Simple Summary
The association of obesity with a host of comorbidities and its role in cancer have been studied extensively, but its influence on cancer treatments is not well understood. In particular, little is known about the interplay between obesity and immune checkpoint inhibitor (ICI)-induced immune-related adverse events (irAEs). This retrospective single-center study explored this relationship in 202 cancer patients with ICI exposure who developed gastrointestinal irAEs and had existing data on their body mass index (BMI) and visceral fat as measured by CT. Lower BMI was interestingly found to correlate with a more severe disease course. Aside from that, obesity was not found to significantly alter the course of ICI-mediated diarrhea and colitis, nor did it impact the overall survival of this population. Importantly, this study also supports the use of BMI as an indicator of adiposity in cancer patients, as higher BMI values were strongly associated with increased visceral fat on CT imaging.
Abstract
Obesity defined by high body mass index (BMI) has traditionally been associated with gastrointestinal inflammatory processes but has recently been correlated with better survival in patients receiving immune checkpoint inhibitors (ICI). We sought to investigate the association between BMI and immune-mediated diarrhea and colitis (IMDC) outcomes and whether BMI reflects body fat content on abdominal imaging. This retrospective, single-center study included cancer patients with ICI exposure who developed IMDC and had BMI and abdominal computed tomography (CT) obtained within 30 days before initiating ICI from April 2011 to December 2019. BMI was categorized as <25, ≥25 but <30, and ≥30. Visceral fat area (VFA), subcutaneous fat area (SFA), total fat area (TFA: VFA+SFA), and visceral to subcutaneous fat (V/S) ratio were obtained from CT at the umbilical level. Our sample comprised 202 patients; 127 patients (62.9%) received CTLA-4 monotherapy or a combination, and 75 (37.1%) received PD-1/PD-L1 monotherapy. Higher BMIs ≥ 30 were associated with a higher incidence of IMDC than BMIs ≤ 25 (11.4% vs. 7.9%, respectively; p = 0.029). Higher grades of colitis (grade 3–4) correlated with lower BMI (p = 0.03). BMI level was not associated with other IMDC characteristics or did not influence overall survival (p = 0.83). BMI is strongly correlated with VFA, SFA, and TFA (p < 0.0001). Higher BMI at ICI initiation was linked to a higher incidence of IMDC but did not appear to affect outcomes. BMI strongly correlated with body fat parameters measured by abdominal imaging, suggesting its reliability as an obesity index.
1. Introduction
The obesity crisis is a longstanding global health issue. In 2015, over a third of the world’s population was found to be overweight. Overweight individuals suffer from a host of sequelae [1]. They are at increased risk of developing conditions such as diabetes mellitus, cardiovascular disease, musculoskeletal disorders, and poor neurological health [2,3,4,5]. Obesity is regarded as a leading cause of preventable deaths globally, causing a predicted 400,000 deaths annually [6]. Obesity has also been found to play a considerable role in the incidence, prognosis, and survival of a less preventable leading cause of death: cancer [7]. To date, there has been much research on the role of obesity in cancer pathophysiology. It has been associated with the development of cancer in at least 13 different anatomical locations and has a variable influence on cancer survival depending on the cancer type [8,9,10,11,12]. However, given the speed at which cancer treatment is evolving in the modern era, there is an unfortunate dearth of research involving the effects of obesity on the efficacy of cancer therapeutics.
Immune checkpoint inhibitors (ICIs)—namely, cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1/programmed death ligand 1 (PD-1/PD-L1) inhibitors—have revolutionized the treatment of advanced cancers by improving patient survival across a wide range of tumors [13]. However, ICIs can give rise to an autoimmune response in the various organ systems of the body through the selective upregulation of tumor antigen-specific T-cells, resulting in immune-related adverse events (irAEs) [14]. irAEs in the gastrointestinal (GI) system, referred to as immune-mediated diarrhea and colitis (IMDC), are typically present as diarrhea that may necessitate hospitalization and further treatment. IMDC is among the most common irAE and a frequent reason for ICI discontinuation [15,16]. Given the success of ICIs and the treatment disruption by IMDC, it is important to identify the risk factors contributing to the development and severity of this disease entity; obesity, in particular, is a highly relevant factor in this regard.
Body mass index (BMI) evaluates an individual’s weight relative to their height and is a widely employed index of obesity [17]. Recent evidence suggests that BMI may serve as an emerging prognostic factor among cancer patients with ICI exposure [18,19,20,21]. A multicenter study of cancer patients treated with anti-PD-1/PD-L1 showed that overweight and obese patients (BMI ≥ 25) experienced significantly more irAEs, particularly GI-related ones, compared to non-overweight patients (BMI < 25) (55.6% vs. 25.2%) [20,21]. This study also found that overweight and obese patients had significantly longer overall survival (OS) and progression-free survival (PFS) compared to non-overweight patients [20]. Similarly, another pooled analysis of 4090 cancer patients treated with ICIs suggested that patients with a BMI of ≥25 had a significantly higher risk of developing irAEs compared to patients with a BMI of <25 [21]. Finally, a meta-analysis of 3768 cancer patients with ICI exposure showed that higher BMI was associated with improved PFS and OS while exhibiting similar mortality and tumor progression rates compared to normal BMI, regardless of tumor type [22].
Despite the high specificity of the standard cut-off value in defining obesity, BMI has a low sensitivity in identifying adiposity and is, therefore, insufficient to delineate visceral fat composition and discriminate between types of adiposities [23,24]. There are established quantitative measures of intra-abdominal fat volume, such as visceral fat area (VFA), subcutaneous fat area (SFA), and total fat area (TFA: sum of VFA and SFA), which can be obtained from computed tomography (CT) imaging and used to evaluate obesity and obesity-related risks [25,26,27]. Emerging evidence also supports the utility of body fat measurement by computed imaging in studying the efficacy of ICI therapy in cancer patients [28,29,30,31]. Notably, higher SFA, VFA/SFA (V/S) ratio, and TFA among advanced cancer patients treated with ICIs were associated with increased OS and PFS [28,30,32]. Meanwhile, VFA was found not to have an effect on OS and PFS in a sample of non-small cell lung cancer patients on ICIs [31].
The current literature on the impact of obesity on IMDC outcomes in cancer patients is still lacking. In this study, we aim to investigate the association between obesity as measured by BMI with IMDC incidence, disease course, and outcome and to assess whether BMI is a valid reflection of adiposity in this population using CT-generated fat measures.
2. Materials and Methods
2.1. Patient and Clinical Data Selection
We retrospectively analyzed patients at a tertiary cancer center who were treated with ICI from April 2011 to December 2019 and met the following inclusion criteria: (1) aged >18 years old, (2) had an established cancer diagnosis and received ICIs (PD-L1 inhibitors, PD-1 inhibitors, or CTLA-4 inhibitors) as monotherapy or as combination therapy, (3) had a diagnosis of IMDC, and (4) underwent abdominal CT imaging within 14 days before initiating ICI therapy. This study was approved by the institutional review board with a waiver of patient’s informed consent.
All patient data were collected through institutional electronic medical databases. Baseline demographics included age, sex, race/ethnicity, comorbidities, and the Charlson comorbidity index [33]. Oncologic information such as cancer type, cancer stage (using the 8th edition American Joint Committee on Cancer staging manual), ICI therapy type (CTLA-4 monotherapy, PD-1/PD-L1 monotherapy, or combination of both), and date of ICI initiation was also gathered, alongside mortality and OS. We broadly categorized malignancies as melanoma, genitourinary cancer, lung cancer, and others (GI cancer, head and neck, endocrine, and hematologic malignancy). Cancer staging for hematological malignancies was not included.
2.2. IMDC Characteristics and Outcomes Assessed
The collected characteristics of IMDC were date of onset, peak diarrhea, and colitis grades at the time of diagnosis based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE; version 5.0), duration of IMDC symptoms, use of intravenous steroids, duration of steroid therapy, and use of non-steroidal immunosuppressants, i.e., infliximab and vedolizumab. The collected outcomes of IMDC included hospitalization, duration of hospitalization, recurrence rate, and clinical remission rate. Clinical remission of IMDC symptoms was defined as a sustained resolution of the symptoms to grade 1 or lower. Clinical response of IMDC was defined as an improvement of symptoms to a lower grade than the initial presentation but not meeting the criteria of clinical remission.
2.3. Obesity Measurement
BMI was calculated from patient height and body weight (BMI = weight in kilograms divided by the square of height in meters) within 30 days before ICI initiation. BMI values were divided into 3 groups: underweight/normal weight (BMI < 25 kg/m2), overweight (BMI ≥ 25 kg/m2 but <30 kg/m2), and obesity (BMI ≥ 30). VFA, SFA, TFA, and the V/S ratio were obtained from CT scans at the level of the umbilicus as an intra-body fat index (Figure 1) based on established standards [34]. MIM Software was used for the measurement of intra-body fat area from radiologic imaging. Subcutaneous fat was defined as the extraperitoneal fat between skin and muscle. Visceral fat was defined as intraperitoneal fat with the same density on imaging as the subcutaneous fat layer. CT scans were analyzed using pre-established thresholds for fat of VFA and SFA (−150 to −50 Hounsfield units). All measurements were carried out by two experienced radiologists.
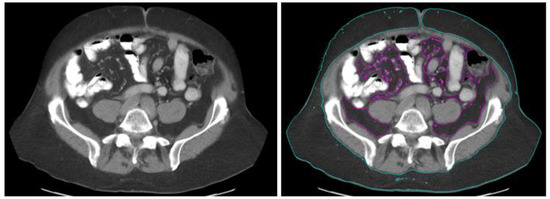
Figure 1.
Measurement of intra-abdominal fat area by use of computed tomography. (Left): Initial computed tomography images. (Right): Intra-abdominal adipose tissue areas. Regions of purple and blue color indicate visceral (89.4 cm2) and subcutaneous (246.6 cm2) adipose tissue, respectively.
2.4. Statistical Analysis
SPSS version 24.0 software was used to perform the statistical analyses. Mann–Whitney U and Kruskal–Wallis tests were used to compare continuous variables between groups. Chi-square and Fisher exact tests were used to evaluate the association between categorical variables. Univariate logistical regression was used to estimate the difference in IMDC incidence among BMI subgroups. The Kaplan–Meier method and log-rank test were used to measure OS. OS was defined as the time from the initiation of ICI therapy to the date of last follow-up or death. Pearson correlation analysis was applied to assess the correlation between BMI and visceral body fat parameters. p values of <0.05 were considered statistically significant.
3. Results
3.1. Patient Baseline Characteristics
A total of 573 IMDC patients had abdominal and pelvic CT scans at the cancer center from 2011 to 2020. Among them, 202 patients were included in this study (patient selection flow chart in Figure 2). Our cohort had a median age of 61 years (interquartile range [IQR] 49–70 years). A majority (89.6%) were white, and a majority were male (67.3%). The most common cancer type was melanoma (44.1%), followed by genitourinary cancer (29.7%) and lung cancer (7.9%). Most patients had a diagnosis of stage IV cancer (87.1%). Regarding therapy, 127 (62.9%) received CTLA-4–based therapy (alone or with PD-1/PD-L1 therapy), and 75 (37.1%) received PD-1/PD-L1 monotherapy. The median follow-up time was 28.8 months (IQR 14.2–48.8 months), and all-cause mortality was 44.1%. The median BMI was 28.1 (IQR 24.4–32.6). Further information regarding patient characteristics can be found in Table 1.
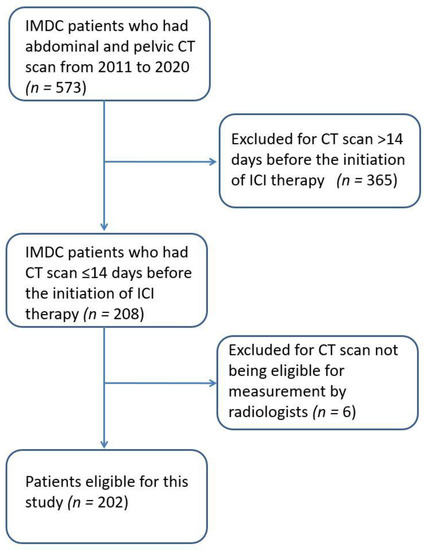
Figure 2.
Patient selection flow chart.

Table 1.
Demographic characteristics.
3.2. Characteristics of IMDC Stratified by BMI
The incidence of IMDC was analyzed and compared among different BMI groups in the base number of 2139 patients who had BMI data available and received ICI, with abdominal CT scan done within 14 days prior to ICI initiation, matching the IMDC cohort. A BMI ≥ 30 was found to have the highest incidence of IMDC at 11.4%, compared to patients with a BMI between 25–30 (9.1%; p = 0.158) and ≤25 (7.9%; p = 0.029) (Supplementary Table S1). Characteristics of IMDC were summarized and compared between the three BMI groups (Table 2). Individuals with lower BMI were found to have higher CTCAE severity of colitis (p = 0.03). The other IMDC characteristics, including time of IMDC onset, duration of symptoms, and use of steroids and non-steroidal immunosuppressants, were similar between BMI groups.

Table 2.
Characteristics and outcomes of IMDC stratified by BMI.
3.3. Outcomes of IMDC Stratified by BMI
The outcomes of IMDC by BMI group are summarized in Table 2. Overall, approximately 60% of patients required hospital admission, with a median length of stay of 5–7 days depending on BMI. Clinical remission of IMDC on medical treatment was achieved in 70% of patients, and the colitis recurrence rate was 15%–25% between groups within the follow-up time of 25–33 months from IMDC diagnosis. There was no significant difference observed in the comparison of these outcome variables between the three BMI groups. On OS analysis, BMI level did not appear to affect the survival duration (p = 0.829) (Figure 3).
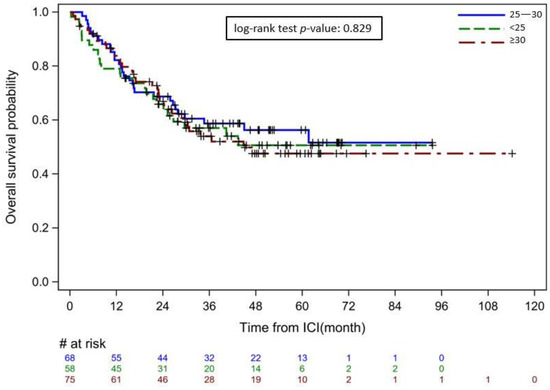
Figure 3.
Overall survival of patients with immune-mediated diarrhea and colitis by BMI group Starting point: immune checkpoint inhibitor initiation. Ending point: last follow-up or death from any cause. # refers to the number of patients at risk, + indicates cases censored due to loss to follow-up.
3.4. Correlation between BMI and Visceral Body Fat Parameters
Correlation analysis demonstrated strong associations between BMI level and the levels of SFA (p < 0.001), VFA (p < 0.0001), and TFA (p < 0.0001) (Table 3).

Table 3.
Correlation analysis between BMI and body fat parameters on imaging.
4. Discussion
Despite the tremendous improvements in survival in a wide range of metastatic solid tumors treated with ICIs, ICI exposure introduces the risk of irAEs, which often require ICI discontinuation and hospitalization [16,35]. An understanding of the potential clinical and demographic risk factors associated with irAEs and their severity is critically needed to improve clinical outcomes. To our knowledge, our study has, for the first time, demonstrated that higher BMIs may be associated with an increased risk for IMDC, with lower rather than higher BMI presenting higher colitis grades. There was no discernable impact of BMI on IMDC outcomes or OS.
Previous studies have investigated the association between BMI and body fat index in different patient populations [36,37,38,39]. BMI compares an individual’s height relative to their weight as a convenient proxy measure for assessing body fat and has been used in pediatric and adult populations to screen for obesity [17,40,41,42]. BMI is a predictive factor for the risk of developing comorbidities such as diabetes mellitus and cardiovascular disease [43,44]. However, BMI has recently come under scrutiny for not being an accurate assessment of obesity [45,46], and whether BMI characterizes body adiposity remains controversial [17,36,47,48]. Body adiposity, particularly visceral adipose tissue, is suggested to be the main culprit in obesity-related complications and is, therefore, an important parameter [49]. In cancer patients specifically, visceral fat and the corresponding insulin resistance and chronic low-grade inflammation play an important role in the disease course, more so than BMI [50,51,52]. Still, most studies on cancer patients continue to use BMI as a measure of obesity despite its contentious association with visceral fat [53,54,55,56,57]. In this study, we compared BMI measures with body fat indices obtained through the gold standard of CT imaging and found strong correlations (p < 0.0001), which supports our use of BMI for the accompanying analyses. However, further validation of BMI as a substitute for CT-generated body fat indices is needed.
High BMI presents an “obesity paradox” for survival among cancer patients whereby it increases the risk of esophageal, colon, and renal cancers while also being a favorable prognostic factor in several solid cancers, such as non–small cell lung cancer, colorectal cancer, and gastric cancer [9,10,11,12]. Recently, BMI was found to be a promising clinical marker for predicting the development of irAEs and their outcomes [18,19,20,21]. Though this tool has extended the possibilities for nutritional assessment in routine clinical care for cancer patients, the relationship between BMI and IMDC—which frequently leads to ICI discontinuation—has not been clear. Our study among cancer patients who developed IMDC importantly showed a possible association between increased BMI ≥30 and the risk of developing colitis compared to patients with a BMI ≤ 25. This association disappears when looking at patients with a BMI in between, likely due to the large variety inherent in that range. This is in line with results from previous research showing an association between obesity and the development of irAEs [19,20]. Furthermore, we found that colitis severity was inversely correlated with BMI. Higher grades of colitis (grade 3–4) were correlated with lower BMI, and BMI had no significant impact on OS, which contradicts previous reports on the association between higher BMI and worse irAE outcomes, including colitis [20,58]. This discrepancy may result from multiple confounding factors, e.g., our unique cohort of patients with IMDC, or the specific type of cancer studied. However, these studies investigated irAEs in all systems, not GI irAEs in particular, which could suggest unique associations between IMDC and obesity. We suspect the benefit to OS from IMDC may overcome the influence of BMI on survival among this cohort, which may also explain the non-significant association of BMI with OS [59]. Further studies, including a control group without IMDC, may clarify the impact of BMI on patients’ survival.
Cancer patients who develop IMDC usually have diarrhea and colitis; this clinical presentation is highly reminiscent of inflammatory bowel disease [60,61]. Particularly in Crohn’s disease, obesity has been associated with a worse disease course [62,63]. Higher VFA was associated with an increased risk of surgery for Crohn’s disease and with early post-operative Crohn’s recurrence [61,64,65]. Moreover, higher VFA was an independent predictive factor for delayed intestinal mucosal healing among patients with Crohn’s disease that required infliximab [66]. Distinct characteristics of adipose distribution may thus contribute to the pathophysiology of Crohn’s disease and possibly IMDC. Further analysis is still needed to evaluate the association between the pattern of adipose distribution and inflammatory bowel conditions.
The impact of obesity on the incidence and mortality of a wide range of cancer types has already been recognized for up to 13 different cancers [13]. The current hypothesis is that the increased volume of visceral fat promotes tumorigenesis and increases cancer risk through an adverse impact on inflammation and metabolism [67,68,69]. This is based on the knowledge that inflammatory cells are more prevalent in visceral fat compared to subcutaneous fat, are more metabolically active, insulin resistant, and sensitive to lipolysis, and are, therefore, more pro-inflammatory and pro-tumorigenic [70,71,72,73,74]. One suggested reason for such phenomena is the existence of non-coding RNA (ncRNA includes long ncRNA and microRNA). It has been discovered that a majority of our genome is transcribed into ncRNA that serves various roles in regulating gene expression, including immune checkpoint genes [75]. The level of expression of these ncRNA has been implicated in the development and prognosis of certain tumors and chemo- and immunotherapy resistance [76]. A few studies have hypothesized that ncRNA expression is altered in obesity [77,78], potentially explaining the current link between obesity and cancer. However, there is no conclusive evidence of this yet. Given the wide range of existing ncRNAs, each with a differential effect on tumorigenesis and immunotherapy resistance, it would be interesting to explore this mechanistic link between BMI and immunotherapy efficacy, adverse event rate, and disease course in the future. Additionally, although the predictive value of various cutoffs for adiposity have been studied for certain medical conditions, e.g., metabolic syndrome, there are no standardized criteria for VFA, SFA, or TFA regarding cancer risk, the effect of cancer treatment, and irAEs, which warrants additional research studies [79,80]. Additionally, it is worth looking into the impact of sarcopenia and its relationship with adiposity, as previous studies have shown that sarcopenia may be associated with worse survival among various cancer types [81,82,83].
Gut microbiota has been associated with obesity and immunotherapy outcomes among cancer patients. Obesity can produce chronic pro-inflammatory states, which can cause a dysfunctional gut barrier and lipopolysaccharide leakage [84]. Immunotherapy also induces gut mucosal damage by compromising barrier integrity, which increases the gut invasion of bacteria. Clinically, multiple studies have demonstrated that certain microbiome compositions (e.g., low Faecalibacterium, Ruminococcaceae, and other Firmicutes with enriched Bacteroides) among cancer patients on ICI are associated with better survival, while other compositions were associated with increased bowel inflammation [85,86,87]. Indeed, the gut microbiome has a significant immunoregulatory effect, and the types of bacteria present may play a crucial role in mediating both bowel inflammatory responses and possibly more distant, systemic inflammatory responses [88,89,90]. Interestingly, previous research has shown that the gut microbiome may also play a role in the development of obesity, and restoration of healthy commensal bacteria via fecal microbiota transplantation has shown promising results for improving outcomes in cancer, refractory IMDC, and obesity [91,92,93,94,95,96,97,98]. Given the complex interplay between obesity, the inflammatory cascade, gut microbiomes, cancer, and cancer treatment, it is especially challenging to delineate a causative relationship and any interactive effects among these variables. Further studies are, therefore, needed to clarify the role of the microbiome and fecal microbiota transplantation in treating obesity in the general population and IMDC in an obese cancer population.
Our study has several limitations. First, this is a retrospective single-center study with all the inherent limitations that stem from this study design. The data availability in patients’ charts could limit the accurate collection of all the details related to IMDC episodes. Second, additional cancer therapies after ICI termination were not included in this study, which could potentially confound the IMDC outcomes and cancer survival. Third, the lack of a control group without IMDC rendered unfeasible the evaluation of the impact of obesity on IMDC incidence and overall cancer outcome. Fourth, we included only patients who had an abdominal CT scan in this study window with images amenable for fat measurement, which could certainly lead to a selection bias. Finally, dietary factors and the microbiome could play a critical role in the parameters investigated in this study; however, we were not able to collect this information retrospectively.
5. Conclusions
IMDC has been recognized as one of the most common irAEs in cancer patients on ICI treatment. The association between obesity and IMDC and its disease course has not been studied. We found that patients with BMIs on the higher end (at or above 30) may be at increased risk of developing IMDC, with lower BMIs (at or below 25) associated with a decreased risk but higher colitis severity. BMI had no impact on survival. BMI categorization has a good correlation with body fat index measurement via imaging and could be a useful and convenient tool in oncologic practice to predict various risks associated with cancer and ICI therapy. Future prospective trials are needed to further elucidate the effect of adiposity on the characteristics and outcomes of IMDC in cancer patients on ICIs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15082329/s1, Table S1: Risk of developing of IMDC among different BMI subgroups.
Author Contributions
Conceptualization, Y.W. and A.S.T.; methodology, W.Q.; validation, Y.W. and A.S.T.; formal analysis, W.Q.; data curation, M.K., M.S., G.X., Y.L., A.M., W.M. and K.V.; writing—original draft preparation, M.K. and M.S.; writing—review and editing, Y.W. and A.S.T.; supervision, Y.W. and A.S.T.; project administration, Y.W; All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported in part by the National Institutes of Health through MD Anderson Cancer Center’s Support Grant CA016672.
Institutional Review Board Statement
This study is conducted under umbrella protocol PA18-0472 at the University of Texas MD Anderson Cancer Center.
Informed Consent Statement
Informed consent is waived for this study, given the retrospective nature of the institutional IRB.
Data Availability Statement
All collected data is available upon request.
Acknowledgments
Medical editing of this paper was provided by Sarah Bronson, Research Medical Library, at the University of Texas MD Anderson Cancer Center.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Anandacoomarasamy, A.; Caterson, I.; Sambrook, P.; Fransen, M.; March, L. The impact of obesity on the musculoskeletal system. Int. J. Obes. 2008, 32, 211–222. [Google Scholar] [CrossRef]
- Anstey, K.J.; Cherbuin, N.; Budge, M.; Young, J. Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obes. Rev. 2011, 12, e426–e437. [Google Scholar] [CrossRef]
- Czernichow, S.; Kengne, A.P.; Stamatakis, E.; Hamer, M.; Batty, G.D. Body mass index, waist circumference and waist-hip ratio: Which is the better discriminator of cardiovascular disease mortality risk?: Evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes. Rev. 2011, 12, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef]
- Hurt, R.T.; Frazier, T.H.; McClave, S.A.; Kaplan, L.M. Obesity epidemic: Overview, pathophysiology, and the intensive care unit conundrum. JPEN J. Parenter. Enteral. Nutr. 2011, 35, 4S–13S. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Peterson, L.L. Obesity and Cancer: Evidence, Impact, and Future Directions. Clin. Chem. 2018, 64, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Sepesi, B.; Gold, K.A.; Correa, A.M.; Heymach, J.V.; Vaporciyan, A.A.; Roszik, J.; Dmitrovsky, E.; Liu, X. The Influence of Body Mass Index on Overall Survival Following Surgical Resection of Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 1280–1287. [Google Scholar] [CrossRef]
- Caan, B.J.; Meyerhardt, J.A.; Kroenke, C.H.; Alexeeff, S.; Xiao, J.; Weltzien, E.; Feliciano, E.C.; Castillo, A.L.; Quesenberry, C.P.; Kwan, M.L.; et al. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol. Biomark. Prev. 2017, 26, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, S.; Song, J.H.; Choi, S.; Cho, M.; Kwon, I.G.; Son, T.; Kim, H.I.; Cheong, J.H.; Hyung, W.J.; et al. Prognostic significance of body mass index and prognostic nutritional index in stage II/III gastric cancer. Eur. J. Surg. Oncol. 2020, 46, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chan, H.L.; Chen, P. Immune Checkpoint Inhibitors: Basics and Challenges. Curr. Med. Chem. 2019, 26, 3009–3025. [Google Scholar] [CrossRef] [PubMed]
- Baxi, S.; Yang, A.; Gennarelli, R.L.; Khan, N.; Wang, Z.; Boyce, L.; Korenstein, D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ 2018, 360, k793. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Ali, F.S.; Naqash, A.R.; Owen, D.H.; Patel, S.; Otterson, G.A.; Kendra, K.; Ricciuti, B.; Chiari, R.; De Giglio, A.; et al. Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. J. Clin. Oncol. 2019, 37, 2738–2745. [Google Scholar] [CrossRef]
- Force, U.S.P.S.T. Screening for obesity in adults: Recommendations and rationale. Ann. Intern. Med. 2003, 139, 930–932. [Google Scholar] [CrossRef]
- Murphy, W.J.; Longo, D.L. The Surprisingly Positive Association Between Obesity and Cancer Immunotherapy Efficacy. JAMA 2019, 321, 1247–1248. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother Cancer 2019, 7, 57. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Santini, D.; Buti, S.; Tiseo, M.; Cannita, K.; Perrone, F.; Giusti, R.; De Tursi, M.; Zoratto, F.; et al. Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/Programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: A multicentre analysis of immune-related adverse events. Eur. J. Cancer 2020, 128, 17–26. [Google Scholar] [CrossRef]
- Xu, H.; Cao, D.; He, A.; Ge, W. The prognostic role of obesity is independent of sex in cancer patients treated with immune checkpoint inhibitors: A pooled analysis of 4090 cancer patients. Int. Immunopharmacol. 2019, 74, 105745. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.C.; Chen, G.M.; Wang, Y.; Yuan, S.Q.; Zhou, J.; Duan, J.L.; Liu, W.W.; Chen, S.; Cai, M.Y.; Li, Y.F. Association between Body Mass Index and Survival Outcomes in Patients Treated with Immune Checkpoint Inhibitors: Meta-analyses of Individual Patient Data. J. Immunother. 2021, 44, 371–375. [Google Scholar] [CrossRef]
- WHO. Physical Status: The Use and Interpretation of Anthropometry, Report of a WHO Expert Committee; WHO Technical Report Series; WHO: Geneva, Switzerland, 1995; Volume 854, pp. 1–452. [Google Scholar]
- Okorodudu, D.O.; Jumean, M.F.; Montori, V.M.; Romero-Corral, A.; Somers, V.K.; Erwin, P.J.; Lopez-Jimenez, F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: A systematic review and meta-analysis. Int. J. Obes. 2010, 34, 791–799. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Despres, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Hiuge-Shimizu, A.; Kishida, K.; Funahashi, T.; Ishizaka, Y.; Oka, R.; Okada, M.; Suzuki, S.; Takaya, N.; Nakagawa, T.; Fukui, T.; et al. Reduction of visceral fat correlates with the decrease in the number of obesity-related cardiovascular risk factors in Japanese with Abdominal Obesity (VACATION-J Study). J. Atheroscler. Thromb. 2012, 19, 1006–1018. [Google Scholar] [CrossRef]
- Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ. J. 2002, 66, 987–992. [Google Scholar] [CrossRef]
- Esposito, A.; Marra, A.; Bagnardi, V.; Frassoni, S.; Morganti, S.; Viale, G.; Zagami, P.; Varano, G.M.; Buccimazza, G.; Orsi, F.; et al. Body mass index, adiposity and tumour infiltrating lymphocytes as prognostic biomarkers in patients treated with immunotherapy: A multi-parametric analysis. Eur. J. Cancer 2021, 145, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.J.; Kline, M.R.; Liu, Y.; Shabto, J.M.; Williams, M.A.; Khan, A.I.; Lewis, C.; Collins, H.; Akce, M.; Kissick, H.T.; et al. Adiposity may predict survival in patients with advanced stage cancer treated with immunotherapy in phase 1 clinical trials. Cancer 2020, 126, 575–582. [Google Scholar] [CrossRef]
- Young, A.C.; Quach, H.T.; Song, H.; Davis, E.J.; Moslehi, J.J.; Ye, F.; Williams, G.R.; Johnson, D.B. Impact of body composition on outcomes from anti-PD1 +/− anti-CTLA-4 treatment in melanoma. J. Immunother. Cancer 2020, 8, e000821. [Google Scholar] [CrossRef]
- Minami, S.; Ihara, S.; Tanaka, T.; Komuta, K. Sarcopenia and Visceral Adiposity Did Not Affect Efficacy of Immune-Checkpoint Inhibitor Monotherapy for Pretreated Patients With Advanced Non-Small Cell Lung Cancer. World J. Oncol. 2020, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.J.; Olsen, T.A.; Goyal, S.; Liu, Y.; Evans, S.T.; Magod, B.; Brown, J.T.; Yantorni, L.; Russler, G.A.; Caulfield, S.; et al. Body Composition Variables as Radiographic Biomarkers of Clinical Outcomes in Metastatic Renal Cell Carcinoma Patients Receiving Immune Checkpoint Inhibitors. Front. Oncol. 2021, 11, 707050. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhu, Y.; Wang, H.; Zhang, H.; Shi, G.; Liu, X.; Ye, D. Prognostic value of components of body composition in patients treated with targeted therapy for advanced renal cell carcinoma: A retrospective case series. PLoS ONE 2015, 10, e0118022. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Thomas, E.L.; Saeed, N.; Hajnal, J.V.; Brynes, A.; Goldstone, A.P.; Frost, G.; Bell, J.D. Magnetic resonance imaging of total body fat. J. Appl. Physiol. 1998, 85, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Grummer-Strawn, L.M.; Pietrobelli, A.; Goulding, A.; Goran, M.I.; Dietz, W.H. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am. J. Clin. Nutr. 2002, 75, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Cahill, F.; Gulliver, W.; Yi, Y.; Xie, Y.; Bridger, T.; Pace, D.; Zhang, H. Concordance of BAI and BMI with DXA in the Newfoundland population. Obesity 2013, 21, 499–503. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, L.; Dong, S.; Zha, X.; Ran, L.; Li, Y.; Chen, S.; Gao, J.; Li, S.; Lu, Y.; et al. CT-derived abdominal adiposity: Distributions and better predictive ability than BMI in a nationwide study of 59,429 adults in China. Metabolism 2021, 115, 154456. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Dorosty, A.R.; Emmett, P.M. Identification of the obese child: Adequacy of the body mass index for clinical practice and epidemiology. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1623–1627. [Google Scholar] [CrossRef]
- Barlow, S.E.; Dietz, W.H. Obesity evaluation and treatment: Expert Committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics 1998, 102, E29. [Google Scholar] [CrossRef] [PubMed]
- Power, C.; Lake, J.K.; Cole, T.J. Measurement and long-term health risks of child and adolescent fatness. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Reeder, B.A.; Elliott, S.; Joffres, M.R.; Pahwa, P.; Raine, K.D.; Kirkland, S.A.; Paradis, G. Body mass index and risk of cardiovascular disease, cancer and all-cause mortality. Can. J. Public. Health 2012, 103, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.; Picone, G.; Sloan, F.; Yashkin, A. Relation between BMI and diabetes mellitus and its complications among US older adults. South Med. J. 2015, 108, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Thomas, R.J.; Collazo-Clavell, M.L.; Korinek, J.; Allison, T.G.; Batsis, J.A.; Sert-Kuniyoshi, F.H.; Lopez-Jimenez, F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 2008, 32, 959–966. [Google Scholar] [CrossRef]
- Franzosi, M.G. Should we continue to use BMI as a cardiovascular risk factor? Lancet 2006, 368, 624–625. [Google Scholar] [CrossRef]
- Shah, N.R.; Braverman, E.R. Measuring adiposity in patients: The utility of body mass index (BMI), percent body fat, and leptin. PLoS ONE 2012, 7, e33308. [Google Scholar] [CrossRef]
- Reilly, J.J.; El-Hamdouchi, A.; Diouf, A.; Monyeki, A.; Somda, S.A. Determining the worldwide prevalence of obesity. Lancet 2018, 391, 1773–1774. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ambrosi, J.; Silva, C.; Galofre, J.C.; Escalada, J.; Santos, S.; Millan, D.; Vila, N.; Ibanez, P.; Gil, M.J.; Valenti, V.; et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int. J. Obes. 2012, 36, 286–294. [Google Scholar] [CrossRef]
- Crudele, L.; Piccinin, E.; Moschetta, A. Visceral Adiposity and Cancer: Role in Pathogenesis and Prognosis. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Harvie, M.; Hooper, L.; Howell, A.H. Central obesity and breast cancer risk: A systematic review. Obes. Rev. 2003, 4, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Komninou, D.; Stephenson, G.D. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes. Rev. 2004, 5, 153–165. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Thun, M.J. Obesity and cancer. Oncogene 2004, 23, 6365–6378. [Google Scholar] [CrossRef] [PubMed]
- Parekh, N.; Chandran, U.; Bandera, E.V. Obesity in cancer survival. Annu. Rev. Nutr. 2012, 32, 311–342. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Chlebowski, R.T. Obesity and Cancer: Insights for Clinicians. J. Clin. Oncol. 2016, 34, 4197–4202. [Google Scholar] [CrossRef]
- Slawinski, C.G.V.; Barriuso, J.; Guo, H.; Renehan, A.G. Obesity and Cancer Treatment Outcomes: Interpreting the Complex Evidence. Clin. Oncol. 2020, 32, 591–608. [Google Scholar] [CrossRef]
- Woodall, M.J.; Neumann, S.; Campbell, K.; Pattison, S.T.; Young, S.L. The Effects of Obesity on Anti-Cancer Immunity and Cancer Immunotherapy. Cancers 2020, 12, 1230. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Abu-Sbeih, H.; Ma, W.; Peng, Y.; Qiao, W.; Wang, J.; Shah, A.Y.; Glitza Oliva, I.C.; Piha-Paul, S.A.; Thompson, J.A.; et al. Association of Chronic Immune-Mediated Diarrhea and Colitis With Favorable Cancer Response. J. Natl. Compr. Canc. Netw. 2020, 19, 700–708. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hebuterne, X.; Klek, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef]
- Van Der Sloot, K.W.; Joshi, A.D.; Bellavance, D.R.; Gilpin, K.K.; Stewart, K.O.; Lochhead, P.; Garber, J.J.; Giallourakis, C.; Yajnik, V.; Ananthakrishnan, A.N.; et al. Visceral Adiposity, Genetic Susceptibility, and Risk of Complications among Individuals with Crohn’s Disease. Inflamm. Bowel. Dis. 2017, 23, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hass, D.J.; Brensinger, C.M.; Lewis, J.D.; Lichtenstein, G.R. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin. Gastroenterol. Hepatol. 2006, 4, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Fink, C.; Karagiannides, I.; Bakirtzi, K.; Pothoulakis, C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm. Bowel. Dis. 2012, 18, 1550–1557. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, X.R.; Remer, E.M.; Lian, L.; Stocchi, L.; Li, Y.; McCullough, A.; Remzi, F.H.; Shen, B. Association between high visceral fat area and postoperative complications in patients with Crohn’s disease following primary surgery. Colorectal. Dis. 2016, 18, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, W.; Gong, J.; Zhang, W.; Gu, L.; Guo, Z.; Cao, L.; Shen, B.; Li, N.; Li, J. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn’s disease. Colorectal. Dis. 2015, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Cao, L.; Li, Y.; Cai, X.; Ge, Y.; Zhu, W. Visceral Fat Is Associated With Mucosal Healing of Infliximab Treatment in Crohn’s Disease. Dis. Colon. Rectum. 2018, 61, 706–712. [Google Scholar] [CrossRef]
- Doyle, S.L.; Donohoe, C.L.; Lysaght, J.; Reynolds, J.V. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc. Nutr. Soc. 2012, 71, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Lysaght, J.; Cathcart, M.C.; Donohoe, C.L.; Cummins, R.; McGarrigle, S.A.; Kay, E.; Reynolds, J.V.; Pidgeon, G.P. MMP9 expression in oesophageal adenocarcinoma is upregulated with visceral obesity and is associated with poor tumour differentiation. Mol. Carcinog. 2013, 52, 144–154. [Google Scholar] [CrossRef]
- Ohki, T.; Tateishi, R.; Shiina, S.; Goto, E.; Sato, T.; Nakagawa, H.; Masuzaki, R.; Goto, T.; Hamamura, K.; Kanai, F.; et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut 2009, 58, 839–844. [Google Scholar] [CrossRef]
- Bruun, J.M.; Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): Implication of macrophages resident in the AT. J. Clin. Endocrinol. Metab. 2005, 90, 2282–2289. [Google Scholar] [CrossRef]
- Curat, C.A.; Wegner, V.; Sengenes, C.; Miranville, A.; Tonus, C.; Busse, R.; Bouloumie, A. Macrophages in human visceral adipose tissue: Increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006, 49, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Marin, P.; Andersson, B.; Ottosson, M.; Olbe, L.; Chowdhury, B.; Kvist, H.; Holm, G.; Sjostrom, L.; Bjorntorp, P. The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism 1992, 41, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Vikram, N.K. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition 2003, 19, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, R.; Almeida, M.; Colombatti, A.; Calin, G.A. Long non-coding RNAs and cancer: A new frontier of translational research? Oncogene 2012, 31, 4577–4587. [Google Scholar] [CrossRef]
- Smolle, M.A.; Prinz, F.; Calin, G.A.; Pichler, M. Current concepts of non-coding RNA regulation of immune checkpoints in cancer. Mol. Asp. Med. 2019, 70, 117–126. [Google Scholar] [CrossRef]
- Yau, M.Y.-C.; Xu, L.; Huang, C.-L.; Wong, C.-M. Long Non-Coding RNAs in Obesity-Induced Cancer. Non-Coding RNA 2018, 4, 19. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Taheri, M. The expression profile and role of non-coding RNAs in obesity. Eur. J. Pharmacol. 2021, 892, 173809. [Google Scholar] [CrossRef]
- Doyle, S.L.; Bennett, A.M.; Donohoe, C.L.; Mongan, A.M.; Howard, J.M.; Lithander, F.E.; Pidgeon, G.P.; Reynolds, J.V.; Lysaght, J. Establishing computed tomography-defined visceral fat area thresholds for use in obesity-related cancer research. Nutr. Res. 2013, 33, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, M.; Tsuchihashi, K.; Saitoh, S.; Odawara, Y.; Hirano, T.; Nakata, T.; Miura, T.; Ura, N.; Hareyama, M.; Shimamoto, K. Visceral obesity in Japanese patients with metabolic syndrome: Reappraisal of diagnostic criteria by CT scan. Hypertens Res. 2007, 30, 315–323. [Google Scholar] [CrossRef]
- Trejo-Avila, M.; Bozada-Gutiérrez, K.; Valenzuela-Salazar, C.; Herrera-Esquivel, J.; Moreno-Portillo, M. Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: A systematic review and meta-analysis. Int. J. Colorectal. Dis. 2021, 36, 1077–1096. [Google Scholar] [CrossRef]
- Ishihara, H.; Kondo, T.; Omae, K.; Takagi, T.; Iizuka, J.; Kobayashi, H.; Hashimoto, Y.; Tanabe, K. Sarcopenia predicts survival outcomes among patients with urothelial carcinoma of the upper urinary tract undergoing radical nephroureterectomy: A retrospective multi-institution study. Int. J. Clin. Oncol. 2017, 22, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, S.; Nau, P.N.; Mezhir, J.J. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J. Surg. Oncol. 2015, 112, 503–509. [Google Scholar] [CrossRef]
- Everard, A.; Geurts, L.; Van Roye, M.; Delzenne, N.M.; Cani, P.D. Tetrahydro iso-alpha acids from hops improve glucose homeostasis and reduce body weight gain and metabolic endotoxemia in high-fat diet-fed mice. PLoS ONE 2012, 7, e33858. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and Anticancer Immunosurveillance. Cell 2016, 165, 276–287. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Impact of the gut microbiome on mucosal inflammation. Trends Immunol 2013, 34, 423–430. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136.e8. [Google Scholar] [CrossRef]
- Qin, Y.; Roberts, J.D.; Grimm, S.A.; Lih, F.B.; Deterding, L.J.; Li, R.; Chrysovergis, K.; Wade, P.A. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome. Biol. 2018, 19, 7. [Google Scholar] [CrossRef]
- Chen, X.; Devaraj, S. Gut Microbiome in Obesity, Metabolic Syndrome, and Diabetes. Curr. Diab. Rep. 2018, 18, 129. [Google Scholar] [CrossRef]
- Sehgal, K.; Khanna, S. Gut microbiome and checkpoint inhibitor colitis. Intest. Res. 2021, 19, 360–364. [Google Scholar] [CrossRef]
- Dai, C.; Liu, W.X. Refractory Immune Checkpoint Inhibitor-induced Colitis Improved by Fecal Microbiota Transplantation: A Case Report. Inflamm. Bowel. Dis. 2022, 28, e43–e44. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wiesnoski, D.H.; Helmink, B.A.; Gopalakrishnan, V.; Choi, K.; DuPont, H.L.; Jiang, Z.D.; Abu-Sbeih, H.; Sanchez, C.A.; Chang, C.C.; et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 2018, 24, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.S.; Halsey, T.; Jiang, Z.-D.; DuPont, H.; Jenq, R.; Wang, Y. S156 Microbiome Alteration via Fecal Microbiota Transplantation (FMT) Is Effective for Immune Checkpoint Inhibitor (ICI) Induced-Colitis (IMC) Refractory to Immunosuppressive Therapy. Am. J. Gastroenterol. 2021, 116, S68–S69. [Google Scholar] [CrossRef]
- Pokrovskaya, E.V.; Sklyanik, I.A.; Shestakova, E.A.; Shestakova, M.V. Prospects for the use of fecal microbiota transplantation in obese patients with Type 2 Diabetes Mellitus for weight loss and improvement of insulin sensitivity. Diabetes Mellit. 2021, 23, 541–547. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clement, K.; Nieuwdorp, M. Fecal Microbiota Transplantation: A Future Therapeutic Option for Obesity/Diabetes? Curr. Diab. Rep. 2019, 19, 51. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojarvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).