Analysis of Local Recurrence Risk in Ductal Carcinoma In Situ and External Validation of the Memorial Sloan Kettering Cancer Center Nomogram
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
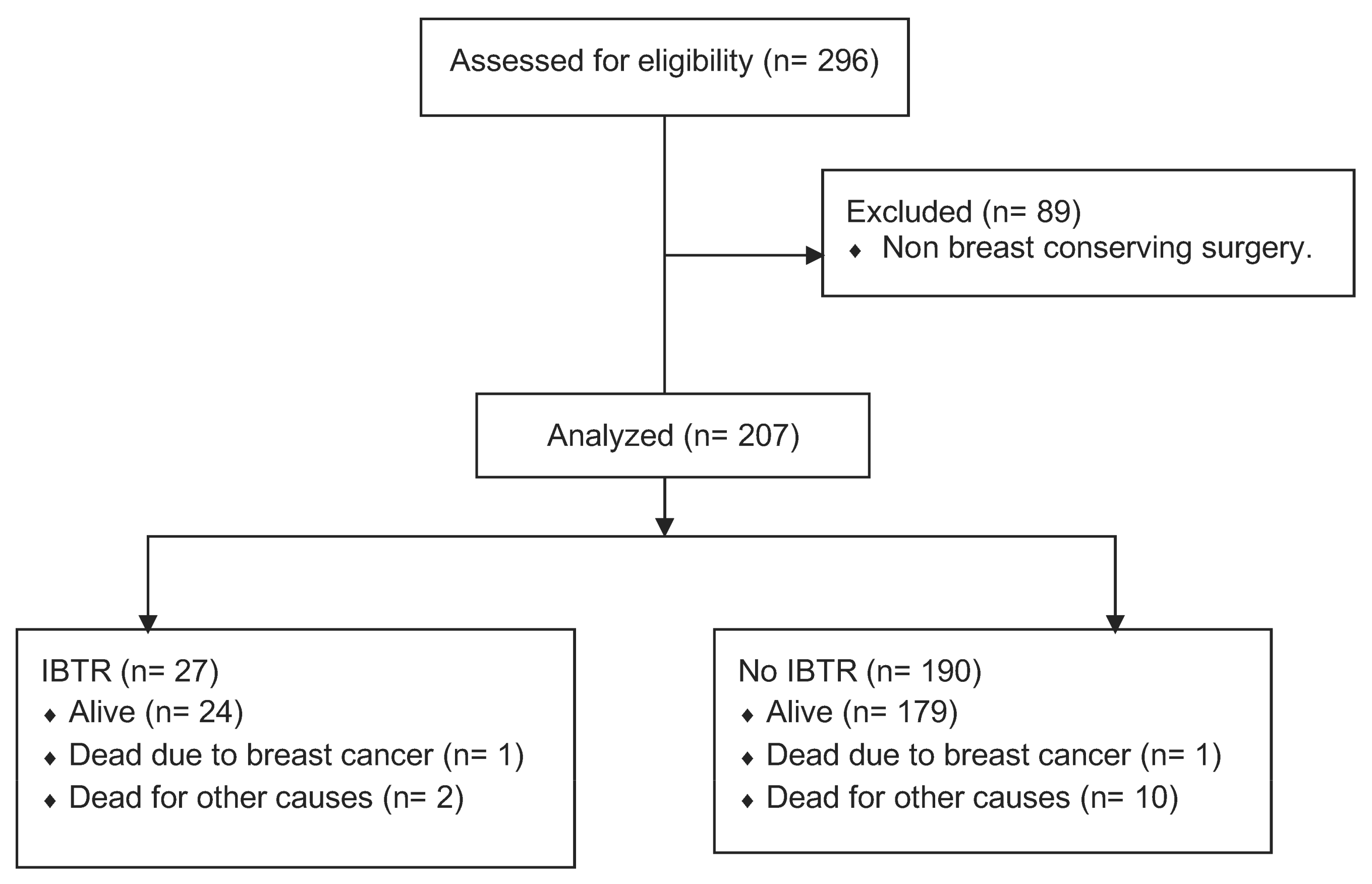
2.1. Design and Subjects
2.2. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ernster, V.L.; Ballard-Barbash, R.; Barlow, W.E.; Zheng, Y.; Weaver, D.L.; Cutter, G.; Yankaskas, B.C.; Rosenberg, R.; Carney, P.A.; Kerlikowske, K.; et al. Detection of Ductal Carcinoma in Situ in Women Undergoing Screening Mammography. Gynecol. Oncol. 2002, 94, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Bijker, N.; Meijnen, P.; Peterse, J.L.; Bogaerts, J.; Van Hoorebeeck, I.; Julien, J.-P.; Gennaro, M.; Rouanet, P.; Avril, A.; Fentiman, I.S.; et al. Breast-Conserving Treatment with or Without Radiotherapy in Ductal Carcinoma-In-Situ: Ten-Year Results of European Organisation for Research and Treatment of Cancer Randomized Phase III Trial 10853—A Study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J. Clin. Oncol. 2006, 24, 3381–3387. [Google Scholar] [CrossRef]
- Correa, C.; McGale, P.; Taylor, C. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. Early Breast Cancer Trialists Collaborative Group (EBCTCG). J. Natl. Cancer Inst. 2010, 41, 162Y177. [Google Scholar]
- Wapnir, I.L.; Dignam, J.J.; Fisher, B.; Mamounas, E.P.; Anderson, S.; Julian, T.B.; Land, S.R.; Margolese, R.G.; Swain, S.; Costantino, J.P.; et al. Long-Term Outcomes of Invasive Ipsilateral Breast Tumor Recurrences After Lumpectomy in NSABP B-17 and B-24 Randomized Clinical Trials for DCIS. Gynecol. Oncol. 2011, 103, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.R.; Dignam, J.; Tan-Chiu, E. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of protocol B-17: Intraductal carcinoma. Cancer 1999, 86, 429Y438. [Google Scholar] [CrossRef]
- Holmberg, L.; Garmo, H.; Granstrand, B.; Ringberg, A.; Arnesson, L.-G.; Sandelin, K.; Karlsson, P.; Anderson, H.; Emdin, S. Absolute Risk Reductions for Local Recurrence After Postoperative Radiotherapy After Sector Resection for Ductal Carcinoma In Situ of the Breast. J. Clin. Oncol. 2008, 26, 1247–1252. [Google Scholar] [CrossRef]
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 366, 2087–2106. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Sestak, I.; Pinder, E.S.; Ellis, I.O.; Forsyth, S.; Bundred, N.J.; Forbes, J.F.; Bishop, H.; Fentiman, I.S.; George, W.D. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: Long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011, 12, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Allred, D.C.; Anderson, S.J.; Paik, S.; Wickerham, D.L.; Nagtegaal, I.D.; Swain, S.M.; Mamounas, E.P.; Julian, T.B.; Geyer, C.E., Jr.; Costantino, J.P.; et al. Adjuvant Tamoxifen Reduces Subsequent Breast Cancer in Women with Estrogen Receptor–Positive Ductal Carcinoma in Situ: A Study Based on NSABP Protocol B-24. J. Clin. Oncol. 2012, 30, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 10 October 2022).
- Ceilley, E.; Jagsi, R.; Goldberg, S.; A Kachnic, L.; Powell, S.N.; Taghian, A.G. The management of ductal carcinoma in situ in North America and Europe. Cancer 2004, 101, 1958–1967. [Google Scholar] [CrossRef]
- Silverstein, M.J. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am. J. Surg. 2003, 186, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, M.J.; Lagios, M.D. Choosing Treatment for Patients with Ductal Carcinoma In Situ: Fine Tuning the University of Southern California/Van Nuys Prognostic Index. JNCI Monogr. 2010, 2010, 193–196. [Google Scholar] [CrossRef]
- De Mascarel, I.; Bonichon, F.; MacGrogan, G. Application of the Van Nuys Prognostic Index in a retrospective series of 367 ductal carcinomas in situ of the breast examined by serial macroscopic sectioning: Practical considerations. Breast Cancer Res. Treat. 2000, 61, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Boland, G.P.; Chan, K.C.; Knox, W.F.; A Roberts, S.; Bundred, N.J. Value of the Van Nuys Prognostic Index in prediction of recurrence of ductal carcinoma in situ after breast-conserving surgery. Br. J. Surg. 2003, 90, 426–432. [Google Scholar] [CrossRef] [PubMed]
- MacAusland, S.G.; Hepel, J.T.; Chong, F.K.; Galper, S.L.; Gass, J.S.; Ruthazer, R.; Wazer, D.E. An attempt to independently verify the utility of the Van Nuys Prognostic Index for ductal carcinoma in situ. Cancer 2007, 110, 2648–2653. [Google Scholar] [CrossRef]
- Gilleard, O.; Goodman, A.; Cooper, M. The significance of the Van Nuys prognostic index in the management of ductal carcinoma in situ. World J. Surg. Oncol. 2008, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Rudloff, U.; Jacks, L.M.; Goldberg, J.I.; Wynveen, C.A.; Brogi, E.; Patil, S.; Van Zee, K.J. Nomogram for Predicting the Risk of Local Recurrence After Breast-Conserving Surgery for Ductal Carcinoma in Situ. J. Clin. Oncol. 2010, 28, 3762–3769. [Google Scholar] [CrossRef]
- Simpson, P.T.; Da Silva, L.M.; Lakhani, S.R. In situ carcinoma: Can we predict which patient will come back with a recurrence? Cancer Cell 2007, 12, 409–411. [Google Scholar] [CrossRef]
- Van Zee, K.J.; Liberman, L.; Samli, B. Long term follow-up of women with ductal carcinoma in situ treated with breast-conserving surgery: The effect of age. Cancer 1999, 86, 1757–1767. [Google Scholar] [CrossRef]
- Fisher, B.; Dignam, J.; Wolmark, N. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet 1999, 353, 1993–2000. [Google Scholar] [CrossRef]
- Vicini, F.A.; Kestin, L.L.; Goldstein, N.S. Impact of young age on outcome in patients with ductal carcinoma-in-situ treated with breast-conserving therapy. J. Clin. Oncol. 2000, 18, 296–306. [Google Scholar] [CrossRef]
- Rudloff, U.; Brogi, E.; Ba, J.P.B.; Ba, J.I.G.; Cranor, M.; Wynveen, C.A.; Nehhozina, T.; Reiner, A.S.; Patil, S.; Van Zee, K.J. Concurrent lobular neoplasia increases the risk of ipsilateral breast cancer recurrence in patients with ductal carcinoma in situ treated with breast-conserving therapy. Cancer 2009, 115, 1203–1214. [Google Scholar] [CrossRef]
- Hiramatsu, H.; Bornstein, B.A.; Recht, A. Local recurrence after conservative surgery and radiation therapy for ductal carcinoma in situ: Possible importance of family history. Cancer J. Sci. Am. 1995, 1, 55–61. [Google Scholar] [PubMed]
- Rakovitch, E.; Pignol, J.P.; Hanna, W. Significance of multifocality in ductal carcinoma in situ: Outcomes of women treated with breast-conserving therapy. J. Clin. Oncol. 2007, 25, 5591–5596. [Google Scholar] [CrossRef]
- Silverstein, M.J.; Lagios, M.D.; Groshen, S. The influence of margin with on local control of ductal carcinoma in situ of the breast. N. Engl. J. Med. 1999, 340, 1455–1461. [Google Scholar] [CrossRef]
- Rudloff, U.; Brogi, E.; Reiner, A. The influence of margin with and volume of disease near margin on benefit of radiation therapy for women with DCIS treated with breast-conserving therapy. Ann. Surg. 2010, 251, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Ringberg, A.; Nordgren, H.; Thorstensson, S. Histopathological risk factors for ipsilateral breast events after breast conserving treatment for ductal carcinoma in situ of the breast: Results from the Swedish randomised trial. Eur. J. Cancer 2007, 43, 291–298. [Google Scholar] [CrossRef]
- Fisher, E.R.; Land, S.R.; Saad, R.S. Pathologic variables predictive of breast events in patients with ductal carcinoma in situ. Am. J. Clin. Pathol. 2007, 128, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Mazouni, C.; Delaloge, S.; Rimareix, F.; Garbay, J.-R. Nomogram for Risk of Relapse After Breast-Conserving Surgery in Ductal Carcinoma in Situ. J. Clin. Oncol. 2011, 29, e44. [Google Scholar] [CrossRef]
- Ballehaninna, U.K.; Chamberlain, R.S. Inclusion of Tumor Biology Molecular Markers to Improve the Ductal Carcinoma In Situ Ipsilateral Breast Tumor Recurrence Nomogram Predictability. J. Clin. Oncol. 2011, 29, e97–e98. [Google Scholar] [CrossRef]
- Yi, M.; Meric-Bernstam, F.; Kuerer, H.M.; Mittendorf, E.A.; Bedrosian, I.; Lucci, A.; Hwang, R.F.; Crow, J.R.; Luo, S.; Hunt, K.K. Evaluation of a Breast Cancer Nomogram for Predicting Risk of Ipsilateral Breast Tumor Recurrences in Patients with Ductal Carcinoma in Situ After Local Excision. J. Clin. Oncol. 2012, 30, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, H.; Tan, P.; Chua, E.; Yeo, R.; Lim, F.; Kim, S.; Tan, D.; Wong, F. Validation of a Nomogram in the Prediction of Local Recurrence Risks after Conserving Surgery for Asian Women with Ductal Carcinoma in Situ of the Breast. Clin. Oncol. 2014, 26, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Sweldens, C.; Peeters, S.; van Limbergen, E. Local relapse after breast-conserving therapy for ductal carcinoma in situ: A European single-center experience and external validation of the Memorial Sloan-Kettering Cancer Center DCIS nomogram. Cancer J. 2014, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thorat, M.A.; Levey, P.M.; Jones, J.L.; Pinder, S.E.; Bundred, N.J.; Fentiman, I.S.; Cuzick, J. Prognostic Value of ER and PgR Expression and the Impact of Multi-clonal Expression for Recurrence in Ductal Carcinoma in situ: Results from the UK/ANZ DCIS Trial. Clin. Cancer Res. 2021, 27, 2861–2867. [Google Scholar] [CrossRef] [PubMed]
| Risk Factors | n = 207 (%) |
| Age | |
| <50 years | 27 (13) |
| >50 years | 180 (87) |
| Family history | |
| No | 107 (52) |
| Yes | 15 (7) |
| Unknown | 85 (41) |
| Clinical presentation | |
| No | 194 (93.7) |
| Yes | 13 (6.3) |
| Nuclear grade | |
| 1 | 68 (32.8) |
| 2–3 | 130 (62.8) |
| Unknown | 9 (4.4) |
| Pathological tumor size | |
| <20 mm | 142 (68.5) |
| >20 mm | 62 (30) |
| Unknown | 3 (1.5) |
| Focality | |
| Unifocal | 176 (85) |
| Multifocal | 31 (15) |
| Comedonecrosis | |
| No | 90 (43.5) |
| Yes | 114 (55) |
| Unknown | 3 (1.5) |
| Estrogen receptors | |
| Positive | 154 (74.4) |
| Negative | 35 (16.9) |
| Unknown | 18 (8.7) |
| Progesterone receptors | |
| Positive | 108 (52.2) |
| Negative | 73 (35.3) |
| Unknown | 26 (12.5) |
| Final surgical resection margin | |
| >2 mm | 166 (80.2) |
| <2 mm | 40 (19.3) |
| Unknown | 1 (0.5) |
| Number of reinterventions | |
| 1 | 181 (87.5) |
| 2 | 23 (11) |
| 3 | 1 (0.5) |
| Unknown | 2 (1) |
| Radiotherapy | |
| No | 33 (15) |
| Yes | 174 (85) |
| Hormonotherapy | |
| No | 148 (71.5) |
| Yes | 59 (28.5) |
| Risk Factors | No IBRT (n = 180) | IBRT (n = 27) | Univariate OR and CI (95%) | Multivariate OR and CI (95%) | p |
|---|---|---|---|---|---|
| Included in MSKCC: | |||||
| Age | |||||
| <50 years | 23 (12.8) | 4 (14.8) | 0.9 (0.9–1.0) | 1.0 (0.9–1.1) | 0.54 |
| >50 years | 157(87.2) | 23 (85.2) | |||
| Family history | |||||
| No | 86 (47.7) | 21 (77.8) | |||
| Yes | 13 (7.2) | 2 (7.4) | 0.6 (0.1–3.0) | 0.7 (0.1–3.6) | 0.54 |
| Unknown | 81 (45.0) | 4 (14.8) | |||
| Clinical presentation | |||||
| No | 171 (95.0) | 23 (85.2) | 3.3 (0.9–11.6) | 7.0 (0.9–52.0) | 0.08 |
| Yes | 9 (5.0) | 4 (14.8) | |||
| Radiotherapy | |||||
| No | 28 (15.6) | 5 (18.5) | 0.8 (0.3–2.8) | 1.3 (0.3–6.2) | 0.69 |
| Yes | 152 (84.4) | 22 (81.5) | |||
| Endocrine Therapy | |||||
| No | 125 (69.4) | 23 (85.1) | |||
| Yes | 55 (30.6) | 4 (14.8) | 0.4 (0.1–1.1) | 0.4 (0.9–1.4) | 0.07 |
| Nuclear grade | |||||
| 1 | 59 (32.8) | 9 (33.3) | |||
| 2–3 | 115 (63.8) | 15 (55.5) | 0.9 (0.3–2.1) | 1.2 (0.4–3.7) | 0.69 |
| Unknown | 6 (3.3) | 3 (11.1) | |||
| Comedonecrosis | |||||
| No | 78 (43.3) | 12 (44.4) | |||
| Yes | 100 (55.5) | 14 (51.8) | 0.9 (0.4–2.1) | 0.7 (0.2–2.2) | 0.82 |
| Unknown | 2 (1) | 1 (3.7) | |||
| Final surgical resection margin | |||||
| >2 mm | 147 (81.7) | 19 (70.3) | |||
| <2 mm | 33 (18.3) | 7 (25.9) | 1.6 (0.6–4.2) | 1.8 (0.5–6.0) | 0.31 |
| Unknown | 0 (0) | 1 (3.7) | |||
| Number of reinterventions | |||||
| 1 | 159 (88.8) | 22 (81.4) | |||
| 2 | 19 (10.6) | 4 (14.8) | 1.5 (0.5–4.8) | 1.6 (0.5–6.0) | 0.49 |
| 3 | 1 (0.0) | 0 (0) | |||
| Unknown | 1 (0.0) | 1 (3.7) | |||
| Other Risk Factors: | |||||
| Pathological tumor size | |||||
| <20 mm | 122 (67.8) | 20 (74.1) | |||
| >20 mm | 56 (31.1) | 6 (22.2) | 0.6 (0.2–1.7) | 0.28 | |
| Unknown | 2 (1.1) | 1 (3.7) | |||
| Focality | |||||
| Unifocal | 153 (85.0) | 23 (85.2) | 1.0 (0.3–3.0) | 0.97 | |
| Multifocal | 27 (15.0) | 4 (14.8) | |||
| Estrogen receptors | |||||
| Positive | 140 (77.8) | 14 (51.8) | |||
| Negative | 25 (13.9) | 10 (37.0) | 0.3 (0.1–0.6) | 0.004 | |
| Unknown | 15 (8.3) | 3 (11.1) | |||
| Progesterone receptors | |||||
| Positive | 95 (52.8) | 13 (48.1) | |||
| Negative | 62 (34.4) | 11 (40.7) | 0.7 (0.3–1.8) | 0.55 | |
| Unknown | 23 (12.8) | 3 (11.1) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oses, G.; Mension, E.; Pumarola, C.; Castillo, H.; Francesc, L.; Torras, I.; Cebrecos, I.; Caparrós, X.; Ganau, S.; Ubeda, B.; et al. Analysis of Local Recurrence Risk in Ductal Carcinoma In Situ and External Validation of the Memorial Sloan Kettering Cancer Center Nomogram. Cancers 2023, 15, 2392. https://doi.org/10.3390/cancers15082392
Oses G, Mension E, Pumarola C, Castillo H, Francesc L, Torras I, Cebrecos I, Caparrós X, Ganau S, Ubeda B, et al. Analysis of Local Recurrence Risk in Ductal Carcinoma In Situ and External Validation of the Memorial Sloan Kettering Cancer Center Nomogram. Cancers. 2023; 15(8):2392. https://doi.org/10.3390/cancers15082392
Chicago/Turabian StyleOses, Gabriela, Eduard Mension, Claudia Pumarola, Helena Castillo, León Francesc, Inés Torras, Isaac Cebrecos, Xavier Caparrós, Sergi Ganau, Belén Ubeda, and et al. 2023. "Analysis of Local Recurrence Risk in Ductal Carcinoma In Situ and External Validation of the Memorial Sloan Kettering Cancer Center Nomogram" Cancers 15, no. 8: 2392. https://doi.org/10.3390/cancers15082392
APA StyleOses, G., Mension, E., Pumarola, C., Castillo, H., Francesc, L., Torras, I., Cebrecos, I., Caparrós, X., Ganau, S., Ubeda, B., Bargallo, X., González, B., Sanfeliu, E., Vidal-Sicart, S., Moreno, R., Muñoz, M., Santamaría, G., & Mollà, M. (2023). Analysis of Local Recurrence Risk in Ductal Carcinoma In Situ and External Validation of the Memorial Sloan Kettering Cancer Center Nomogram. Cancers, 15(8), 2392. https://doi.org/10.3390/cancers15082392





