Baveno VII Criteria Is an Accurate Risk Stratification Tool to Predict High-Risk Varices Requiring Intervention and Hepatic Events in Patients with Advanced Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design Overview and Subjects
2.2. Clinical and Laboratory Evaluation
2.3. Transient Elastography Examination
2.4. Upper Endoscopy
2.5. Systemic Therapies for HCC
2.6. Clinical Assessments during Follow-Up
2.7. Clinical Outcomes
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Liver Stiffness Measurement
3.3. Baveno Criteria and Other Combinations of LSM and PLT
3.4. Varices in Patients Who Did or Did Not Receive Systemic Therapies
3.5. VNT and Baveno VII Criteria
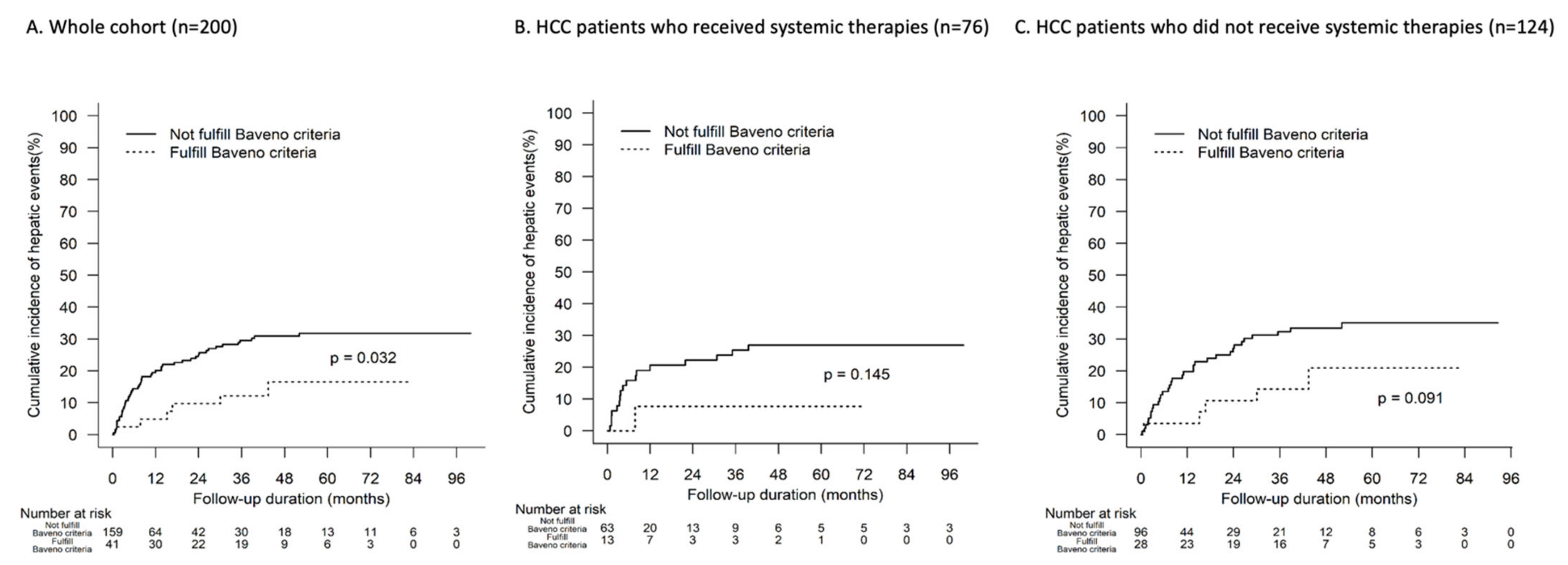
3.6. Hepatic Events and Baveno VII Criteria
3.7. Predictors of VNT and Hepatic Events
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giannini, E.G.; Risso, D.; Testa, R.; Trevisani, F.; Di Nolfo, M.A.; Del Poggio, P.; Benvegnù, L.; Rapaccini, G.L.; Farinati, F.; Zoli, M.; et al. Prevalence and prognostic significance of the presence of esophageal varices in patients with hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2006, 4, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Garcia-Tsao, G.; Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006, 44, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.Y.; Chen, P.H.; Lin, I.Y.; Su, C.W.; Chao, Y.; Huo, T.I.; Huang, Y.-H.; Hou, M.-C.; Lin, H.-C.; Wu, J.-C. The impact of esophagogastric varices on the prognosis of patients with hepatocellular carcinoma. Sci. Rep. 2017, 7, 42577. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Iavarone, M.; Primignani, M.; Vavassori, S.; Sangiovanni, A.; La Mura, V.; Romeo, R.; Colombo, M. Determinants of esophageal varices bleeding in patients with advanced hepatocellular carcinoma treated with sorafenib. United Eur. Gastroenterol. J. 2016, 4, 363–370. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef]
- Govalan, R.; Luu, M.; Lauzon, M.; Kosari, K.; Ahn, J.C.; Rich, N.E.; Nissen, N.; Roberts, L.; Singal, A.; Yang, J.D. Therapeutic Underuse and Delay in Hepatocellular Carcinoma: Prevalence, Associated Factors, and Clinical Impact. Hepatol. Commun. 2022, 6, 223–236. [Google Scholar] [CrossRef]
- Alvi, H.; Zuberi, B.F.; Rasheed, T.; Ibrahim, M.A. Evaluation of endoscopic variceal band ligation sessions in obliteration of esophageal varices. Pak. J. Med. Sci. Q. 2020, 36, 37–41. [Google Scholar]
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J. Hepatol. 2021, 75, 960–974. [Google Scholar] [CrossRef]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Baveno VII Faculty. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Chen, S.; Li, W. Prognostic value of liver stiffness measurement in patients with hepatocellular carcinoma (HCC) treated by radiofrequency ablation: A meta-analysis. Int. J. Hyperthermia. 2021, 38, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, J.; Zhou, T.; Cao, H.; Tan, B. Prognostic value of liver stiffness measurement for the liver-related surgical outcomes of patients under hepatic resection: A meta-analysis. PLoS ONE 2018, 13, e0190512. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address: Easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Yip, T.C.F.; Lee, H.W.; Chan, W.K.; Wong, G.L.H.; Wong, V.W.S. Asian perspective on NAFLD-associated HCC. J. Hepatol. 2022, 76, 726–734. [Google Scholar] [CrossRef]
- Castéra, L.; Le Bail, B.; Roudot-Thoraval, F.; Bernard, P.H.; Foucher, J.; Merrouche, W.; Couzigou, P.; de Lédinghen, V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: Comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J. Hepatol. 2009, 50, 59–68. [Google Scholar] [CrossRef]
- Wong, G.L.H. Non-invasive assessments for liver fibrosis: The crystal ball we long for. J. Gastroenterol. Hepatol. 2018, 33, 1009–1015. [Google Scholar] [CrossRef]
- Wong, G.L.H.; Kwok, R.; Hui, A.J.; Tse, Y.K.; Ho, K.T.; Lo, A.O.S.; Lam, K.L.Y.; Chan, H.C.H.; Lui, R.A.; Au, K.H.D.; et al. A new screening strategy for varices by liver and spleen stiffness measurement (LSSM) in cirrhotic patients: A randomized trial. Liver Int. 2018, 38, 636–644. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Sanyal, A.J.; Grace, N.D.; Carey, W. Practice Guidelines Committee of the American Association for the Study of Liver Diseases, Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007, 46, 922–938. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017, 38, e16–e47. [Google Scholar]
- Allaire, M.; Rudler, M.; Thabut, D. Portal hypertension and hepatocellular carcinoma: Des liaisons dangereuse. Liver Int. 2021, 41, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.H.; Yip, T.C.F.; Wong, A.; Chan, B.; Fong, M.; Wong, V.W.S.; Chan, S.L.; Chan, B.; Ng, K. Baveno VII criteria identify varices needing treatment in patients with hepatocellular carcinoma of different BCLC stages undergoing curative hepatectomy. J. Hepatol. 2022, 77, S933–S934. [Google Scholar] [CrossRef]
- Szakács, Z.; Erőss, B.; Soós, A.; Mátrai, P.; Szabó, I.; Pétervári, E.; Bajor, J.; Farkas, N.; Hegyi, P.; Illés, A.; et al. Baveno Criteria Safely Identify Patients with Compensated Advanced Chronic Liver Disease Who Can Avoid Variceal Screening Endoscopy: A Diagnostic Test Accuracy Meta-Analysis. Front. Physiol. 2019, 10, 1028. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.H.; Liang, L.Y.; Kwok, R.; Hui, A.J.; Tse, Y.K.; Chan, H.L.Y.; Wong, V.W.-S. Low Risk of Variceal Bleeding in Patients with Cirrhosis After Variceal Screening Stratified by Liver/Spleen Stiffness. Hepatology 2019, 70, 971–981. [Google Scholar] [CrossRef]
- Lim, J.; Kim, H.I.; Kim, E.; Kim, J.; An, J.; Chang, S.; Kim, S.-O.; Lee, H.C.; Lee, Y.S.; Shim, J.H. Variceal bleeding is aggravated by portal venous invasion of hepatocellular carcinoma: A matched nested case-control study. BMC Cancer 2021, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Chan, H.L.Y.; Chien, R.N.; Chuang, W.L.; Fung, J.; Goh, G.B.B.; Hu, T.; Huang, J.-F.; Jang, B.; Jun, D.; et al. Modelling NAFLD disease burden in four Asian regions-2019–2030. Aliment. Pharmacol. Ther. 2020, 51, 801–811. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, H.; Tian, M.; Chu, J.; Tian, C.; Yang, Y.; Lin, S. Validation and Refinement of the Baveno VI Criteria for Ruling Out High-Risk Varices. Gastroenterol. Res. Pract. 2020, 2020, 4217512. [Google Scholar] [CrossRef]
- Pons, M.; Augustin, S.; Scheiner, B.; Guillaume, M.; Rosselli, M.; Rodrigues, S.G.; Stefanescu, H.; Ma, M.; Mandorfer, M.; Mergeay-Fabre, M.; et al. Noninvasive Diagnosis of Portal Hypertension in Patients with Compensated Advanced Chronic Liver Disease. Am. J. Gastroenterol. 2021, 116, 723–732. [Google Scholar] [CrossRef]
- Perazzo, H.; Veloso, V.G.; Grinsztejn, B.; Hyde, C.; Castro, R. Factors That Could Impact on Liver Fibrosis Staging by Transient Elastography. Int. J. Hepatol. 2015, 2015, 624596. [Google Scholar] [CrossRef]
- Poynard, T.; Lebrec, D.; Hillon, P.; Sayegh, R.; Bernuau, J.; Naveau, S.; Chaput, J.C.; Lepping, C.K.; Rueff, B.; Benhamou, J.P. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: A prospective study of factors associated with rebleeding. Hepatology 1987, 7, 447–451. [Google Scholar] [CrossRef]

| All Patients N = 200 | Systemic Therapies N = 76 | No Systemic Therapies N = 124 | p-Value | |
|---|---|---|---|---|
| Follow-up duration (months) | 11.9 (4.6–39.6) | 8.5 (4.7–21.3) | 15.6 (4.5–42.2) | 0.066 |
| Male gender | 161 (80.5%) | 64 (84.2%) | 97 (78.2%) | 0.3 |
| Age (year) | 61.3 (11.3) | 58.0 (10.4) | 63.4 (11.3) | <0.001 |
| Chronic hepatitis B | 139 (69.5%) | 54 (71.1%) | 85 (68.5%) | 0.709 |
| Chronic hepatitis C | 15 (7.5%) | 7 (9.2%) | 8 (6.5%) | 0.472 |
| Platelet (×109/L) | 204.1 (113.6) | 214.2 (117.9) | 197.9 (110.8) | 0.328 |
| ≥150 × 109/L | 125 (62.5%) | 53 (69.7%) | 72 (58.1%) | 0.098 |
| ≥110 × 109/L | 159 (79.5%) | 64 (84.2%) | 95 (76.6%) | 0.196 |
| Prothrombin time (s) | 11.9 (1.6) | 11.9 (1.0) | 12.0 (1.9) | 0.738 |
| Creatinine (μmol/L) | 82.9 (31.2) | 80.8 (38.4) | 84.2 (26.0) | 0.455 |
| Albumin (g/l) | 37.0 (5.3) | 37.3 (5.1) | 36.9 (5.4) | 0.574 |
| Total bilirubin (μmol/L) | 20.4 (16.2) | 19.3 (13.2) | 20.9 (17.8) | 0.626 |
| ALT (IU/L) | ||||
| Median (IQR) | 45 (32–67) | 42 (33.5–52.5) | 46.5 (30.5–75) | 0.160 |
| AFP (μg/L) | ||||
| Median (IQR) | 213 (11–7757) | 1526 (22–44,248) | 56 (7.5–2085) | 0.005 |
| CTP grade | ||||
| A | 135 (67.5%) | 54 (71.1%) | 81 (65.3%) | 0.401 |
| B | 64 (32.0%) | 22 (28.9%) | 42 (33.9%) | 0.469 |
| C | 1 (0.5%) | 1 (0.8%) | 0 (0%) | 1.000 |
| CTP score | 6 (5–7) | 6 (5–7) | 6 (5–7) | 0.272 |
| BCLC | ||||
| A | 25 (12.5%) | 7 (9.2%) | 18 (14.5%) | 0.271 |
| Fulfil Baveno criteria (n = 41) | 3 (7.3%) | 1 (7.7%) | 2 (7.1%) | 1.000 |
| Not fulfil Baveno criteria (n = 159) | 22 (13.8%) | 6 (9.5%) | 16 (16.7%) | 0.202 |
| B | 51 (25.5%) | 19 (25%) | 32 (25.8%) | 0.899 |
| Fulfil Baveno criteria (n = 41) | 14 (34.1%) | 3 (23.1%) | 11 (39.3%) | 0.481 |
| Not fulfil Baveno criteria (n = 159) | 37 (23.3%) | 16 (25.4%) | 21 (21.9%) | 0.607 |
| C | 124 (62%) | 50 (65.8%) | 74 (59.7%) | 0.387 |
| Fulfil Baveno criteria (n = 41) | 24 (58.5%) | 9 (69.2%) | 15 (53.6%) | 0.344 |
| Not fulfil Baveno criteria (n = 159) | 100 (62.9%) | 41 (65.1%) | 59 (61.5%) | 0.644 |
| Combination with immunotherapy | 3 (1.5%) | 3 (3.9%) | 0 (0%) | 0.054 |
| LSM (kPa) Missing (%) | 25.4 (11.9–66.8) 12% | 32.4 (16.5–69.1) 14.5% | 21.8 (11.1–65.2) 10.5% | 0.117 |
| IQR/LSM (%) Missing (%) | 15.2 (7.3–22.6) 21.2% | 14.2 (7.7–22.8) 23.7% | 15.5 (7.2–21.7) 20.2% | 0.850 |
| With right-sided involvement (n = 91) | 17.2 (10–46.7) | 19.4 (9.0–24.7) | 16.5 (10.0–56.9) | 0.457 |
| IQR/LSM (%) Missing (%) | 16.4 (9.4–22.4) 24.2% | 16.9(13.9–22.9) 34.6% | 16.4 (8.0–21.3) 20% | 0.419 |
| Without right-sided involvement (n = 109) | 35.8 (14.1–75) | 48.4 (24.8–75) | 24.6 (13.5–75) | 0.087 |
| IQR/LSM (%) Missing (%) | 14.0 (5.4–22.6) 19.3% | 13.5 (0–22.7) 18% | 14.4 (6.4–21.7) 20.3 | 0.641 |
| ≤15 kPa | 60 (30%) | 16 (21.1%) | 44 (35.5%) | 0.042 |
| ≤20 kPa | 73 (36.5%) | 21 (27.6%) | 52 (41.9%) | 0.059 |
| ≤25 kPa | 88 (44%) | 27 (35.5%) | 61 (49.2%) | 0.086 |
| LSM and platelet criteria | ||||
| LSM ≤ 15 kPa and PLT > 150 × 109/L | 37 (18.5%) | 12 (15.8%) | 25 (20.2%) | 0.440 |
| LSM ≤ 15 kPa and PLT > 110 × 109/L | 47 (23.5%) | 14 (18.2%) | 34 (27.0%) | 0.185 |
| LSM ≤ 20 kPa and PLT > 150 × 109/L | 41 (20.5%) | 13 (17.1%) | 28 (22.6%) | 0.352 |
| LSM ≤ 20 kPa and PLT > 110 × 109/L | 54 (27%) | 15 (19.7%) | 39 (31.5%) | 0.07 |
| LSM ≤ 25 kPa and PLT > 150 × 109/L | 53 (26.5%) | 17 (22.4%) | 36 (29.0%) | 0.3 |
| LSM ≤ 25 kPa and PLT > 110 × 109/L | 67 (33.5%) | 20 (26.3%) | 47 (37.9%) | 0.092 |
| All Patients N = 200 | Systemic Therapies N = 76 | No Systemic Therapies N = 124 | p-Value | |
|---|---|---|---|---|
| Any varices | 50 (25%) | 18 (23.7%) | 32 (25.8%) | 0.737 |
| Oesophageal varices | 48 (24%) | 18 (23.7%) | 30 (24.2%) | 0.935 |
| Gastric varices | 4 (2%) | 2 (2.6%) | 2 (1.6%) | 0.626 |
| Acute variceal bleeding (AVB) | 11 (5.5%) | 3 (3.9%) | 8 (6.5%) | 0.353 |
| Bleeding oesophageal varices | 8 (3.5%) | 1 (1.3%) | 7 (5.6%) | 0.138 |
| Bleeding gastric varices | 3 (1.5%) | 2 (2.6%) | 1 (0.8%) | 0.559 |
| Endoscopic therapy | 22 (11%) | 9 (11.8%) | 13 (10.5%) | 0.766 |
| Varices needing treatment (VNT) | 45 (22.5%) | 15 (19.7%) | 30 (24.2%) | 0.464 |
| Fulfil Baveno criteria (n = 41) | 7 (17.1%) | 2 (15.4%) | 5 (15.4%) | 1.000 |
| Not fulfil Baveno criteria (n = 159) | 38 (23.9%) | 13 (20.6%) | 25 (26.0%) | 0.434 |
| CTP grade for VNT | ||||
| A | 24 (12%) | 10 (13.2%) | 14 (11.3%) | 0.693 |
| B | 21 (10.5%) | 5 (6.6%) | 16 (12.9%) | 0.157 |
| BCLC | ||||
| A | 8 (4%) | 2 (2.6%) | 6 (4.8%) | 0.712 |
| B | 16 (8%) | 6 (7.9%) | 10 (8.1%) | 0.966 |
| C | 21 (10.5%) | 7 (9.2%) | 14 (11.3%) | 0.641 |
| Portal vein thrombosis (PVT) ** | 75 (37.5%) | 36 (47.4%) | 39 (31.5%) | 0.024 |
| VNT in those with PVT (n = 75) | 12 (16.0%) | 7 (19.4%) | 5 (12.8%) | 0.434 |
| Bleeding risk | 4 (5.3%) | 1 (2.8%) | 3 (7.7%) | 0.616 |
| Without PVT | 125 (62.5%) | 40 (52.6%) | 85 (68.5%) | 0.024 |
| VNT in those without PVT (n = 125) | 33 (26.4%) | 8 (20%) | 25 (29.4%) | 0.265 |
| Bleeding risk | 6 (4.8%) | 2 (5%) | 4 (4.7%) | 1.000 |
| VNT in following subgroups | ||||
| LSM ≤ 15 kPa and PLT ≥ 150 × 109/L | 6 (3%) | 2 (2.6%) | 4 (3.2%) | 1.000 |
| LSM ≤ 15 kPa and PLT ≥ 110 × 109/L | 9 (4.5%) | 3 (3.9%) | 6 (4.8%) | 1.000 |
| LSM ≤ 20 kPa and PLT ≥ 150 × 109/L | 7 (3.5%) | 2 (2.6%) | 5 (4.0%) | 1.000 |
| LSM ≤ 20 kPa and PLT ≥ 110 × 109/L | 10 (5%) | 3 (3.9%) | 7 (5.6%) | 0.745 |
| LSM ≤ 25 kPa and PLT ≥ 150 × 109/L | 7 (3.5%) | 2 (2.6%) | 5 (4.0%) | 0.711 |
| LSM ≤ 25 kPa and PLT ≥ 110 × 109/L | 11 (5.5%) | 4 (5.3%) | 7 (5.6%) | 1.000 |
| Time to VNT (months) | 11.3 (3.9–37.4) | 7.9 (4.2–21.1) | 15.4 (3.8–41.8) | 0.067 |
| All Patients N = 200 | Systemic Therapies N = 76 | No Systemic Therapies N = 124 | p-Value | |
|---|---|---|---|---|
| Any hepatic events * | 56 (28%) | 18 (23.7%) | 38 (30.6%) | 0.287 |
| Acute variceal bleed (AVB) ^ | 11 (5.5%) | 3 (3.9%) | 8 (6.5%) | 0.353 |
| Ascites ^ | 44 (22%) | 13 (17.1%) | 31 (25%) | 0.191 |
| Spontaneous bacterial peritonitis (SBP) ^ | 4 (2%) | 0 (0%) | 4 (3.2%) | 0.300 |
| Hepatic encephalopathy (HE) ^ | 17 (8.5%) | 5 (6.6%) | 12 (9.7%) | 0.446 |
| Hepatorenal syndrome (HRS) ^ | 6 (3%) | 1 (1.3%) | 5 (4.0%) | 0.411 |
| Liver-related death | 128 (64%) | 54 (71.1%) | 74 (59.7%) | 0.104 |
| Fulfil Baveno criteria (n = 41) | 16 (39.0%) | 7 (53.8%) | 9 (32.1%) | 0.185 |
| Not fulfil Baveno criteria (n = 159) | 112 (70.4%) | 47 (74.6%) | 65 (67.7%) | 0.351 |
| Hepatic events in following subgroups | ||||
| LSM ≤ 15 kPa and PLT ≥ 150 × 109/L | 4 (2%) | 1 (1.3%) | 3 (2.4%) | 1.000 |
| LSM ≤ 15 kPa and PLT ≥ 110 × 109/L | 8 (4%) | 1 (1.3%) | 7 (5.6%) | 0.159 |
| LSM ≤ 20 kPa and PLT ≥ 150 × 109/L | 6 (3%) | 1 (1.3%) | 5 (4.0%) | 0.411 |
| LSM ≤ 20 kPa and PLT ≥ 110 × 109/L | 10 (5%) | 1 (1.3%) | 9 (7.3%) | 0.093 |
| LSM ≤ 25 kPa and PLT ≥ 150 × 109/L | 7 (3.5%) | 2 (2.6%) | 5 (4.0%) | 0.711 |
| LSM ≤ 25 kPa and PLT ≥ 110 × 109/L | 12 (6%) | 3 (3.9%) | 9 (7.3%) | 0.541 |
| LSM > 25 kPa | 28 (14%) | 10 (13.2%) | 18 (14.5%) | 0.786 |
| Fulfil Baveno criteria (n =41) | 6 (14.6%) | 1 (7.7%) | 5 (17.9%) | 0.645 |
| Not fulfil Baveno criteria (n = 159) | 50 (31.4%) | 17 (27.0%) | 33 (34.4%) | 0.326 |
| Parameters | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| SHR (95% CI) | p-Value | Adjusted SHR (95% CI) | p-Value | |
| Use of systemic therapy | 0.81 (0.44–1.49) | 0.508 | 1.01 (0.41–2.45) | 0.989 |
| Age | 0.99 (0.97–1.01) | 0.373 | 0.97 (0.94–1.01) | 0.125 |
| Male gender | 1.76 (0.79–3.95) | 0.168 | 1.58 (0.79–3.18) | 0.198 |
| ||||
| Referent | Referent | ||
| 0.97 (0.44–2.14) | 0.945 | 1.17 (0.58–2.38) | 0.663 |
| 0.48 (0.22–1.03) | 0.059 | 0.87 (0.39–1.90) | 0.718 |
| ||||
| Referent | Referent | ||
| 0.63 (0.30–1.32) | 0.224 | 0.83 (0.38–1.83) | 0.646 |
| Presence of PVT | 0.55 (0.29–1.03) | 0.064 | 0.77 (0.34–1.77) | 0.542 |
| Previous hepatectomy or TACE | 1.35 (0.62–2.95) | 0.451 | 0.70 (0.21–2.30) | 0.555 |
| LSM | 0.99 (0.98–1.00) | 0.128 | 1.00 (0.98–1.01) | 0.852 |
| Platelet | 0.99 (0.99–0.99) | <0.001 | 0.99 (0.98–1.00) | 0.001 |
| Baveno criteria | 0.72 (0.33–1.59) | 0.418 | 1.14 (0.33–3.97) | 0.842 |
| Parameters | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| SHR (95% CI) | p-Value | Adjusted SHR (95% CI) | p-Value | |
| Use of systemic therapy | 0.76 (0.44–1.33) | 0.333 | 1.13 (0.55–2.34) | 0.731 |
| Age | 1.02 (0.99–1.04) | 0.2 | 1.01 (0.97–1.04) | 0.672 |
| Male gender | 1.00 (0.52–1.90) | 0.992 | 1.21 (0.62–2.38) | 0.57 |
| ||||
| Referent | Referent | ||
| 0.90 (0.39–2.11) | 0.815 | 1.14 (0.48–2.71) | 0.769 |
| 0.78 (0.37–1.67) | 0.525 | 1.13 (0.47–2.68) | 0.785 |
| ||||
| Referent | Referent | ||
| 1.46 (0.83–2.55) | 0.187 | 1.48 (0.75–2.91) | 0.261 |
| Presence of PVT | 0.81 (0.47–1.39) | 0.448 | 0.83 (0.42–1.64) | 0.589 |
| previous hepatectomy or TACE | 0.61 (0.26–1.45) | 0.264 | 0.48 (0.15–1.48) | 0.199 |
| LSM | 1.00 (0.99–1.01) | 0.668 | 1.01 (0.99–1.02) | 0.349 |
| Platelet | 0.99 (0.99–1.00) | 0.004 | 0.99 (0.99–1.00) | 0.005 |
| Baveno criteria | 0.41 (0.18–0.94) | 0.036 | 0.78 (0.26–2.33) | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.W.-K.; Lui, R.N.-S.; Wong, V.W.-S.; Yam, T.-F.; Yip, T.C.-F.; Liu, K.; Lai, J.C.-T.; Tse, Y.-K.; Mok, T.S.-K.; Chan, H.L.-Y.; et al. Baveno VII Criteria Is an Accurate Risk Stratification Tool to Predict High-Risk Varices Requiring Intervention and Hepatic Events in Patients with Advanced Hepatocellular Carcinoma. Cancers 2023, 15, 2480. https://doi.org/10.3390/cancers15092480
Wu CW-K, Lui RN-S, Wong VW-S, Yam T-F, Yip TC-F, Liu K, Lai JC-T, Tse Y-K, Mok TS-K, Chan HL-Y, et al. Baveno VII Criteria Is an Accurate Risk Stratification Tool to Predict High-Risk Varices Requiring Intervention and Hepatic Events in Patients with Advanced Hepatocellular Carcinoma. Cancers. 2023; 15(9):2480. https://doi.org/10.3390/cancers15092480
Chicago/Turabian StyleWu, Claudia Wing-Kwan, Rashid Nok-Shun Lui, Vincent Wai-Sun Wong, Tsz-Fai Yam, Terry Cheuk-Fung Yip, Ken Liu, Jimmy Che-To Lai, Yee-Kit Tse, Tony Shu-Kam Mok, Henry Lik-Yuen Chan, and et al. 2023. "Baveno VII Criteria Is an Accurate Risk Stratification Tool to Predict High-Risk Varices Requiring Intervention and Hepatic Events in Patients with Advanced Hepatocellular Carcinoma" Cancers 15, no. 9: 2480. https://doi.org/10.3390/cancers15092480
APA StyleWu, C. W.-K., Lui, R. N.-S., Wong, V. W.-S., Yam, T.-F., Yip, T. C.-F., Liu, K., Lai, J. C.-T., Tse, Y.-K., Mok, T. S.-K., Chan, H. L.-Y., Ng, K. K.-C., Wong, G. L.-H., & Chan, S. L. (2023). Baveno VII Criteria Is an Accurate Risk Stratification Tool to Predict High-Risk Varices Requiring Intervention and Hepatic Events in Patients with Advanced Hepatocellular Carcinoma. Cancers, 15(9), 2480. https://doi.org/10.3390/cancers15092480





