The Spinal Cord as Organ of Risk: Assessment for Acute and Subacute Neurological Adverse Effects after Microbeam Radiotherapy in a Rodent Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Small Animal Study
2.2. Dose Calculation, Dose Measurements and Simulation
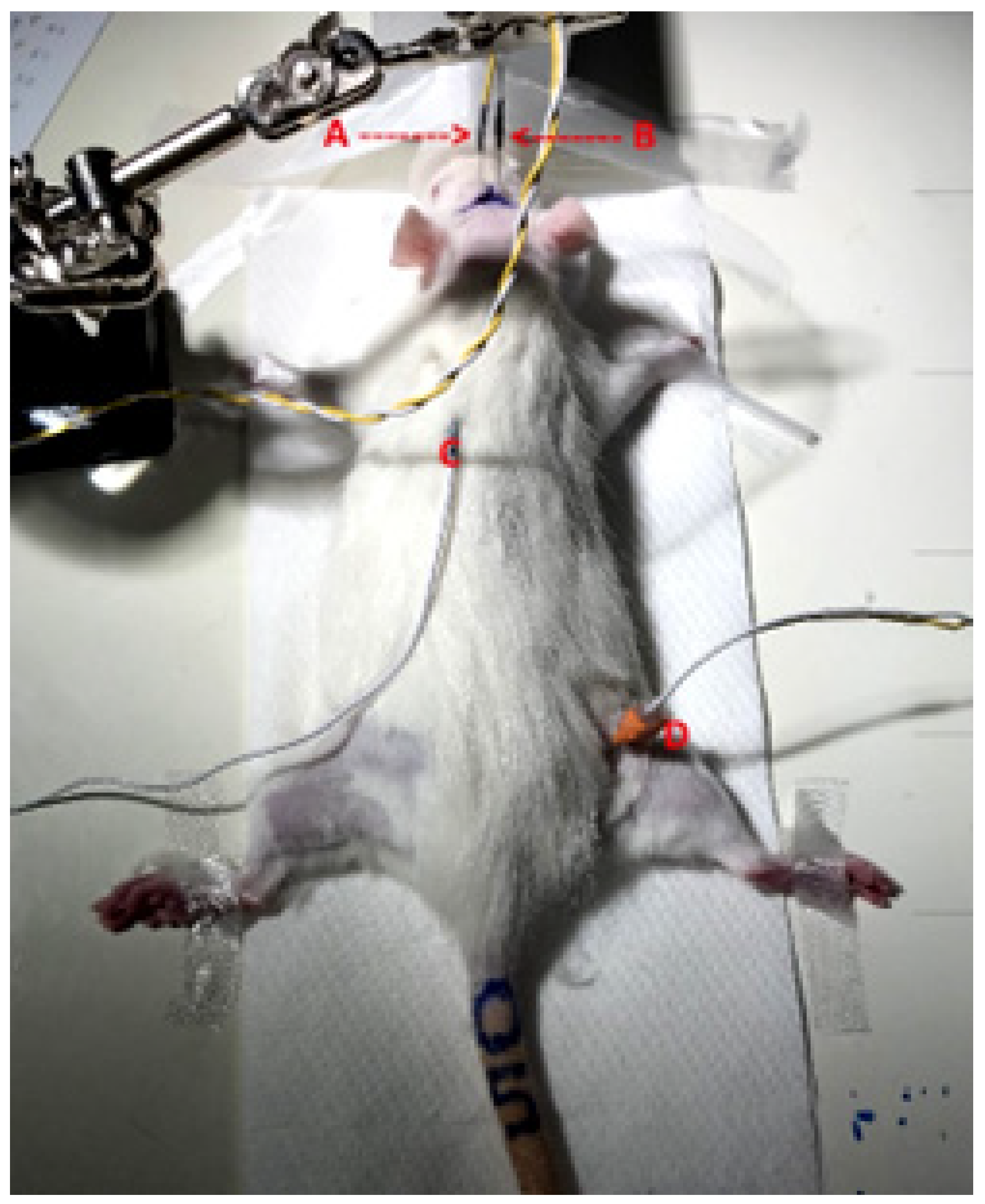
2.3. Microbeam Irradiation Setup
2.4. Neurobehavioural Testing
2.4.1. BBB Scores Assessing Hind Limb Movement and Trunk Stability
2.4.2. Rotarod Performance Test to Assess Balance, Coordination and Endurance
2.4.3. Hind Paw Sensitivity Assessment with Von Frey Filaments
2.5. Electrophysiology
2.6. MRI Imaging
2.7. Statistical Analysis
3. Results
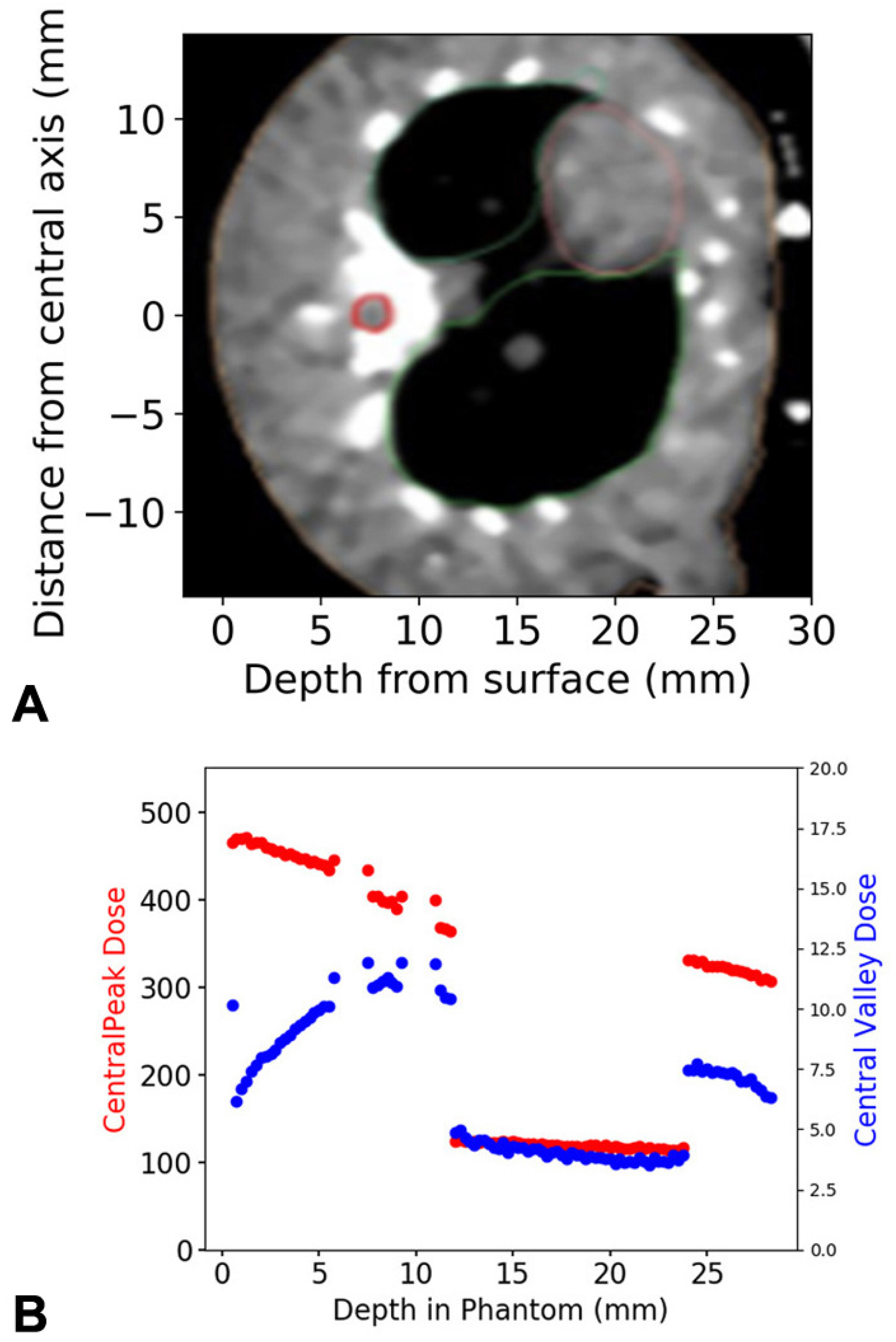
3.1. Dose Calculations, Measurements and Simulations
3.2. Neurologic Signs and Acute Adverse Effects—Toxicity Study
3.3. Motor and Sensitivity Testing of Animals Treated with MRT Peak Doses of 40 Gy and 400 Gy
3.3.1. Rotarod: Assessment of Motor Abilities and Endurance
3.3.2. Von Frey Filaments: Sensitivity Assessment of the Hind Paws
3.4. SSEP
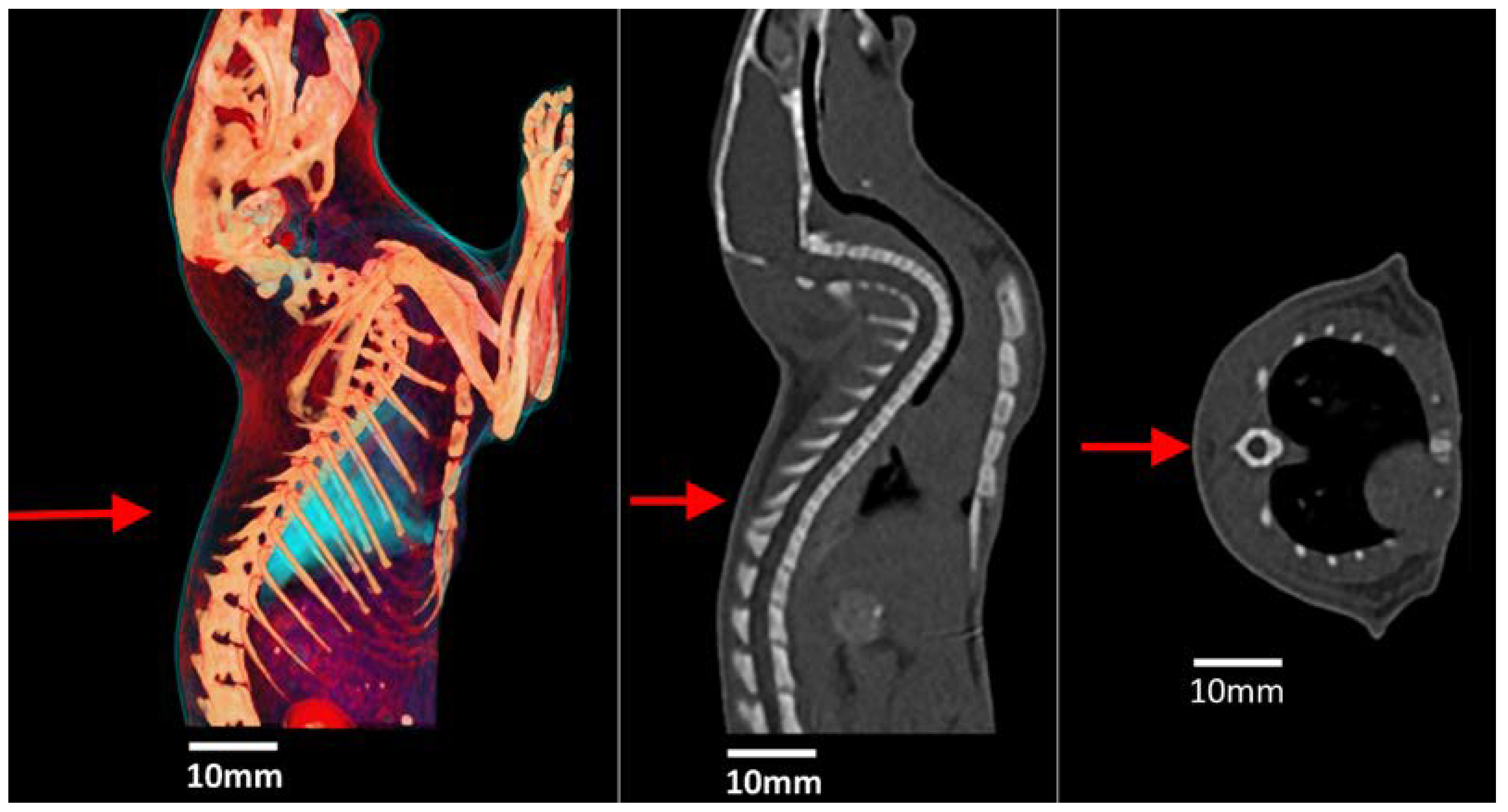
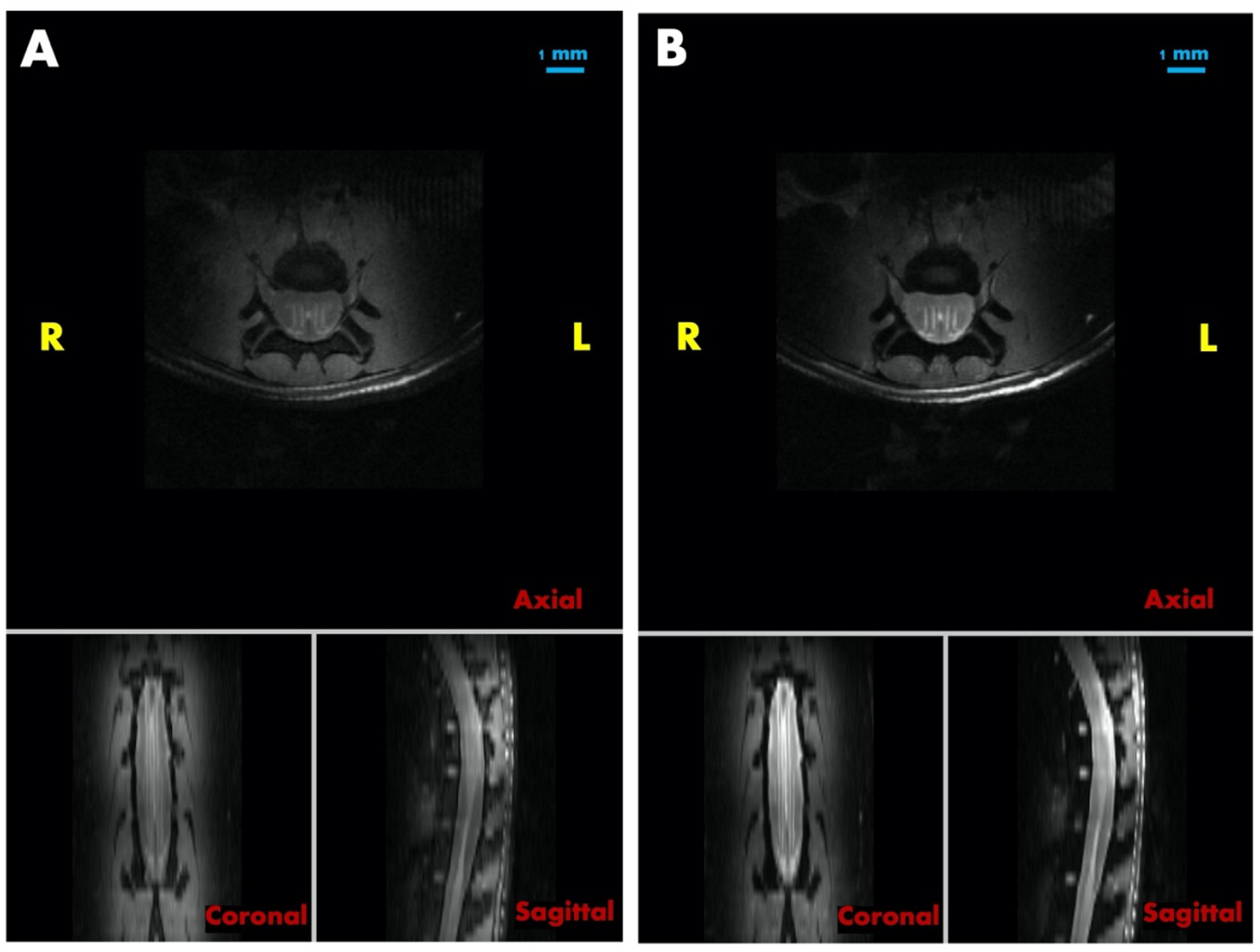
3.5. Post-Mortem MRI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schültke, E.; Balosso, J.; Breslin, T.; Cavaletti, G.; Djonov, V.; Esteve, F.; Grotzer, M.; Hildebrandt, G.; Valdman, A.; Laissue, J. Microbeam radiation therapy—Grid therapy and beyond: A clinical perspective. BJR 2017, 90, 20170073. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.; Li, N.; Davis, J.; Paino, J.; Cameron, M.; Dipuglia, A.; Vogel, S.; Valceski, M.; Khochaiche, A.; O’Keefe, A.; et al. Toward personalized synchrotron microbeam radiation therapy. Sci. Rep. 2020, 10, 8833. [Google Scholar] [CrossRef] [PubMed]
- Potez, M.; Bouchet, A.; Flaender, M.; Rome, C.; Collomb, N.; Grotzer, M.; Krisch, M.; Djonov, V.; Balosso, J.; Brun, E.; et al. Synchrotron X-Ray Boost Delivered by Microbeam Radiation Therapy After Conventional X-Ray Therapy Fractionated in Time Improves F98 Glioma Control. Int. J. Radiat. Oncol. 2020, 107, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Schültke, E.; Bräuer-Krisch, E.; Blattmann, H.; Requardt, H.; Laissue, J.A.; Hildebrandt, G. Survival of rats bearing advanced intracerebral F 98 tumors after glutathione depletion and microbeam radiation therapy: Conclusions from a pilot project. Radiat. Oncol. 2018, 13, 89. [Google Scholar] [CrossRef]
- Bouchet, A.; Bräuer-Krisch, E.; Prezado, Y.; El Atifi, M.; Rogalev, L.; Le Clec’H, C.; Laissue, J.A.; Pelletier, L.; Le Duc, G. Better Efficacy of Synchrotron Spatially Microfractionated Radiation Therapy Than Uniform Radiation Therapy on Glioma. Int. J. Radiat. Oncol. 2016, 95, 1485–1494. [Google Scholar] [CrossRef]
- Trappetti, V.; Fernandez-Palomo, C.; Smyth, L.; Klein, M.; Haberthür, D.; Butler, D.; Barnes, M.; Shintani, N.; de Veer, M.; Laissue, J.A.; et al. Synchrotron Microbeam Radiation Therapy for the Treatment of Lung Carcinoma: A Preclinical Study. Int. J. Radiat. Oncol. 2021, 111, 1276–1288. [Google Scholar] [CrossRef]
- Jaekel, F.; Bräuer-Krisch, E.; Bartzsch, S.; Laissue, J.; Blattmann, H.; Scholz, M.; Soloviova, J.; Hildebrandt, G.; Schültke, E. Microbeam Irradiation as a Simultaneously Integrated Boost in a Conventional Whole-Brain Radiotherapy Protocol. Int. J. Mol. Sci. 2022, 23, 8319. [Google Scholar] [CrossRef]
- Schültke, E.; Jaekel, F.; Bartzsch, S.; Bräuer-Krisch, E.; Requardt, H.; Laissue, J.A.; Blattmann, H.; Hildebrandt, G. Good Timing Matters: The Spatially Fractionated High Dose Rate Boost Should Come First. Cancers 2022, 14, 5964. [Google Scholar] [CrossRef]
- Adam, J.-F.; Balosso, J.; Bayat, S.; Berkvens, P.; Berruyer, G.; Bräuer-Krisch, E.; Brochard, T.; Chamel, G.; Desagneaux, A.; Drevon-Gaud, R.; et al. Toward Neuro-Oncologic Clinical Trials of High-Dose-Rate Synchrotron Microbeam Radiation Therapy: First Treatment of a Spontaneous Canine Brain Tumor. Int. J. Radiat. Oncol. 2022, 113, 967–973. [Google Scholar] [CrossRef]
- Lange, F.; Kirschstein, T.; Davis, J.; Paino, J.; Barnes, M.; Klein, M.; Porath, K.; Stöhlmacher, P.; Fiedler, S.; Frank, M.; et al. Microbeam irradiation of the beating rodent heart: An ex vivo study of acute and subacute effects on cardiac function. Int. J. Rad. Oncol. Biol. Phys. 2022, 114, 143–152. [Google Scholar] [CrossRef]
- Schültke, E.; Lerch, M.; Kirschstein, T.; Lange, F.; Porath, K.; Fiedler, S.; Davis, J.; Paino, J.; Engels, E.; Barnes, M.; et al. Modification of the Langendorff system of the isolated beating heart for experimental radiotherapy at a synchrotron: 4000 Gy in a heart beat. J. Synchrotron Rad. 2022, 29, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Frerker, B.; Fiedler, S.; Kirschstein, T.; Lange, F.; Porath, K.; Sellmann, T.; Kutzner, L.; Wilde, F.; Moosmann, J.; Köhling, R.; et al. Effects of Microbeam Irradiation on Rodent Esophageal Smooth Muscle Contraction. Cells 2022, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, A.W.; Crosbie, J.C.; Hall, C.J.; Häusermann, D.; Livingstone, J.; Lye, J.E. Quantitative characterization of the X-ray beam at the Australian Synchrotron Imaging and Medical Beamline (IMBL). J. Synchrotron Rad. 2017, 24, 110–141. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.; Cornelius, I.; Donzelli, M.; Requardt, H.; Nemoz, C.; Petasecca, M.; Bräuer-Krisch, E.; Rosenfeld, A.; Lerch, M. X-Tream quality assurance in synchrotron X-ray microbeam radiation therapy. J. Synchrotron Rad. 2016, 23, 1180–1190. [Google Scholar] [CrossRef]
- Dipuglia, A.; Cameron, M.; Davis, J.A.; Cornelius, I.M.; Stevenson, A.W.; Rosenfeld, A.B.; Petasecca, M.; Corde, S.; Guatelli, S.; Lerch, M.L.F. Validation of a Monte Carlo simulation for Microbeam Radiation Therapy on the Imaging and Medical Beamline at the Australian Synchrotron. Sci. Rep. 2019, 9, 17696. [Google Scholar] [CrossRef]
- Schültke, E.; Bayat, S.; Bartzsch, S.; Bräuer-Krisch, E.; Djonov, V.; Fiedler, S.; Fernandez-Palomo, C.; Jaekel, F.; Pellicioli, P.; Trappetti, V.; et al. A Mouse Model for Microbeam Radiation Therapy of the Lung. Int. J. Radiat. Oncol. 2020, 110, 521–525. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef]
- Vogel, P. Kursbuch Klinische Neurophysiologie, 3rd ed.; Thieme-Verlag: Lübeck, Germany, 2011; ISBN 9783131888037. [Google Scholar] [CrossRef]
- Sakatani, K.; Iizuka, H.; Young, W. Somatosensory evoked potentials in rat cerebral cortex before and after middle cerebral artery occlusion, 1990. Stroke 1990, 21, 124–132. [Google Scholar] [CrossRef]
- ICRP Publication 110. Adult Reference Computational Phantoms, Annals of the ICRP 39(2), 1st ed.; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 9780702041860. [Google Scholar]
- Wong, C.S.; Fehlings, M.G.; Sahgal, A. Pathobiology of radiation myelopathy and strategies to mitigate injury. Spinal Cord 2015, 53, 574–580. [Google Scholar] [CrossRef]
- Kortelainen, J.; Vipi, A.; Yuan, T.X.; Mir, H.; Thakor, N.; Al-Nashash, H.; All, A. Effect of isoflurane on somatosensory evoked potentials in a rat model. IEFE 2014, 2014, 4286–4289. [Google Scholar] [CrossRef]
- Schultheiss, T.E.; Stephens, L.C.; Peters, L.J. Survival in radiation myelopathy. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 1765–1769. [Google Scholar] [CrossRef] [PubMed]
- Khan, L.; Chiang, A.; Zhang, L.; Thibault, I.; Bedard, G.; Wong, E.; Loblaw, A.; Soliman, H.; Fehlings, M.G.; Chow, E.; et al. Prophylactic dexamethasone effectively reduces the incidence of pain flare following spine stereotactic body radiotherapy (SBRT): A prospective observational study. Support Care Cancer 2015, 3, 2937–2943. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Kristiansen, C.; Keil, D.; Schild, S.E.; Janssen, S. Current Radiotherapy Concepts Regarding Brain and Bone Metastases in Centers Participating in the German-Danish Interreg-Project TreaT. In Vivo 2023, 37, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Grosu, A.L.; Andratschke, N.; Nieder, C.; Molls, M. Retreatment of the spinal cord with palliative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.; Zeng, L.; Zhang, L.; Lochray, F.; Korol, R.; Loblaw, A.; Chow, E.; Sahgal, A. Pain flare is a common adverse event in steroid-naïve patients after spine stereotactic body radiation therapy: A prospective clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 638–642. [Google Scholar] [CrossRef] [PubMed]


| MRT Peak Dose | MRT Valley Dose | Dose Integrated over The Spinal Cord |
|---|---|---|
| 400 Gy | 13.3 Gy | 65.6 Gy |
| 500 Gy | 16.9 Gy | 82.0 Gy |
| 600 Gy | 20.0 Gy | 98.4 Gy |
| 800 Gy | 26.6 Gy | 131.2 Gy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaekel, F.; Paino, J.; Engels, E.; Klein, M.; Barnes, M.; Häusermann, D.; Hall, C.; Zheng, G.; Wang, H.; Hildebrandt, G.; et al. The Spinal Cord as Organ of Risk: Assessment for Acute and Subacute Neurological Adverse Effects after Microbeam Radiotherapy in a Rodent Model. Cancers 2023, 15, 2470. https://doi.org/10.3390/cancers15092470
Jaekel F, Paino J, Engels E, Klein M, Barnes M, Häusermann D, Hall C, Zheng G, Wang H, Hildebrandt G, et al. The Spinal Cord as Organ of Risk: Assessment for Acute and Subacute Neurological Adverse Effects after Microbeam Radiotherapy in a Rodent Model. Cancers. 2023; 15(9):2470. https://doi.org/10.3390/cancers15092470
Chicago/Turabian StyleJaekel, Felix, Jason Paino, Elette Engels, Mitzi Klein, Micah Barnes, Daniel Häusermann, Christopher Hall, Gang Zheng, Hongxin Wang, Guido Hildebrandt, and et al. 2023. "The Spinal Cord as Organ of Risk: Assessment for Acute and Subacute Neurological Adverse Effects after Microbeam Radiotherapy in a Rodent Model" Cancers 15, no. 9: 2470. https://doi.org/10.3390/cancers15092470
APA StyleJaekel, F., Paino, J., Engels, E., Klein, M., Barnes, M., Häusermann, D., Hall, C., Zheng, G., Wang, H., Hildebrandt, G., Lerch, M., & Schültke, E. (2023). The Spinal Cord as Organ of Risk: Assessment for Acute and Subacute Neurological Adverse Effects after Microbeam Radiotherapy in a Rodent Model. Cancers, 15(9), 2470. https://doi.org/10.3390/cancers15092470





