A Comprehensive Narrative Review on the History, Current Landscape, and Future Directions of Hepatocellular Carcinoma (HCC) Systemic Therapy
Abstract
Simple Summary
Abstract
1. Introduction
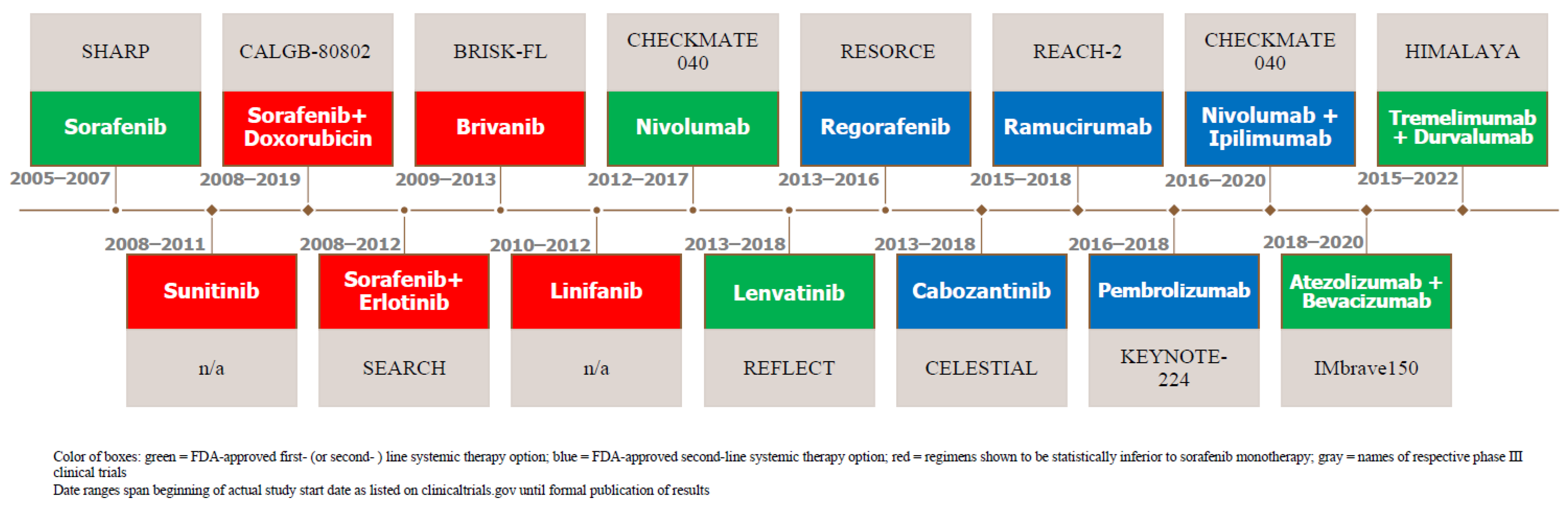
2. Evolution of Systemic Therapy
2.1. Systemic Therapy as First-Line Therapy
2.1.1. For HCC Patients with Preserved Liver Function and Functional Status
2.1.2. Sorafenib
2.1.3. Unsuccessful TKI Challengers of Sorafenib
2.1.4. Unsuccessful Combination Therapy: Sorafenib and Doxorubicin
2.1.5. Unsuccessful Combination Therapy: Sorafenib and an EGFR Inhibitor
2.1.6. Lenvatinib, the First Approved Alternative to Sorafenib
2.1.7. Bevacizumab and the Emergence of Anti-VEGF Monoclonal Antibodies
2.1.8. Immune Checkpoint Inhibitors (ICIs): Origin in Treatment for Advanced HCC
2.1.9. Combination Therapy: Atezolizumab and Bevacizumab
2.1.10. Combination Therapy: Sintilimab and Bevacizumab
2.1.11. Combination Therapy: Tremelimumab and Durvalumab
| Regimen | Trial Name | Authors | Year | Study Arm | # of Pts | Primary Endpoint Results | |
|---|---|---|---|---|---|---|---|
| “Preferred Regimens:” | Atezolizumab (Checkpoint inhibitor: anti-PD-L1 monoclonal Ab) + Bevacizumab (anti-VEGF-A monoclonal Ab) combination therapy | IMbrave150 | Finn et al. [51] | 2020 | Atezolizumab + bevacizumab vs. sorafenib (first-line setting) | 501 | OS: at 12 months, 67.2% (95% CI, 61.3 to 73.1) with atezolizumab—bevacizumab and 54.6% (95% CI, 45.2 to 64.0) with sorafenib. mPFS: 6.8 months (95% CI, 5.7 to 8.3) and 4.3 months (95% CI, 4.0 to 5.6) in the respective groups (hazard ratio for disease progression or death, 0.59; 95% CI, 0.47 to 0.76; p < 0.001). |
| STRIDE (Tremelimumab (anti-CTLA-4 monoclonal Ab) + Durvalumab (checkpoint inhibitor: anti-PD-L1 monoclonal Ab)) combination therapy | HIMALAYA | Abou-Alfa et al. [54] | 2022 | STRIDE vs. durvalumab vs. sorafenib (first-line setting) | 1171 | OS: 16.4 months (14.2–19.6) w/STRIDE (single tremelimumab + regular interval durvalumab) and 13.8 months (12.3–16.1) with sorafenib (HR for death, 0.78; 96% CI, 0.65–0.92; p = 0.0035) | |
| “Other Recommended Regimens:” | Sorafenib (TKI of VEGF-R1–3, PDGF beta and serine-threonine kinase inhibitor of Raf-1 and B-Raf) monotherapy | SHARP | Lovet et al. [8] | 2008 | Sorafenib vs. placebo (first-line setting) | 602 | OS: 10.7 months with sorafenib and 7.9 months with placebo (HR, 0.69; 95% CI, 0.55 to 0.87; p < 0.001) mPFS: 4.1 months vs. 4.9 months (p = 0.77) |
| Lenvatinib (TKI of VEGF-R1–3, FGF receptors 1–4, PDGF receptor alpha, RET, KIT) monotherapy | REFLECT | Kudo et al. [28] | 2018 | Lenvatinib vs. sorafenib (first-line setting) | 954 | OS: 13.6 months (95% CI, 12.1–14.9) for lenvatinib was non-inferior to 12.3 months (10.4–13.9; HR, 0.92; 95% CI, 0.79–1.06) for sorafenib | |
| Durvalumab (checkpoint inhibitor: anti-PD-L1 monoclonal Ab) monotherapy | HIMALAYA | Abou-Alfa et al. [54] | 2022 | STRIDE vs. durvalumab vs. sorafenib (first-line setting) | 1171 | OS: 16.6 months for durvalumab monotherapy vs. 13.8 months for sorafenib monotherapy (HR, 0.86; 95% CI, 0.73–1.03). Judged to be non-inferior. | |
| Pembrolizumab (checkpoint inhibitor: anti-PD-1 Monoclonal Ab) monotherapy | KEYNOTE-224, 2021 update of Cohort 2 | Verset et al. [46] | 2022 | Phase II study of pembrolizumab | 51 | ORR: 16% (95% CI, 7–29) for pembrolizumab monotherapy | |
| “Useful in Certain Circumstances:” | Nivolumab (checkpoint inhibitor: anti-PD-1 monoclonal Ab) monotherapy | CheckMate 459 | Yau et al. [47] | 2021 | Nivolumab vs. sorafenib (first-line setting) | 743 | OS: 16.4 months (95% CI 13.9–18.4) with nivolumab and 14.7 months (11.9–17.2) with sorafenib (HR, 0.85; 95% CI, 0.72–1.02; p = 0.075) |
2.1.12. Other Trials Combining TKIs and ICIs
2.2. Second-Line Systemic Therapies
2.2.1. Approved Second-Line TKI Therapies
2.2.2. Second-Line Monoclonal Antibody Therapies
2.2.3. Second-Line Combination Therapy: Nivolumab and Ipilimumab
2.2.4. Unsuccessful Second-Line Therapies
| Regimen | Trial Name | Authors | Year | Study Arm | # of Pts | Primary Endpoint Results | |
|---|---|---|---|---|---|---|---|
| “Preferred Regimens:” | Regorafenib (a multikinase inhibitor targeting VEGFR 1–3, FGFR 1–2, angiopoietin-1 receptor (TIE2) and PDFRs alpha and beta) | RESORCE | Bruix et al. [58] | 2017 | Regorafenib vs. placebo (second-line setting) | 573 | OS: 10.6 months (95% CI 9.1–12.1) for regorafenib versus 7.8 months (6.3–8.8) for placebo (HR of 0.63; 95% CI, 0.50–0.79; one-sided p < 0·0001) |
| Cabozantinib (a multikinase inhibitor (TKI) of kinases involved in tumor pathogenesis including VEGF, MET and the TAM family (TYRO3, AXL, MER) | CELESTIAL | Kelley et al. [59] | 2020 | Cabozantinib vs. placebo (second-line setting) | 707 | OS: Cabozantinib improved OS relative to placebo in the overall second-line population who had received only prior sorafenib (median 11.3 vs. 7.2 months; HR, 0.70; 95% CI, 0.55 to 0.88) | |
| Ramucirumab (humanized monoclonal antibody directed against VEGF 2) monotherapy | REACH-2 | Zhu et al. [60] | 2019 | Ramucirumab vs. placebo (second-line setting) | 292 | OS: At a median follow-up of 7.6 months (IQR 4.0–12.5), mOS was 8.5 months (95% CI 7.0–10.6) in the ramucirumab group vs. 7.3 months (5.4–9.1) in the placebo group (HR, 0.710; 95% CI, 0.531–0.949; p = 0.0199) | |
| Lenvatinib (TKI of VEGF-R1–3, FGF receptors 1–4, PDGF receptor alpha, RET, and KIT) monotherapy | REFLECT | Kudo et al. [28] | 2018 | Lenvatinib vs. sorafenib (first-line setting) | 954 | OS: 13.6 months (95% CI, 12.1–14.9) for Lenvatinib was non-inferior to 12.3 months (10.4–13.9; HR, 0.92; 95% CI 0.79–1.06) for sorafenib | |
| Sorafenib (TKI of VEGF-R1–3, PDGF beta and serine-threonine kinase inhibitor of Raf-1 and B-Raf) monotherapy | SHARP | Lovet et al. [8] | 2008 | Sorafenib vs. placebo (first-line setting) | 602 | OS: 10.7 months with sorafenib and 7.9 months with placebo; HR, 0.69; 95% CI, 0.55 to 0.87; p < 0.001 mPFS: 4.1 months vs. 4.9 months; p = 0.77 | |
| “Other Recommended Regimens:” | Nivolumab (checkpoint inhibitor: anti-PD-1 monoclonal antibody) + Ipilimumab (anti-CTLA-4 humanized monoclonal antibody) | CheckMate 040 | Yau et al. [63] | 2020 | Phase I/II, three-arm study of nivolumab and ipilimumab (second-line setting) | 148 | ORR: 32% (95% CI, 20–47%) in arm A, 27% (95% CI, 15–41%) in arm B, and 29% (95% CI, 17–43%) in arm C, with the respective arms differing in quantity and time for the administration of drugs |
| Pembrolizumab (checkpoint inhibitor: anti-PD-1 monoclonal antibody monotherapy | Keynote-240 | Finn et al. [61] | 2020 | Pembrolizumab vs. placebo (second-line setting) | 413 | OS: 13.9 months (95% CI, 11.6 to 16.0 months) for pembrolizumab versus 10.6 months (95% CI, 8.3 to 13.5 months) for placebo (HR, 0.781; 95% CI, 0.611 to 0.998; p = 0.0238). mPFS (with predefined one-sided significance thresholds; p = 0.0174 for final analysis) for pembrolizumab was 3.0 months (95% CI, 2.8 to 4.1 months) versus 2.8 months (95% CI, 1.6 to 3.0 months) at final analysis (HR, 0.718; 95% CI, 0.570 to 0.904; p = 0.0022). | |
| “Useful in Certain Circumstances:” | Nivolumab monotherapy | CheckMate 040 | El-Khoueiry et al. [62] | 2017 | Phase I/II dose escalation and expansion trial assessing safety and efficacy of nivolumab monotherapy | 262 | ORR: 20% (95% CI 15–26) in patients treated with nivolumab 3 mg/kg in the dose-expansion phase and 15% (95% CI 6–28) in the dose-escalation phase |
| Dostarlimab-gxly (humanized anti-PD-1 monoclonal antibody) monotherapy | GARNET | Andre et al. [77] | N/a | Phase I study evaluating safety of dostarlimab | N/a | N/a | |
| Selpercatinib (highly selective RET kinase inhibitor) monotherapy | LIBRETTO-001 | Subbiah et al. [78] | 2022 | Phase ½ study evaluating safety and efficacy of selpercatinib in RET fusion-positive advanced solid non-lung or thyroid tumors | N/a | N/a |
2.2.5. For Advanced, Refractory HCC Patients with MSI-H/dMMR Tumors
2.2.6. For RET Gene Fusion-Positive Tumors
3. Our Current Approach to Systemic Therapy for HCC (or “How We Treat HCC”)
4. Future Directions
4.1. Novel VEGF Monotherapies and Combination Therapies
4.2. Approved Systemic Therapies in China
4.3. Novel Targeted Therapies and ICIs in Development
4.4. Alternative Combination Regimens
4.5. Chemoprevention of HCC with Non-Antitumoral Agents
4.6. Emerging Treatment Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [PubMed]
- Singal, A.; Volk, M.L.; Waljee, A.; Salgia, R.; Higgins, P.; Rogers, M.A.M.; Marrero, J.A. Meta-analysis: Surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment. Pharmacol. Ther. 2009, 30, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; McGlynn, K.A.; Reichman, M.E. Hepatocellular Carcinoma Incidence, Mortality, and Survival Trends in the United States From 1975 to 2005. J. Clin. Oncol. 2009, 27, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236, Erratum in J. Hepatol. 2019, 70, 817. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Yu, S.J. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clin. Mol. Hepatol. 2016, 22, 7–17. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Lopez, P.M.; Villanueva, A.; Llovet, J.M. Systematic review: Evidence-based management of hepatocellular carcinoma—An updated analysis of randomized controlled trials. Aliment. Pharm. Ther. 2006, 23, 1535–1547. [Google Scholar] [CrossRef]
- Hwang, J.P.; Feld, J.J.; Hammond, S.P.; Wang, S.H.; Alston-Johnson, D.E.; Cryer, D.R.; Hershman, D.L.; Loehrer, A.P.; Sabichi, A.L.; Symington, B.E.; et al. Hepatitis B Virus Screening and Management for Patients with Cancer Prior to Therapy: ASCO Provisional Clinical Opinion Update. J. Clin. Oncol. 2020, 38, 3698–3715. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib Blocks the RAF/MEK/ERK Pathway, Inhibits Tumor Angiogenesis, and Induces Tumor Cell Apoptosis in Hepatocellular Carcinoma Model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2008, 10, 25–34. [Google Scholar] [CrossRef]
- Johnson, P.J.; Qin, S.; Park, J.-W.; Poon, R.T.; Raoul, J.-L.; Philip, P.A.; Hsu, C.-H.; Hu, T.-H.; Heo, J.; Xu, J.; et al. Brivanib Versus Sorafenib as First-Line Therapy in Patients With Unresectable, Advanced Hepatocellular Carcinoma: Results From the Randomized Phase III BRISK-FL Study. J. Clin. Oncol. 2013, 31, 3517–3524. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; Lin, D.-Y.; Park, J.-W.; Kudo, M.; Qin, S.; Chung, H.-C.; Song, X.; Xu, J.; Poggi, G.; et al. Sunitinib Versus Sorafenib in Advanced Hepatocellular Cancer: Results of a Randomized Phase III Trial. J. Clin. Oncol. 2013, 31, 4067–4075. [Google Scholar] [CrossRef]
- Cainap, C.; Qin, S.; Huang, W.-T.; Chung, I.J.; Pan, H.; Cheng, Y.; Kudo, M.; Kang, Y.-K.; Chen, P.-J.; Toh, H.-C.; et al. Linifanib Versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma: Results of a Randomized Phase III Trial. J. Clin. Oncol. 2015, 33, 172–179. [Google Scholar] [CrossRef]
- Richly, H.; Henning, B.F.; Kupsch, P.; Passarge, K.; Grubert, M.; Hilger, R.A.; Christensen, O.; Brendel, E.; Schwartz, B.; Ludwig, M.; et al. Results of a Phase I trial of sorafenib (BAY 43-9006) in combination with doxorubicin in patients with refractory solid tumors. Ann. Oncol. 2006, 17, 866–873. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Johnson, P.; Knox, J.J.; Capanu, M.; Davidenko, I.; Lacava, J.; Leung, T.; Gansukh, B.; Saltz, L. Doxorubicin Plus Sorafenib vs. Doxorubicin Alone in Patients with Advanced Hepatocellular Carcinoma. JAMA 2010, 304, 2154–2160. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Abrams, S.L.; Lee, J.T.; Chang, F.; Bertrand, F.E.; Navolanic, P.M.; Terrian, D.M.; Franklin, R.A.; D’assoro, A.B.; et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv. Enzym. Regul. 2006, 46, 249–279. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Groschner, K. Vascular actions of anthracycline antibiotics. Curr. Med. Chem. 2003, 10, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Shi, Q.; Knox, J.J.; Kaubisch, A.; Niedzwiecki, D.; Posey, J.; Tan, B.R., Jr.; Kavan, P.; Goel, R.; Lammers, P.E.; et al. Assessment of Treatment with Sorafenib Plus Doxorubicin vs Sorafenib Alone in Patients With Advanced Hepatocellular Carcinoma: Phase 3 CALGB 80802 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1582–1588, Erratum in JAMA Oncol. 2019, 5, 1643. [Google Scholar] [CrossRef] [PubMed]
- El Dika, I.; Capanu, M.; Chou, J.F.; Harding, J.J.; Ly, M.; Hrabovsky, A.D.; Do, R.K.G.; Shia, J.; Millang, B.; Ma, J.; et al. Phase II trial of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma after disease progression on sorafenib. Cancer Med. 2020, 9, 7453–7459. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Nicou, A.; Garcia-Irigoyen, O.; Latasa, M.U.; Urtasun, R.; Elizalde, M.; Salis, F.; Perugorría, M.J.; Prieto, J.; Recio, J.A.; et al. Epidermal Growth Factor Receptor Signaling in Hepatocellular Carcinoma: Inflammatory Activation and a New Intracellular Regulatory Mechanism. Dig. Dis. 2012, 30, 524–531. [Google Scholar] [CrossRef]
- Blivet-Van Eggelpoël, M.-J.; Chettouh, H.; Fartoux, L.; Aoudjehane, L.; Barbu, V.; Rey, C.; Priam, S.; Housset, C.; Rosmorduc, O.; Desbois-Mouthon, C. Epidermal growth factor receptor and HER-3 restrict cell response to sorafenib in hepatocellular carcinoma cells. J. Hepatol. 2012, 57, 108–115. [Google Scholar] [CrossRef]
- Duran, I.; Hotté, S.J.; Hirte, H.; Chen, E.X.; MacLean, M.; Turner, S.; Duan, L.; Pond, G.R.; Lathia, C.; Walsh, S.; et al. Phase I Targeted Combination Trial of Sorafenib and Erlotinib in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2007, 13, 4849–4857. [Google Scholar] [CrossRef]
- Zhu, A.X.; Rosmorduc, O.; Evans, T.J.; Ross, P.J.; Santoro, A.; Carrilho, F.J.; Bruix, J.; Qin, S.; Thuluvath, P.J.; Llovet, J.M.; et al. SEARCH: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial of Sorafenib Plus Erlotinib in Patients with Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2015, 33, 559–566. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef]
- Siegel, A.B.; Cohen, E.I.; Ocean, A.; Lehrer, D.; Goldenberg, A.; Knox, J.J.; Chen, H.; Clark-Garvey, S.; Weinberg, A.; Mandeli, J.; et al. Phase II Trial Evaluating the Clinical and Biologic Effects of Bevacizumab in Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2008, 26, 2992–2998. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Yang, T.-S.; Toh, H.C.; Epstein, R.J.; Hsiao, L.-T.; Chen, P.-J.; Lin, Z.-Z.; Chao, T.-Y.; Cheng, A.-L. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br. J. Cancer 2010, 102, 981–986. [Google Scholar] [CrossRef]
- Sun, W.; Sohal, D.; Haller, D.G.; Mykulowycz, K.; Rosen, M.; Soulen, M.C.; Caparro, M.; Teitelbaum, U.R.; Giantonio, B.; O’Dwyer, P.J.; et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer 2011, 117, 3187–3192. [Google Scholar] [CrossRef]
- Thomas, M.B.; Morris, J.S.; Chadha, R.; Iwasaki, M.; Kaur, H.; Lin, E.; Kaseb, A.; Glover, K.; Davila, M.; Abbruzzese, J. Phase II Trial of the Combination of Bevacizumab and Erlotinib in Patients Who Have Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2009, 27, 843–850, Erratum in J. Clin. Oncol. 2009, 27, 3263. [Google Scholar] [CrossRef]
- Kaseb, A.; Garrett-Mayer, E.; Morris, J.; Xiao, L.; Lin, E.; Onicescu, G.; Hassan, M.; Hassabo, H.; Iwasaki, M.; Deaton, F.; et al. Efficacy of Bevacizumab plus Erlotinib for Advanced Hepatocellular Carcinoma and Predictors of Outcome: Final Results of a Phase II Trial. Oncology 2012, 82, 67–74. [Google Scholar] [CrossRef]
- Voron, T.; Colussi, O.; Marcheteau, E.; Pernot, S.; Nizard, M.; Pointet, A.-L.; Latreche, S.; Bergaya, S.; Benhamouda, N.; Tanchot, C.; et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015, 212, 139–148. [Google Scholar] [CrossRef]
- Finn, R.S.; Bentley, G.; Britten, C.D.; Amado, R.; Busuttil, R.W. Targeting vascular endothelial growth factor with the monoclonal antibody bevacizumab inhibits human hepatocellular carcinoma cells growing in an orthotopic mouse model. Liver Int. 2009, 29, 284–290. [Google Scholar] [CrossRef]
- Boige, V.; Malka, D.; Bourredjem, A.; Dromain, C.; Baey, C.; Jacques, N.; Pignon, J.-P.; Vimond, N.; Bouvet-Forteau, N.; De Baere, T.; et al. Efficacy, Safety, and Biomarkers of Single-Agent Bevacizumab Therapy in Patients with Advanced Hepatocellular Carcinoma. Oncologist 2012, 17, 1063–1072. [Google Scholar] [CrossRef]
- Fang, P.; Hu, J.-H.; Cheng, Z.-G.; Liu, Z.-F.; Wang, J.-L.; Jiao, S.-C. Efficacy and Safety of Bevacizumab for the Treatment of Advanced Hepatocellular Carcinoma: A Systematic Review of Phase II Trials. PLoS ONE 2012, 7, e49717. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Park, J.-J.; Omiya, R.; Matsumura, Y.; Sakoda, Y.; Kuramasu, A.; Augustine, M.M.; Yao, S.; Tsushima, F.; Narazaki, H.; Anand, S.; et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 2010, 116, 1291–1298. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126, Erratum in J. Clin. Oncol. 2022, 40, 315. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Chan, S.L.; Meyer, T.; Reig, M.; El-Khoueiry, A.; Galle, P.R. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. 2020, 72, 320–341. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Faleck, D.M.; Ricciuti, B.; Mendelsohn, R.B.; Naqash, A.R.; Cohen, J.V.; Sellers, M.C.; Balaji, A.; Ben-Betzalel, G.; Hajir, I.; et al. Immune Checkpoint Inhibitor Therapy in Patients with Preexisting Inflammatory Bowel Disease. J. Clin. Oncol. 2020, 38, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.P.; Gergich, K.; Lubiniecki, G.M.; de Alwis, D.P.; Chen, C.; Tice, M.A.B.; Rubin, E.H. Pembrolizumab KEYNOTE-001: An adaptive study leading to accelerated approval for two indications and a companion diagnostic. Ann. Oncol. 2017, 28, 1388–1398. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Verset, G.; Borbath, I.; Karwal, M.; Verslype, C.; Van Vlierberghe, H.; Kardosh, A.; Zagonel, V.; Stal, P.; Sarker, D.; Palmer, D.H.; et al. Pembrolizumab Monotherapy for Previously Untreated Advanced Hepatocellular Carcinoma: Data from the Open-Label, Phase II KEYNOTE-224 Trial. Clin. Cancer Res. 2022, 28, 2547–2554. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2021, 23, 77–90. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723, Erratum in N. Engl. J. Med. 2010, 363, 1290. [Google Scholar] [CrossRef]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef]
- Lee, M.; Ryoo, B.Y.; Hsu, C.H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Randomised efficacy and safety results for atezolizumab (Atezo) + bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Ann. Oncol. 2019, 30, v875. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Finn, S.Q.R.S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.V.; Merle, P.; Kaseb, A.O.; et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39 (Suppl. S33), 267. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990, Erratum in Lancet Oncol. 2021, 22, e347. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Chan, S.L.; Kudo, M.; Lau, G.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Dao, T.V.; De Toni, E.N.; et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J. Clin. Oncol. 2022, 40, 379. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.-L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef]
- Finn, R.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.-Y.; Ren, Z.; et al. LBA34 Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2022, 33, S1401. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66, Erratum in Lancet 2017, 389, 36. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ryoo, B.-Y.; Merle, P.; Park, J.-W.; Bolondi, L.; Chan, S.L.; Lim, H.Y.; Baron, A.D.; Parnis, F.; Knox, J.; et al. Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: A subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open 2020, 5, e000714. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564, Erratum in JAMA Oncol. 2021, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.C.; Maulik, G.; Christensen, J.; Salgia, R. c-Met: Structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003, 22, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Takami, T.; Kaposi-Novak, P.; Uchida, K.; Gomez-Quiroz, L.E.; Conner, E.A.; Factor, V.M.; Thorgeirsson, S.S. Loss of Hepatocyte Growth Factor/c-Met Signaling Pathway Accelerates Early Stages of N-nitrosodiethylamine–Induced Hepatocarcinogenesis. Cancer Res. 2007, 67, 9844–9851. [Google Scholar] [CrossRef]
- Ueki, T.; Fujimoto, J.; Suzuki, T.; Yamamoto, H.; Okamoto, E. Expression of hepatocyte growth factor and its receptor, the c-met proto-oncogene, in hepatocellular carcinoma. Hepatology 1997, 25, 619–623. [Google Scholar] [CrossRef]
- Martell, R.E.; Puzanov, I.; Ma, W.W.; Santoro, A.; Dy, G.K.; Goff, L.W.; Fetterly, G.J.; Michael, S.A.; Means-Powell, J.A.; Chai, F.; et al. Safety and efficacy of MET inhibitor tivantinib (ARQ 197) combined with sorafenib in patients (pts) with hepatocellular carcinoma (HCC) from a phase I study. J. Clin. Oncol. 2012, 30, 4117. [Google Scholar] [CrossRef]
- Santoro, A.; Rimassa, L.; Borbath, I.; Daniele, B.; Salvagni, S.; Van Laethem, J.L.; Van Vlierberghe, H.; Trojan, J.; Kolligs, F.T.; Weiss, A.; et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: A randomised, placebo-controlled phase 2 study. Lancet Oncol. 2012, 14, 55–63. [Google Scholar] [CrossRef]
- Rimassa, L.; Assenat, E.; Peck-Radosavljevic, M.; Pracht, M.; Zagonel, V.; Mathurin, P.; Caremoli, E.R.; Porta, C.; Daniele, B.; Bolondi, L.; et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): A final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018, 19, 682–693. [Google Scholar] [CrossRef]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef]
- Wong, C.-M.; Ng, I.O.L. Molecular pathogenesis of hepatocellular carcinoma. Liver Int. 2007, 28, 160–174. [Google Scholar] [CrossRef]
- Schmidt, C.; McKillop, I.H.; Cahill, P.; Sitzmann, J.V. Increased MAPK Expression and Activity in Primary Human Hepatocellular Carcinoma. Biochem. Biophys. Res. Commun. 1997, 236, 54–58. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Ichida, T.; Sugitani, S.; Genda, T.; Inayoshi, J.; Takamura, M.; Matsuda, Y.; Nomoto, M.; Aoyagi, Y. Overexpression of extracellular signal-regulated protein kinase and its correlation with proliferation in human hepatocellular carcinoma. Liver Int. 2004, 24, 432–436. [Google Scholar] [CrossRef]
- Schmieder, R.; Puehler, F.; Neuhaus, R.; Kissel, M.; A Adjei, A.; Miner, J.N.; Mumberg, D.; Ziegelbauer, K.; Scholz, A. Allosteric MEK1/2 Inhibitor Refametinib (BAY 86-9766) in Combination with Sorafenib Exhibits Antitumor Activity in Preclinical Murine and Rat Models of Hepatocellular Carcinoma. Neoplasia 2013, 15, 1161–1171. [Google Scholar] [CrossRef]
- Adjei, A.A.; Richards, D.A.; El-Khoueiry, A.; Braiteh, F.; Becerra, C.H.; Stephenson, J.J., Jr.; Hezel, A.F.; Sherman, M.; Garbo, L.; Leffingwell, D.P.; et al. A Phase I Study of the Safety, Pharmacokinetics, and Pharmacodynamics of Combination Therapy with Refametinib plus Sorafenib in Patients with Advanced Cancer. Clin. Cancer Res. 2016, 22, 2368–2376. [Google Scholar] [CrossRef]
- Lim, H.Y.; Merle, P.; Weiss, K.H.; Yau, T.; Ross, P.; Mazzaferro, V.; Blanc, J.-F.; Ma, Y.T.; Yen, C.J.; Kocsis, J.; et al. Phase II Studies with Refametinib or Refametinib plus Sorafenib in Patients with RAS-Mutated Hepatocellular Carcinoma. Clin. Cancer Res. 2018, 24, 4650–4661. [Google Scholar] [CrossRef]
- Andre, T.; Berton, D.; Curigliano, G.; Ellard, S.; Pérez, J.M.T.; Arkenau, H.-T.; Abdeddaim, C.; Moreno, V.; Guo, W.; Im, E.; et al. Safety and efficacy of anti–PD-1 antibody dostarlimab in patients (pts) with mismatch repair-deficient (dMMR) solid cancers: Results from GARNET study. J. Clin. Oncol. 2021, 39, 9. [Google Scholar] [CrossRef]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef]
- Referenced from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Guideline Name V5.2022. Hepatocellular Caricinoma © National Comprehensive Cancer Network, Inc. 2022. All Rights Reserved. Available online: https://www.NCCN.org (accessed on 10 February 2023).
- Sonbol, M.B.; Bin Riaz, I.; Naqvi, S.A.A.; Almquist, D.R.; Mina, S.; Almasri, J.; Shah, S.; Almader-Douglas, D.; Junior, P.L.S.U.; Mahipal, A.; et al. Systemic Therapy and Sequencing Options in Advanced Hepatocellular Carcinoma. JAMA Oncol. 2020, 6, e204930. [Google Scholar] [CrossRef]
- Kudo, M.; Matilla, A.; Santoro, A.; Melero, I.; Gracián, A.C.; Acosta-Rivera, M.; Choo, S.-P.; El-Khoueiry, A.B.; Kuromatsu, R.; El-Rayes, B.; et al. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J. Hepatol. 2021, 75, 600–609. [Google Scholar] [CrossRef]
- Yamazaki, H.; Iwasaki, H.; Takasaki, H.; Suganuma, N.; Sakai, R.; Masudo, K.; Nakayama, H.; Rino, Y.; Masuda, M. Efficacy and tolerability of initial low-dose lenvatinib to treat differentiated thyroid cancer. Medicine 2019, 98, e14774. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Kudo, M.; Ye, S.; Bronowicki, J.; Chen, X.; Dagher, L.; Furuse, J.; Geschwind, J.F.; de Guevara, L.L.; Papandreou, C.; et al. GIDEON (Global Investigation of therapeutic DE cisions in hepatocellular carcinoma and of its treatment with sorafeNib): Second interim analysis. Int. J. Clin. Pract. 2013, 68, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, C.; Gupta, M.; Lee, S.; Krishnamurthi, S.; Estfan, B.; Wang, K.; Attwood, K.; Wilton, J.; Bies, R.; Bshara, W.; et al. A multicentre phase 1b/2 study of tivozanib in patients with advanced inoperable hepatocellular carcinoma. Br. J. Cancer 2020, 122, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.V.; Li, D.; Dayyani, F.; Rowe, J.H.; Beg, M.S.; Kasturi, V.; Abrams, T.A. Phase 1b/2 study of tivozanib in combination with durvalumab in subjects with advanced hepatocellular carcinoma (Deductive): Efficacy results in previously untreated patients. J. Clin. Oncol. 2022, 40, 462. [Google Scholar] [CrossRef]
- Qin, S.; Bi, F.; Gu, S.; Bai, Y.; Chen, Z.; Wang, Z.; Ying, J.; Lu, Y.; Meng, Z.; Pan, H.; et al. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J. Clin. Oncol. 2021, 39, 3002–3011. [Google Scholar] [CrossRef]
- Qin, S.; Li, Q.; Gu, S.; Chen, X.; Lin, L.; Wang, Z.; Xu, A.; Chen, X.; Zhou, C.; Ren, Z.; et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 559–568. [Google Scholar] [CrossRef]
- BeiGene Announces Positive Global Phase 3 Trial Results for PD-1 Inhibitor Tislelizumab in First-Line Unresectable Hepatocellular Cancer. News Release. BeiGene. 9 August 2022. Available online: https://bwnews.pr/3JMx3D2 (accessed on 10 August 2022).
- Zhang, H.; Zhang, W.; Jiang, L.; Chen, Y. Recent advances in systemic therapy for hepatocellular carcinoma. Biomark. Res. 2022, 10, 3. [Google Scholar] [CrossRef]
- Peng, Z.; Fan, W.; Zhu, B.; Wang, G.; Sun, J.; Xiao, C.; Huang, F.; Tang, R.; Cheng, Y.; Huang, Z.; et al. Lenvatinib Combined with Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J. Clin. Oncol. 2023, 41, 117–127. [Google Scholar] [CrossRef]
- Dawson, L.A.; Winter, K.A.; Knox, J.J.; Zhu, A.X.; Krishnan, S.; Guha, C.; Kachnic, L.A.; Gillin, M.; Hong, T.S.; Craig, T.; et al. NRG/RTOG 1112: Randomized phase III study of sorafenib vs. stereotactic body radiation therapy (SBRT) followed by sorafenib in hepatocellular carcinoma (HCC). J. Clin. Oncol. 2023, 41, 489. [Google Scholar] [CrossRef]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.-Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.-H.; Tak, W.Y.; et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef]
- Demierre, M.-F.; Higgins, P.D.R.; Gruber, S.B.; Hawk, E.T.; Lippman, S.M. Statins and cancer prevention. Nat. Rev. Cancer 2005, 5, 930–942. [Google Scholar] [CrossRef]
- Facciorusso, A.; Aziz, M.A.A.E.; Singh, S.; Pusceddu, S.; Milione, M.; Giacomelli, L.; Sacco, R. Statin Use Decreases the Incidence of Hepatocellular Carcinoma: An Updated Meta-Analysis. Cancers 2020, 12, 874. [Google Scholar] [CrossRef]
- Sun, L.; Gao, F.; Gao, Z.; Ao, L.; Li, N.; Ma, S.; Jia, M.; Lu, P.; Sun, B.; Ho, M.; et al. Shed antigen-induced blocking effect on CAR-T cells targeting Glypican-3 in Hepatocellular Carcinoma. J. Immunother. Cancer 2021, 9, e001875. [Google Scholar] [CrossRef]
- Shi, D.; Shi, Y.; Kaseb, A.O.; Qi, X.; Zhang, Y.; Chi, J.; Lu, Q.; Gao, H.; Jiang, H.; Wang, H.; et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin. Cancer Res. 2020, 26, 3979–3989. [Google Scholar] [CrossRef]
- Komatsu, S.-I.; Kayukawa, Y.; Miyazaki, Y.; Kaneko, A.; Ikegami, H.; Ishiguro, T.; Nakamura, M.; Frings, W.; Ono, N.; Sakata, K.; et al. Determination of starting dose of the T cell-redirecting bispecific antibody ERY974 targeting glypican-3 in first-in-human clinical trial. Sci. Rep. 2022, 12, 12312. [Google Scholar] [CrossRef]
- Flores, Y.N.; Datta, G.D.; Yang, L.; Corona, E.; Devineni, D.; Glenn, B.A.; Bastani, R.; May, F.P. Disparities in Hepatocellular Carcinoma Incidence, Stage, and Survival: A Large Population-Based Study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1193–1199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzaro, A.; Hartshorn, K.L. A Comprehensive Narrative Review on the History, Current Landscape, and Future Directions of Hepatocellular Carcinoma (HCC) Systemic Therapy. Cancers 2023, 15, 2506. https://doi.org/10.3390/cancers15092506
Lazzaro A, Hartshorn KL. A Comprehensive Narrative Review on the History, Current Landscape, and Future Directions of Hepatocellular Carcinoma (HCC) Systemic Therapy. Cancers. 2023; 15(9):2506. https://doi.org/10.3390/cancers15092506
Chicago/Turabian StyleLazzaro, Alexander, and Kevan L. Hartshorn. 2023. "A Comprehensive Narrative Review on the History, Current Landscape, and Future Directions of Hepatocellular Carcinoma (HCC) Systemic Therapy" Cancers 15, no. 9: 2506. https://doi.org/10.3390/cancers15092506
APA StyleLazzaro, A., & Hartshorn, K. L. (2023). A Comprehensive Narrative Review on the History, Current Landscape, and Future Directions of Hepatocellular Carcinoma (HCC) Systemic Therapy. Cancers, 15(9), 2506. https://doi.org/10.3390/cancers15092506






