | −3.490 | 12.744 | −4.978 | 4.101 | 8.809 | −2.439 | 3.628 | −14.714 | |
| 1996 | male | 9.618 | 0.872 | 1.758 | −5.059 | 5.700 | −5.734 | −12.389 | 18.874 | −0.071 | 0.141 | −0.988 | −12.363 | 9.045 | −11.853 |
| female | −56.473 | 1.117 | 22.647 | −31.317 | 3.720 | −6.850 | −3.545 | 8.440 | −4.148 | 4.241 | 5.513 | −0.585 | 2.932 | −10.783 | |
| 1997 | male | −94.246 | 1.954 | 3.120 | −6.945 | 4.508 | −4.683 | −8.745 | 20.708 | 1.269 | −0.797 | −0.188 | −20.494 | 9.530 | −12.183 |
| female | −96.660 | 1.278 | 9.078 | −33.229 | 2.126 | −0.451 | −1.749 | 11.208 | −3.755 | 2.632 | 13.506 | −21.721 | 5.097 | −3.399 | |
| 1998 | male | −32.252 | 0.561 | 2.169 | −7.243 | 5.182 | −5.865 | −13.616 | 22.988 | −1.599 | 1.944 | −1.483 | −5.422 | 3.685 | −6.985 |
| female | −37.969 | 0.557 | −7.175 | −10.224 | 2.163 | 1.081 | −11.111 | 19.389 | −0.563 | 0.514 | 6.965 | −11.517 | 3.245 | −6.156 | |
| 1999 | male | −5.952 | 1.129 | 0.715 | −4.426 | 1.680 | −1.093 | −11.828 | 17.892 | 0.558 | −1.087 | 3.525 | −0.223 | 3.986 | −12.779 |
| female | −26.719 | 1.748 | −1.343 | −9.035 | 2.177 | −1.362 | −9.978 | 20.515 | 2.171 | −3.352 | 5.048 | −10.480 | 3.825 | −20.287 | |
| 2000 | male | −29.915 | 0.923 | 2.455 | −7.747 | 1.563 | −1.043 | −8.303 | 20.630 | −0.607 | 1.014 | 7.804 | −17.363 | 3.555 | −14.564 |
| female | −39.605 | 1.847 | 12.567 | −41.681 | 2.746 | −5.276 | −4.936 | 13.145 | −0.855 | 1.912 | 4.270 | −2.180 | 2.893 | −18.859 | |
| 2001 | male | −38.963 | 1.855 | 1.018 | −3.271 | −1.300 | 1.041 | −12.073 | 20.943 | 1.592 | −1.820 | 12.951 | −8.922 | 1.660 | −19.124 |
| female | 18.507 | 1.338 | 16.250 | −34.572 | 3.576 | −6.774 | −0.777 | 5.655 | −1.210 | 2.698 | −0.557 | 1.324 | 4.074 | −23.876 | |
| 2002 | male | 19.396 | 1.549 | 0.836 | −3.610 | 1.166 | −2.129 | −5.670 | 11.174 | 0.743 | −1.298 | 3.321 | 1.438 | 3.962 | −16.634 |
| female | −16.541 | 1.303 | −5.946 | −7.025 | 1.714 | −1.249 | −9.222 | 15.499 | 3.788 | −3.499 | 2.034 | 0.132 | 3.120 | −15.125 | |
| 2003 | male | −36.198 | 1.661 | 1.269 | −4.213 | 1.303 | −2.315 | −11.210 | 21.755 | 0.093 | 0.007 | 6.853 | −11.102 | 4.360 | −17.865 |
| female | −49.031 | 2.062 | 8.035 | −30.333 | −0.815 | 0.901 | −2.931 | 9.645 | 2.487 | −1.995 | 6.264 | −4.302 | 3.934 | −17.503 | |
| 2004 | male | −116.733 | 2.246 | 1.445 | −3.340 | 0.876 | −0.927 | −4.320 | 12.592 | 0.728 | −1.632 | 4.920 | −6.637 | 1.442 | 1.171 |
| female | −7.967 | 1.699 | 5.265 | −23.117 | 0.487 | −1.518 | −7.087 | 12.579 | 0.968 | −0.703 | 4.178 | 1.804 | 2.561 | −18.420 | |
| 2005 | male | 42.673 | 1.852 | −0.747 | −1.919 | 0.545 | −1.705 | −5.665 | 10.510 | 1.162 | −2.156 | 8.148 | −2.974 | 0.582 | −12.209 |
| female | 104.668 | 0.320 | 7.684 | −29.843 | 1.935 | −3.953 | −4.446 | 6.146 | 1.143 | 0.608 | −4.073 | 6.664 | 1.383 | −14.167 | |
| 2006 | male | 3.442 | 1.680 | 0.197 | −2.906 | 0.275 | −0.783 | −6.154 | 11.322 | 0.831 | −1.545 | 6.360 | −2.124 | 1.879 | −10.043 |
| female | 116.613 | −0.145 | 16.017 | −29.256 | 0.054 | −5.638 | 0.757 | 1.945 | −1.081 | 3.344 | 1.585 | 4.492 | 2.825 | −17.164 | |
| 2007 | male | −5.330 | 2.288 | 3.011 | −3.088 | 2.924 | −8.204 | −8.741 | 11.896 | −0.260 | 0.814 | −2.529 | −0.351 | 6.771 | −13.588 |
| female | 43.679 | 0.768 | 12.990 | −13.262 | 1.518 | −6.431 | −5.110 | 8.034 | −0.780 | 1.245 | 0.191 | 6.034 | 2.190 | −13.623 | |
| 2008 | male | −81.044 | 2.888 | 1.465 | −3.861 | 1.118 | −3.415 | −5.868 | 13.455 | 0.656 | −0.273 | 6.476 | −16.566 | 3.226 | −7.560 |
| female | 66.250 | 0.739 | 8.846 | −23.246 | 1.930 | −6.707 | −3.930 | 5.942 | −1.588 | 2.587 | 2.009 | 7.477 | 3.394 | −17.845 | |
| 2009 | male | 19.698 | 1.888 | 1.396 | −3.147 | 1.468 | −4.220 | −4.620 | 9.338 | −0.041 | −0.033 | 3.104 | −4.412 | 2.086 | −10.465 |
| female | 82.513 | 0.026 | 3.904 | −50.314 | −0.765 | 2.024 | −0.414 | 8.013 | −0.310 | 1.906 | 4.430 | 0.149 | 3.532 | −22.820 | |
| 2010 | male | 56.652 | 0.510 | 1.419 | −4.605 | 1.471 | −3.714 | −6.695 | 10.964 | 0.457 | −0.530 | 1.867 | 5.245 | 3.829 | −17.403 |
| female | 25.205 | 2.198 | 3.593 | −21.986 | −2.084 | 1.169 | −3.381 | 8.552 | 3.584 | −3.307 | 2.398 | −3.721 | 0.798 | −10.002 | |
| 2011 | male | 51.611 | 2.969 | −1.674 | −1.844 | −0.111 | 1.111 | −1.123 | 1.990 | 1.907 | −3.392 | 4.723 | −2.753 | 0.090 | −4.840 |
| female | −42.105 | 2.831 | 7.311 | −11.801 | 0.679 | −3.300 | −2.632 | 4.691 | 0.091 | 0.539 | 3.080 | −4.113 | 0.970 | −6.995 | |
| 2012 | male | 48.878 | 3.586 | −1.720 | −2.030 | −0.744 | 1.655 | −2.094 | 4.542 | 1.643 | −2.893 | 3.161 | −2.328 | −0.199 | −7.405 |
| female | 54.287 | 2.613 | 0.614 | −23.699 | −1.136 | 0.132 | −3.943 | 7.208 | 3.670 | −3.014 | 2.157 | −5.702 | 0.590 | −10.592 | |
| 2013 | male | 70.774 | 1.185 | 1.591 | −3.096 | 1.231 | −3.246 | −1.247 | 3.379 | −0.611 | 0.286 | 2.614 | −2.551 | 1.445 | −7.872 |
| female | 96.762 | 0.582 | 9.179 | −40.160 | 0.384 | −2.880 | 1.487 | −0.780 | −1.027 | 2.897 | 2.821 | 3.826 | 0.901 | −12.936 | |
| 2014 | male | 93.700 | 2.509 | −0.177 | −3.681 | −0.674 | 1.009 | 4.355 | −2.388 | 0.167 | −0.759 | 4.871 | −8.760 | 0.258 | −6.890 |
| female | 45.717 | 2.882 | 2.639 | −9.473 | 0.107 | −4.089 | −3.350 | 2.326 | 2.789 | −2.306 | 3.025 | −0.865 | 0.269 | −9.693 | |
| 2015 | male | 113.616 | 1.537 | −0.038 | −2.571 | 1.122 | −2.813 | −1.202 | 0.852 | −0.196 | −0.749 | 2.187 | 3.739 | 0.845 | −7.351 |
| female | 79.077 | 1.045 | 4.542 | −18.028 | 0.598 | −5.639 | −0.582 | 2.338 | −0.191 | 1.444 | 3.393 | 1.661 | 1.095 | −10.155 | |
| 2016 | male | 19.049 | 2.854 | −1.088 | −1.149 | 0.785 | −1.216 | −1.027 | 2.351 | −0.240 | −1.325 | 4.311 | 0.792 | −0.569 | −0.994 |
| female | 82.521 | 1.354 | 4.609 | −28.109 | 0.362 | −3.424 | −0.852 | 2.799 | 0.447 | 0.824 | 2.745 | −0.917 | 1.135 | −10.526 | |
| 2017 | male | 120.242 | 1.713 | −0.020 | −1.248 | 1.306 | −3.504 | −2.945 | 1.190 | 0.325 | −1.058 | 1.403 | 5.343 | 1.721 | −11.735 |
| female | 126.934 | 0.748 | 4.403 | −26.365 | −0.169 | −0.279 | −2.484 | 5.123 | 1.253 | −0.745 | 3.002 | −5.392 | 1.610 | −15.938 | |
| 2018 | male | 147.363 | 0.796 | 0.838 | −1.763 | 0.757 | −2.592 | −2.690 | 2.310 | 0.240 | −0.906 | −1.058 | 7.665 | 2.015 | −12.197 |
| female | 117.359 | 0.041 | 4.355 | −25.694 | −0.225 | −2.066 | 0.505 | 2.524 | 0.435 | 0.649 | 3.003 | −1.844 | 1.371 | −10.418 | |
| 2019 | male | 137.066 | 0.985 | −0.011 | −1.879 | 0.127 | 0.095 | −1.506 | 1.125 | 0.636 | −1.727 | 1.681 | 3.233 | 0.881 | −8.689 |
| female | 96.788 | 0.583 | 5.191 | −4.548 | −0.075 | −3.179 | −0.931 | 0.366 | 1.125 | −0.455 | 5.054 | −4.998 | 0.698 | −7.639 | |
| 2020 | male | 117.468 | 1.161 | 0.853 | −1.677 | 1.035 | −3.135 | −1.438 | 0.934 | 0.207 | −0.527 | 0.682 | 0.023 | 1.153 | −7.555 |
| female | 84.225 | 1.569 | 0.009 | −6.108 | 0.967 | −4.732 | −2.706 | 2.450 | 1.186 | −0.788 | 2.742 | −0.025 | 0.727 | −9.642 | |
| 2021 | male | 122.991 | 1.457 | 0.381 | −1.132 | 1.034 | −2.947 | −1.925 | 0.056 | 0.271 | −0.793 | −0.070 | 2.973 | 1.191 | −6.258 |
| female | 105.850 | 1.439 | −0.682 | −6.697 | 1.212 | −4.981 | −2.690 | 2.016 | 1.014 | −0.633 | 1.872 | 2.332 | 0.727 | −10.126 | |
| ANOVA | Female | Male | Compared Models Male vs. Female | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F | Sig. | Sum of Squares | df | Mean Square | F | Sig. | Sum of Squares (M/F%) | F (M/F%) | H0 Male (a < 0.01) | H0 Female (a < 0.01) | ||
| 1993 | Regression | 82,959.652 | 13 | 6381.5117 | 3.8758706 | 0.015 | 87,628.094 | 13 | 6740.6226 | 5.5157508 | 0.004 | 105.63% | 142.31% | R.NHyp 2 | |
| Residual | 18,111.19 | 11 | 1646.4718 | 13,442.748 | 11 | 1222.068 | 74.22% | ||||||||
| 1994 | Regression | 85,331.736 | 13 | 6563.9797 | 7.0726407 | 0.001 | 79,472.486 | 13 | 6113.2682 | 4.1850501 | 0.012 | 93.13% | 59.17% | R.NHyp | |
| Residual | 10,208.885 | 11 | 928.08046 | 16,068.135 | 11 | 1460.7396 | 157.39% | ||||||||
| 1995 | Regression | 78,623.689 | 13 | 6047.9761 | 5.5389873 | 0.004 | 70,936.577 | 13 | 5456.6598 | 3.0471869 | 0.036 | 90.22% | 55.01% | R.NHyp | |
| Residual | 12,010.812 | 11 | 1091.892 | 19,697.925 | 11 | 1790.7204 | 164.00% | ||||||||
| 1996 | Regression | 77,477.752 | 13 | 5959.8271 | 7.4608524 | 0.001 | 77,365.488 | 13 | 5951.1914 | 7.3560596 | 0.001 | 99.86% | 98.60% | R.NHyp | R.NHyp |
| Residual | 8786.9447 | 11 | 798.81315 | 8899.2081 | 11 | 809.01892 | 101.28% | ||||||||
| 1997 | Regression | 66,849.014 | 13 | 5142.2319 | 3.6458185 | 0.019 | 70,363.701 | 13 | 5412.5924 | 4.9614504 | 0.006 | 105.26% | 136.09% | R.NHyp | |
| Residual | 15,514.911 | 11 | 1410.4465 | 12,000.224 | 11 | 1090.9295 | 77.35% | ||||||||
| 1998 | Regression | 65,278.451 | 13 | 5021.4193 | 4.0623023 | 0.013 | 68,922.472 | 13 | 5301.7286 | 5.8593821 | 0.003 | 105.58% | 144.24% | R.NHyp | |
| Residual | 13,597.12 | 11 | 1236.1018 | 9953.0997 | 11 | 904.82725 | 73.20% | ||||||||
| 1999 | Regression | 66,181.039 | 13 | 5090.8492 | 5.8577214 | 0.003 | 62,412.215 | 13 | 4800.9396 | 3.9621392 | 0.014 | 94.31% | 67.64% | R.NHyp | |
| Residual | 9559.9188 | 11 | 869.08353 | 13,328.743 | 11 | 1211.7039 | 139.42% | ||||||||
| 2000 | Regression | 66,701.862 | 13 | 5130.9125 | 9.0762499 | 0 | 64,208.128 | 13 | 4939.0868 | 6.2361025 | 0.002 | 96.26% | 68.71% | R.NHyp | R.NHyp |
| Residual | 6218.4314 | 11 | 565.31195 | 8712.1652 | 11 | 792.01501 | 140.10% | ||||||||
| 2001 | Regression | 65,728.255 | 13 | 5056.0196 | 11.965925 | 0 | 64,034.994 | 13 | 4925.7688 | 8.5447449 | 0.001 | 97.42% | 71.41% | R.NHyp | R.NHyp |
| Residual | 4647.8828 | 11 | 422.5348 | 6341.1438 | 11 | 576.46762 | 136.43% | ||||||||
| 2002 | Regression | 58,054.951 | 13 | 4465.7654 | 4.9007422 | 0.006 | 56,982.771 | 13 | 4383.2901 | 4.3454258 | 0.01 | 98.15% | 88.67% | R.NHyp | |
| Residual | 10,023.669 | 11 | 911.24268 | 11,095.849 | 11 | 1008.7136 | 110.70% | ||||||||
| 2003 | Regression | 60,543.935 | 13 | 4657.2257 | 9.3988606 | 0 | 58,714.191 | 13 | 4516.4762 | 6.8240186 | 0.002 | 96.98% | 72.60% | R.NHyp | R.NHyp |
| Residual | 5450.6057 | 11 | 495.50961 | 7280.3493 | 11 | 661.84993 | 133.57% | ||||||||
| 2004 | Regression | 58,072.298 | 13 | 4467.0999 | 8.1452879 | 0.001 | 50,267.019 | 13 | 3866.6938 | 3.0736876 | 0.035 | 86.56% | 37.74% | R.NHyp | |
| Residual | 6032.7025 | 11 | 548.4275 | 13,837.982 | 11 | 1257.9983 | 229.38% | ||||||||
| 2005 | Regression | 54,862.385 | 13 | 4220.1834 | 6.1695484 | 0.002 | 54,154.671 | 13 | 4165.7439 | 5.5664079 | 0.004 | 98.71% | 90.22% | R.NHyp | R.NHyp |
| Residual | 7524.3786 | 11 | 684.03442 | 8232.0922 | 11 | 748.37202 | 109.41% | ||||||||
| 2006 | Regression | 56,156.079 | 13 | 4319.6984 | 10.176813 | 0 | 54,602.703 | 13 | 4200.2079 | 7.4250505 | 0.001 | 97.23% | 72.96% | R.NHyp | R.NHyp |
| Residual | 4669.1123 | 11 | 424.46476 | 6222.488 | 11 | 565.68073 | 133.27% | ||||||||
| 2007 | Regression | 55,005.414 | 13 | 4231.1857 | 10.596327 | 0 | 51,868.907 | 13 | 3989.9159 | 5.8294274 | 0.003 | 94.30% | 55.01% | R.NHyp | R.NHyp |
| Residual | 4392.3753 | 11 | 399.30684 | 7528.8827 | 11 | 684.44388 | 171.41% | ||||||||
| 2008 | Regression | 52,120.287 | 13 | 4009.2528 | 7.3796384 | 0.001 | 52,762.664 | 13 | 4058.6665 | 8.3703202 | 0.001 | 101.23% | 113.42% | R.NHyp | R.NHyp |
| Residual | 5976.1439 | 11 | 543.28581 | 5333.7663 | 11 | 484.88784 | 89.25% | ||||||||
| 2009 | Regression | 50,326.305 | 13 | 3871.2542 | 6.4737687 | 0.002 | 48,474.149 | 13 | 3728.7807 | 4.8655183 | 0.006 | 96.32% | 75.16% | R.NHyp | R.NHyp |
| Residual | 6577.899 | 11 | 597.99082 | 8430.0552 | 11 | 766.36865 | 128.16% | ||||||||
| 2010 | Regression | 48,120.658 | 13 | 3701.589 | 5.290958 | 0.005 | 50,877.153 | 13 | 3913.6272 | 8.716007 | 0.001 | 105.73% | 164.73% | R.NHyp | R.NHyp |
| Residual | 7695.6725 | 11 | 699.60659 | 4939.1767 | 11 | 449.01606 | 64.18% | ||||||||
| 2011 | Regression | 47,640.891 | 13 | 3664.6839 | 5.6170233 | 0.004 | 47831.947 | 13 | 3679.3805 | 5.7937905 | 0.003 | 100.40% | 103.15% | R.NHyp | R.NHyp |
| Residual | 7176.6701 | 11 | 652.42456 | 6985.6143 | 11 | 635.05585 | 97.34% | ||||||||
| 2012 | Regression | 48,296.923 | 13 | 3715.1479 | 8.7832306 | 0 | 49,038.244 | 13 | 3772.1726 | 10.608233 | 0 | 101.53% | 120.78% | R.NHyp | R.NHyp |
| Residual | 4652.8014 | 11 | 422.98194 | 3911.4806 | 11 | 355.58915 | 84.07% | ||||||||
| 2013 | Regression | 48,929.908 | 13 | 3763.839 | 8.5112537 | 0.001 | 43,004.683 | 13 | 3308.0525 | 3.3725495 | 0.026 | 87.89% | 39.62% | R.NHyp | |
| Residual | 4864.4102 | 11 | 442.21911 | 10,789.635 | 11 | 980.87589 | 221.81% | ||||||||
| 2014 | Regression | 46,327.916 | 13 | 3563.6858 | 6.0676754 | 0.003 | 44,895.055 | 13 | 3453.4658 | 4.8126349 | 0.007 | 96.91% | 79.32% | R.NHyp | R.NHyp |
| Residual | 6460.5539 | 11 | 587.32309 | 7893.4148 | 11 | 717.58316 | 122.18% | ||||||||
| 2015 | Regression | 44,170.079 | 13 | 3397.6984 | 5.2517248 | 0.005 | 43,805.729 | 13 | 3369.6715 | 4.9547373 | 0.006 | 99.18% | 94.34% | R.NHyp | R.NHyp |
| Residual | 7116.649 | 11 | 646.96809 | 7480.9992 | 11 | 680.09084 | 105.12% | ||||||||
| 2016 | Regression | 46,061.312 | 13 | 3543.1778 | 9.5686186 | 0 | 41570.068 | 13 | 3197.6975 | 4.1070554 | 0.012 | 90.25% | 42.92% | R.NHyp | |
| Residual | 4073.2062 | 11 | 370.29147 | 8564.4504 | 11 | 778.5864 | 210.26% | ||||||||
| 2017 | Regression | 42,092.459 | 13 | 3237.8815 | 7.6141015 | 0.001 | 41,718.972 | 13 | 3209.1517 | 6.988549 | 0.001 | 99.11% | 91.78% | R.NHyp | R.NHyp |
| Residual | 4677.728 | 11 | 425.248 | 5051.2157 | 11 | 459.20143 | 107.98% | ||||||||
| 2018 | Regression | 39,670.453 | 13 | 3051.5733 | 7.9672801 | 0.001 | 37,460.119 | 13 | 2881.5476 | 4.9345567 | 0.006 | 94.43% | 61.94% | R.NHyp | R.NHyp |
| Residual | 4213.1451 | 11 | 383.01319 | 6423.4794 | 11 | 583.95267 | 152.46% | ||||||||
| 2019 | Regression | 36,490.28 | 13 | 2806.9446 | 6.1589549 | 0.002 | 33,311.988 | 13 | 2562.4606 | 3.4409961 | 0.024 | 91.29% | 55.87% | R.NHyp | |
| Residual | 5013.2516 | 11 | 455.75015 | 8191.5428 | 11 | 744.68571 | 163.40% | ||||||||
| 2020 | Regression | 36,152.692 | 13 | 2780.9763 | 4.6221106 | 0.008 | 34,349.946 | 13 | 2642.3035 | 3.4514915 | 0.023 | 95.01% | 74.67% | R.NHyp | |
| Residual | 6618.3487 | 11 | 601.66806 | 8421.0953 | 11 | 765.55411 | 127.24% | ||||||||
| 2021 | Regression | 35,304.427 | 13 | 2715.7251 | 5.0810538 | 0.005 | 33,404.997 | 13 | 2569.6152 | 3.6337311 | 0.02 | 94.62% | 71.52% | R.NHyp | |
| Residual | 5879.2875 | 11 | 534.48069 | 7778.7173 | 11 | 707.15612 | 132.31% | ||||||||
| Pearson Correlation | Gender | COFOG | Lip | Colon | Pancreatic | Lung | Leukaemia | Brain | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence | Mortality | Incidence | Mortality | Incidence | Mortality | Incidence | Mortality | Incidence | Mortality | Incidence | Mortality | |||
| PRCPPP1993 | M | 0.473 | −0.039 | −0.327 | 0.562 | 0.467 | −0.131 | 0.196 | −0.184 | −0.120 | 0.558 | 0.433 | 0.413 | 0.061 |
| F | 0.473 | 0.412 | 0.255 | 0.616 | 0.535 | 0.386 | 0.729 | 0.409 | 0.374 | 0.442 | 0.461 | 0.431 | 0.074 | |
| PRCPPP1994 | M | 0.488 | 0.016 | −0.344 | 0.533 | 0.415 | −0.071 | 0.230 | −0.191 | −0.197 | 0.514 | 0.351 | 0.468 | 0.129 |
| F | 0.488 | 0.470 | 0.185 | 0.586 | 0.511 | 0.380 | 0.639 | 0.444 | 0.401 | 0.259 | 0.429 | 0.391 | 0.064 | |
| PRCPPP1995 | M | 0.503 | 0.007 | −0.379 | 0.509 | 0.441 | −0.099 | 0.150 | −0.233 | −0.218 | 0.471 | 0.426 | 0.471 | 0.158 |
| F | 0.503 | 0.506 | 0.191 | 0.615 | 0.537 | 0.404 | 0.648 | 0.438 | 0.414 | 0.267 | 0.573 | 0.429 | 0.037 | |
| PRCPPP1996 | M | 0.518 | −0.005 | −0.409 | 0.491 | 0.419 | −0.020 | 0.210 | −0.252 | −0.250 | 0.412 | 0.154 | 0.435 | 0.138 |
| F | 0.518 | 0.752 | 0.192 | 0.569 | 0.496 | 0.336 | 0.658 | 0.407 | 0.405 | 0.214 | 0.297 | 0.441 | 0.086 | |
| PRCPPP1997 | M | 0.533 | −0.079 | −0.399 | 0.489 | 0.383 | −0.136 | 0.092 | −0.223 | −0.231 | 0.560 | 0.459 | 0.445 | 0.074 |
| F | 0.533 | 0.347 | 0.234 | 0.560 | 0.515 | 0.360 | 0.623 | 0.444 | 0.431 | 0.326 | 0.421 | 0.457 | 0.014 | |
| PRCPPP1998 | M | 0.547 | −0.061 | −0.418 | 0.417 | 0.324 | −0.200 | 0.084 | −0.288 | −0.260 | 0.454 | 0.340 | 0.390 | −0.068 |
| F | 0.547 | 0.269 | 0.160 | 0.565 | 0.503 | 0.352 | 0.659 | 0.455 | 0.449 | 0.340 | 0.442 | 0.394 | −0.052 | |
| PRCPPP1999 | M | 0.561 | −0.152 | −0.458 | 0.410 | 0.335 | −0.136 | 0.160 | −0.319 | −0.298 | 0.361 | 0.388 | 0.328 | −0.100 |
| F | 0.561 | 0.406 | 0.157 | 0.557 | 0.484 | 0.299 | 0.611 | 0.440 | 0.425 | 0.145 | 0.458 | 0.398 | −0.076 | |
| PRCPPP2000 | M | 0.575 | −0.123 | −0.433 | 0.320 | 0.243 | −0.197 | 0.218 | −0.325 | −0.309 | 0.433 | 0.326 | 0.343 | −0.179 |
| F | 0.575 | 0.490 | 0.221 | 0.520 | 0.471 | 0.211 | 0.603 | 0.437 | 0.442 | 0.133 | 0.426 | 0.346 | −0.068 | |
| PRCPPP2001 | M | 0.588 | −0.141 | −0.485 | 0.300 | 0.221 | −0.239 | 0.031 | −0.312 | −0.369 | 0.473 | 0.354 | 0.238 | −0.321 |
| F | 0.588 | 0.443 | 0.013 | 0.495 | 0.467 | 0.255 | 0.588 | 0.461 | 0.452 | 0.152 | 0.230 | 0.275 | −0.197 | |
| PRCPPP2002 | M | 0.602 | −0.116 | −0.493 | 0.255 | 0.108 | −0.153 | −0.051 | −0.360 | −0.401 | 0.403 | 0.340 | 0.365 | −0.341 |
| F | 0.602 | 0.434 | 0.098 | 0.498 | 0.401 | 0.283 | 0.592 | 0.479 | 0.456 | 0.223 | 0.354 | 0.308 | −0.313 | |
| PRCPPP2003 | M | 0.613 | −0.105 | −0.478 | 0.286 | 0.094 | −0.244 | −0.016 | −0.380 | −0.407 | 0.379 | 0.248 | 0.252 | −0.416 |
| F | 0.613 | 0.438 | 0.152 | 0.454 | 0.381 | 0.284 | 0.506 | 0.485 | 0.431 | 0.120 | 0.307 | 0.298 | −0.432 | |
| PRCPPP2004 | M | 0.625 | −0.152 | −0.499 | 0.268 | 0.060 | −0.068 | 0.139 | −0.370 | −0.410 | 0.369 | 0.201 | 0.299 | −0.210 |
| F | 0.625 | 0.502 | 0.078 | 0.411 | 0.296 | 0.235 | 0.533 | 0.487 | 0.470 | 0.167 | 0.170 | 0.332 | −0.443 | |
| PRCPPP2005 | M | 0.635 | −0.169 | −0.561 | 0.208 | −0.045 | −0.188 | 0.003 | −0.391 | −0.434 | 0.457 | 0.249 | 0.352 | −0.317 |
| F | 0.635 | 0.539 | −0.104 | 0.522 | 0.318 | 0.256 | 0.500 | 0.508 | 0.483 | 0.266 | 0.316 | 0.265 | −0.573 | |
| PRCPPP2006 | M | 0.643 | −0.082 | −0.583 | 0.251 | −0.047 | −0.125 | 0.159 | −0.382 | −0.472 | 0.475 | 0.197 | 0.336 | −0.129 |
| F | 0.643 | 0.675 | −0.022 | 0.422 | 0.239 | 0.110 | 0.399 | 0.502 | 0.480 | 0.135 | 0.128 | 0.280 | −0.492 | |
| PRCPPP2007 | M | 0.650 | −0.115 | −0.602 | 0.226 | −0.168 | −0.129 | −0.004 | −0.413 | −0.502 | 0.354 | 0.201 | 0.277 | −0.283 |
| F | 0.650 | 0.654 | −0.026 | 0.435 | 0.142 | 0.240 | 0.507 | 0.525 | 0.465 | 0.118 | 0.106 | 0.287 | −0.442 | |
| PRCPPP2008 | M | 0.654 | −0.054 | −0.586 | 0.197 | −0.166 | −0.162 | −0.047 | −0.406 | −0.508 | 0.309 | 0.118 | 0.289 | −0.315 |
| F | 0.654 | 0.513 | −0.005 | 0.374 | 0.134 | 0.105 | 0.363 | 0.545 | 0.473 | 0.165 | 0.055 | 0.297 | −0.444 | |
| PRCPPP2009 | M | 0.655 | −0.044 | −0.635 | 0.147 | −0.249 | −0.180 | −0.077 | −0.424 | −0.497 | 0.379 | 0.082 | 0.226 | −0.398 |
| F | 0.655 | 0.464 | −0.029 | 0.422 | 0.133 | 0.152 | 0.354 | 0.512 | 0.456 | 0.167 | 0.101 | 0.256 | −0.457 | |
| PRCPPP2010 | M | 0.653 | −0.091 | −0.596 | 0.094 | −0.247 | −0.063 | −0.064 | −0.438 | −0.499 | 0.360 | 0.125 | 0.262 | −0.366 |
| F | 0.653 | 0.617 | −0.161 | 0.343 | 0.053 | 0.169 | 0.366 | 0.519 | 0.453 | 0.152 | 0.034 | 0.234 | −0.541 | |
| PRCPPP2011 | M | 0.646 | −0.075 | −0.635 | 0.084 | −0.290 | −0.089 | −0.040 | −0.453 | −0.542 | 0.345 | 0.179 | 0.224 | −0.223 |
| F | 0.646 | 0.683 | −0.061 | 0.362 | 0.032 | 0.106 | 0.274 | 0.498 | 0.426 | 0.118 | 0.035 | 0.193 | −0.573 | |
| PRCPPP2012 | M | 0.690 | 0.038 | −0.660 | 0.062 | −0.348 | −0.176 | −0.062 | −0.462 | −0.571 | 0.292 | 0.033 | 0.223 | −0.430 |
| F | 0.690 | 0.516 | −0.110 | 0.277 | −0.134 | 0.098 | 0.350 | 0.506 | 0.411 | 0.108 | 0.087 | 0.205 | −0.591 | |
| PRCPPP2013 | M | 0.537 | −0.003 | −0.642 | 0.022 | −0.424 | −0.024 | −0.126 | −0.491 | −0.569 | 0.323 | 0.056 | 0.155 | −0.461 |
| F | 0.537 | 0.654 | −0.116 | 0.280 | −0.134 | 0.212 | 0.302 | 0.488 | 0.398 | 0.077 | −0.117 | 0.162 | −0.570 | |
| PRCPPP2014 | M | 0.581 | 0.045 | −0.667 | 0.068 | −0.408 | 0.196 | −0.066 | −0.494 | −0.586 | 0.295 | −0.075 | 0.192 | −0.442 |
| F | 0.581 | 0.771 | −0.174 | 0.267 | −0.208 | 0.322 | 0.247 | 0.500 | 0.390 | 0.108 | −0.017 | 0.107 | −0.674 | |
| PRCPPP2015 | M | 0.459 | −0.016 | −0.664 | 0.044 | −0.452 | 0.212 | 0.020 | −0.522 | −0.599 | 0.218 | −0.064 | 0.130 | −0.485 |
| F | 0.459 | 0.770 | −0.108 | 0.257 | −0.302 | 0.341 | 0.271 | 0.482 | 0.380 | 0.143 | −0.003 | 0.156 | −0.516 | |
| PRCPPP2016 | M | 0.540 | 0.070 | −0.622 | 0.007 | −0.430 | 0.196 | 0.032 | −0.531 | −0.594 | 0.235 | −0.019 | 0.160 | −0.293 |
| F | 0.540 | 0.751 | −0.239 | 0.232 | −0.260 | 0.278 | 0.160 | 0.482 | 0.371 | 0.042 | −0.108 | 0.105 | −0.622 | |
| PRCPPP2017 | M | 0.510 | 0.015 | −0.655 | −0.046 | −0.513 | 0.035 | −0.036 | −0.533 | −0.594 | 0.324 | 0.013 | 0.176 | −0.475 |
| F | 0.510 | 0.677 | −0.187 | 0.191 | −0.272 | 0.180 | 0.205 | 0.480 | 0.319 | 0.083 | −0.157 | 0.173 | −0.555 | |
| PRCPPP2018 | M | 0.470 | 0.058 | −0.668 | −0.044 | −0.534 | 0.084 | 0.008 | −0.526 | −0.605 | 0.173 | −0.008 | 0.107 | −0.513 |
| F | 0.470 | 0.748 | −0.189 | 0.124 | −0.372 | 0.207 | 0.166 | 0.495 | 0.314 | 0.074 | −0.147 | 0.094 | −0.660 | |
| PRCPPP2019 | M | 0.436 | −0.041 | −0.618 | −0.108 | −0.420 | −0.117 | −0.095 | −0.545 | −0.642 | 0.200 | −0.089 | 0.113 | −0.517 |
| F | 0.436 | 0.630 | −0.179 | 0.020 | −0.167 | 0.080 | 0.124 | 0.502 | 0.371 | 0.069 | −0.182 | 0.093 | −0.657 | |
| PRCPPP2020 | M | 0.303 | 0.005 | −0.627 | −0.110 | −0.573 | −0.103 | −0.081 | −0.554 | −0.654 | 0.179 | −0.109 | 0.121 | −0.500 |
| F | 0.303 | 0.580 | −0.092 | 0.085 | −0.453 | 0.044 | 0.119 | 0.511 | 0.380 | 0.078 | −0.224 | 0.101 | −0.638 | |
| PRCPPP2021 | M | 0.324 | 0.022 | −0.609 | −0.127 | −0.591 | −0.089 | −0.090 | −0.566 | −0.667 | 0.157 | −0.128 | 0.110 | −0.498 |
| F | 0.324 | 0.528 | −0.118 | 0.065 | −0.481 | 0.026 | 0.095 | 0.507 | 0.375 | 0.081 | −0.227 | 0.085 | −0.641 | |
| Min | M | 0.303 | −0.169 | −0.668 | −0.127 | −0.591 | −0.244 | −0.126 | −0.566 | −0.667 | 0.157 | −0.128 | 0.107 | −0.517 |
| F | 0.303 | 0.269 | −0.239 | 0.020 | −0.481 | 0.026 | 0.095 | 0.407 | 0.314 | 0.042 | −0.227 | 0.085 | −0.674 | |
| Max | M | 0.690 | 0.070 | −0.327 | 0.562 | 0.467 | 0.212 | 0.230 | −0.184 | −0.120 | 0.560 | 0.459 | 0.471 | 0.158 |
| F | 0.690 | 0.771 | 0.255 | 0.616 | 0.537 | 0.404 | 0.729 | 0.545 | 0.483 | 0.442 | 0.573 | 0.457 | 0.086 | |
| Std Dev | M | 0.096 | 0.069 | 0.109 | 0.208 | 0.358 | 0.123 | 0.109 | 0.115 | 0.157 | 0.113 | 0.181 | 0.115 | 0.216 |
| F | 0.096 | 0.136 | 0.153 | 0.175 | 0.343 | 0.109 | 0.196 | 0.034 | 0.045 | 0.094 | 0.240 | 0.119 | 0.266 | |
| Average | M | 0.552 | −0.048 | −0.543 | 0.201 | −0.083 | −0.083 | 0.030 | −0.399 | −0.449 | 0.361 | 0.165 | 0.272 | −0.266 |
| F | 0.552 | 0.560 | 0.001 | 0.384 | 0.133 | 0.231 | 0.420 | 0.481 | 0.417 | 0.165 | 0.146 | 0.262 | −0.378 | |
| Amplitude | M | 0.387 | 0.239 | 0.342 | 0.689 | 1.058 | 0.456 | 0.357 | 0.382 | 0.546 | 0.404 | 0.587 | 0.364 | 0.674 |
| F | 0.387 | 0.502 | 0.494 | 0.596 | 1.018 | 0.378 | 0.634 | 0.139 | 0.169 | 0.400 | 0.800 | 0.372 | 0.760 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negoita, S.I.; Ionescu, R.V.; Zlati, M.L.; Antohi, V.M.; Nechifor, A. New Regional Dynamic Cancer Model across the European Union. Cancers 2023, 15, 2545. https://doi.org/10.3390/cancers15092545
Negoita SI, Ionescu RV, Zlati ML, Antohi VM, Nechifor A. New Regional Dynamic Cancer Model across the European Union. Cancers. 2023; 15(9):2545. https://doi.org/10.3390/cancers15092545
Chicago/Turabian StyleNegoita, Silvius Ioan, Romeo Victor Ionescu, Monica Laura Zlati, Valentin Marian Antohi, and Alexandru Nechifor. 2023. "New Regional Dynamic Cancer Model across the European Union" Cancers 15, no. 9: 2545. https://doi.org/10.3390/cancers15092545
APA StyleNegoita, S. I., Ionescu, R. V., Zlati, M. L., Antohi, V. M., & Nechifor, A. (2023). New Regional Dynamic Cancer Model across the European Union. Cancers, 15(9), 2545. https://doi.org/10.3390/cancers15092545







