Stereotactic Body Radiation Therapy versus Surgical Resection for Stage I/II Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
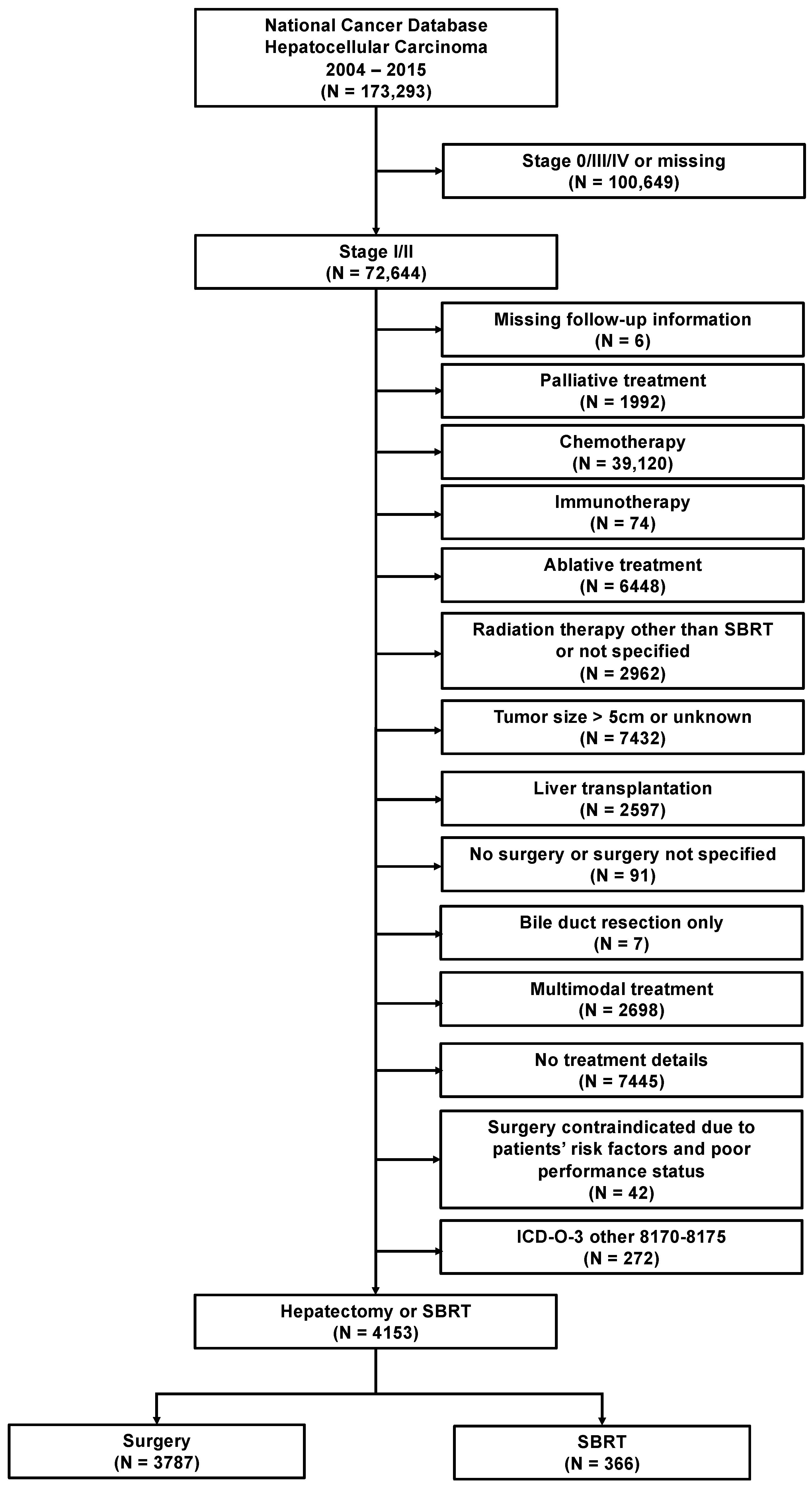
2. Materials and Methods
3. Results
3.1. Study Cohort Characteristics
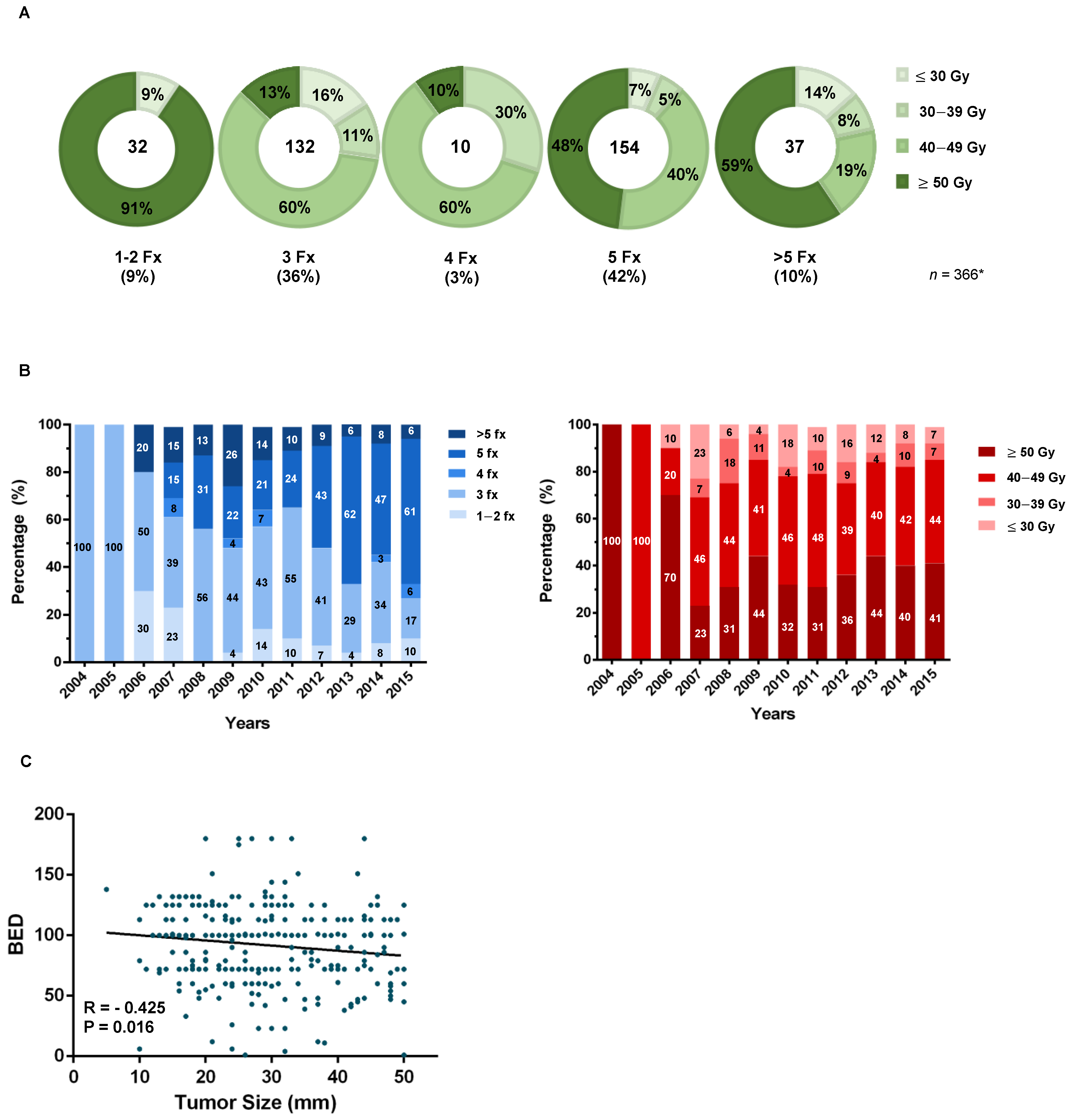
3.2. SBRT Dose Selection and Clinical Practice Patterns
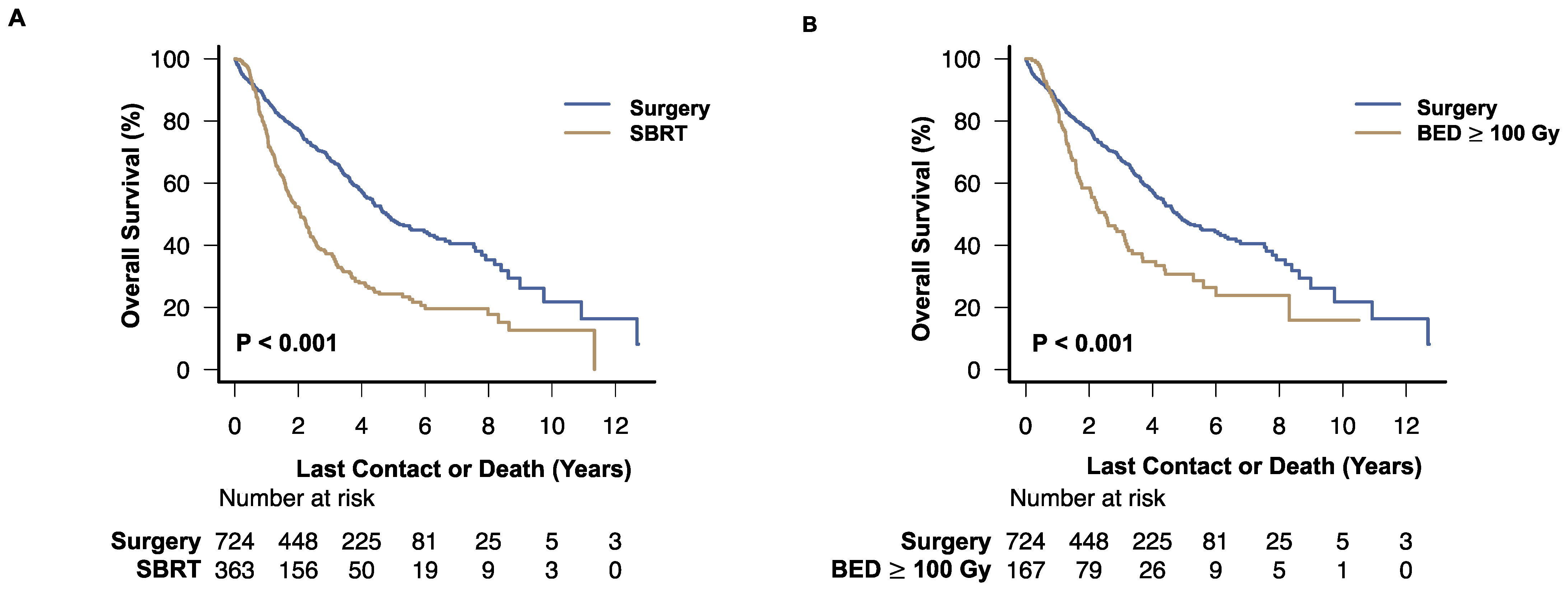
3.3. Survival Analysis
3.4. Effect of Treatment Details on Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lin, L.; Yan, L.; Liu, Y.; Qu, C.; Ni, J.; Li, H. The Burden and Trends of Primary Liver Cancer Caused by Specific Etiologies from 1990 to 2017 at the Global, Regional, National, Age, and Sex Level Results from the Global Burden of Disease Study 2017. Liver Cancer 2020, 9, 563–582. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Mehrabi, A.; Mollberg, N.M.; Muller, S.A.; Koch, M.; Buchler, M.W.; Weitz, J. Hepatocellular Carcinoma: Current Management and Perspectives for the Future. Ann. Surg. 2011, 253, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address, easloffice easloffice eu, and Liver European Association for the Study of the. “Easl Clinical Practice Guidelines: Management of Hepatocellular Carcinoma”. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Abrams, T.A.; Alberts, S.R.; Anaya, D.A.; Anders, R.; Are, C.; Brown, D.; Chang, D.T.; et al. Nccn Guidelines Insights: Hepatobiliary Cancers, Version 2.2019: Featured Updates to the Nccn Guidelines. J. Natl. Compr. Cancer Netw. 2019, 17, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.M.; Lawrence, T.S.; Dworzanin, L.M.; Andrews, J.C.; Walker, S.; Kessler, M.L.; DuRoss, D.J.; Ensminger, W.D. Treatment of Primary Hepatobiliary Cancers with Conformal Radiation Therapy and Regional Chemotherapy. J. Clin. Oncol. 1993, 11, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Birgin, E.; Rasbach, E.; Seyfried, S.; Rathmann, N.; Diehl, S.J.; Schoenberg, S.O.; Reissfelder, C.; Rahbari, N.N. Contralateral Liver Hypertrophy and Oncological Outcome Following Radioembolization with (90)Y-Microspheres: A Systematic Review. Cancers 2020, 12, 294. [Google Scholar] [CrossRef]
- Murray, L.; Dawson, L.A. Advances in Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Semin. Radiat. Oncol. 2017, 27, 247–255. [Google Scholar] [CrossRef]
- Mathew, A.S.; Atenafu, E.G.; Owen, D.; Maurino, C.; Brade, A.; Brierley, J.; Dinniwell, R.; Kim, J.; Cho, C.; Ringash, J.; et al. Long Term Outcomes of Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma without Macrovascular Invasion. Eur. J. Cancer 2020, 134, 41–51. [Google Scholar] [CrossRef]
- Long, Y.; Liang, Y.; Li, S.; Guo, J.; Wang, Y.; Luo, Y.; Wu, Y. Therapeutic Outcome and Related Predictors of Stereotactic Body Radiotherapy for Small Liver-Confined Hcc: A Systematic Review and Meta-Analysis of Observational Studies. Radiat. Oncol. 2021, 16, 68. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Q.; Hong, Z.X.; Li, W.G.; He, W.P.; Zhang, T.; Zhang, A.M.; Fan, Y.Z.; Sun, Y.Z.; Zheng, L.; et al. Stereotactic Body Radiotherapy Versus Hepatic Resection for Hepatocellular Carcinoma (</= 5 cm): A Propensity Score Analysis. Hepatol. Int. 2020, 14, 788–797. [Google Scholar]
- Nakano, R.; Ohira, M.; Kobayashi, T.; Ide, K.; Tahara, H.; Kuroda, S.; Shimizu, S.; Kimura, T.; Nagata, Y.; Aikata, H.; et al. Hepatectomy Versus Stereotactic Body Radiotherapy for Primary Early Hepatocellular Carcinoma: A Propensity-Matched Analysis in a Single Institution. Surgery 2018, 164, 219–226. [Google Scholar] [CrossRef]
- Su, T.-S.; Liang, P.; Liang, J.; Lu, H.-Z.; Jiang, H.-Y.; Cheng, T.; Huang, Y.; Tang, Y.; Deng, X. Long-Term Survival Analysis of Stereotactic Ablative Radiotherapy Versus Liver Resection for Small Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 639–646. [Google Scholar] [CrossRef]
- Xu, X.-L.; Liu, X.-D.; Liang, M.; Luo, B.-M. Radiofrequency Ablation versus Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology 2018, 287, 461–472. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Y. Comparison of Percutaneous Microwave Ablation and Laparoscopic Resection in the Prognosis of Liver Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11665–11669. [Google Scholar]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. Bclc Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef]
- Winchester, D.P.; Stewart, A.K.; Phillips, J.L.; Ward, E.E. The National Cancer Data Base: Past, Present, and Future. Ann. Surg. Oncol. 2010, 17, 4–7. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Mayne, N.R.; Lin, B.K.; Darling, A.J.; Raman, V.; Patel, D.C.; Liou, D.Z.; D’amico, T.A.; Yang, C.-F.J. Stereotactic Body Radiotherapy Versus Delayed Surgery for Early-Stage Non-Small-Cell Lung Cancer. Ann. Surg. 2020, 272, 925–929. [Google Scholar] [CrossRef]
- Fowler, J.F. 21 years of Biologically Effective Dose. Br. J. Radiol. 2010, 83, 554–568. [Google Scholar] [CrossRef]
- Robbins, J.R.; Schmid, R.K.; Hammad, A.Y.; Gamblin, T.C.; Erickson, B.A. Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Practice Patterns, Dose Selection and Factors Impacting Survival. Cancer Med. 2019, 8, 928–938. [Google Scholar] [CrossRef]
- Schaub, S.; Hartvigson, P.E.; Lock, M.; Høyer, M.; Brunner, T.B.; Cardenes, H.R.; Dawson, L.; Kim, E.Y.; Mayr, N.A.; Lo, S.S.; et al. Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Current Trends and Controversies. Technol. Cancer Res. Treat. 2018, 17, 1533033818790217. [Google Scholar] [CrossRef]
- Mokdad, A.A.; Minter, R.M.; Zhu, H.; Augustine, M.M.; Porembka, M.; Wang, S.; Yopp, A.C.; Mansour, J.C.; Choti, M.A.; Polanco, P.M. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J. Clin. Oncol. 2017, 35, 515–522. [Google Scholar] [CrossRef]
- Rajyaguru, D.J.; Borgert, A.J.; Smith, A.L.; Thomes, R.M.; Conway, P.D.; Halfdanarson, T.; Truty, M.J.; Kurup, A.N.; Go, R.S. Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Localized Hepatocellular Carcinoma in Nonsurgically Managed Patients: Analysis of the National Cancer Database. J. Clin. Oncol. 2018, 36, 600–608. [Google Scholar] [CrossRef]
- Austin, P.C. The Use of Propensity Score Methods with Survival or Time-to-Event Outcomes: Reporting Measures of Effect Similar to Those Used in Randomized Experiments. Stat. Med. 2013, 33, 1242–1258. [Google Scholar] [CrossRef]
- Yoon, S.M.; Kim, S.Y.; Lim, Y.S.; Kim, K.M.; Shim, J.H.; Lee, D.; An, J.; Jung, J.; Kim, J.H.; Lee, H.C. Stereotactic Body Radiation Therapy for Small (≤5 cm) Hepatocellular Carcinoma Not Amenable to Curative Treatment: Results of a Single-Arm, Phase Ii Clinical Trial. Clin. Mol. Hepatol. 2020, 26, 506–515. [Google Scholar] [CrossRef]
- Beaton, L.; Dunne, E.; Yeung, R.; Rackley, T.; Weber, B.; Mar, C.; Yong-Hing, C.; Yoshida, E.; DeVries, K.; Lee, R.; et al. Stereotactic Body Radiotherapy for Large Unresectable Hepatocellular Carcinomas—A Single Institution Phase II Study. Clin. Oncol. (R. Coll. Radiol.) 2020, 32, 423–432. [Google Scholar] [CrossRef]
- Durand-Labrunie, J.; Baumann, A.-S.; Ayav, A.; Laurent, V.; Boleslawski, E.; Cattan, S.; Bogart, E.; Le Deley, M.-C.; Steen, V.; Lacornerie, T.; et al. Curative Irradiation Treatment of Hepatocellular Carcinoma: A Multicenter Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 116–125. [Google Scholar] [CrossRef]
- Kimura, T.; Takeda, A.; Sanuki, N.; Ariyoshi, K.; Yamaguchi, T.; Imagumbai, T.; Katoh, N.; Eriguchi, T.; Oku, Y.; Ozawa, S.; et al. Multicenter Prospective Study of Stereotactic Body Radiotherapy for Previously Untreated Solitary Primary Hepatocellular Carcinoma: The Strsph Study. Hepatol. Res. 2020, 51, 461–471. [Google Scholar] [CrossRef]
- Lee, J.; Shin, I.-S.; Yoon, W.S.; Koom, W.S.; Rim, C.H. Comparisons between Radiofrequency Ablation and Stereotactic Body Radiotherapy for Liver Malignancies: Meta-Analyses and a Systematic Review. Radiother. Oncol. 2020, 145, 63–70. [Google Scholar] [CrossRef]
- Pan, Y.-X.; Fu, Y.-Z.; Hu, D.-D.; Long, Q.; Wang, J.-C.; Xi, M.; Liu, S.-L.; Xu, L.; Liu, M.-Z.; Chen, M.-S.; et al. Stereotactic Body Radiotherapy vs. Radiofrequency Ablation in the Treatment of Hepatocellular Carcinoma: A Meta-Analysis. Front. Oncol. 2020, 10, 1639. [Google Scholar] [CrossRef]
- Kim, N.; Cheng, J.; Jung, I.; Liang, J.; Shih, Y.L.; Huang, W.Y.; Kimura, T.; Lee, V.H.F.; Zeng, Z.C.; Zhenggan, R.; et al. Stereotactic Body Radiation Therapy Vs. Radiofrequency Ablation in Asian Patients with Hepatocellular Carcinoma. J. Hepatol. 2020, 73, 121–129. [Google Scholar] [CrossRef]
- Kim, N.; Kim, H.J.; Won, J.Y.; Kim, D.Y.; Han, K.-H.; Jung, I.; Seong, J. Retrospective Analysis of Stereotactic Body Radiation Therapy Efficacy over Radiofrequency Ablation for Hepatocellular Carcinoma. Radiother. Oncol. 2019, 131, 81–87. [Google Scholar] [CrossRef]
- Sanuki, N.; Takeda, A.; Oku, Y.; Mizuno, T.; Aoki, Y.; Eriguchi, T.; Iwabuchi, S.; Kunieda, E. Stereotactic Body Radiotherapy for Small Hepatocellular Carcinoma: A Retrospective Outcome Analysis in 185 Patients. Acta Oncol. 2013, 53, 399–404. [Google Scholar] [CrossRef]
- Wang, P.-M.; Hsu, W.-C.; Chung, N.-N.; Chang, F.-L.; Jang, C.-J.; Fogliata, A.; Scorsetti, M.; Cozzi, L. Feasibility of Stereotactic Body Radiation Therapy with Volumetric Modulated Arc Therapy and High Intensity Photon Beams for Hepatocellular Carcinoma Patients. Radiat. Oncol. 2014, 9, 18. [Google Scholar] [CrossRef]
- Jang, W.I.; Bae, S.H.; Kim, M.; Han, C.J.; Park, S.C.; Kim, S.B.; Cho, E.; Choi, C.W.; Kim, K.S.; Hwang, S.; et al. A Phase 2 Multicenter Study of Stereotactic Body Radiotherapy for Hepatocellular Carcinoma: Safety and Efficacy. Cancer 2019, 126, 363–372. [Google Scholar] [CrossRef]
- Takeda, A.; Sanuki, N.; Tsurugai, Y.; Iwabuchi, S.; Matsunaga, K.; Ebinuma, H.; Imajo, K.; Aoki, Y.; Saito, H.; Kunieda, E. Phase 2 Study of Stereotactic Body Radiotherapy and Optional Transarterial Chemoembolization for Solitary Hepatocellular Carcinoma Not Amenable to Resection and Radiofrequency Ablation. Cancer 2016, 122, 2041–2049. [Google Scholar] [CrossRef]
- Feng, M.; Suresh, K.; Schipper, M.J.; Bazzi, L.; Ben-Josef, E.; Matuszak, M.M.; Parikh, N.D.; Welling, T.H.; Normolle, D.; Haken, R.K.T.; et al. Individualized Adaptive Stereotactic Body Radiotherapy for Liver Tumors in Patients at High Risk for Liver Damage: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 40–47. [Google Scholar] [CrossRef]
- Su, T.-S.; Liu, Q.-H.; Zhu, X.-F.; Liang, P.; Liang, S.-X.; Lai, L.; Zhou, Y.; Huang, Y.; Cheng, T.; Li, L.-Q. Optimal Stereotactic Body Radiotherapy Dosage for Hepatocellular Carcinoma: A Multicenter Study. Radiat. Oncol. 2021, 16, 79. [Google Scholar] [CrossRef]
- Kuo, H.-T.; Que, J.; Lin, L.-C.; Yang, C.-C.; Koay, L.-B.; Lin, C.-H. Impact of Tumor Size on Outcome after Stereotactic Body Radiation Therapy for Inoperable Hepatocellular Carcinoma. Medicine 2017, 96, e9249. [Google Scholar] [CrossRef]
- Chi, A.; Fang, W.; Sun, Y.; Wen, S. Comparison of Long-Term Survival of Patients with Early-Stage Non-Small Cell Lung Cancer after Surgery Vs Stereotactic Body Radiotherapy. JAMA Netw. Open 2019, 2, e1915724. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, S.M.; Jiang, J.; Chang, J.Y.; Welsh, J.; Likhacheva, A.; Buchholz, T.A.; Swisher, S.G.; Smith, B.D. Lobectomy, Sublobar Resection, and Stereotactic Ablative Radiotherapy for Early-Stage Non-Small Cell Lung Cancers in the Elderly. JAMA Surg. 2014, 149, 1244–1253. [Google Scholar] [CrossRef]
- Birgin, E.; Kaslow, S.R.; Hetjens, S.; Correa-Gallego, C.; Rahbari, N.N. Minimally Invasive versus Open Liver Resection for Stage I/Ii Hepatocellular Carcinoma. Cancers 2021, 13, 4800. [Google Scholar] [CrossRef]
- Méndez Romero, A.; de Man, R.A. Stereotactic Body Radiation Therapy for Primary and Metastatic Liver Tumors: From Technological Evolution to Improved Patient Care. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 603–616. [Google Scholar] [CrossRef]
- Birgin, E.; Mehrabi, A.; Sturm, D.; Reißfelder, C.; Weitz, J.; Rahbari, N.N. Infrahepatic Inferior Vena Cava Clamping Does Not Increase the Risk of Pulmonary Embolism Following Hepatic Resection. World J. Surg. 2021, 45, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Birgin, E.; Tesfazgi, W.; Knoth, M.; Wilhelm, T.; Post, S.; Rückert, F. Evaluation of the New Isgls Definitions of Typical Posthepatectomy Complications. Scand. J. Surg. 2018, 108, 130–136. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Birgin, E.; Sturm, D.; Schwanebeck, U.; Weitz, J.; Reissfelder, C. Randomized Clinical Trial of Biofoam(R) Surgical Matrix to Achieve Hemostasis after Liver Resection. HPB (Oxford) 2020, 22, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T. Stereotactic Body Radiotherapy for Hepatocellular Carcinoma—Still Searching for a Role. J. Hepatol. 2020, 73, 15–16. [Google Scholar] [CrossRef]

| Unmatched Dataset | Matched Dataset | |||||
|---|---|---|---|---|---|---|
| SBRT | Surgery | SBRT | Surgery | |||
| Characteristics | n = 366 | n = 3787 | p | n = 363 | n = 724 | p |
| Age, years | <0.001 | 0.960 | ||||
| ≤49 | 10 (3) | 277 (7) | 10 (3) | 21 (3) | ||
| 50–59 | 92 (25) | 1035 (27) | 91 (25) | 173 (24) | ||
| 60–70 | 126 (34) | 1458 (39) | 125 (34) | 246 (34) | ||
| ≥71 | 138 (38) | 1017 (27) | 137 (38) | 284 (39) | ||
| Sex | 0.077 | 0.945 | ||||
| Male | 249 (68) | 2742 (72) | 248 (68) | 492 (68) | ||
| Female | 117 (32) | 1045 (28) | 115 (32) | 232 (32) | ||
| Race | <0.001 | 0.478 | ||||
| White | 340 (93) | 3099 (82) | 337 (93) | 685 (95) | ||
| Black | 20 (6) | 647 (17) | 20 (5) | 31 (4) | ||
| Other/Unknown | 6 (1) | 41 (1) | 6 (2) | 8 (1) | ||
| Comorbidity Index | <0.001 | 0.970 | ||||
| 0 | 253 (69) | 2179 (58) | 251 (69) | 489 (67) | ||
| 1 | 40 (11) | 990 (26) | 40 (11) | 84 (12) | ||
| 2 | 20 (6) | 305 (8) | 20 (6) | 43 (6) | ||
| 3 | 53 (14) | 313 (8) | 52 (14) | 108 (15) | ||
| Era of Diagnosis | <0.001 | 0.398 | ||||
| 2004–2009 | 69 (19) | 1081 (28) | 69 (19) | 122 (17) | ||
| 2010–2015 | 297 (81) | 2706 (72) | 294 (81) | 602 (83) | ||
| Facility Type | 0.007 | 0.967 | ||||
| Community | 8 (2) | 83 (2) | 8 (2) | 13 (2) | ||
| Comprehensive Community | 66 (18) | 702 (18) | 66 (18) | 133 (18) | ||
| Academic/research | 235 (64) | 2523 (67) | 233 (64) | 467 (65) | ||
| Integrated network | 57 (16) | 418 (11) | 56 (16) | 111 (15) | ||
| Missing | 0 | 61 (2) | 0 | 0 | ||
| Distance to Facility | 0.082 | 0.902 | ||||
| <12.5 miles | 166 (45) | 1832 (48) | 166 (46) | 342 (47) | ||
| 12.5–50 miles | 114 (31) | 1146 (30) | 113 (31) | 213 (29) | ||
| 50–250 miles | 83 (22) | 723 (19) | 81 (22) | 164 (23) | ||
| >250 miles | 2 (1) | 79 (2) | 2 (1) | 2 (1) | ||
| Missing | 1 (1) | 7 (1) | 1 (1) | 3 (1) | ||
| High School Education | <0.001 | 0.939 | ||||
| ≥29% | 72 (20) | 1043 (28) | 72 (20) | 155 (21) | ||
| 20–29% | 102 (28) | 918 (24) | 102 (28) | 188 (26) | ||
| 14–20% | 125 (34) | 987 (26) | 122 (34) | 250 (35) | ||
| <14% | 62 (17) | 790 (21) | 62 (17) | 121 (17) | ||
| Missing | 5 (1) | 49 (1) | 5 (1) | 10 (1) | ||
| Residence Area | 0.135 | 0.997 | ||||
| Metro | 305 (83) | 3263 (86) | 303 (84) | 601 (83) | ||
| Urban | 49 (13) | 371 (10) | 48 (13) | 96 (13) | ||
| Rural | 2 (1) | 47 (1) | 2 (1) | 5 (1) | ||
| Missing | 10 (3) | 106 (3) | 10 (3) | 22 (3) | ||
| Insurance | <0.001 | 0.991 | ||||
| Not insured | 9 (3) | 122 (3) | 9 (3) | 22 (3) | ||
| Private | 91 (25) | 1378 (36) | 91 (25) | 178 (25) | ||
| Medicaid | 39 (11) | 452 (12) | 39 (11) | 78 (11) | ||
| Medicare | 220 (60) | 1725 (46) | 217 (60) | 431 (60) | ||
| Other | 6 (2) | 59 (2) | 6 (2) | 14 (2) | ||
| Unknown | 1 (1) | 51 (1) | 1 (1) | 1 (1) | ||
| Median Household Income, USD | 0.001 | 0.997 | ||||
| <40,227 | 77 (21) | 813 (22) | 77 (21) | 151 (21) | ||
| 40,227–50,353 | 105 (29) | 791 (21) | 104 (29) | 214 (30) | ||
| 50,354–63,332 | 91 (25) | 890 (24) | 90 (25) | 180 (25) | ||
| >63,333 | 87 (24) | 1243 (33) | 87 (24) | 168 (23) | ||
| Missing | 6 (1) | 50 (1) | 5 (1) | 11 (1) | ||
| Tumor Stage | 0.018 | 0.230 | ||||
| Stage I | 250 (68) | 2809 (74) | 248 (68) | 521 (72) | ||
| Stage II | 116 (32) | 978 (26) | 115 (32) | 203 (28) | ||
| Tumor Size, cm | 0.002 | 0.666 | ||||
| <2 | 93 (25) | 769 (20) | 91 (25) | 173 (24) | ||
| 2–3 | 129 (35) | 1136 (30) | 128 (35) | 236 (33) | ||
| 3–4 | 81 (22) | 1032 (27) | 81 (22) | 182 (25) | ||
| 4–5 | 63 (17) | 850 (22) | 63 (17) | 133 (18) | ||
| Surgery | - | - | ||||
| Segmentectomy | 2734 (72) | 535 (74) | ||||
| Lobectomy | 896 (24) | 162 (22) | ||||
| Hepatectomy (NOS) | 157 (4) | 27 (4) | ||||
| Adjusted HR a | 95% CI | ||
|---|---|---|---|
| Variable | p | ||
| Treatment | |||
| SBRT | Reference | ||
| Surgery | 0.44 | 0.37–0.53 | <0.001 |
| Tumor Stage | |||
| Stage I | Reference | ||
| Stage II | 1.76 | 1.47–2.11 | <0.001 |
| Tumor Size, cm | |||
| <2 | Reference | ||
| 2–3 | 1.40 | 1.09–1.79 | 0.008 |
| 3–4 | 1.45 | 1.11–1.88 | 0.006 |
| 4–5 | 1.65 | 1.25–2.18 | 0.001 |
| Grading | |||
| Well differentiated | Reference | ||
| Moderately differentiated | 0.84 | 0.66–1.09 | 0.191 |
| Poorly differentiated | 1.53 | 1.14–2.12 | 0.005 |
| Undifferentiated | 1.16 | 0.36–3.72 | 0.796 |
| Not determined | 1.83 | 1.43–2.34 | <0.001 |
| BED b | |||
| <100 Gray | Reference | ||
| ≥100 Gray | 0.58 | 0.43–0.77 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birgin, E.; Hetjens, S.; Tam, M.; Correa-Gallego, C.; Rahbari, N.N. Stereotactic Body Radiation Therapy versus Surgical Resection for Stage I/II Hepatocellular Carcinoma. Cancers 2023, 15, 2330. https://doi.org/10.3390/cancers15082330
Birgin E, Hetjens S, Tam M, Correa-Gallego C, Rahbari NN. Stereotactic Body Radiation Therapy versus Surgical Resection for Stage I/II Hepatocellular Carcinoma. Cancers. 2023; 15(8):2330. https://doi.org/10.3390/cancers15082330
Chicago/Turabian StyleBirgin, Emrullah, Svetlana Hetjens, Moses Tam, Camilo Correa-Gallego, and Nuh N. Rahbari. 2023. "Stereotactic Body Radiation Therapy versus Surgical Resection for Stage I/II Hepatocellular Carcinoma" Cancers 15, no. 8: 2330. https://doi.org/10.3390/cancers15082330
APA StyleBirgin, E., Hetjens, S., Tam, M., Correa-Gallego, C., & Rahbari, N. N. (2023). Stereotactic Body Radiation Therapy versus Surgical Resection for Stage I/II Hepatocellular Carcinoma. Cancers, 15(8), 2330. https://doi.org/10.3390/cancers15082330





