Patient-Related Characteristics Associated with Treatment Modifications and Suboptimal Relative Dose Intensity of Neoadjuvant Chemotherapy in Patients with Breast Cancer—A Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Neoadjuvant Chemotherapy
2.3. Data Extraction
2.4. Outcomes
2.5. Statistical Analyses
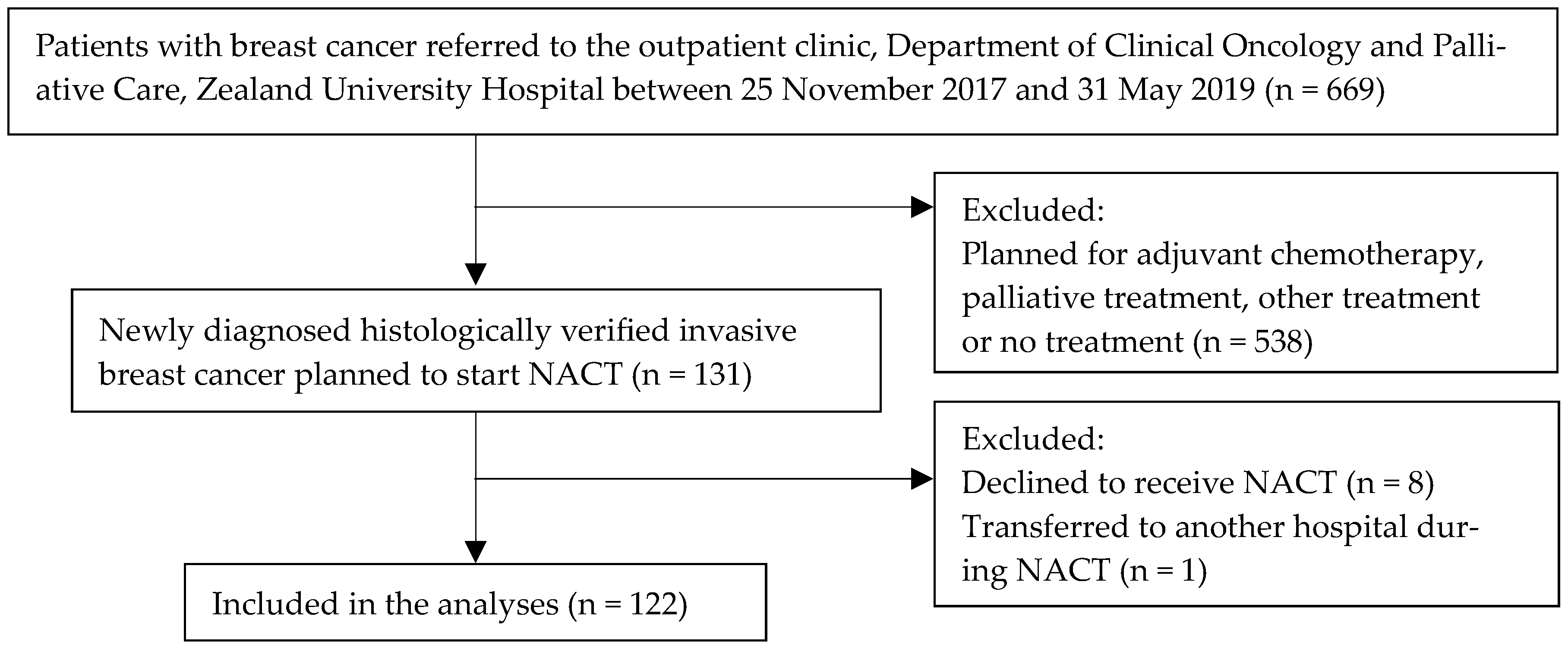
3. Results
3.1. Descriptive Characteristics
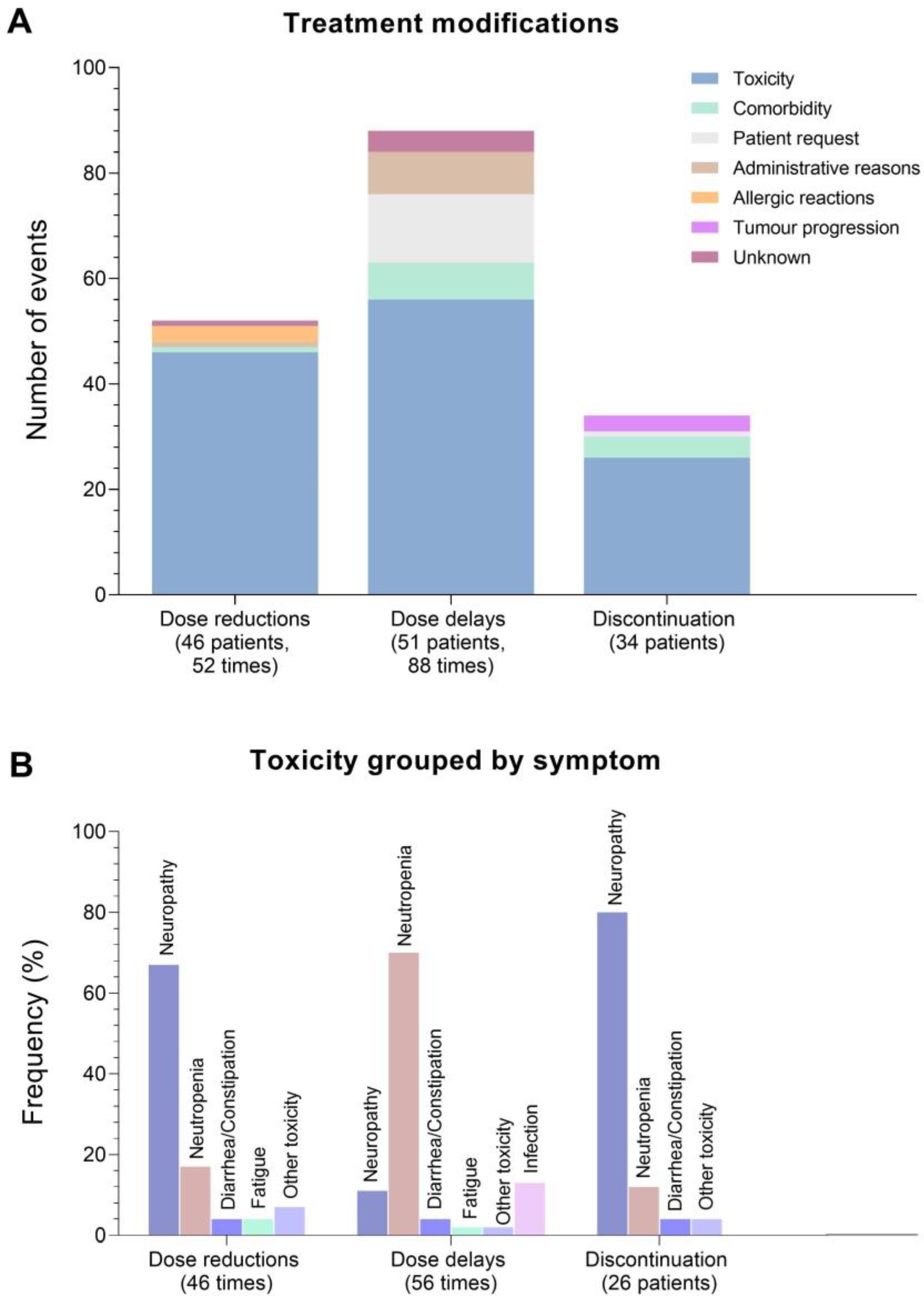
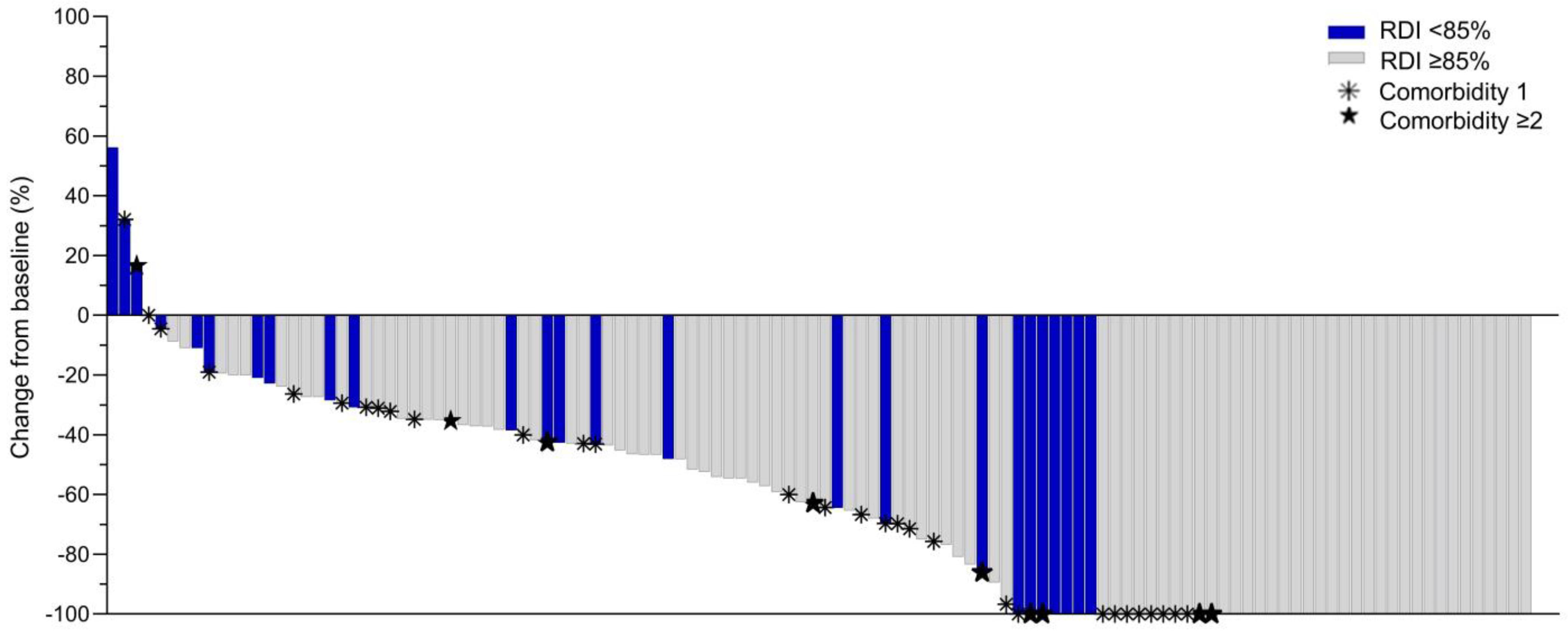
3.2. Treatment Modifications and Relative Dose Intensity
3.3. Tumour Response to Neoadjuvant Chemotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Suppan, C.; Posch, F.; Mueller, H.D.; Mischitz, N.; Steiner, D.; Klocker, E.V.; Setaffy, L.; Bargfrieder, U.; Hammer, R.; Hauser, H.; et al. Patterns of Recurrence after Neoadjuvant Therapy in Early Breast Cancer, according to the Residual Cancer Burden Index and Reductions in Neoadjuvant Treatment Intensity. Cancers 2021, 13, 2492. [Google Scholar] [CrossRef] [PubMed]
- Denduluri, N.; Lyman, G.H.; Wang, Y.; Morrow, P.K.; Barron, R.; Patt, D.; Bhowmik, D.; Li, X.; Bhor, M.; Fox, P.; et al. Chemotherapy Dose Intensity and Overall Survival Among Patients With Advanced Breast or Ovarian Cancer. Clin. Breast Cancer 2018, 18, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Weycker, D.; Barron, R.; Edelsberg, J.; Kartashov, A.; Lyman, G.H. Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res. Treat. 2012, 133, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Skacel, T.; Nekljudova, V.; Luck, H.J.; Schwenkglenks, M.; Brodowicz, T.; Zielinski, C.; von Minckwitz, G. Evaluating the impact of Relative Total Dose Intensity (RTDI) on patients’ short and long-term outcome in taxane- and anthracycline-based chemotherapy of metastatic breast cancer—a pooled analysis. BMC Cancer 2011, 11, 131. [Google Scholar] [CrossRef]
- Veitch, Z.; Khan, O.F.; Tilley, D.; Tang, P.A.; Ribnikar, D.; Stewart, D.A.; Kostaras, X.; King, K.; Lupichuk, S. Impact of Cumulative Chemotherapy Dose on Survival With Adjuvant FEC-D Chemotherapy for Breast Cancer. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 957–967. [Google Scholar] [CrossRef]
- Nielson, C.M.; Bylsma, L.C.; Fryzek, J.P.; Saad, H.A.; Crawford, J. Relative Dose Intensity of Chemotherapy and Survival in Patients with Advanced Stage Solid Tumor Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e1609–e1618. [Google Scholar] [CrossRef] [PubMed]
- Shayne, M.; Crawford, J.; Dale, D.C.; Culakova, E.; Lyman, G.H. Predictors of reduced dose intensity in patients with early-stage breast cancer receiving adjuvant chemotherapy. Breast Cancer Res. Treat. 2006, 100, 255–262. [Google Scholar] [CrossRef]
- Garg, P.; Rana, F.; Gupta, R.; Buzaianu, E.M.; Guthrie, T.H. Predictors of toxicity and toxicity profile of adjuvant chemotherapy in elderly breast cancer patients. Breast J. 2009, 15, 404–408. [Google Scholar] [CrossRef]
- Lyman, G.H.; Dale, D.C.; Tomita, D.; Whittaker, S.; Crawford, J. A retrospective evaluation of chemotherapy dose intensity and supportive care for early-stage breast cancer in a curative setting. Breast Cancer Res. Treat. 2013, 139, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Ladwa, R.; Kalas, T.; Pathmanathan, S.; Woodward, N.; Wyld, D.; Sanmugarajah, J. Maintaining Dose Intensity of Adjuvant Chemotherapy in Older Patients With Breast Cancer. Clin. Breast Cancer 2018, 18, e1181–e1187. [Google Scholar] [CrossRef] [PubMed]
- Schraa, S.J.; Frerichs, K.A.; Agterof, M.J.; Hunting, J.C.B.; Los, M.; de Jong, P.C. Relative dose intensity as a proxy measure of quality and prognosis in adjuvant chemotherapy for breast cancer in daily clinical practice. Eur. J. Cancer 2017, 79, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Cespedes Feliciano, E.M.; Chen, W.Y.; Lee, V.; Albers, K.B.; Prado, C.M.; Alexeeff, S.; Xiao, J.; Shachar, S.S.; Caan, B.J. Body Composition, Adherence to Anthracycline and Taxane-Based Chemotherapy, and Survival After Nonmetastatic Breast Cancer. JAMA Oncol. 2020, 6, 264–270. [Google Scholar] [CrossRef]
- Usiskin, I.; Li, F.; Irwin, M.L.; Cartmel, B.; Sanft, T. Association of relative dose intensity with BMI and pathologic complete response in patients treated with neoadjuvant chemotherapy for breast cancer. Breast Cancer Res. Treat. 2021, 186, 191–197. [Google Scholar] [CrossRef] [PubMed]
- DBCG. Neoadjuverende Kemoterapi Ved Brystkræft, Klinisk Retningslinje. 2016. Available online: https://www.dbcg.dk/PDF%20Filer/Kap7_Neoadj_KT_ved_brystkraeft_mhp_down-sizing_og_down-staging_04.10.2016.pdf (accessed on 8 November 2022).
- Jensen, M.B.; Laenkholm, A.V.; Offersen, B.V.; Christiansen, P.; Kroman, N.; Mouridsen, H.T.; Ejlertsen, B. The clinical database and implementation of treatment guidelines by the Danish Breast Cancer Cooperative Group in 2007–2016. Acta Oncol. 2018, 57, 13–18. [Google Scholar] [CrossRef]
- Patanwala, A.E. A practical guide to conducting and writing medical record review studies. Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. Health-Syst. Pharm. 2017, 74, 1853–1864. [Google Scholar] [CrossRef]
- Lalami, Y.; Klastersky, J. Impact of chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) on cancer treatment outcomes: An overview about well-established and recently emerging clinical data. Crit. Rev. Oncol./Hematol. 2017, 120, 163–179. [Google Scholar] [CrossRef]
- Wang, L.; Baser, O.; Kutikova, L.; Page, J.H.; Barron, R. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: A systematic review and meta-analysis of randomized controlled trials. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2015, 23, 3131–3140. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, Q.; Wu, X.C.; Hsieh, M.C.; Loch, M.; Chen, V.W.; Fontham, E.; Ferguson, T. Impact of chemotherapy relative dose intensity on cause-specific and overall survival for stage I–III breast cancer: ER+/PR+, HER2- vs. triple-negative. Breast Cancer Res. Treat. 2018, 169, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.Q.; Newcomer, B.J.; Cheung, W.Y. Real-World Use of Granulocyte-Colony Stimulating Factor in Patients with Breast Cancer from Alberta, Canada. Cancers 2022, 14, 6197. [Google Scholar] [CrossRef]
- Bhatnagar, B.; Gilmore, S.; Goloubeva, O.; Pelser, C.; Medeiros, M.; Chumsri, S.; Tkaczuk, K.; Edelman, M.; Bao, T. Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: A single-center experience. SpringerPlus 2014, 3, 366. [Google Scholar] [CrossRef] [PubMed]
- Usiskin, I.; Li, F.; Irwin, M.L.; Cartmel, B.; Sanft, T. Association between pre-diagnosis BMI, physical activity, pathologic complete response, and chemotherapy completion in women treated with neoadjuvant chemotherapy for breast cancer. Breast Cancer 2019, 26, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Sedrak, M.S.; Sun, C.L.; Ji, J.; Cohen, H.J.; Gross, C.P.; Tew, W.P.; Klepin, H.D.; Wildes, T.M.; Dotan, E.; Freedman, R.A.; et al. Low-Intensity Adjuvant Chemotherapy for Breast Cancer in Older Women: Results From the Prospective Multicenter HOPE Trial. J. Clin. Oncol. 2022, 41, 316–326. [Google Scholar] [CrossRef]
- Qi, W.; Wang, X.; Gan, L.; Li, Y.; Li, H.; Cheng, Q. The effect of reduced RDI of chemotherapy on the outcome of breast cancer patients. Sci. Rep. 2020, 10, 13241. [Google Scholar] [CrossRef]
- Hourdequin, K.C.; Schpero, W.L.; McKenna, D.R.; Piazik, B.L.; Larson, R.J. Toxic effect of chemotherapy dosing using actual body weight in obese versus normal-weight patients: A systematic review and meta-analysis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 2952–2962. [Google Scholar] [CrossRef]
- Shayne, M.; Harvey, R.D.; Lyman, G.H. Prophylaxis and treatment strategies for optimizing chemotherapy relative dose intensity. Expert Rev. Anticancer Ther. 2021, 21, 1145–1159. [Google Scholar] [CrossRef]
- Solans, B.P.; Garrido, M.J.; Trocóniz, I.F. Drug Exposure to Establish Pharmacokinetic-Response Relationships in Oncology. Clin Pharm. 2020, 59, 123–135. [Google Scholar] [CrossRef]
- Denduluri, N.; Patt, D.A.; Wang, Y.; Bhor, M.; Li, X.; Favret, A.M.; Morrow, P.K.; Barron, R.L.; Asmar, L.; Saravanan, S.; et al. Dose Delays, Dose Reductions, and Relative Dose Intensity in Patients With Cancer Who Received Adjuvant or Neoadjuvant Chemotherapy in Community Oncology Practices. J. Natl. Compr. Cancer Netw. JNCCN 2015, 13, 1383–1393. [Google Scholar] [CrossRef]
- Gampenrieder, S.P.; Peer, A.; Weismann, C.; Meissnitzer, M.; Rinnerthaler, G.; Webhofer, J.; Westphal, T.; Riedmann, M.; Meissnitzer, T.; Egger, H.; et al. Radiologic complete response (rCR) in contrast-enhanced magnetic resonance imaging (CE-MRI) after neoadjuvant chemotherapy for early breast cancer predicts recurrence-free survival but not pathologic complete response (pCR). Breast Cancer Res. BCR 2019, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- De Los Santos, J.F.; Cantor, A.; Amos, K.D.; Forero, A.; Golshan, M.; Horton, J.K.; Hudis, C.A.; Hylton, N.M.; McGuire, K.; Meric-Bernstam, F.; et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer 2013, 119, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Macaskill, P.; von Minckwitz, G.; Marinovich, M.L.; Mamounas, E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur. J. Cancer 2012, 48, 3342–3354. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Sim, S.H.; Park, B.; Chae, I.H.; Han, J.H.; Jung, S.Y.; Lee, S.; Kwon, Y.; Park, I.H.; Ko, K.; et al. Criteria for identifying residual tumours after neoadjuvant chemotherapy of breast cancers: A magnetic resonance imaging study. Sci. Rep. 2021, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, M.; Basch, E.; Denis, F.; Fallowfield, L.J.; Ganz, P.A.; Howell, D.; Kowalski, C.; Perrone, F.; Stover, A.M.; Sundaresan, P.; et al. The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO Clinical Practice Guideline. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennett, A.V.; Dueck, A.C.; Atkinson, T.M.; et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Schauer, T.; Henriksson, A.; Strandberg, E.; Lindman, H.; Berntsen, S.; Demmelmaier, I.; Raastad, T.; Nordin, K.; Christensen, J.F. Pre-treatment levels of inflammatory markers and chemotherapy completion rates in patients with early-stage breast cancer. Int. J. Clin. Oncol. 2023, 28, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Bland, K.A.; Zadravec, K.; Landry, T.; Weller, S.; Meyers, L.; Campbell, K.L. Impact of exercise on chemotherapy completion rate: A systematic review of the evidence and recommendations for future exercise oncology research. Crit. Rev. Oncol./Hematol. 2019, 136, 79–85. [Google Scholar] [CrossRef]
- Courneya, K.S.; Segal, R.J.; Mackey, J.R.; Gelmon, K.; Reid, R.D.; Friedenreich, C.M.; Ladha, A.B.; Proulx, C.; Vallance, J.K.; Lane, K.; et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J. Clin. Oncol. 2007, 25, 4396–4404. [Google Scholar] [CrossRef]
- van Waart, H.; Stuiver, M.M.; van Harten, W.H.; Geleijn, E.; Kieffer, J.M.; Buffart, L.M.; de Maaker-Berkhof, M.; Boven, E.; Schrama, J.; Geenen, M.M.; et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J. Clin. Oncol. 2015, 33, 1918–1927. [Google Scholar] [CrossRef]
- Mijwel, S.; Bolam, K.A.; Gerrevall, J.; Foukakis, T.; Wengstrom, Y.; Rundqvist, H. Effects of Exercise on Chemotherapy Completion and Hospitalization Rates: The OptiTrain Breast Cancer Trial. Oncologist 2020, 25, 23–32. [Google Scholar] [CrossRef] [PubMed]

| n | % | Median (Range) | |
|---|---|---|---|
| Age (years) | |||
| All | 50 (25–78) | ||
| 25–49 | 54 | (44) | |
| 50–64 | 44 | (36) | |
| 65–78 | 24 | (20) | |
| Cohabitation status | |||
| Living with partner | 85 | (70) | |
| Living alone | 37 | (30) | |
| Work market affiliation | |||
| Working | 71 | (58) | |
| Not working due to illness | 19 | (15) | |
| Age retirement | 29 | (24) | |
| Missing data | 3 | (2) | |
| Body Mass Index (kg/m2) | |||
| All | 26 (17–45) | ||
| Under- and normal weight (<25) | 53 | (44) | |
| Overweight (25–29.9) | 39 | (32) | |
| Obese (≥30) | 30 | (24) | |
| Body surface area for chemotherapy dosing (m2) | |||
| All | 1.9 (1.5–2.5) | ||
| <2.0 | 88 | (72) | |
| ≥2.0 | 34 | (28) | |
| Doses capped at 2.0 m2 (yes, no) | 8, 114 | (7, 93) | |
| Smoking status | |||
| Never smoker | 56 | (46) | |
| Previous smoker | 27 | (22) | |
| Current smoker | 31 | (25) | |
| Missing data | 8 | (6) | |
| Alcohol consumption | |||
| No consumption | 52 | (43) | |
| Any consumption | 52 | (43) | |
| Missing data | 18 | (15) | |
| WHO Performance status | |||
| 0 | 114 | (93) | |
| 1–2 | 8 | (7) | |
| Kidney function (eGFR) | |||
| <90 mL/min/1.73 m2 | 41 | (34) | |
| ≥90 mL/min/1.73 m2 | 81 | (66) | |
| Comorbidity (chronic diseases) | |||
| None * | 81 | (66) | |
| 1 | 32 | (26) | |
| ≥2 | 9 | (7) | |
| Long-term medications | |||
| None * | 54 | (44) | |
| 1–4 different medications | 54 | (44) | |
| ≥5 different medications (polypharmacy) | 14 | (11) | |
| Menopausal status | |||
| Premenopausal | 57 | (47) | |
| Postmenopausal | 65 | (53) | |
| Breast cancer subtype | |||
| ER+HER2 normal | 59 | (48) | |
| ER+HER2+ | 20 | (16) | |
| ER-HER2+ | 20 | (16) | |
| ER-HER2 normal | 23 | (19) | |
| Positive lymph node involvement | |||
| None | 40 | (33) | |
| Suspected | 13 | (11) | |
| Diagnosed by MRI and/or biopsy | 69 | (56) | |
| Planned chemotherapy treatment regimen | |||
| EC (3 cycles) + PAC (3 cycles) | 59 | (48) | |
| EC (4 cycles) + PAC (4 cycles) | 61 | (50) | |
| Other/part of a regimen | 2 | (2) | |
| Concomitant HER2 blockade (trastuzumab and pertuzumab) | |||
| Yes | 40 | (33) | |
| No | 82 | (67) | |
| n | % | Median (Range) | |
|---|---|---|---|
| Relative Dose Intensity | |||
| All | 122 | (100) | 95.23 (8.33–100) |
| <70% | 11 | (9) | |
| 70 to 84.99% | 19 | (16) | |
| 85 to 94.99% | 29 | (24) | |
| 95 to 99.99% | 28 | (23) | |
| 100% | 35 | (28) |
| Dose Reductions (Yes vs. No) | Dose Delays (Yes vs. No) | Discontinuation (Yes vs. No) | RDI (<85% vs. ≥85%) | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | ||||||||
| Covariate | Crude | Adjusted a | Crude | Adjusted a | Crude | Adjusted a | Crude | Adjusted a |
| Age in years | ||||||||
| 25–64 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 65–78 | 3.3 (1.3–8.4) * | 2.4 (0.9–6.8) | 1.0 (0.4–2.4) | 1.1 (0.4–3.1) | 1.4 (0.5–3.6) | 1.2 (0.4–3.3) | 3.5 (1.4–9.0) * | 3.0 (1.1–8.7) * |
| Cohabitation status | ||||||||
| Living with partner | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Living alone | 1.6 (0.7–3.4) | 1.5 (0.6–3.7) | 0.6 (0.2–1.3) | 0.7 (0.3–1.6) | 0.8 (0.3–1.9) | 0.7 (0.3–1.7) | 1.2 (0.5–2.9) | 1.1 (0.4–3.0) |
| Work market affiliation | ||||||||
| Working | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Not working due to illness | 2.5 (0.9–6.9) | 1.8 (0.6–5.4) | 1.4 (0.5–3.8) | 1.3 (0.4–3.9) | 2.5 (0.8–7.3) | 2.3 (0.8–6.9) | 1.8 (0.5–6.3) | 1.5 (0.4–5.3) |
| Age retirement | 4.9 (1.9–12.6) * | 1.1 (0.2–4.8) | 1.4 (0.6–3.4) | 2.7 (0.6–12.2) | 1.8 (0.7–4.7) | 1.0 (0.2–4.8) | 5.4 (2.0–14.5) * | 2.3 (0.4–11.2) |
| Body Mass Index b | ||||||||
| Underweight/Normal | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Overweight | 1.2 (0.5–2.8) | 1.7 (0.6–4.4) | 0.6 (0.2–1.4) | 0.8 (0.3–1.9) | 2.6 (1.0–6.7) * | 2.8 (1.1–7.4) * | 1.2 (0.5–3.0) | 1.6 (0.6–4.4) |
| Obese | 0.7 (0.3–1.9) | 1.5 (0.5–4.4) | 0.4 (0.1–1.0) | 0.7 (0.2–2.1) | 1.2 (0.4–3.4) | 1.4 (0.4–4.4) | 0.4 (0.1–1.4) | 0.8 (0.2–2.9) |
| Body surface area | ||||||||
| <2 m2 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| ≥2 m2 | 1.2 (0.5–2.7) | 2.0 (0.8–5.0) | 1.0 (0.4–2.1) | 1.5 (0.6–3.6) | 2.0 (0.8–4.6) | 2.2 (0.9–1.1) | 1.1 (0.5–2.8) | 1.7 (0.6–4.9) |
| Comorbidity | ||||||||
| None | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 1 | 3.3 (1.4–7.8) * | 3.4 (1.3–8.9) * | 1.3 (0.6–3.0) | 1.7 (0.7–4.2) | 0.8 (0.3–2.1) | 0.7 (0.3–2.0) | 1.3 (0.5–3.6) | 1.1 (0.4–3.2) |
| ≥2 | 4.0 (0.9–17.2) | 2.1 (0.4–11.0) | 1.2 (0.3–4.9) | 1.4 (0.3–6.8) | 5.7 (1.3–24.9) * | 4.8 (1.0–22.8) * | 8.1 (1.8–36.0) * | 5.1 (1.0–26.4) * |
| Long term medications | ||||||||
| None | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 1–4 different medications | 4.4 (1.9–10.0) * | 4.1 (1.6–10.8) * | 0.6 (0.3–1.3) | 0.5 (0.2–1.3) | 1.7 (0.7–4.2) | 1.8 (0.7–4.6) | 1.2 (0.5–3.2) | 0.8 (0.3–2.4) |
| ≥5 different medications | 12.8 (3.0–53.5) * | 8.4 (1.8–39.9) * | 1.5 (0.5–5.0) | 1.6 (0.4–6.4) | 7.9 (2.2–28.8) * | 8.2 (1.9–34.3) * | 5.9 (1.7–20.7) * | 3.0 (0.7–12.5) |
| Breast cancer subtype | ||||||||
| ER+HER2 normal | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| ER+HER2+ | 1.0 (0.4–2.9) | 0.6 (0.2–2.0) | 1.9 (0.7–5.4) | 1.4 (0.4–4.3) | 2.4 (0.8–6.9) | 2.3 (0.8–6.8) | 3.3 (1.1–10.0) * | 2.4 (0.7–8.2) |
| ER-HER2+ | 1.0 (0.4–2.9) | 1.5 (0.4–4.8) | 1.9 (0.7–5.4) | 1.3 (0.4–4.3) | 0.7 (0.2–2.5) | 0.8 (0.2–3.1) | 1.2 (0.3–4.4) | 1.6 (0.4–6.8) |
| ER-HER2 normal | 0.8 (0.3–2.2) | 0.9 (0.3–2.7) | 1.8 (0.7–4.8) | 1.5 (0.5–4.2) | 1.0 (0.3–3.1) | 1.1 (0.4–3.4) | 2.6 (0.9–7.8) | 3.4 (0.9–11.1) |
| Tumour size | ||||||||
| <50 mm | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| ≥50 mm | 2.2 (0.7–6.2) | 1.5 (0.5–4.9) | 1.9 (0.6–5.3) | 1.7 (0.5–5.2) | 1.2 (0.4–3.7) | 1.0 (0.3–3.4) | 3.1 (1.0–9.5) * | 2.3 (0.7–7.6) |
| Positive lymph node involvement c | ||||||||
| No | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes | 1.4 (0.7–3.1) | 1.3 (0.5–3.0) | 2.1 (0.9–4.7) | 2.3 (1.0–5.6) * | 1.5 (0.6–3.6) | 1.4 (0.6–3.5) | 1.5 (0.6–3.7) | 1.3 (0.5–3.5) |
| rCR | Non-rCR | pCR (Grade I) | Non-pCR (Grade II–IV) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | p-Value * | n | (%) | n | (%) | p-Value * | |
| All (n = 118) | 43 | (36) | 75 | (64) | 0.35 | 41 | (35) | 75 | (65) | >0.99 |
| RDI ≥ 85% (n = 92) | 36 | (39) | 56 | (61) | 32 | (35) | 58 | (65) | ||
| RDI < 85% (n = 26) | 7 | (27) | 19 | (73) | 9 | (35) | 17 | (65) | ||
| ER+HER2 normal (n = 59) ** | 10 | (17) | 49 | (83) | >0.99 | 7 | (12) | 50 | (88) | >0.99 |
| RDI ≥ 85% (n = 49) | 9 | (18) | 40 | (82) | 6 | (13) | 41 | (87) | ||
| RDI < 85% (n = 10) | 1 | (10) | 9 | (90) | 1 | (10) | 9 | (90) | ||
| ER+HER2+ (n = 20) | 10 | (50) | 10 | (50) | 0.65 | 9 | (45) | 11 | (55) | >0.99 |
| RDI ≥ 85% (n = 12) | 7 | (58) | 5 | (42) | 5 | (42) | 7 | (58) | ||
| RDI < 85% (n = 8) | 3 | (38) | 5 | (62) | 4 | (50) | 4 | (50) | ||
| ER-HER2 normal (n = 21) | 9 | (43) | 12 | (57) | 0.65 | 10 | (48) | 11 | (52) | 0.63 |
| RDI ≥ 85% (n = 15) | 7 | (47) | 8 | (53) | 8 | (53) | 7 | (47) | ||
| RDI < 85% (n = 6) | 2 | (33) | 4 | (67) | 2 | (33) | 4 | (67) | ||
| ER-HER2+ (n = 18) | 14 | (78) | 4 | (22) | 0.40 | 15 | (83) | 3 | (17) | >0.99 |
| RDI ≥ 85% (n = 16) | 13 | (81) | 3 | (19) | 13 | (81) | 3 | (19) | ||
| RDI < 85% (n = 2) | 1 | (50) | 1 | (50) | 2 | (100) | 0 | (0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kjeldsted, E.; Gehl, J.; Sørensen, D.M.; Lodin, A.; Ceballos, S.G.; Dalton, S.O. Patient-Related Characteristics Associated with Treatment Modifications and Suboptimal Relative Dose Intensity of Neoadjuvant Chemotherapy in Patients with Breast Cancer—A Retrospective Study. Cancers 2023, 15, 2483. https://doi.org/10.3390/cancers15092483
Kjeldsted E, Gehl J, Sørensen DM, Lodin A, Ceballos SG, Dalton SO. Patient-Related Characteristics Associated with Treatment Modifications and Suboptimal Relative Dose Intensity of Neoadjuvant Chemotherapy in Patients with Breast Cancer—A Retrospective Study. Cancers. 2023; 15(9):2483. https://doi.org/10.3390/cancers15092483
Chicago/Turabian StyleKjeldsted, Eva, Julie Gehl, Dina Melanie Sørensen, Alexey Lodin, Silvia Gonzalez Ceballos, and Susanne Oksbjerg Dalton. 2023. "Patient-Related Characteristics Associated with Treatment Modifications and Suboptimal Relative Dose Intensity of Neoadjuvant Chemotherapy in Patients with Breast Cancer—A Retrospective Study" Cancers 15, no. 9: 2483. https://doi.org/10.3390/cancers15092483
APA StyleKjeldsted, E., Gehl, J., Sørensen, D. M., Lodin, A., Ceballos, S. G., & Dalton, S. O. (2023). Patient-Related Characteristics Associated with Treatment Modifications and Suboptimal Relative Dose Intensity of Neoadjuvant Chemotherapy in Patients with Breast Cancer—A Retrospective Study. Cancers, 15(9), 2483. https://doi.org/10.3390/cancers15092483







