The Relationship between Oxidative Status and Radioiodine Treatment Qualification among Papillary Thyroid Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Sample Collection and Measurement
2.3. Statistical Analysis
2.4. Institutional Review Board Statement
3. Results
3.1. Studied Population Characteristics
3.2. Biochemical Profiling of the PTC Patients
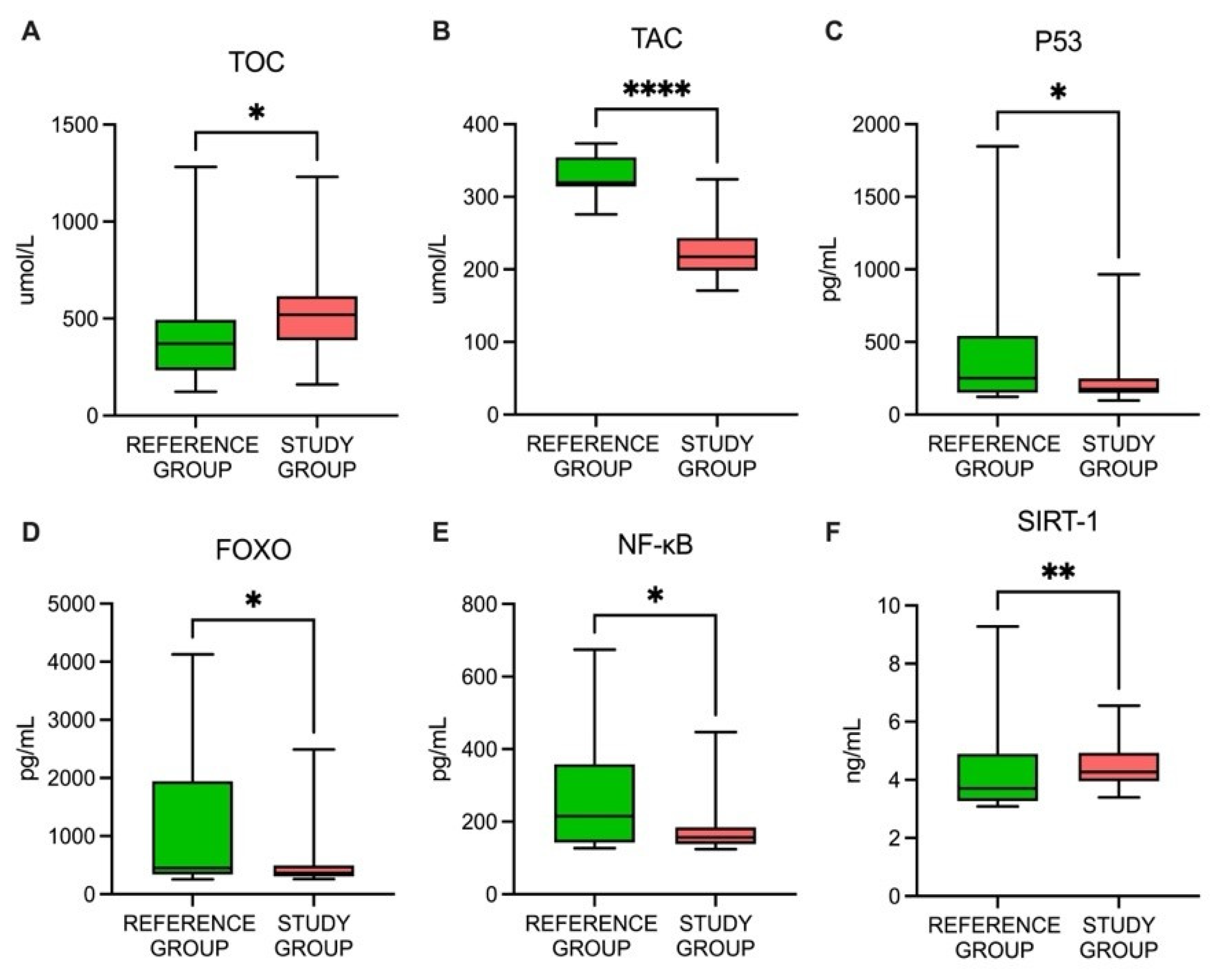
3.3. A Comparison of the Oxidative Status-Related Parameters between the Study and Reference Groups
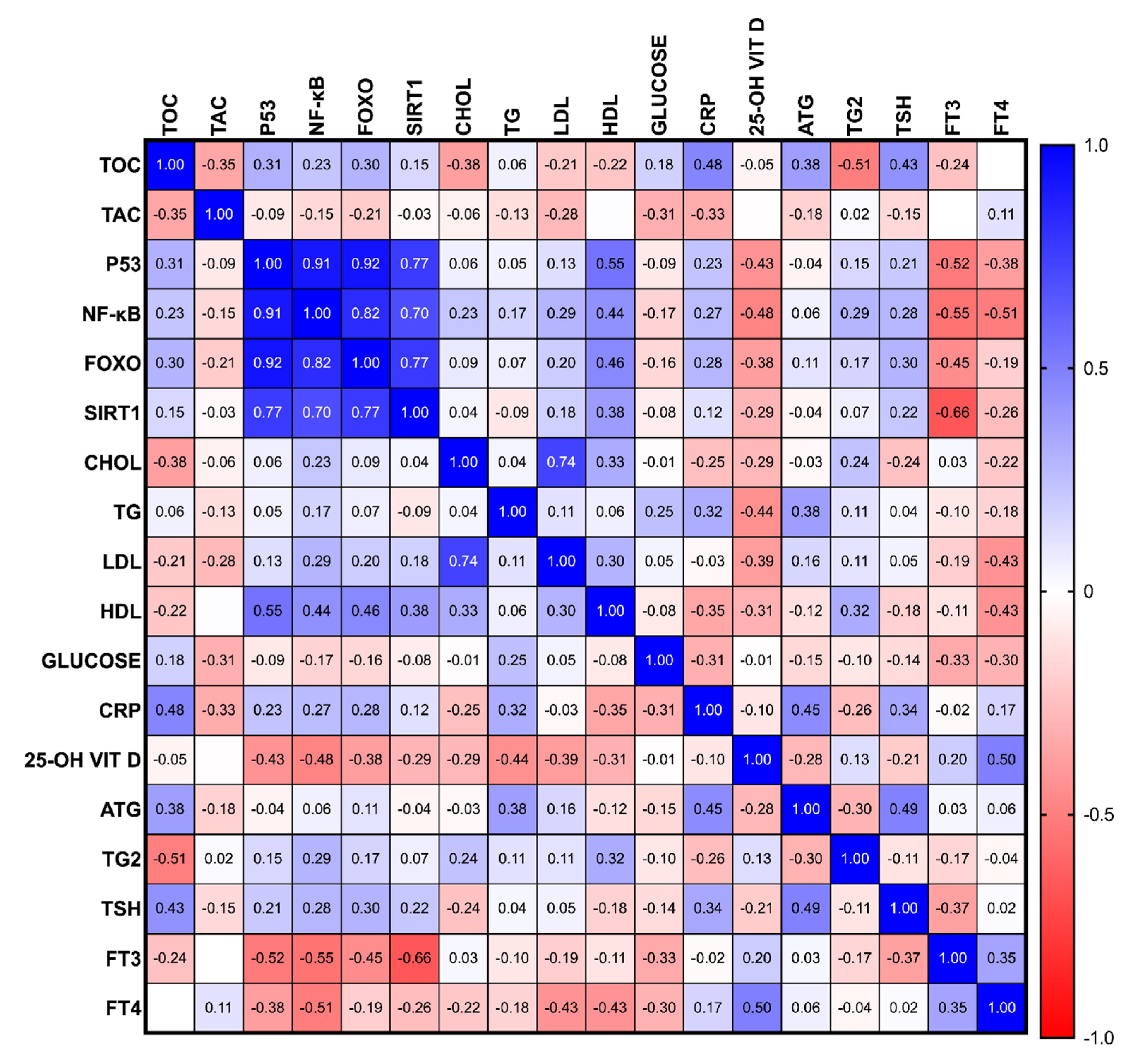
3.4. The Association of the Oxidative Status-Related Parameters in PTC Patients
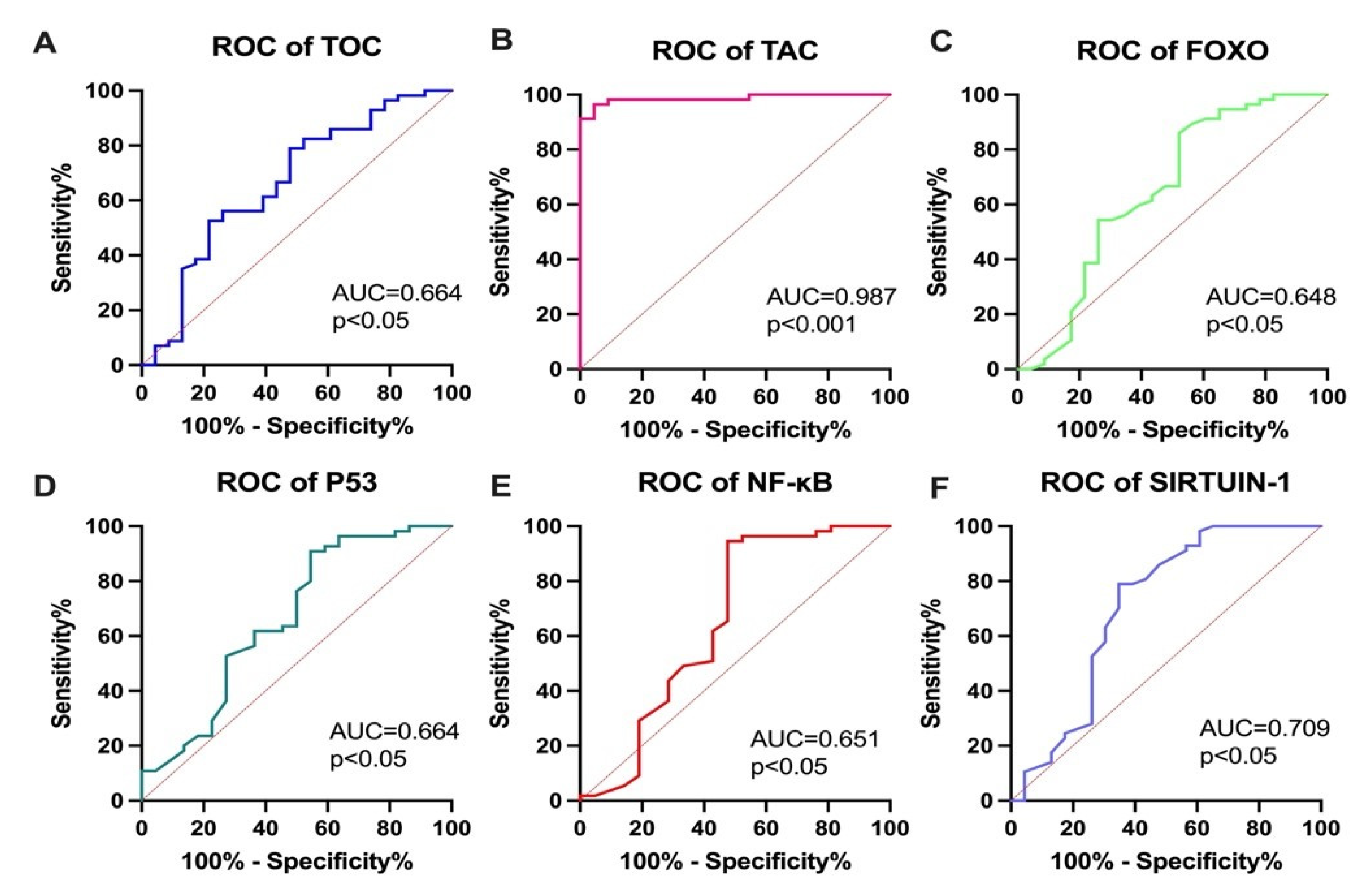
3.5. Diagnostic Utility of the Studied Parameters for RAI Qualification, According to Current Recommendations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 25-OH VIT D | 25-OH vitamin D |
| AUC | area under the ROC curve |
| CHOL | cholesterol |
| CRP | C-reactive protein |
| FNAB | fine aspiration needle biopsy |
| fT3 | free triiodothyronine |
| fT4 | free thyroxine |
| FOXO | forkhead box protein O1 |
| HDL | high-density lipoprotein |
| LDL | low-density lipoprotein |
| NF-κB | nuclear factor kappa B |
| p53 | tumor protein 53 |
| SIRT1 | sirtuin 1 |
| PTC | papillary thyroid cancer |
| ROC | received operatic characteristics |
| TAC | total antioxidant capacity |
| TOC | total oxidative capacity |
| TG | triglyceride |
| Tg | thyroglobulin |
| TgAb | thyroglobulin antibody |
| TSH | thyroid-stimulating hormone |
References
- Miranda-Filho, A.; Lortet-Tieulent, J.; Bray, F.; Cao, B.; Franceschi, S.; Vaccarella, S.; Dal Maso, L. Thyroid cancer incidence trends by histology in 25 countries: A population-based study. Lancet Diabetes Endocrinol. 2021, 9, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; Pantanowitz, L.; Hornick, J.L. A worldwide journey of thyroid cancer incidence centred on tumour histology. Lancet Diabetes Endocrinol. 2021, 9, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Che, W.; Yu, Z.; Zheng, S.; Xie, S.; Chen, C.; Qiao, M.; Lyu, J. The Incidence Trend of Papillary Thyroid Carcinoma in the United States During 2003–2017. Cancer Control 2022, 29, 10732748221135447. [Google Scholar] [CrossRef] [PubMed]
- Rago, T.; Vitti, P. Risk Stratification of Thyroid Nodules: From Ultrasound Features to TIRADS. Cancers 2022, 14, 717. [Google Scholar] [CrossRef]
- Peng, S.; Liu, Y.; Lv, W.; Liu, L.; Zhou, Q.; Yang, H.; Ren, J.; Liu, G.; Wang, X.; Zhang, X.; et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: A multicentre diagnostic study. Lancet Digit. Health 2021, 3, e250–e259. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. THYROID 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Wang, T.S.; Sosa, J.A. Thyroid surgery for differentiated thyroid cancer—Recent advances and future directions. Nat. Rev. Endocrinol. 2018, 14, 670–683. [Google Scholar] [CrossRef]
- Schlumberger, M.; Leboulleux, S. Current practice in patients with differentiated thyroid cancer. Nat. Rev. Endocrinol. 2020, 17, 176–188. [Google Scholar] [CrossRef]
- Li, M.; Zheng, R.; Dal Maso, L.; Zhang, S.; Wei, W.; Vaccarella, S. Mapping overdiagnosis of thyroid cancer in China. Lancet Diabetes Endocrinol. 2021, 9, 330–332. [Google Scholar] [CrossRef]
- Worden, F. Treatment strategies for radioactive iodine-refractory differentiated thyroid cancer. Ther. Adv. Med. Oncol. 2014, 6, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Adamska, A.; Tomczuk-Bobik, P.; Popławska-Kita, A.B.; Siewko, K.; Buczyńska, A.; Szumowski, P.; Żukowski, Ł.; Myśliwiec, J.; Zbucka-Krętowska, M.; Adamski, M.; et al. Assessment of different markers of ovarian reserve in women with papillary thyroid cancer treated with radioactive iodine. Endocr. Connect. 2021, 10, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Samimi, H.; Haghpanah, V. Molecular evidence reveals thyrotropin intervention enhances the risk of developing radioiodine-refractory differentiated thyroid carcinoma. Cancer Cell Int. 2022, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Fuhrer, D.; Elisei, R.; Handkiewicz-Junak, D.; Leboulleux, S.; Luster, M.; Schlumberger, M.; Smit, J.W. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur. Thyroid. J. 2022, 11, e210046. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Gosnell, J.E.; Roman, S.A. Geographic influences in the global rise of thyroid cancer. Nat. Rev. Endocrinol. 2019, 16, 17–29. [Google Scholar] [CrossRef]
- Buczyńska, A.; Sidorkiewicz, I.; Krętowski, A.J.; Zbucka-Krętowska, M.; Adamska, A. Metformin Intervention-A Panacea for Cancer Treatment? Cancers 2022, 14, 1336. [Google Scholar] [CrossRef]
- Muzza, M.; Pogliaghi, G.; Colombo, C.; Carbone, E.; Cirello, V.; Palazzo, S.; Frattini, F.; Gentilini, D.; Gazzano, G.; Persani, L.; et al. Oxidative Stress Correlates with More Aggressive Features in Thyroid Cancer. Cancers 2022, 14, 5857. [Google Scholar] [CrossRef]
- Buczyńska, A.; Sidorkiewicz, I.; Rogucki, M.; Siewko, K.; Adamska, A.; Kościuszko, M.; Maliszewska, K.; Kozłowska, G.; Szumowski, P.; Myśliwiec, J.; et al. Oxidative stress and radioiodine treatment of differentiated thyroid cancer. Sci. Rep. 2021, 11, 17126. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Konukoǧlu, D.; Hüsrev Hatemi, H.; Arikan, S.; Demir, M.; Akçay, T. Radioiodine treatment and oxidative stress in thyroidectomised patients for differentiated thyroid cancers. Pharmacol. Res. 1998, 38, 311–315. [Google Scholar] [CrossRef]
- Xing, M. Oxidative stress: A new risk factor for thyroid cancer. Endocr. Relat. Cancer 2012, 19, C7–C11. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Q.; Liu, B.; Wang, J.; Chen, C.; Sun, S. Blood Profiles in the Prediction of Radioiodine Refractory Papillary Thyroid Cancer: A Case-Control Study. J. Multidiscip. Healthc. 2023, 16, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Rosário, P.W.; Batista, K.C.S.; Calsolari, M.R. Radioiodine-induced oxidative stress in patients with differentiated thyroid carcinoma and effect of supplementation with vitamins C and E and selenium (Antioxidants). Arch. Endocrinol. Metab. 2016, 60, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Sengoz, T.; Kilic-Toprak, E.; Yaylali, O.; Kilic-Erkek, O.; Ozdemir, Y.; Oymak, B.; Senol, H.; Yuksel, D.; Kucukatay, V.; Bor-Kucukatay, M. Hemorheology and oxidative stress in patients with differentiated thyroid cancer following I-131 ablation/metastasis treatment. Clin. Hemorheol. Microcirc. 2019, 74, 209–221. [Google Scholar] [CrossRef]
- Klubo-Gwiezdzinska, J.; Jensen, K.; Bauer, A.; Patel, A.; Costello, J.; Burman, K.D.; Wartofsky, L.; Hardwick, M.J.; Vasko, V.V. The expression of translocator protein in human thyroid cancer and its role in the response of thyroid cancer cells to oxidative stress. J. Endocrinol. 2012, 214, 207–216. [Google Scholar] [CrossRef]
- Ameziane El Hassani, R.; Buffet, C.; Leboulleux, S.; Dupuy, C. Oxidative stress in thyroid carcinomas: Biological and clinical significance. Endocr. Relat. Cancer 2019, 26, R131–R143. [Google Scholar] [CrossRef]
- Brehar, A.C.; Brehar, F.M.; Bulgar, A.C.; Dumitrache, C. Genetic and epigenetic alterations in differentiatedthyroid carcinoma. J. Med. Life 2013, 6, 403. [Google Scholar]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef]
- Li, W.; Ming, H.; Sun, D.; Li, W.; Wang, D.; Zhang, G.; Tan, J. The relationship between BRAFV600E, NF-κB and TgAb expression in papillary thyroid carcinoma. Pathol. Res. Pract. 2017, 213, 183–188. [Google Scholar] [CrossRef]
- Saljooghi, S.; Heidari, Z.; Saravani, M.; Rezaei, M.; Salimi, S. Association of FOXO1 Rs17592236 Polymorphism and Tumor Size in Papillary Thyroid Carcinoma. Rep. Biochem. Mol. Biol. 2022, 11, 216. [Google Scholar]
- Harb, O.A.; Kaf, R.M.; Taha, H.F.; Balata, S.A.; Hemeda, R.; Yehia, A.M.; Gertallah, L.M.; Embaby, A. Clinical, pathological and prognostic implications of USP22, SIRT1 and E-cadherin expression in papillary thyroid cancer (PTC) and adjacent non-neoplastic tissue. Surg. Exp. Pathol. 2019, 2, 22. [Google Scholar] [CrossRef]
- Morita, N.; Ikeda, Y.; Takami, H. Clinical significance of p53 protein expression in papillary thyroid carcinoma. World J. Surg. 2008, 32, 2617–2622. [Google Scholar] [CrossRef]
- Momesso, D.P.; Vaisman, F.; Yang, S.P.; Bulzico, D.A.; Corbo, R.; Vaisman, M.; Michael Tuttle, R. Dynamic Risk Stratification in Patients with Differentiated Thyroid Cancer Treated Without Radioactive Iodine. J. Clin. Endocrinol. Metab. 2016, 101, 2692. [Google Scholar] [CrossRef]
- Jarząb, B.; Dedecjus, M.; Lewiński, A.; Adamczewski, Z.; Bakuła-Zalewska, E.; Bałdys-Waligórska, A.; Barczyński, M.; Biskup-Frużyńska, M.; Bobek-Billewicz, B.; Bossowski, A.; et al. Diagnosis and treatment of thyroid cancer in adult patients—Recommendations of Polish Scientific Societies and the National Oncological Strategy. 2022 Update [Diagnostyka i leczenie raka tarczycy u chorych dorosłych—Rekomendacje Polskich Towarzystw Naukowych oraz Narodowej Strategii Onkologicznej. Aktualizacja na rok 2022]. Endokrynol. Pol. 2022, 73, 173–300. [Google Scholar] [CrossRef]
- Sapuppo, G.; Tavarelli, M.; Pellegriti, G. The new AJCC/TNM Staging System (VIII ed.) in papillary thyroid cancer: Clinical and molecular impact on overall and recurrence free survival. Ann. Transl. Med. 2020, 8, 838. [Google Scholar] [CrossRef]
- Spencer, C.; Petrovic, I.; Fatemi, S. Current Thyroglobulin Autoantibody (TgAb) Assays Often Fail to Detect Interfering TgAb that Can Result in the Reporting of Falsely Low/Undetectable Serum Tg IMA Values for Patients with Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2011, 96, 1283–1291. [Google Scholar] [CrossRef]
- Spencer, C.A.; Petrovic, I.; Fatemi, S.; LoPresti, J. Serum Thyroglobulin (Tg) Monitoring of Patients with Differentiated Thyroid Cancer Using Sensitive (Second-Generation) Immunometric Assays Can Be Disrupted by False-Negative and False-Positive Serum Thyroglobulin Autoantibody Misclassifications. J. Clin. Endocrinol. Metab. 2014, 99, 4589. [Google Scholar] [CrossRef]
- Van Kinschot, C.M.J.; Peeters, R.P.; Van Den Berg, S.A.A.; Verburg, F.A.; Van Noord, C.; Van Ginhoven, T.M.; Visser, W.E. Thyroglobulin and thyroglobulin antibodies: Assay-dependent management consequences in patients with differentiated thyroid carcinoma. Clin. Chem. Lab. Med. 2022, 60, 756–765. [Google Scholar] [CrossRef]
- Algeciras-Schimnich, A. Thyroglobulin measurement in the management of patients with differentiated thyroid cancer. Crit. Rev. Clin. Lab. Sci. 2018, 55, 205–218. [Google Scholar] [CrossRef]
- Cazarin, J.; Dupuy, C.; Pires de Carvalho, D. Redox Homeostasis in Thyroid Cancer: Implications in Na+/I- Symporter (NIS) Regulation. Int. J. Mol. Sci. 2022, 23, 6129. [Google Scholar] [CrossRef]
- Kowaltowski, A.J. Strategies to detect mitochondrial oxidants. Redox Biol. 2019, 21, 101065. [Google Scholar] [CrossRef]
- Chan, X.C.Y.; Black, C.M.; Lin, A.J.; Ping, P.; Lau, E. Mitochondrial protein turnover: Methods to measure turnover rates on a large scale. J. Mol. Cell. Cardiol. 2015, 78, 54. [Google Scholar] [CrossRef]
- Young, O.; Crotty, T.; O’Connell, R.; O’Sullivan, J.; Curran, A.J. Levels of oxidative damage and lipid peroxidation in thyroid neoplasia. Head Neck 2010, 32, 750–756. [Google Scholar] [CrossRef]
- Song, H.M.; Song, J.L.; Li, D.F.; Hua, K.Y.; Zhao, B.K.; Fang, L. Inhibition of FOXO1 by small interfering RNA enhances proliferation and inhibits apoptosis of papillary thyroid carcinoma cells via Akt/FOXO1/Bim pathway. Onco. Targets. Ther. 2015, 8, 3565. [Google Scholar] [CrossRef]
- Palona, I.; Namba, H.; Mitsutake, N.; Starenki, D.; Podtcheko, A.; Sedliarou, I.; Ohtsuru, A.; Saenko, V.; Nagayama, Y.; Umezawa, K.; et al. BRAFV600E promotes invasiveness of thyroid cancer cells through nuclear factor kappaB activation. Endocrinology 2006, 147, 5699–5707. [Google Scholar] [CrossRef]
- Li, T.; Wang, G.; Li, Z.; Zhang, N.; Li, Y.; Zhao, Y.; Tian, X. SIRT1 Expression and BRAF V600E Mutation in Papillary Thyroid Cancer: Implications for Diagnosis and Prognosis. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, X.; Li, M. Correlation analysis between the pre-operative contrast-enhanced ultrasound parameters and biological characteristics of papillary thyroid carcinoma and associated risk factors for prognosis after radiofrequency ablation. Exp. Ther. Med. 2020, 20, 1575–1581. [Google Scholar] [CrossRef]
- McFadden, D.G.; Vernon, A.; Santiago, P.M.; Martinez-McFaline, R.; Bhutkar, A.; Crowley, D.M.; McMahon, M.; Sadow, P.M.; Jacks, T. p53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. Proc. Natl. Acad. Sci. USA 2014, 111, E1600–E1609. [Google Scholar] [CrossRef]
- Maiese, K. Forkhead transcription factors: New considerations for alzheimer’s disease and dementia. J. Transl. Sci. 2016, 2, 241. [Google Scholar] [CrossRef]
- Liu, L.; Tao, Z.; Zheng, L.D.; Brooke, J.P.; Smith, C.M.; Liu, D.; Long, Y.C.; Cheng, Z. FoxO1 interacts with transcription factor EB and differentially regulates mitochondrial uncoupling proteins via autophagy in adipocytes. Cell Death Discov. 2016, 2, 16066. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef]
- Myatt, S.S.; Lam, E.W.F. The emerging roles of forkhead box (fox) proteins in cancer. Nat. Rev. Cancer 2007, 7, 847–859. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Ioannilli, L.; Aquilano, K.; Ciccarone, F.; Rosina, M.; Ciriolo, M.R. FoxO1 localizes to mitochondria of adipose tissue and is affected by nutrient stress. Metabolism 2019, 95, 84–92. [Google Scholar] [CrossRef]
- Jerome, M.S.; Kuthethur, R.; Kabekkodu, S.P.; Chakrabarty, S. Regulation of mitochondrial function by forkhead transcription factors. Biochimie 2022, 198, 96–108. [Google Scholar] [CrossRef]
- Ao, N.; Wang, L.; Liu, Y. Prognostic and clinicopathological significance of ubiquitin-specific protease 22 overexpression in cancers: Evidence from a meta-analysis. Onco. Targets. Ther. 2017, 10, 5533. [Google Scholar] [CrossRef]
- Yao, L.; Wang, Y. Bioinformatic Analysis of the Effect of the Sirtuin Family on Differentiated Thyroid Carcinoma. Biomed Res. Int. 2022, 2022, 5794118. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, H.; Kong, Q.; Li, J.; Lee, S.M.; Gao, B.; Dong, H.; Wei, J.; Song, J.; Zhang, D.D.; et al. USP22 antagonizes p53 transcriptional activation by deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development. Mol. Cell 2012, 46, 484–494. [Google Scholar] [CrossRef]
- Marcello, M.A.; Morari, E.C.; Cunha, L.L.; De Nadai Silva, A.C.; Carraro, D.M.; Carvalho, A.L.; Soares, F.A.; Vassallo, J.; Ward, L.S. P53 and expression of immunological markers may identify early stage thyroid tumors. Clin. Dev. Immunol. 2013, 2013, 846584. [Google Scholar] [CrossRef]
- Manzella, L.; Stella, S.; Pennisi, M.S.; Tirrò, E.; Massimino, M.; Romano, C.; Puma, A.; Tavarelli, M.; Vigneri, P. New Insights in Thyroid Cancer and p53 Family Proteins. Int. J. Mol. Sci. 2017, 18, 1325. [Google Scholar] [CrossRef]
- Dai, C.Q.; Luo, T.T.; Luo, S.C.; Wang, J.Q.; Wang, S.M.; Bai, Y.H.; Yang, Y.L.; Wang, Y.Y. p53 and mitochondrial dysfunction: Novel insight of neurodegenerative diseases. J. Bioenerg. Biomembr. 2016, 48, 337. [Google Scholar] [CrossRef]
- Madan, E.; Gogna, R.; Kuppusamy, P.; Bhatt, M.; Mahdi, A.A.; Pati, U. SCO2 Induces p53-Mediated Apoptosis by Thr845 Phosphorylation of ASK-1 and Dissociation of the ASK-1–Trx Complex. Mol. Cell. Biol. 2013, 33, 1285. [Google Scholar] [CrossRef] [PubMed]
- Omur, O.; Baran, Y. An update on molecular biology of thyroid cancers. Crit. Rev. Oncol. Hematol. 2014, 90, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mu, Y.; Greene, W.C. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J. 2002, 21, 6539. [Google Scholar] [CrossRef] [PubMed]
- Albensi, B.C. What is nuclear factor kappa B (NF-κB) doing in and to the mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef]
- Pacifico, F.; Mauro, C.; Barone, C.; Crescenzi, E.; Mellone, S.; Monaco, M.; Chiappetta, G.; Terrazzano, G.; Liguoro, D.; Vito, P.; et al. Oncogenic and anti-apoptotic activity of NF-kappa B in human thyroid carcinomas. J. Biol. Chem. 2004, 279, 54610–54619. [Google Scholar] [CrossRef]
- Shim, S.R.; Kitahara, C.M.; Cha, E.S.; Kim, S.J.; Bang, Y.J.; Lee, W.J. Cancer Risk After Radioactive Iodine Treatment for Hyperthyroidism: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2125072. [Google Scholar] [CrossRef]
- Rogucki, M.; Buczyńska, A.; Krętowski, A.J.; Popławska-Kita, A. The Importance of miRNA in the Diagnosis and Prognosis of Papillary Thyroid Cancer. J. Clin. Med. 2021, 10, 4738. [Google Scholar] [CrossRef]
- Finnberg, N.; El-Deiry, W.S. Activating FOXO3a, NF-kappaB and p53 by targeting IKKs: An effective multi-faceted targeting of the tumor-cell phenotype? Cancer Biol. Ther. 2004, 3, 614–616. [Google Scholar] [CrossRef]
- Krajewska, J.; Jarząb, M.; Czarniecka, A.; Roskosz, J.; Kukulska, A.; Handkiewicz-Junak, D.; Puch, Z.; Wygoda, Z.; Paliczka-Cieślik, E.; Kropińska, A.; et al. Ongoing risk stratification for differentiated thyroid cancer (DTC)—Stimulated serum thyroglobulin (Tg) before radioiodine (RAI) ablation, the most potent risk factor of cancer recurrence in M0 patients. Endokrynol. Pol. 2016, 67, 2–11. [Google Scholar] [CrossRef]
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in stress related diseases/disorders: Diagnostic, prognostic, and therapeutic values. Front. Media SA 2019, 6, 91. [Google Scholar] [CrossRef]
- Sparano, C.; Moog, S.; Hadoux, J.; Dupuy, C.; Al Ghuzlan, A.; Breuskin, I.; Guerlain, J.; Hartl, D.; Baudin, E.; Lamartina, L. Strategies for Radioiodine Treatment: What’s New. Cancers 2022, 14, 3800. [Google Scholar] [CrossRef] [PubMed]
| Study Group | Reference Group | p-Value | |
|---|---|---|---|
| Number of patients | 60 | 25 | |
| Median age (upper and lower quartiles) | 54 (51.41; 64.22) | 51 (50.21; 62.58) | 0.054 |
| Sex | M: 18 | M: 8 | 0.052 |
| F: 42 | F: 17 | 0.051 | |
| Menopausal status | |||
| Premenopausal | 9 | 5 | 0.064 |
| Postmenopausal | 33 | 12 | 0.082 |
| Stage (TNM) | pT1a(m): 11 pT1b: 15 pT1b(m): 6 pT2: 16 pT3/pT4: 12 | pT1a: 25 | <0.001 |
| Study Group | Reference Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unit | 25% Percentile | Median | 75% Percentile | Range | 25% Percentile | Median | 75% Percentile | Range | p-Value | |
| CHOL | mg/dL | 189.51 | 216.00 | 240.00 | 142–463 | 179.23 | 142.05 | 225.01 | 101–272 | 0.018 |
| LDL | mg/dL | 118.50 | 138.04 | 168.52 | 70–314 | 74.30 | 107.92 | 137.42 | 55.7–164.8 | <0.001 |
| TG | mg/dL | 78.52 | 103.07 | 161.45 | 40–499 | 84.41 | 107.91 | 166.00 | 45–411 | 0.510 |
| HDL | mg/dL | 45.06 | 53.01 | 62.14 | 31–114 | 53.73 | 58.94 | 72.57 | 39.26–102.8 | 0.048 |
| CRP | mg/L | 15.85 | 22.41 | 29.35 | 7.2–82.6 | 1.00 | 1.34 | 2.83 | 0.38–4.12 | 0.053 |
| GLUCOSE | mg/dL | 88.07 | 94.14 | 99.53 | 69–248 | 85.12 | 92.22 | 97.15 | 77–117 | 0.297 |
| 25-OH VIT D | ng/mL | 15.81 | 22.17 | 29.35 | 7.2–82.6 | 23.34 | 25.92 | 36.86 | 17.4–54.2 | 0.019 |
| TSH | µIU/mL | 0.15 | 0.60 | 2.32 | 0.1–68.55 | 0.12 | 0.36 | 0.79 | 0.075–1.55 | 0.318 |
| fT3 | pg/mL | 2.21 | 2.56 | 3.01 | 1–6.27 | 2.47 | 2.63 | 2.86 | 1–3.27 | 0.754 |
| fT4 | ng/dL | 0.96 | 1.19 | 1.37 | 0.4–2.14 | 1.05 | 1.19 | 1.23 | 0.4–1.8 | 0.991 |
| Tg | ng/mL | 0.51 | 1.10 | 2.53 | 0.04–37.05 | 0.04 | 0.09 | 0.29 | 0.04–1.13 | 0.078 |
| TgAb | IU/mL | 0.85 | 2.02 | 6.67 | 0–185 | 0.61 | 1.82 | 2.89 | 0.1–5.23 | 0.131 |
| Unit | Study Group | Reference Group | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25% Percentile | Median | 75% Percentile | Range | 25% Percentile | Median | 75% Percentile | Range | |||
| TOC | Umol/L | 387.5 | 519 | 615.5 | 161–1231 | 233 | 371 | 493 | 123–1282 | 0.020 |
| TAC | Umol/L | 198.7 | 217.4 | 243.4 | 170.8–324.3 | 313.9 | 317.2 | 353.3 | 271.4–373.3 | <0.001 |
| p53 | pg/mL | 149 | 176 | 249 | 98–966 | 150.5 | 250 | 543.3 | 123–1847 | 0.025 |
| NF-κB | pg/mL | 138 | 157 | 184 | 124–447 | 142 | 215 | 358 | 127–674 | 0.043 |
| FOXO | ng/mL | 0.3 | 0.37 | 0.5 | 0.26–2.49 | 0.34 | 0.45 | 1.94 | 0.26–4.13 | 0.039 |
| SIRT1 | ng/mL | 3.96 | 4.26 | 4.93 | 3.4–6.56 | 3.27 | 3.71 | 4.9 | 3.09–9.28 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buczyńska, A.; Sidorkiewicz, I.; Kościuszko, M.; Adamska, A.; Siewko, K.; Dzięcioł, J.; Szumowski, P.; Myśliwiec, J.; Popławska-Kita, A.; Krętowski, A.J. The Relationship between Oxidative Status and Radioiodine Treatment Qualification among Papillary Thyroid Cancer Patients. Cancers 2023, 15, 2436. https://doi.org/10.3390/cancers15092436
Buczyńska A, Sidorkiewicz I, Kościuszko M, Adamska A, Siewko K, Dzięcioł J, Szumowski P, Myśliwiec J, Popławska-Kita A, Krętowski AJ. The Relationship between Oxidative Status and Radioiodine Treatment Qualification among Papillary Thyroid Cancer Patients. Cancers. 2023; 15(9):2436. https://doi.org/10.3390/cancers15092436
Chicago/Turabian StyleBuczyńska, Angelika, Iwona Sidorkiewicz, Maria Kościuszko, Agnieszka Adamska, Katarzyna Siewko, Janusz Dzięcioł, Piotr Szumowski, Janusz Myśliwiec, Anna Popławska-Kita, and Adam Jacek Krętowski. 2023. "The Relationship between Oxidative Status and Radioiodine Treatment Qualification among Papillary Thyroid Cancer Patients" Cancers 15, no. 9: 2436. https://doi.org/10.3390/cancers15092436
APA StyleBuczyńska, A., Sidorkiewicz, I., Kościuszko, M., Adamska, A., Siewko, K., Dzięcioł, J., Szumowski, P., Myśliwiec, J., Popławska-Kita, A., & Krętowski, A. J. (2023). The Relationship between Oxidative Status and Radioiodine Treatment Qualification among Papillary Thyroid Cancer Patients. Cancers, 15(9), 2436. https://doi.org/10.3390/cancers15092436





